Probiotic self-coated with biofilms achieve strikingly enhanced oral availability and intestinal colonization in a porcine model.
Abstract
Transplanting beneficial bacteria to the gut microbiome can positively modulate the bacterial composition and remains of great interest in prevention and treatment. However, environmental assaults and rapid transit times in the gastrointestinal (GI) tract result in low oral bioavailability and limited intestinal colonization. Here, we describe a bioinspired strategy of self-coating with biofilms that endows the transplanted gut microbiota with superior resistance and adhesion capacity. Using clinical Bacillus subtilis as a model probiotic bacterium, biofilm-coated probiotics demonstrate substantially improved GI tract tolerance and mucoadhesion in mice and swine. In particular, coated probiotics exhibit a 125-fold higher oral bioavailability and a 17 times greater intestinal colonization than uncoated bacteria in the porcine model. With notable ability to survive and reside in the GI tract, coated bacteria further show a significantly enhanced decolonization effect in mice colonized with Staphylococcus aureus. Self-coating with biofilms suggests a robust platform for oral doses of gut microbiota.
INTRODUCTION
The gut microbiota, a reservoir for a large community of microorganisms, plays an increasingly essential role in human health (1). Disorders of the gut microbiota cause various diseases by either direct pathogen invasion or metabolism-mediated interference (2). For instance, bacterial translocation and lack of intestinal commensal bacteria can damage intestinal epithelial barrier by toxins and cause severe pathogen infections (3). In addition, a multitude of intractable diseases, such as Alzheimer’s disease, diabetes, and some cancers, has proven to be associated with the metabolism of intestinal bacteria (4–6). With the ability to inhibit pathogen colonization and exert beneficial effects, probiotic supplement is an effective strategy to positively modulate the balance of the gut microbiome (7, 8). While fecal microbiota transplantation has been successful in prevention and treatment, the implementation has been largely restricted by invasive operation and indeterminate composition, which inevitably lead to low patient compliance as well as gastrointestinal (GI) irritation and potential complication (9, 10). As a noninvasive method, oral delivery of probiotic species to the gut microbiome is of pronounced interest and can provide an alternative to overcome these limitations (11, 12). Unfortunately, environmental complexity and a continuous flow within the GI tract render low oral bioavailability and limited intestinal colonization. Previously, bacteria with increased resistance have been engineered to improve stomach survival (13). Instead of focusing on genetic engineering, surface modification endows an effective approach to decorate bacteria with functional motifs (14–16). More recently, we have encapsulated probiotics with protective coatings to tune bacterial behaviors (17, 18). However, these attempts only succeed in protecting bacteria from environmental insults. Methods capable of simultaneously withstanding the GI tract stressors and slowing bowel transit would be of great use for oral delivery of gut microbiota but remain extremely challenging.
In nature, to survive in different extreme conditions, bacteria produce biofilms to combat physical threats such as displacement by physical forces and removal by environmental attacks (Fig. 1A) (19). Biofilms not only act as an adhesive that attaches the colony to a surface and prevents removal by a flowing fluid but also defend against external threats such as antibiotics and the host immune system by preventing penetration (20, 21). Inspired by the dual physical adhesion and chemical barrier functions of biofilms, we speculate that gut microbiota wrapped up with an extra biofilm coating might be able to markedly promote resistance and adherence in the GI tract. Here, we report that self-coating with biofilms endows the transplanted gut microbiota with superior oral bioavailability and intestinal colonization. Different from previous encapsulation that only provides a temporary coating before bacterial division, self-produced biofilms enable the bacteria to be coated during growth, which can provide a long-lasting effect for protection and mucoadhesion (Fig. 1B). During in vivo swine studies, clinical Bacillus subtilis (BS) probiotics self-coated with biofilms exhibit a 125-fold greater oral bioavailability and a 17 times higher intestinal colonization than uncoated bacteria. Coated probiotics also show a long-term yet substantially enhanced decolonization effect in a murine model of intestinal colonization with Staphylococcus aureus. Given the protective and mucoadhesive properties of the long-lasting coating, we anticipate the application of biofilm self-coated bacteria in a broad set of GI biomedical applications.
Fig. 1. Design and characterization of self-coating with biofilms.
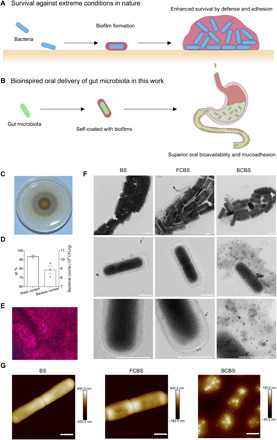
(A and B) Schematic illustration of (A) biofilm formation of bacteria in nature to enhance survival by defense and adhesion under extreme conditions and (B) bioinspired oral delivery of gut microbiota with superior oral bioavailability and mucoadhesion by self-coating with biofilms. (C) Typical digital photo of a BS biofilm formed on an MSgg plate after 48-hour incubation. (D) Contents of water loading and bacteria in the biofilms. Error bars represent SD (n = 3). (E) Representative microscopic image of Gram staining of the biofilm. (F and G) Typical (F) TEM and (G) AFM images of BS, FCBS, and BCBS. Scale bars, 1 μm. A drop of bacterial suspension was deposited onto a carbon-coated copper grid for TEM observation or a mica disc for AFM measurement. The samples were dried completely in air before observation.
RESULTS AND DISCUSSION
Preparation and characterization of bacteria self-coated with biofilms
For proof of principle, we chose BS, a useful and important gut probiotic, which works in concert with other beneficial GI bacteria to support digestion, enzyme production, and immune and digestive system health (22). BS supplementation is clinically applied to treat a variety of symptoms, such as general stomach discomfort, irregular bowel patterns, and pathogen infections (23). Particularly, BS can adapt between two mutually exclusive lifestyles: flagellum-mediated swimming motility and biofilm formation (24). Under a suitable condition, BS can secrete a large quantity of exopolysaccharide and proteins including TasA and BslA, collectively forming biofilms (25). The exopolysaccharide bundles the colony, while BslA forms a hydrophobic outer layer and TasA self-assembles into fibers that can anchor to the cell wall (26). By culturing on solid minimal salts glycerol glutamate (MSgg) plates, we found that BS formed a robust biofilm with a water loading content of 93.6 weight % (wt %) and a bacterial number of 8.9 × 109 colony-forming units (CFUs) per gram (Fig. 1, C and D). The MSgg plates acted as attachment points for the formation of the solid biofilm. Gram staining displayed that BS well embedded within biofilms (Fig. 1E). Individual biofilm-coated BS (BCBS) were prepared by homogenizing the films with phosphate-buffered saline (PBS). Given that the bacteria colonized inside biofilms, an entire coating could be formed once a macroscopic solid biofilm was produced. Transmission electron microscopy (TEM) images showed an entire coating with a thickness ranging from nanometers to micrometers on BCBS (Fig. 1F, right). Oppositely, culturing BS in regular lysogeny broth (LB) medium provoked the generation of bacteria without biofilms (Fig. 1F, left). BS were able to form an unconsolidated biofilm in a liquid MSgg medium, in which bacteria were coated with biofilm fragments (FCBS) (Fig. 1F, middle). Atom force microscopy (AFM) images further confirmed a consolidated biofilm for BCBS, as displayed by the unsharp edges and decreased heights of the bacteria in comparison with those of BS and FCBS (Fig. 1G). In addition, the zeta potential of the bacteria decreased by 3.4 ± 1.0 mV upon coating with biofilms (fig. S1).
Resistance of biofilm-coated bacteria against environmental assaults
The first challenge encountered following oral administration is to safely survive low pH balance in the early GI tract, which can deactivate probiotics (27). BCBS were incubated with simulated gastric fluid (SGF; pH 1.2) to assess the protective effect of the biofilm coating. Quantitative survival after incubation was determined by bacterial counting at different time points. BCBS (72.4%) survived after 0.5-hour culture in SGF (Fig. 2A). With cultivation time extended from 1 to 2 hours, the survival decreased from 39.6 to 6.3%. A small portion of the bacteria successfully survived even after a 4-hour exposure to SGF. By contrast, complete death was observed from both BS and FCBS only after 0.5 hour of incubation in SGF, indicating the significantly enhanced tolerance of BCBS against acidic stomach conditions. TEM images of BS and FCBS after exposure to SGF showed that stomach pH rapidly promoted the permeability of bacterial cell wall and subsequently deactivated the bacteria by diffusion (Fig. 2B and fig. S2A). Coating with biofilms could effectively protect BCBS from acidic insults, as intact coatings were observed even after 4-hour culture in SGF, suggesting that an entire coating was necessary to resist environmental threats. The resistance of coated bacteria against bile acids was further evaluated considering the fact that passage through the intestines involves an encounter with bile salts, which are recognized to solubilize lipids and thereby kill probiotics (28, 29). As shown in Fig. 2C, survival rates of both BS and FCBS markedly reduced to 1.7% following a short incubation of 1 hour in bile acids (0.3 mg/ml). Notably, the survival rate of BCBS was maintained consistently after exposure to bile salts, which stood at a high level of 28.2% even with incubation time that prolonged to 4 hours. TEM observation revealed that bile salts dissolved the lipid membranes and then destructed the bacteria (Fig. 2D and fig. S2B). Similarly, the biofilm coating could prevent the penetration of bile acids and retain the integrity of coated bacteria. In addition, coated bacteria were able to resist antibiotics, such as ampicillin (10 mg/ml) (fig. S2C). The protective effect might be derived from nonwetting nature of biofilms, which impeded the diffusion of toxic substances (30). The wettability was measured by contact angle goniometer. As expected, the tested biofilm exhibited a contact angle of 130°, verifying its high hydrophobicity (Fig. 2E). The result was in stark contrast with that of a regular polyacrylamide hydrogel with a similar water content, which showed a low contact angle of 30°. The large contact angle could be attributed to the presence of hydrophobic BslA, one of the main components that are essential for the formation of BS biofilms (25).
Fig. 2. Resistance of biofilm-coated bacteria against environmental assaults.
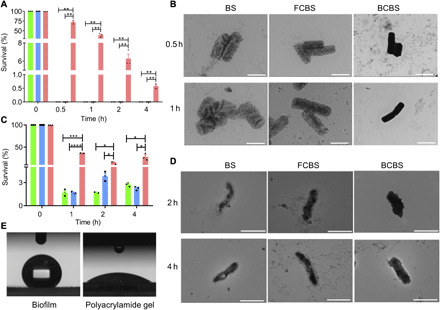
(A and C) Equal amounts of BS (circle), FCBS (square), and BCBS (triangle) were separately exposed to (A) SGF (pH 1.2, supplemented with pepsin) and (C) bile salts (0.3 mg/ml). After incubation at 37°C for the indicated time points, 50 μl of each sample was washed twice with PBS, spread onto LB agar plates, and incubated at 37°C for 24 hours before bacterial plate counting. Error bars represent SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test. (B and D) Typical TEM images of BS, FCBS, and BCBS after incubation in (B) SGF and (D) bile salts at 37°C for the indicated time points. Scale bars, 2 μm. A drop of bacterial suspension was deposited onto a carbon-coated copper grid. The samples were further rinsed with double-distilled H2O twice and subsequently dried completely in air before observation. (E) Contact angle of the biofilms. A polyacrylamide hydrogel with a similar water content was used as a control.
Mucoadhesion of self-coated bacteria in mouse intestines
To determine whether the biofilm coating could improve mucoadhesion, the number of BCBS that adhered to intestinal mucosal surface was quantified at different time intervals following oral administration of 1.0 × 107 CFUs of coated bacteria by gavage. Small intestine, large intestine, and cecum were separately harvested, and the number of BCBS that attached to the bowel wall was evaluated by plate counting. Markedly, a large number of BCBS attached to the small intestinal mucosa 4 hours after administration, which was 480 times higher than BS and FCBS (Fig. 3A). Very similar results were obtained from the large intestine and cecum, which were 69- and 191-fold greater than those of uncoated and partially coated bacteria, respectively (Fig. 3, B and C). The increased quantities of BCBS in the mucosae of the small intestine (P < 0.01), large intestine (P < 0.001), and cecum (P < 0.05) remained up to 120 hours. Gram staining further evidenced that self-coating with biofilms could concentrate the bacteria in the intestinal mucosae (Fig. 3D and fig. S3). On the contrary, negligible mucoadhesion was observed from BS and FCBS, verifying the extraordinary mucoadhesion capacity of BCBS. The emergence of the strengthened mucoadhesion was primarily attributed to the presence of TasA, a protein that assists the adhesion (31). In comparison with BS and FCBS, viability assay and TEM images validated that BCBS not only grew faster but also produced biofilms during growth in intestinal fluids (Fig. 3, E to G, and fig. S4). The continuous secretion of biofilms led to a long-lasting mucoadhesion of BCBS in the intestines. These results were in good agreement with a previous report, where biofilm-forming BS could persist in the mouse gut for a longer period than the strain without biofilms (32). Other than conventional probiotic encapsulation that only afforded a temporary coating, self-secreted biofilm coating could be retained during bacterial growth, which implied long-lasting effects on protection and mucoadhesion.
Fig. 3. Mucoadhesion of self-coated bacteria in mouse intestines.
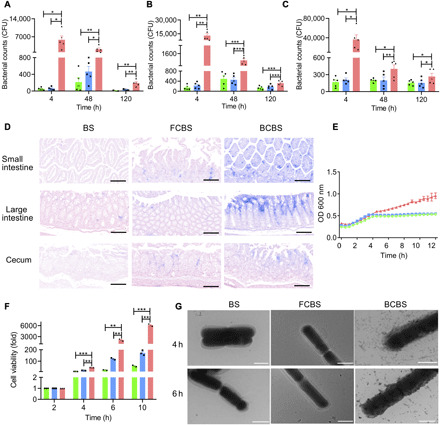
(A to C) Numbers of BS, FCBS, and BCBS that adhered to the (A) small intestine, (B) large intestine, and (C) cecum at 4, 48, and 120 hours after administration, respectively. Each mouse was fed with 1 × 107 CFUs of bacteria by oral gavage and then sacrificed at the indicated time points. Bacteria were quantified by plate counting. Error bars represent SD (n = 5). (D) Representative microscopic images of Gram staining of the intestinal tissues harvested from mice orally delivered with 1 × 107 CFUs of bacteria at 24 hours after administration. Scale bars, 100 μm. (E) Growth curves of bacteria cultured in SIF at 37°C, and OD600 was recorded at 30-min intervals using a microplate reader. (F) Bacterial viability in SIF (pH 6.8). Error bars represent SD (n = 3). (G) Typical TEM images of bacteria after culture in SIF at 37°C for the indicated time points. Scale bars, 1 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test. Circle, square, and triangle represent BS, FCBS, and BCBS, respectively.
Enhanced oral bioavailability in mice
The elevated resistance and mucoadhesion encouraged us to further evaluate the oral bioavailability of BCBS. Following oral gavage of 1.0 × 107 CFUs of coated bacteria, the numbers of BCBS in stomach, small intestine, large intestine, and cecum were calculated individually at different time points. Both uncoated and partially coated bacteria were used as controls. As given in Fig. 4 (A to D), the counts of BCBS in all these locations far exceeded those of BS and FCBS. Representatively, the amounts of BCBS in the large intestine and cecum were about 67 and 49 times higher than those of the control groups 4 hours after administration (Fig. 4, C and D). Even with the period prolonged up to 120 hours, the colonization of BCBS in the small intestine (P < 0.0001), large intestine (P < 0.001), and cecum (P < 0.05) was largely increased in comparison with those of the controls. The total BCBS colonized in the GI tract was further quantified to determine oral bioavailability, which were 68- and 36-fold higher than BS and FCBS 4 hours after gavage, respectively (Fig. 4E). The quantity of BCBS was threefold higher, with increasing time to 48 hours. Compared with BS (P < 0.0001) and FCBS (P < 0.001), the enhanced reservation of BCBS in the GI tract was maintained for up to 120 hours, testifying the greatly improved oral bioavailability. It is worth noting that no detectable side effect was associated with the oral administration of BCBS. As shown in Fig. 4F and fig. S5, hematoxylin and eosin (H&E) staining illuminated that no histological damage and morphology difference emerged in the intestinal tissues compared with the control groups at day 5 after administration. In addition, cytokine assays using commercially available enzyme-linked immunosorbent assay (ELISA) kits were conducted to measure the levels of cytokines in serum collected from mice administrated with BCBS. Similar to the control groups including PBS, BS, and FCBS, no inflammatory response was perceived, as confirmed by the comparable levels of inflammatory factors including interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor–α (TNF-α) (Fig. 4, G to I).
Fig. 4. Oral bioavailability and biosafety in mice.
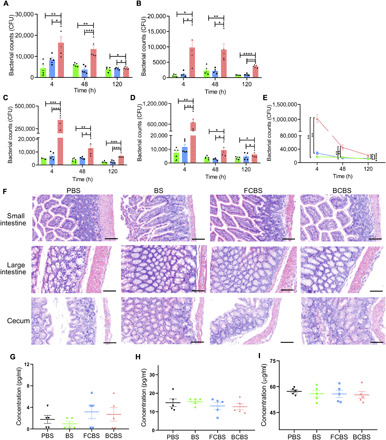
(A to D) Colonization of BS, FCBS, and BCBS in the (A) stomach, (B) small intestine, (C) large intestine, and (D) cecum at 4, 48, and 120 hours after administration, respectively. Each mouse was fed with 1 × 107 CFUs of bacteria by oral gavage and then sacrificed at the indicated time points for bacterial plate counting. (E) Total amounts of BS, FCBS, and BCBS retained in the GI tract. (F to I) Results of (F) H&E staining of the intestine and cytokine assays including (G) TNF-α, (H) IL-6, and (I) CRP in serum measured by commercially available ELISA kits. The samples were obtained from mice at 120 hours after administration of 1 × 107 CFUs of BS, FCBS, and BCBS. PBS was used as a control. Scale bars, 100 μm. Error bars represent SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test. Triangle (down), circle, square, and triangle (up) represent PBS, BS, FCBS, and BCBS, respectively.
Enhanced mucoadhesion and oral bioavailability in swine
We further evaluated the validity of self-coating with biofilms by means of a porcine model. Swine, which is arguably one of the most powerful models of human organ systems, in particular the GI tract, has provided important tools and a number of translational advantages (33). Here, both the oral bioavailability and intestinal colonization of BCBS were further assessed in swine. Bama miniature pigs with body weight around 20 kg were orally administrated with 3.5 × 108 CFUs of BCBS by gavage, and the intestines including duodenum, jejunum, ileum, cecum, colon, and rectum were harvested at day 2 after administration to examine the mucoadhesion of the bacteria. Gram staining showed that a great number of BS were attached to the intestinal mucosa after coating with biofilms, while very limited mucoadhesion was observed for both uncoated and partially coated bacteria (Fig. 5A). The notable difference was further manifested by bacterial counting, especially for the duodenum, jejunum, cecum, and rectum (Fig. 5B). To investigate the survival of BCBS, the excretion by stool was monitored for five consecutive days after oral gavage. The amount of BCBS was found to be as high as 2.6 × 106 CFUs/g at day 1 after administration, which was approximately 60-fold of the control groups (Fig. 5C). Notably, the counts further increased to 2071 and 73 times higher than those of BS and FCBS at day 2 after gavage, respectively. The raised quantities of BCBS in stool were maintained for 4 days (P < 0.01). To further study the retention of BCBS in the intestines, bacteria located in each part of the intestines were counted at day 2 after dosing. In accord with the contents of the stool, the numbers of BCBS in the duodenum, jejunum, colon, and rectum were significantly higher than those of the control groups (Fig. 5D). Overall colonization of BCBS reached 3.5 × 105 CFUs/g, while almost negligible bacterial colonization appeared to both BS and FCBS (Fig. 5E). The amplified availability in the porcine model further attested that self-coating with biofilms could endow orally delivered gut microbiota with superior oral bioavailability and intestinal colonization. H&E staining together with cytokine assays also ensured that no detectable inflammatory response was induced after the implementation of biofilm-coated bacteria (figs. S6 and S7).
Fig. 5. Mucoadhesion and oral bioavailability in swine.
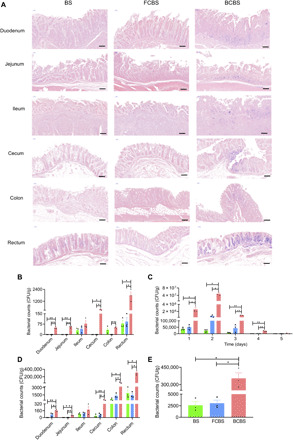
(A) Representative Gram staining images of BS, FCBS, and BCBS that adhered to the intestines. (B) Counts of the bacteria attached to the intestines. Scale bars, 200 μm. Each swine was fed with 3.5 × 108 CFUs of bacteria by oral gavage and then sacrificed at 48 hours after administration. After the removal of the inside contents, the intestinal tissues were sectioned for Gram staining and homogenization, respectively. The homogenized samples were serially diluted with PBS for bacterial counting. (C) Bacterial counts of BS, FCBS, and BCBS in feces. Feces were collected daily and resuspended in sterile PBS (1 g of fecal material in 2 ml of PBS) for counting. (D) Quantities of bacteria colonized in the intestines at 48 hours after administration. The intestinal tissues together with the inside contents were homogenized, and the bacterial numbers were similarly counted. (E) Total amounts of the bacteria retained in the GI tract. Error bars represent SD (n = 3). *P < 0.05, **P < 0.01, Student’s t test. Circle, square, and triangle represent BS, FCBS, and BCBS, respectively.
Treatment of intestinal colonization of S. aureus
Having confirmed the resistance and adhesion behaviors of coated bacteria, we turned our attention to further evaluate whether they could be exploited as enhanced therapeutics for treatment. S. aureus, a widespread and dangerous human pathogen, can colonize in the intestinal tract and cause a variety of infectious diseases (34). Unfortunately, the treatment of S. aureus infections is severely impeded by antibiotic resistance and lack of working vaccine (35). The fengycins, a class of lipopeptides secreted by BS, have been recently reported to be able to exclude intestinal colonization of S. aureus (36). We tested the potency of BCBS on the inhibition of S. aureus growth by an in vitro competitive experiment. In contrast to BS and FCBS, BCBS exhibited the highest potency of inhibition, which could be explained by the fastest growth in simulated intestinal fluid (SIF) (Fig. 6A). The reinforced inhibition was further validated by coculturing S. aureus with BCBS in bile salts supplemented SIF, a condition more relevant to in vivo intestinal fluids (Fig. 6B). We then investigated the potential of BCBS to exclude S. aureus in a murine model of intestinal colonization (Fig. 6C). The colonization was developed by oral gavage of 1.0 × 109 CFUs of S. aureus (fig. S8). Mice were treated with 1.0 × 107 CFUs of BCBS 24 hours after infection. BS and FCBS were dosed as controls. The excluded S. aureus in feces were enumerated at the indicated time points. As expected, the number of S. aureus declined markedly following the treatment with BCBS, showing a 10 times higher reduction than mice treated with BS and FCBS at day 2 after treatment (Fig. 6D). With time extended to 6 days after treatment, the count further descended to 684 times lower than those of the controls. The enhanced inhibition was further verified by the limited colonization of S. aureus in the intestines, including the small intestine, cecum, and large intestine (Fig. 6, E to H, and fig. S9). The robust yet long-term inhibition against S. aureus was ascribed to the adequate reservation of BCBS in the intestines (fig. S10, A to C). Correspondingly, the translocation of S. aureus was retarded after the treatment with BCBS, as suggested by the lessened blood invasion (fig. S10D). The enhanced elimination of intestinal colonization of S. aureus also indicated the limited influence of the coating on the benefits of BS, suggesting a specific transportation of active substances across biofilms.
Fig. 6. Treatment of intestinal colonization of S. aureus.
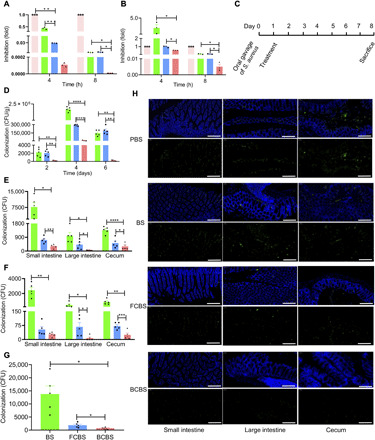
(A and B) In vitro inhibition efficiencies of BS, FCBS, and BCBS against S. aureus in (A) SIF and (B) SIF supplemented with bile salts (0.3 mg/ml). S. aureus were co-incubated with the bacteria at a ratio of 1:25. (C) Experimental design for treating intestinal colonization of S. aureus. Mice were fed with 1 × 109 CFUs of S. aureus at day 0 by gavage and then treated with 1 × 107 CFUs of the bacteria at day 1. (D) Bacterial counts of S. aureus in feces. At days 2, 4, and 6 after treatment, feces were collected and resuspended in PBS (0.1 g of fecal material in 1 ml of PBS) for counting. (E and F) S. aureus colonized in the intestinal (E) contents and (F) mucosa 7 days after treatment. (G) Total amounts of S. aureus colonized in the intestinal tract. (H) Typical microscopic images of S. aureus colonized in the bowel walls 7 days after treatment. Green fluorescence represents the labeled S. aureus. Scale bars, 200 μm. Error bars represent SD (n = 3 to 5). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test. Circle, square, and triangle represent BS, FCBS, and BCBS, respectively.
In summary, we have described a bioinspired strategy of self-coating with biofilms for oral delivery of gut microbiota, with aims to overcome environmental assaults and rapid transit times in the GI tract. During in vivo studies in mice and swine, self-coated probiotic bacteria demonstrate extraordinary GI tract tolerance and mucoadhesion, which can substantially improve oral bioavailability and intestinal colonization. The characteristic of self-producing, coated bacteria can generate a long-term beneficial effect for the treatment of GI infections. The majority of bacteria, such as probiotic Streptococcus thermophilus and Lactobacillus bulgaricus, can form biofilms under appropriate conditions (37). Meanwhile, many probiotics have been genetically programmed to self-produce specific biofilms, suggesting that coating with biofilms is a versatile strategy for oral delivery of gut microbiota. With the ability to survive and reside in a complicated environment, we anticipate the use of probiotics self-coated with biofilms to treat other diseases, such as respiratory and reproductive tract infections.
MATERIALS AND METHODS
Materials and strains
S. aureus was purchased from China General Microbiological Culture Collection Center (CGMCC). BS was purchased from Hanmi Pharmaceutical Co. Ltd. All other reagents were purchased from domestic suppliers and used as received.
Preparation of BS biofilms
The extracted BS was grown on solid LB plates. Seed colonies were first cultured in 4 ml of LB medium overnight at 37°C to obtain BS without biofilms. Then, 100 μl of seed culture medium was resuspended and grown in 4 ml of MSgg medium overnight at 37°C to generate FCBS. Cell pellets collected from the FCBS suspension were resuspended in 1 ml of PBS, and 10 μl of the resulted solution was spread on solid MSgg plates. Robust BS biofilms were produced after 2 days of culture at 30°C. Individually coated bacteria BCBS were prepared by homogenizing the films with PBS.
Physical characterization
Water loading was measured by calculating the weight changes of the biofilms after 24-hour dehydration at 65°C. To determine bacterial loading, a certain amount of the biofilm was dispersed in PBS and incubated overnight at 37°C for bacterial plate counting. A drop of Gram stain solution was deposited onto a biofilm to label the bacteria for optical microscope observation (H550S, Nikon, Japan) to observe bacteria embedded in the biofilms. TEM (Talos L120C G2, Thermo Fisher Scientific, USA) was used to visualize bacteria coated with biofilms. A carbon-coated copper grid was applied as a carrier for 10-μl bacterial suspension, which was rinsed by double-distilled H2O and air-dried before observation. The surface morphology of the bacteria was observed by AFM (Dimension FastScan Bio, Bruker, USA). A drop of bacterial suspension was deposited onto a mica disc and air-dried before observation. Contact angle was analyzed with a contact angle analyzer (DSA100, KRÜSS, Germany). A solid biofilm (~1 cm2) was placed onto a glass slide for analysis.
Resistance assessment in vitro
BS, FCBS, or BCBS (3.8 × 105 CFUs) were resuspended into 1 ml of SGF (pH 1.2, per 1000 ml of water containing 0.2 g of sodium chloride, 0.32 g of pepsin, and 700 μl of hydrochloric acid) and further incubated at 37°C with gentle shaking. At predetermined time points, 50-μl solution was withdrawn from the medium and washed with PBS. After spreading on solid LB plates, the bacteria were incubated overnight at 37°C for bacterial counting. Simultaneously, 10-μl bacteria suspension was withdrawn at each time point for TEM observation (Hitachi, Japan). Similarly, 6 × 106 CFUs of BS, FCBS, and BCBS were separately resuspended into 1 ml of culture medium supplemented with bile salts (0.3 mg/ml) or ampicillin (10 mg/ml) for resistance assessment.
Viability assay
BS, FCBS, or BCBS (3.8 × 105 CFUs) were cultured in 1 ml of SIF (pH 6.8, per 1000 ml of water containing 0.2 M sodium hydroxide solution, 6.8 g of potassium dihydrogen phosphate, and 10 g of trypsin) at 37°C with gentle shaking. A series of 50-μl droplets were withdrawn at 2, 4, 6, and 10 hours, which were spread on solid LB plates and further incubated at 37°C overnight for bacterial counting. Similarly, the bacteria were sampled at each time point for TEM observation. Growth curves of the bacteria cultured in SIF were further recorded. SIF medium (200 μl) added to 7.6 × 104 CFUs of BS, FCBS, or BCBS was placed in a 96-well plate. The optical density value at 600 nm (OD600) was recorded at 0.5-hour intervals with a microplate reader (HIMF, BioTek, USA).
Animal studies
All the animal procedures complied with the guidelines of the Shanghai Medical Experimental Animal Care. Animal protocols were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
Intestinal adhesion and oral bioavailability in mice
Female mice, which are widely used as experimental animal models, were chosen considering the less aggression. Six-week-old female ICR mice (five mice per group) were fed with 100 μl of PBS containing 1 × 107 CFUs of BCBS by oral gavage. Both BS and FCBS were used as controls. At predetermined time points, the mice were sacrificed and the intestinal tracts were harvested. To evaluate the mucoadhesion and colonization of the bacteria, the intestinal tract tissues including stomach, small intestine, large intestine, and cecum as well as their inside contents were separately collected. The tissues were grinded and diluted with 1 ml of PBS. Meanwhile, the contents of the stomach, small intestine, large intestine, and cecum were soaked in 1 ml of PBS for 2 hours. The suspensions (50 μl) from both the tissues and the contents were withdrawn and spread on solid LB plates. The bacteria were incubated overnight at 37°C before counting. The mucoadhesion of the bacteria was further observed with a three-dimensional slice scanner (Pannoramic MIDI, 3DHISTECH, Hungary). The tissue samples were infiltrated in universal tissue fixation fluid containing 4% paraformaldehyde (1 ml per sample) for at least 24 hours and processed to longitudinal sections. The samples were embedded and subsequently subjected to Gram staining.
Intestinal adhesion and oral bioavailability in swine
Bama miniature pigs with a body weight of around 20 kg and with no gender limitations were used to assess the intestinal adhesion and oral bioavailability of the bacteria in swine. Swine (three animals per group) were orally delivered with 3.5 × 108 CFUs of BCBS by gavage. Both BS and FCBS were used as controls. The feces were collected each day in the following 5 days to trace the retention of the bacteria in vivo. In detail, 1 g of stool was soaked in 2 ml of PBS for 2 hours and then 50 μl of each PBS solution was spread on solid LB plates. The bacteria were further cultured at 37°C overnight for plate counting. To determine the mucoadhesion and the GI tract colonization of the bacteria, the swine were sacrificed 2 days after administration, and the intestines including duodenum, jejunum, ileum, cecum, colon, and rectum were harvested. One gram of the intestinal tissues as well as their inside contents were separately collected, immersed in 2 ml of PBS, and incubated for 24 hours. Afterward, 50 μl of each soaking solution was spread on solid LB plates and incubated at 37°C overnight before bacterial counting. To directly observe the attachment of the bacteria to the bowel walls, the collected tissue samples were infiltrated in universal tissue fixation fluid containing 4% paraformaldehyde (1 ml per sample) for at least 24 hours and then subjected to Gram staining.
Biosafety assessment
At day 5 after administration of 1 × 107 CFUs of BCBS, the treated mice were dissected, and the blood and intestinal tract tissues were sampled. Both uncoated and partially coated bacteria were used as controls. The samples were immersed in universal tissue fixation fluid containing 4% paraformaldehyde (1 ml per sample) for more than 24 hours followed by H&E staining. Serum (200 μl) was separated from the blood samples by centrifugation at 3000 rpm for 10 min. Cytokine assays were conducted with the related ELISA kits including IL-6, CRP, and TNF-α. To assess the biosafety of the bacteria in swine, the corresponding blood and intestinal tissue samples were collected at day 8 after administration. Both H&E staining and cytokine assays were performed similarly to those of the mice.
In vitro bacterial competition
Mixtures of 6 × 108 CFUs of BCBS and 2.4 × 107 CFUs of S. aureus (25:1) were cocultured at 37°C in 1 ml of SIF or SIF supplemented with bile salts (0.3 mg/ml). At predetermined time points, 50-μl suspension was rinsed by PBS, spread on solid LB plates, and incubated at 37°C overnight for bacterial counting.
Mouse intestinal colonization of S. aureus model
Six-week-old female mice including C57BL/6 and BALB/c were used to study the intestinal colonization of S. aureus. At days 2, 4, and 6 after administration of 1 × 109 CFUs of S. aureus, 0.1 g of stool was collected and soaked in 1 ml of PBS for 2 hours. Then, 50 μl of each suspension was spread on solid LB plates and incubated at 37°C overnight for bacterial calculating. At day 7 after administration, mice were sacrificed and the intestinal tissues were harvested. The tissues were grinded and diluted with 4 ml of PBS. Fifty microliters of each suspension was spread on solid LB plates and incubated at 37°C overnight for counting.
Treatment of intestinal colonization of S. aureus
Six-week-old female C57BL/6 mice were inoculated by oral gavage with 1 × 109 CFUs of S. aureus. CFUs (1 × 107) of BCBS were administered intragastrically for treatment on the following day. Both BS and FCBS were used as controls. At days 2, 4, and 6 after treatment, 0.1 g of stool was collected, diluted in 1 ml of PBS, and soaked for 2 hours. Fifty microliters of each suspension was smeared on solid LB plates and incubated at 37°C overnight for counting. At day 7 after treatment, the mice were sacrificed for harvesting the GI tract tissues and collecting blood samples. Intestinal tissues including small intestine, large intestine, and cecum were processed for fluorescence staining. 4′,6-Diamidino-2-phenylindole was applied to stain the intestinal epithelial cells, and SAU Probe was used to label the colonized S. aureus. In addition, 50 μl of each blood sample was spread on solid LB plates and cultured at 37°C overnight to calculate S. aureus translocation.
Supplementary Material
Acknowledgments
We thank the Institute of Molecular Medicine, Renji Hospital for instrumental support. Funding: This work was financially supported by the National Natural Science Foundation of China (21875135), the Recruitment Program of Global Youth Experts of China (D1410022), the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20181704), and the innovative research team of high-level local universities in Shanghai (SSMU-ZLCX20180701). Author contributions: J.L. conceived and designed the experiments. X.W., Z.C., M.Z., L.M., and Z.M. performed all experiments. All authors analyzed and discussed the data. X.W. and J.L. wrote the paper. Competing interests: X.W., Z.C., and J.L. are co-inventors on patent application number 201911034435.7 describing biofilm-coated bacteria, which was filed on 29 October 2019. The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eabb1952/DC1
REFERENCES AND NOTES
- 1.Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I., Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Dethlefsen L., McFall-Ngai M., Relman D. A., An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449, 811–818 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffie C. G., Pamer E. G., Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knip M., Siljander H., The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 12, 154–167 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Fung T. C., Olson C. A., Hsiao E. Y., Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z., Tang H., Chen P., Xie H., Tao Y., Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 4, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bárcena C., Valdés-Mas R., Mayoral P., Garabaya C., Durand S., Rodríguez F., Fernández-García M. T., Salazar N., Nogacka A. M., Garatachea N., Bossut N., Aprahamian F., Lucia A., Kroemer G., Freije J. M. P., Quirós P. M., López-Otín C., Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Gadir A., Stephen-Victor E., Gerber G. K., Noval Rivas M., Wang S., Harb H., Wang L., Li N., Crestani E., Spielman S., Secor W., Biehl H., DiBenedetto N., Dong X., Umetsu D. T., Bry L., Rachid R., Chatila T. A., Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 25, 1164–1174 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pamer E. G., Fecal microbiota transplantation: Effectiveness, complexities, and lingering concerns. Mucosal Immunol. 7, 210–214 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Cammarota G., Ianiro G., Kelly C. R., Mullish B. H., Allegretti J. R., Kassam Z., Putignani L., Fischer M., Keller J. J., Costello S. P., Sokol H., Kump P., Satokari R., Kahn S. A., Kao D., Arkkila P., Kuijper E. J., Vehreschild M. J. G. T., Pintus C., Lopetuso L., Masucci L., Scaldaferri F., Terveer E. M., Nieuwdorp M., López-Sanromán A., Kupcinskas J., Hart A., Tilg H., Gasbarrini A., International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 68, 2111–2121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anselmo A. C., Gokarn Y., Mitragotri S., Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 18, 19–40 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Anselmo A. C., McHugh K. J., Webster J., Langer R., Jaklenec A., Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 28, 9486–9490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay R., Ghodasra M., Schardt J., Quan D., Pottash A. E., Shang W., Jay S. M., Payne G. F., Chang M. W., March J. C., Bentley W. E., A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics: Toward applications for Crohn’s disease. Bioeng. Transl. Med. 3, 209–221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W., Wang Y., Qin M., Zhang X., Zhang Z., Sun X., Gu Z., Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy. ACS Nano 12, 5995–6005 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Zheng D.-W., Chen Y., Li Z.-H., Xu L., Li C.-X., Li B., Fan J.-X., Cheng S.-X., Zhang X.-Z., Optically-controlled bacterial metabolite for cancer therapy. Nat. Commun. 9, 1680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F., Zang Z., Chen Z., Cui L., Chang Z., Ma A., Yin T., Liang R., Han Y., Wu Z., Zheng M., Liu C., Cai L., Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 214, 119226 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Cao Z., Wang X., Pang Y., Cheng S., Liu J., Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 10, 5783 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z., Cheng S., Wang X., Pang Y., Liu J., Camouflaging bacteria by wrapping with cell membranes. Nat. Commun. 10, 3452 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tallawi M., Opitz M., Lieleg O., Modulation of the mechanical properties of bacterial biofilms in response to environmental challenges. Biomater. Sci. 5, 887–900 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Flemming H. C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S., Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Yan J., Bassler B. L., Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 26, 15–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong H. A., Duc le H., Cutting S. M., The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29, 813–835 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Kong W., Huang C., Tang Y., Zhang D., Wu Z., Chen X., Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella). Sci. Rep. 7, 1588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kampf J., Gerwig J., Kruse K., Cleverley R., Dormeyer M., Grünberger A., Kohlheyer D., Commichau F. M., Lewis R. J., Stülke J., Selective pressure for biofilm formation in Bacillus subtilis: Differential effect of mutations in the master regulator SinR on bistability. mBio 9, e01464-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mielich-Süss B., Lopez D., Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ. Microbiol. 17, 555–565 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolker-Nielsen T., Biofilm development. Microbiol. Spectr. 3, MB-0001-2014 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Schubert M. L., Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr. Opin. Gastroenterol. 33, 430–438 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Chand D., Avinash V. S., Yadav Y., Pundle A. V., Suresh C. G., Ramasamy S., Molecular features of bile salt hydrolases and relevance in human health. Biochim. Biophys. Acta Gen. Subj. 1861, 2981–2991 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Begley M., Gahan C. G., Hill C., The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Vlamakis H., Chai Y., Beauregard P., Losick R., Kolter R., Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 11, 157–168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sicard J. F., Le Bihan G., Vogeleer P., Jacques M., Harel J., Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell. Infect. Microbiol. 7, 387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam N. K., Uyen N. Q., Hong H. A., Duc le H., Hoa T. T., Serra C. R., Henriques A. O., Cutting S. M., The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 188, 2692–2700 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillman E. T., Lu H., Yao T., Nakatsu C. H., Microbial ecology along the gastrointestinal tract. Microbes Environ. 32, 300–313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins A., Diep B. A., Mai T. T., Vo N. H., Warrener P., Suzich J., Stover C. K., Sellman B. R., Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 6, e02272-02214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster T. J., Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 41, 430–449 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Piewngam P., Zheng Y., Nguyen T. H., Dickey S. W., Joo H.-S., Villaruz A. E., Glose K. A., Fisher E. L., Hunt R. L., Li B., Chiou J., Pharkjaksu S., Khongthong S., Cheung G. Y. C., Kiratisin P., Otto M., Pathogen elimination by probiotic Bacillus via signalling interference. Nature 562, 532–537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpino S., Randazzo C. L., Pino A., Russo N., Rapisarda T., Belvedere G., Caggia C., Influence of PDO Ragusano cheese biofilm microbiota on flavour compounds formation. Food Microbiol. 61, 126–135 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eabb1952/DC1


