Abstract
Beta amyloid (Aβ) accumulation is the earliest pathological marker of Alzheimer’s disease (AD), but early AD pathology also affects white matter (WM) integrity. We performed a cross-sectional study including 44 subjects (23 healthy controls and 21 MCI or early AD) that underwent simultaneous MR-PET using 18F-Florbetapir, and were categorized into three groups based on Aβ burden: Aβ− [mean mSUVr ≤ 1.00], Aβi [1.00 < mSUVr < 1.17], Aβ+ [mSUVr ≥ 1.17]. Inter-group comparisons of diffusion MRI metrics revealed significant differences across multiple WM tracts. Aβi group displayed more restricted diffusion (higher fractional anisotropy, radial kurtosis, axonal water fraction and lower radial diffusivity) than both Aβ− and Aβ+ groups. This non-monotonic trend was confirmed by significant continuous correlations between mSUVr and diffusion metrics going in opposite direction for two cohorts: pooled Aβ−/Aβi and pooled Aβi/Aβ+. The transient period of increased diffusion restriction may be due to inflammation that accompanies rising Aβ burden. In the later stages of Aβ accumulation, neurodegeneration is the predominant factor affecting diffusion.
Keywords: Diffusion MRI, kurtosis, white matter, white matter tract integrity, Amyloid, Alzheimer
INTRODUCTION
Alzheimer’s disease (AD) is neuropathologically defined by the accumulation of beta amyloid (Aβ) plaques and tau protein hyperphosphorylation in gray matter structures particular the cerebral cortex and hippocampus. While classic macroscopic structural changes such as hippocampal atrophy and parenchymal volume loss can be detected in the later stages of mild cognitive impairment (MCI) and AD using conventional magnetic resonance imaging (MRI), the pathogenesis of AD long precedes its clinical symptoms, often by decades (Jack et al., 2013), making preclinical diagnosis difficult. One of the earliest pathological findings in AD is the accumulation of Aβ plaques in the cerebral cortex, which can be detected in vivo with Aβ PET imaging radiotracers such as florbetapir before the onset of clinical manifestations (Clark et al., 2011; Sperling et al., 2011; Wong et al., 2010) with high sensitivity (88–98%) and specificity (88–100%) (Clark et al., 2012; Curtis et al., 2015; Sabri et al., 2015). Aβ plaques are a necessary but insufficient marker for clinical deterioration, consistent with recent evidence of synergistic effects between Aβ burden and hippocampal atrophy (Bilgel et al., 2018), and with the Research Framework of AD considering the presence of amyloid, tau, and neurodegeneration to stage cases along a pathobiological continuum (Jack et al., 2018).
While the pathogenesis of AD was historically considered a disease of gray matter, white matter WM damage has been observed histologically in post-mortem patients with early AD as partial loss of myelin, axons, and oligodendroglial cells, as well as the presence of reactive astrocytic gliosis (Brun and Englund, 1986; Englund et al., 1988; Gottfries et al., 1996; Kemper, 1994; Kobayashi et al., 2002; Malone and Szoke, 1985; Roher et al., 2002; Svennerholm and Gottfries, 1994). WM structure can be monitored in vivo using diffusion MRI. The most widely-used diffusion MRI method is diffusion tensor imaging (DTI) (Basser and Pierpaoli, 1996), which quantifies the Gaussian part of the diffusion displacement distribution, characterized by the diffusion tensor and its derived metrics including the mean, axial and radial diffusivity (RD), and fractional anisotropy (FA). The FA is a marker of orientation coherence for diffusion, whereas the RD is the diffusivity transverse to the main fiber orientation, with both being used as empirical markers of WM integrity. Multiple studies have consistently shown that subjects with MCI and early AD exhibit WM alterations indicative of reduced integrity/complexity with reduced FA, and increased mean, axial and radial diffusivities in normal-appearing WM tracts of the temporal, parietal, and frontal lobes compared to healthy elderly control subjects (Agosta et al., 2011; Madden et al., 2009; Mayo et al., 2017; Wurtman, 2015). Several DTI studies also have highlighted a strong correlation between WM alterations and cognitive performance (Chang et al., 2015; Di Paola et al., 2015; Kantarci et al., 2014; Mielke et al., 2009), as well as high prediction value for future memory decline (Mielke et al., 2012).
Despite these findings of reduced WM integrity in AD, the exact underlying pathologic changes of these diffusion markers and their timeline in the clinical course of AD are undefined, and the temporal relation of WM changes with respect to amyloid deposition is not well characterized. In particular, there are conflicting studies of WM changes as measured with DTI with respect to amyloid burden, reporting either increased diffusion restriction (reflected by a respectively lower mean diffusivity (MD) and higher FA (Racine et al., 2014), or reduced diffusion restriction (reflected by a higher MD (Chao et al., 2013; Pietroboni et al., 2017) or lower FA (Chao et al., 2013)) in WM with increasing amyloid load. A decoupling between amyloid status and WM degeneration also has been reported (Kantarci et al., 2014; Mito et al., 2018).
In this study, we employ Diffusion Kurtosis Imaging (DKI), a clinically feasible extension of DTI that accounts also for non-Gaussian diffusion properties of nervous tissue and thus provides additional information on tissue complexity (Jensen and Helpern, 2010). DKI includes standard DTI metrics such as FA and RD, as well as additional kurtosis metrics such as radial kurtosis (RK). DKI also allows for estimating the white matter tract integrity (Fieremans, E. et al., 2011) model including quantification of the axonal water fraction (AWF), which is the relative (T2-weighted) signal fraction from water inside the axons (and potentially glial processes) over the total water fraction (from water both inside and outside axons, excluding myelin water due to its short T2). Using the cuprizone-fed mouse model (Guglielmetti et al., 2016; Jelescu et al., 2016b), the diffusion MRI-derived AWF has been recently validated against AWF derived from electron microscopy images, and has been shown to be sensitive to both patchy demyelination and axonal degeneration taking place during the acute (6 weeks) and chronic (12 weeks) phases of cuprizone intoxication, respectively.
As applied to the study of aging and AD, both DKI metrics and AWF have been shown previously to differentiate AD from MCI (Fieremans et al., 2013) and to be altered first in vulnerable late-myelinating WM tracts compared to early myelinating tracts (Benitez et al., 2013; Benitez et al., 2018). This is indicative of demyelination and potential axonal degeneration in these bundles during the AD pathologic cascade.
The goals of the current study are to employ DKI metrics and AWF to identify WM tracts affected by AD pathology, and to characterize the relationship between WM microstructural changes and Aβ deposition in both cognitively healthy and cognitively impaired populations. This way, we aim to better understand the mechanisms that may be involved in the pathogenesis of AD, and in its prodromal stage, MCI.
METHODS
Subjects
This study was approved by the local Institutional Review Board. 52 subjects were recruited from an Alzheimer Disease Center in a cross-sectional study design of cognitively-normal or early cognitively-impaired patients. Per protocol, all subjects received neurological and neuropsychological evaluations in addition to integrated PET/MR imaging. PET and MR were separately reviewed by a nuclear medicine physician and a board-certified neuroradiologist, respectively, prior to inclusion in this study. Of the 52 subjects initially recruited, 8 subjects in total were excluded either due to motion degradation (n = 1), co-morbidities (traumatic brain injury or severe depression, n = 2) or alternative explanations for cognitive impairment (primary progressive aphasia, posterior cortical atrophy, severe ischemic WM disease, frontotemporal dementia or cerebellar ataxia, n = 5) as suggested by medical history and/or imaging examination. Ultimately, 44 subjects (mean age 69.0 ± 5.1 years, range 56 – 79 years; 24 females) were included in this study.
All participants were comprehensively evaluated by board certified neurologists, psychiatrists, and neuropsychologists using the Uniform Data Set from the National Institute on Aging Alzheimer Disease Center Program (Morris et al., 2006; Weintraub et al., 2009). Clinical diagnosis was derived at consensus conference using standard criteria for AD (McKhann et al., 2011) and MCI thought to be due to underlying AD (Albert et al., 2011; Petersen et al., 2001). Global cognitive status was staged using the Global Deterioration Scale (GDS) (Reisberg et al., 1982). A GDS score of 1 corresponds with normal cognition, GDS 2 corresponds to subjective cognitive impairment, GDS score of 3 corresponds with MCI, and GDS score of 4 represented mild dementia. Although developed in 1982, the GDS staging closely aligns with the research clinical stages of the AD continuum proposed by Jack and colleagues (Jack et al 2018). In our analyses, GDS 1 and 2 combine to represent normal controls, GDS 3 represents MCI, and GDS 4 represents mild AD.
Acquisition
Subjects were scanned on a 3-T integrated PET-MRI system (Siemens Biograph mMR, VB20) after obtaining informed consent. 9 mCi of 18F-Florbetapir (Eli Lilly) was injected intravenously and PET list-mode data were acquired for 20 minutes starting at 40 min post-injection. One static uptake image was reconstructed (Wong et al., 2010) using the Siemens e7tools combined with a VB20 Siemens Ultrashort Echo Time (UTE)-based attenuation map (TE1 = 0.07ms, TE2 = 2.46 ms, Resolution = 1.6 mm isotropic). This attenuation correction was performed to account for Standardized Uptake Value (SUV) inaccuracy in air, bone, and soft tissue, and has been shown to exhibit robust reduction of attenuation-related artifacts (Aasheim et al., 2015). PET reconstruction parameters were – algorithm: OP-OSEM (ordinary Poisson ordered subset expectation maximization) with 3 iterations and 21 subsets; matrix: 344×344; 2 mm-kernel Gaussian filter; zoom 2.
MRI data were acquired using a 12-channel phased array RF coil. An anatomical MP-RAGE was acquired (TE = 2.98ms, TR = 2.3s, TI = 900ms, flip angle = 9°, resolution = 1 mm isotropic) for cortical segmentation. A FLAIR image (FLuid Attenuated Inversion Recovery) was acquired to evaluate WM lesion load (32 axial slices, slice thickness = 5mm, in-plane resolution = 750×692 μm2, TE = 91ms, TR = 8s, inversion time = 2.37s, flip angle = 150°). For the derivation of diffusion tensor, kurtosis tensor, and AWF (Fieremans, E. et al., 2011), a total of 140 diffusion-weighted images were acquired as follows: 4 b = 0 images, b = 250 s/mm2 – 6 directions, b = 1000 s/mm2 – 20 directions, b = 1500 s/mm2 – 20 directions, b = 2000 s/mm2 – 30 directions, b = 2500 s/mm2 – 60 directions. Twice refocused spin-echo single shot echo planar imaging (EPI) was performed with TE = 96ms, TR = 8.2s, 50 slices, resolution = 2.5mm isotropic, GRAPPA acceleration factor of 2. To correct for EPI distortions secondary to magnetic field inhomogeneity (combined with eddy current correction in FSL’s EDDY tool (Andersson and Sotiropoulos, 2016)), six additional b = 0 images were acquired with reverse phase-encode direction (posterior-anterior instead of anterior-posterior).
Image Processing
Structural volume
Automatic cortical and subcortical segmentation was performed on the MP-RAGE sequence using FreeSurfer v5.3.0 (http://surfer.nmr.mgh.harvard.edu/). For each subject, the volume of the following gray matter regions of interest were extracted: hippocampus, medial temporal lobe structures (MTL, comprised of the entorhinal cortex, fusiform and parahippocampal gyri), parietal lobe (PL, comprised of the inferior parietal, superior parietal and precuneus), anterior cingulate cortex (AC), posterior cingulate cortex (PC), and medio-orbital frontal lobe (MOF). Each region volume was normalized to the estimated total intra-cranial volume (TIV) of each subject.
WM lesions were automatically segmented on the FLAIR images using FireVoxel (Mikheev et al., 2008) and accuracy of segmentation was reviewed by a board-certified neuroradiologist. The total WM lesion volume was subsequently calculated and normalized to the total intra-cranial volume (Bilello et al., 2015) computed using the FreeSurfer anatomical segmentation for each subject.
Florbetapir uptake
The reconstructed PET image was registered to the MR anatomical image using FSL’s linear registration function “FLIRT” (Jenkinson et al., 2002) with a mutual information cost function. Using the structural masks obtained from FreeSurfer (see above section), the mean SUV was calculated in five cortical regions known for pathological uptake of florbetapir (anterior cingulate, posterior cingulate, medial orbito-frontal lobe, parietal lobe and medial temporal lobe), normalized to the mean SUV in the cerebellum to yield relative SUV (SUVr) (Clark et al., 2011). Averaging across all five regions yielded the mean SUVr (mSUVr) for each subject. Each region was given the same weight upon averaging (i.e. not accounting for region size), per initial definition of the mSUVr (Clark et al., 2012). SUVr values below 1 are possible given non-pathological uptake of Florbetapir in cerebellar WM (Joshi et al., 2012).
Subject classification
Using a lower mSUVr threshold of 1.00 and upper mSUVr threshold of 1.17, subjects were categorized into Aβ low, Aβ intermediate, or Aβ high (denoted as Aβ−, Aβi or Aβ+ respectively). A mSUVr less than or equal to 1.00 denoted that the amyloid load in the cerebral cortex was equal or less than that the reference region. A mSUVr equal to or greater than 1.17 has been shown to be an accurate threshold used to reflect pathological levels of amyloid based on postmortem neuropathology data (Fleisher As and et al., 2011). Values of mSUVr between 1.00 and 1.17 were considered equivocal for pathological level of amyloid.
Diffusion metrics
Processing and tensor estimation were performed using the DESIGNER pipeline (Ades-Aron et. al. 2018) (https://github.com/NYU-DiffusionMRI/DESIGNER) and included “Marchenko-Pastur Principle Component Analysis” (MP-PCA) denoising (Veraart et al., 2016a; Veraart et al., 2016b), Gibbs artifacts correction (Kellner et al., 2016), Rician bias correction, eddy current and motion correction (Andersson and Sotiropoulos, 2016), followed by CSF-excluded smoothing using a Gaussian filter with full-width-half-maximum of 2.5mm. The kurtosis tensor was estimated in each voxel using a weighted linear least squares fit (Veraart et al., 2013) in MATLAB (The MathWorks, Natick, MA). Parametric maps of FA, RD and RK were derived from the diffusion and kurtosis tensors. We focused on the radial metrics given their sensitivity to myelin and axonal changes in the white matter (Jelescu et al., 2016b). In addition, the White Matter Tract Integrity (WMTI) parameter estimation framework was used to derive AWF based on the kurtosis tensor (Fieremans, E. et al., 2011). AWF estimated from WMTI was shown to correlate with tissue axonal volume fraction from histology (Jelescu et al., 2016b). Its accuracy relies on the fact that it does not rely on the “branch choice” assumptions based on the compartment diffusivity values (Fieremans, Els et al., 2011), that are an active topic of investigation (Jelescu et al., 2015; Jelescu et al., 2016a; Kunz et al., 2018; Novikov et al., 2019; Novikov et al., 2018). Parametric maps from a representative subject from each group are provided as Supplementary Figures 1–3.
Voxel-Wise Analysis
Using FSL’s Tract-Based Spatial Statistics (TBSS), standardized skeletonized voxel-wise analyses were performed to identify areas where significant differences in diffusion metrics (Smith et al., 2006) occurred between groups. Briefly, the Johns Hopkins University (JHU) White Matter FA template (Mori et al., 2008) was used as a target for registration of each subject’s FA map using FSL’s non-linear registration tool (FNIRT) (Andersson et al., 2007; Jenkinson et al., 2012), from which the mean FA map was computed and projected onto a WM skeleton, representing the center of WM tracts. A threshold of FA > 0.4 was chosen to ensure that the skeleton represented areas of high fiber unidirectionality, which is a regime where the WMTI model is applicable and where the AWF estimation has been shown to agree with that from more advanced models that can account for fiber dispersion, such as Fiber Ball Imaging (McKinnon et al., 2018). Finally, each diffusion metric parametric map (FA, RD, RK, AWF) was projected onto the thresholded WM skeleton prior to statistical analysis.
Region of Interest (ROI) Analysis
For each subject, the FA map was registered to the JHU template (Mori et al., 2008) using the non-linear transformation computed during TBSS analysis. All WM ROIs were then warped from the JHU Atlas into native subject space. For each subject, mean and standard deviations for FA, RD, RK, and AWF were extracted in nine WM ROIs that were chosen based on their (early) involvement in AD pathogenesis (Chao et al., 2013; Oishi and Lyketsos, 2014; Racine et al., 2014; Wolf et al., 2015): fornix, genu and body of the corpus callosum (cc), anterior limb of the internal capsule (left and right), anterior corona radiata (left and right), and superior corona radiata (left and right). Quantitative comparison of these four metrics among the three Aβ groups was performed for each WM ROI of interest.
Statistical Methods
For both voxel-wise and ROI based approaches, we performed a one-way ANCOVA analysis between Aβ−, Aβi, and Aβ+ groups for each diffusion parameter of interest, with group membership acting as the independent variable and controlling for patient age and sex. Similar ANCOVA analyses were also performed controlling for age, sex and GDS. For the voxel-wise approach, a non-parametric statistical analysis was performed using FSL’s ‘randomise’ (Winkler et al., 2014), with 5000 permutations along with threshold-free cluster enhancement (Smith and Nichols, 2009) to correct for multiple comparisons between groups, and obtain group differences between voxels at a significance level of P < 0.05. Significant differences in voxel-based clusters were projected onto the WM skeleton in order to visualize statistical comparison results. For the ROI approach, the threshold for statistically significant group differences was P < 0.05 after applying Tukey’s Honest Significant Difference criterion (Tukey’s HSD) for comparing three independent groups. To further confirm our findings, we additionally performed partial Pearson correlations (covarying for age, sex and GDS) relative to mSUVr in two groups, created by combining the Aβ−/Aβi groups into a single cohort, and Aβi/Aβ+ groups into a single cohort.
RESULTS
Participant Characteristics
The subject demographics categorized by Aβ level are listed in Table 1. Of the 44 subjects, 13 (30%) were classified as Aβ−, 22 (50%) as Aβi, and 9 (20%) as Aβ+. There were no significant differences among Aβ groups in terms of age (p = 0.61, ANOVA) or sex (χ2 = 3.4, p = 0.18, χ2 test). Statistical difference was observed among the Aβ groups based on cognitive status (χ2 = 12.6, p = 0.01, χ2 test). Among the Aβ− cohort, 10/13 (77%) were cognitively normal, and 3/13 had MCI. Among the Aβi cohort, 11/22 (50%) were cognitively normal and 11/22 had MCI. Among the Aβ+ cohort, 2/9 (22%) were cognitively normal, 5/9 (56%) had mild cognitive impairment, and 2/9 had clinical AD.
Table 1:
Mean demographics and clinical characteristics for Aβ−, Aβi, and Aβ+ groups (N = 44). Subjects are age- and sex- matched across groups. Mental status is significantly different across groups (p = 0.013). ANOVA test for age, Chi-square test for sex, Chi-square test for cognitive status (healthy control vs MCI vs AD).
| Parameter | Aβ− (n = 13) | Aβi (n = 22) | Aβ+ (n = 9) | χ2/F-test | P Value |
|---|---|---|---|---|---|
| Age | 67.7± 5.8 | 69.5 ± 5.0 | 69.8 ± 4.7 | N/A | 0.616 |
| # of Females | 5/13 (38%) | 15/22 (68%) | 4/9 (44%) | 3.4 | 0.185 |
| # of Cognitive Normal | 10/13 (77%) | 11/22 (50%) | 2/9 (22%) | 12.6 | 0.013 |
| # of MCI | 3/13 (23%) | 11/22 (50%) | 5/9 (56%) | ||
| # of AD | 0/13 (0%) | 0/22 (0%) | 2/9 (22%) |
The mean hippocampal volume [% of TIV] was 0.49, 0.54, and 0.40, respectively, for Aβ−, Aβi, Aβ+ groups (p = 0.06 for Aβ−/Aβi comparison, p = 0.05 for Aβ−/Aβ+ comparison, and p < 0.01 for Aβi/ Aβ+ comparison, ANCOVA covarying for age and corrected for multiple comparisons using Tukey’s HSD). There was no difference in WM lesion load among the three groups (p = 0.80 for Aβ−/Aβi comparison, p = 0.56 for Aβ−/Aβ+ comparison, p = 0.84 for Aβi/Aβ+ comparison, ANCOVA covarying for age and corrected for multiple comparison using Tukey’s HSD). These neuroimaging characteristics are summarized in Table 2. A plot of mSUVr versus age is shown in Figure 1.
Table 2:
Neuroimaging characteristics of Aβ−, Aβi, and Aβ+ groups (N = 44). Standardized hippocampal volume differed significantly only in the Aβi/Aβ+ comparison. ANCOVA, covarying for age, corrected for multiple comparisons (e.g. Aβ−/Aβi, Aβ−/Aβ+, and Aβi/Aβ+ comparisons) using Tukey’s HSD. TTV = total intracranial volume.
| Parameter | Aβ− (n = 13) | Aβi (n = 22) | Aβ+ (n = 9) | P Value (Aβ−/Aβi) | P Value (Aβ−/Aβ+) | P Value (Aβi/Aβ+) |
|---|---|---|---|---|---|---|
| mSUVr | 0.95 ± 0.04 | 1.07 ± 0.05 | 1.43 ± 0.11 | <0.01 | <0.01 | <0.001 |
| Standardized Hippocampal Volume [% of TIV] | 0.49 ± 0.05 | 0.54 ± 0.08 | 0.40 ± 0.07 | 0.06 | 0.05 | <0.001 |
| WM Lesion Load [% of TIV] | 0.14 ± 0.29 | 0.10 ± 0.19 | 0.07 ± 0.09 | 0.80 | 0.56 | 0.844 |
Figure 1:
Plot of mSUVr vs Age, categorized by cognitive status. Subjects with lower amyloid burden correlates with lower prevalence of impaired cognitive status (77% of Aβ− subjects are cognitively normal, compared to 50% in Aβi, and 22% in Aβ+, p=0.013, Chi-square test). Aβ−: mSUVr ≤ 1.1, Aβi: 1.1 < mSUVr < 1.17, Aβ+: mSUVr ≥ 1.17.
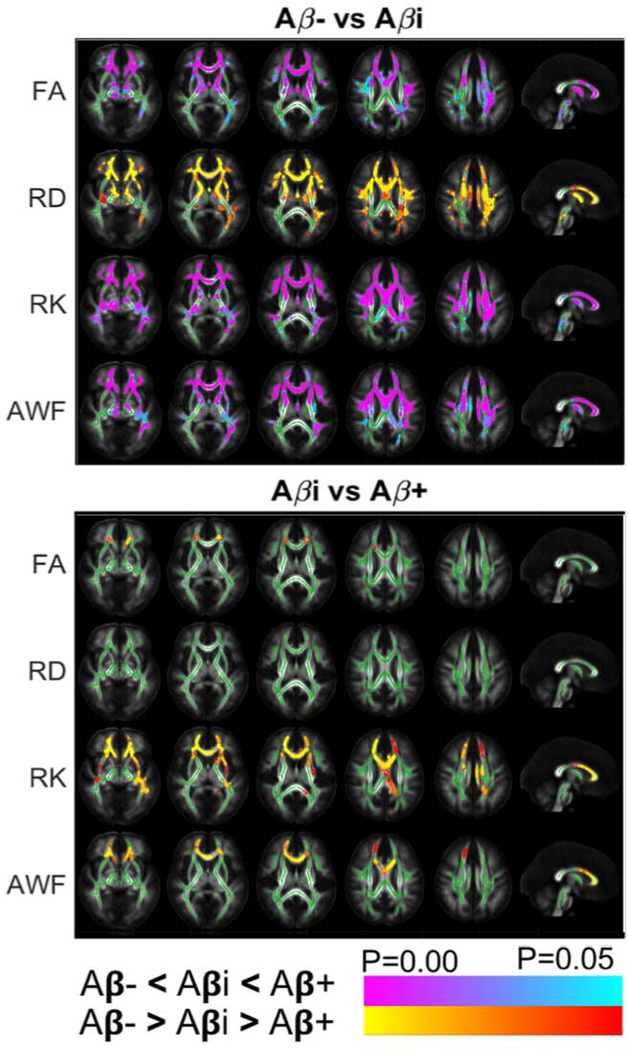
TBSS Voxel-Wise Analyses are summarized in Figure 2. TBSS detected differences in FA, RD, RK, AWF between Aβ− versus Aβi, and in FA, RK, AWF between Aβi and Aβ+ groups. Most notably, group differences were found in the genu of the corpus callosum between Aβ−/Aβi for all diffusion parameters and between Aβi/Aβ+ for RK, and AWF (p < 0.01). From Aβ− to Aβi, there was an overall increase in diffusion restriction as shown by decreasing RD, and increasing FA, RK, and AWF. Interestingly, from Aβi to Aβ+, the diffusion differences implied an overall decrease in diffusion restriction, as shown by lower FA, RK, and AWF.
Figure 2:
TBSS Results: Axial and mid-sagittal views show significant differences between Aβ−/Aβi groups and Aβi/Aβ+ groups for FA (row 1), RD (row 2), RK (row 3), and AWF (row 4). Clusters of increased (red/orange) and lower (blue/purple) are overlaid on the FA template, together with the mean skeleton (green). The observed difference between Aβ−/Aβi comparison are in the opposite direction of those in Aβi/Aβ+ comparison. TBSS revealed significant differences involving the genu of the corpus callosum and the anterior corona radiata. Directions of changes are consistent as those observed in the ROI analysis.
While these opposing changes were predominantly observed in the genu corpus callosum and anterior corona radiata, we also found similar differences in the fornix. The latter however disappeared after cluster-wise correction for multiple comparisons, possibly because of the small size of this region and increased partial volume effects with CSF due to AD-related fornix atrophy (Oishi and Lyketsos, 2014). The uncorrected TBSS results are shown as Supplementary Figure 4.
ROI Analysis: Group comparisons
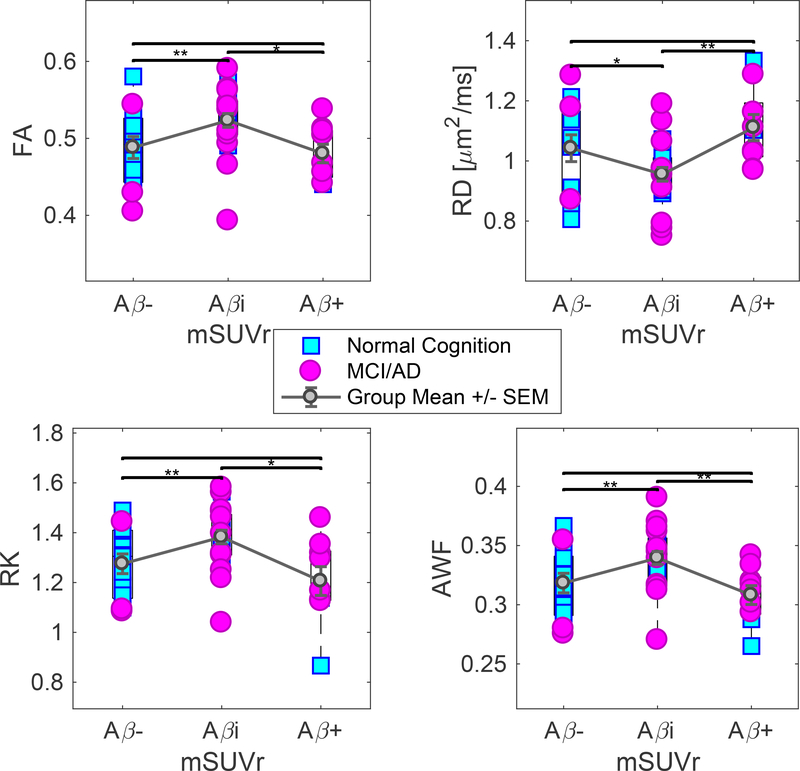
The nonmonotonic diffusion metric changes between Aβ−/Aβi and Aβi/Aβ+ as observed in the TBSS analysis also were observed in independent ROI analyses of the WM tracts defined based on the JHU WM Atlas, summarized in Table 3. The most notable significant changes were found in the fornix and the genu of the corpus callosum, but the nonmonotonic trend also appeared diffusely, including the body of corpus callosum and anterior corona radiata. Corresponding boxplots are shown in Figure 3 for the fornix and Figure 4 for the genu of corpus callosum. Results were not altered by inclusion of GDS as a covariate (not shown), which suggests the relationship between amyloid burden and white matter integrity is not likely to be directly driven by underlying relationships between amyloid burden and cognitive status on the one hand, and cognitive status and white matter integrity on the other. In addition, to FA, RD, RK, and AWF, we observed similar changes across diffusion metrics such as mean diffusivity (MD) and kurtosis (MK), as well as axial diffusivity (AxD) and kurtosis (AK), provided in Supplementary Table 1.
Table 3:
Independent region of interest analysis demonstrated non-linearity in diffusion metrics with respect to amyloid burden. This is noted particularly in the fornix and genu of the corpus callosum. Widespread changes were observed in multiple white matter tracts. ANCOVA, covarying for age and sex, corrected for multiple comparisons of combinations of two groups using Tukey’s HSD. Statistical significance is denoted by *.
| ROI | Metric | Aβ− (AVG+/−STD) | Aβi (AVG+/−STD) | Aβ+ (AVG+/−STD) | Aβ−/Aβi | Aβ−/Aβ+ | Aβi/Aβ+ |
|---|---|---|---|---|---|---|---|
| Fornix | FA | 0.32 +/− 0.06 | 0.37 +/− 0.06 | 0.26 +/− 0.06 | *0.036 | 0.117 | *<0.001 |
| RD | 2.05 +/− 0.44 | 1.62 +/− 0.44 | 2.6 +/− 0.52 | *0.008 | 0.086 | *<0.001 | |
| RK | 0.76 +/− 0.14 | 0.88 +/− 0.16 | 0.58 +/− 0.13 | *0.018 | *0.037 | *<0.001 | |
| AWF | 0.21 +/− 0.03 | 0.24 +/− 0.04 | 0.17 +/− 0.03 | *0.018 | 0.0533 | *<0.001 | |
| Genu of CC | FA | 0.49 +/− 0.05 | 0.52 +/− 0.04 | 0.48 +/− 0.04 | *0.004 | 0.986 | *0.023 |
| RD | 1.04 +/− 0.16 | 0.96 +/− 0.11 | 1.11 +/− 0.13 | *0.013 | 0.784 | *0.007 | |
| RK | 1.28 +/− 0.14 | 1.38 +/− 0.13 | 1.21 +/− 0.17 | *0.002 | 0.860 | *0.013 | |
| AWF | 0.32 +/− 0.03 | 0.34 +/− 0.03 | 0.31 +/− 0.02 | *0.002 | 0.958 | *0.005 | |
| Body of CC | FA | 0.59 +/− 0.05 | 0.60 +/− 0.05 | 0.56 +/− 0.05 | 0.503 | 0.612 | 0.142 |
| RD | 0.72 +/− 0.11 | 0.68 +/− 0.10 | 0.76 +/− 0.11 | 0.268 | 0.808 | 0.148 | |
| RK | 1.68 +/− 0.14 | 1.79 +/− 0.17 | 1.58 +/− 0.32 | *0.027 | 0.833 | 0.179 | |
| AWF | 0.39 +/− 0.03 | 0.40 +/− 0.03 | 0.38 +/− 0.04 | 0.070 | 0.974 | 0.210 | |
| Anterior limb of internal capsule (right) | FA | 0.52 +/− 0.03 | 0.54 +/− 0.03 | 0.53 +/− 0.02 | 0.211 | 0.362 | 0.952 |
| RD | 0.64 +/− 0.06 | 0.58 +/− 0.05 | 0.57 +/− 0.05 | *0.025 | *0.016 | 0.754 | |
| RK | 1.56 +/− 0.09 | 1.60 +/− 0.09 | 1.52 +/− 0.19 | 0.304 | 0.948 | 0.561 | |
| AWF | 0.39 +/− 0.02 | 0.40 +/− 0.02 | 0.39 +/− 0.03 | 0.225 | 0.994 | 0.476 | |
| Anterior limb of internal capsule (left) | FA | 0.50 +/− 0.04 | 0.52 +/− 0.04 | 0.50 +/− 0.03 | 0.148 | 0.982 | 0.263 |
| RD | 0.63 +/− 0.10 | 0.58 +/− 0.07 | 0.60 +/− 0.07 | 0.121 | 0.537 | 0.634 | |
| RK | 1.51 +/− 0.12 | 1.55 +/− 0.11 | 1.40 +/− 0.13 | 0.261 | 0.316 | *0.015 | |
| AWF | 0.38 +/− 0.03 | 0.39 +/− 0.02 | 0.37 +/− 0.02 | 0.162 | 0.841 | *0.040 | |
| Anterior corona radiata (right) | FA | 0.37 +/− 0.04 | 0.39 +/− 0.03 | 0.38 +/− 0.02 | *0.029 | 0.415 | 0.455 |
| RD | 0.81 +/− 0.09 | 0.75 +/− 0.05 | 0.80 +/− 0.09 | *0.009 | 0.608 | 0.178 | |
| RK | 1.16 +/− 0.10 | 1.20 +/− 0.08 | 1.10 +/− 0.16 | *0.034 | 0.729 | 0.075 | |
| AWF | 0.31 +/− 0.02 | 0.32 +/− 0.02 | 0.31 +/− 0.02 | 0.053 | 1.00 | 0.194 | |
| Anterior corona radiata (left) | FA | 0.37 +/− 0.04 | 0.39 +/− 0.03 | 0.36 +/− 0.02 | *0.004 | 0.989 | *<0.001 |
| RD | 0.84 +/− 0.13 | 0.75 +/− 0.06 | 0.84 +/− 0.10 | *0.003 | 0.908 | *0.049 | |
| RK | 1.13 +/− 0.10 | 1.20 +/− 0.08 | 1.08 +/− 0.11 | *0.007 | 0.721 | *0.008 | |
| AWF | 0.31 +/− 0.02 | 0.32 +/− 0.02 | 0.30 +/− 0.02 | *0.002 | 0.970 | *0.011 | |
| Superior corona radiata (right) | FA | 0.45 +/− 0.03 | 0.46 +/− 0.03 | 0.48 +/− 0.04 | 0.820 | 0.093 | 0.141 |
| RD | 0.65 +/− 0.06 | 0.64 +/− 0.10 | 0.69 +/− 0.10 | 0.915 | 0.773 | 0.621 | |
| RK | 1.46 +/− 0.07 | 1.46 +/− 0.08 | 1.45 +/− 0.13 | 0.993 | 0.980 | 0.991 | |
| AWF | 0.38 +/− 0.02 | 0.38 +/− 0.02 | 0.37 +/− 0.02 | 0.887 | 0.996 | 0.783 | |
| Superior corona radiata (left) | FA | 0.45 +/− 0.02 | 0.46 +/− 0.03 | 0.48 +/− 0.03 | 0.484 | *0.046 | 0.218 |
| RD | 0.68 +/− 0.07 | 0.65 +/− 0.08 | 0.71 +/− 0.09 | 0.254 | 0.796 | 0.178 | |
| RK | 1.42 +/− 0.04 | 1.46 +/− 0.09 | 1.43 +/− 0.10 | 0.187 | 0.870 | 0.797 | |
| AWF | 0.37 +/− 0.01 | 0.38 +/− 0.02 | 0.37 +/− 0.02 | 0.113 | 0.942 | 0.380 |
Figure 3:
ROI analysis of the fornix. Observed changes in FA, RD, RK, and AWF are in opposite directions between Aβ−/Aβi and between Aβi/Aβ+ groups. There is lower RD and higher FA, RK, and AWF between the Aβ− and Aβi groups. There is higher RD and lower FA, RK and AWF between Aβi and Aβ+ groups. Statistical significance is denoted by * (p ≤ 0.05), and *** (p ≤ 0.001). Statistics is performed using ANCOVA, co-varying for age, with correction for multiple comparison. Group means ± standard errors of the mean are displayed in error bars.
Figure 4:
ROI analysis of the genu corpus callosum. Observed changes in FA, RD, RK, and AWF are in opposite directions between Aβ−/Aβi and between Aβi/Aβ+ groups. There is lower RD and higher FA, RK, and AWF between the Aβ− and Aβi groups. There is higher RD and lower FA, RK and AWF between Aβi and Aβ+ groups. Statistical significance is denoted by * (p ≤ 0.05), and *** (p ≤ 0.001). Statistics is performed using ANCOVA, co-varying for age, with correction for multiple comparison. Group means ± standard errors of the mean are displayed in error bars.
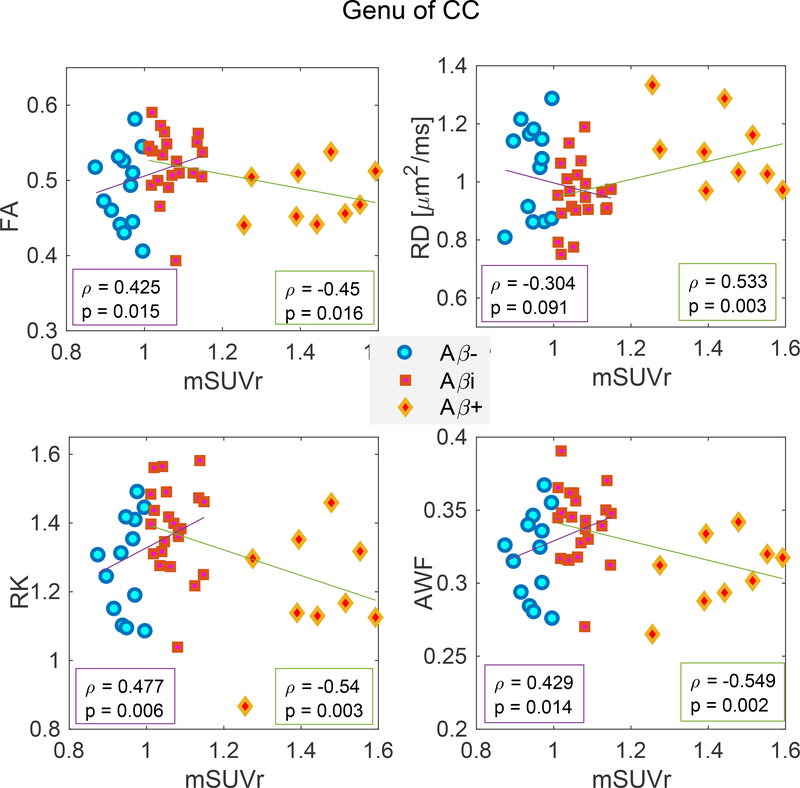
ROI analysis: Correlation Analysis of mSUVr vs Diffusion Metrics
To examine the overall trend between amyloid low and high groups, we additionally combined the Aβ−/Aβi groups into a single cohort, and Aβi/Aβ+ groups into a single cohort. Separately for each cohort, we analyzed the correlations between each diffusion metric (FA, RD, DK, AWF) and to mSUVr (with amyloid burden as a continuous variable) using age-, sex- and GDS-controlled Pearson regression in two selected region of interests known to be involved (fornix and genu of corpus callosum) in AD pathology. With the exception of RD in the Aβ−/Aβi cohort in the genu of corpus callosum, we found significant correlations between all diffusion metrics and mSUVr among both cohorts, and in both ROIs (Figures 5 and 6). Furthermore, the sign of the correlation coefficient was opposite between the two cohorts, e.g. AWF correlated positively with SUVr in the Aβ−/Aβi cohort and negatively in the Aβi/Aβ+ cohort. This further validates the observed nonmonotonic changes and highlights that the voxel-wise and ROI analyses presented previously do not depend strongly on the precise SUVr threshold chosen to separate Aβ−, Aβi and Aβ+ groups.
Figure 5:
Correlation analysis of diffusion metrics in the fornix vs mSUVr in combined Aβ− and Aβi groups (cohort 1), and combined Aβi and Aβ+ groups (cohort 2), which demonstrate that correlation coefficients (corrected for age, sex and GDS) are significant and in opposite directions for the two cohorts. Each plot illustrates the correlation with mSUVr for FA (top left), RD (top right), RK (bottom left), and AWF (bottom right).
Figure 6:
Correlation analysis of diffusion metrics in the genu vs mSUVr in combined Aβ− and Aβi groups (cohort 1), and combined Aβi and Aβ+ groups (cohort 2), which demonstrate that correlation coefficients (corrected for age, sex and GDS) are significant and in opposite directions for the two cohorts. Each plot illustrates the correlation with mSUVr for FA (top left), RD (top right), RK (bottom left), and AWF (bottom right).
DISCUSSION
We reported diffusion metrics in cognitively normal and impaired subjects with varying Aβ levels using integrated PET/MR imaging. Subjects were grouped into low, intermediate, and high Aβ levels, based on binding of 18F-Florbetapir in selected cortical regions known for pathological uptake (Clark et al., 2011). Using two independent methods – Tract-Based Spatial Statistics (TBSS), and ROI analysis of WM tracts defined by the JHU WM Atlas – we measured the group means of diffusion metrics as a function of Aβ levels, and found changes in opposing directionality that were most notable in the genu of corpus callosum and fornix, and further supported by correlational analyses in these regions. Findings also were more extensively spread in anterior cerebral WM tracts such as the anterior corona radiata. We found a pattern suggesting more diffusion restriction at intermediate amyloid burden (between Aβ− and Aβi groups) – as indicated by higher FA, RK, and AWF, and lower RD – and less diffusion restriction at higher amyloid burden (between Aβi and Aβ+ groups) – as indicated by lower FA, RK, and AWF and higher RD. RK and AWF parameters showed the most robust and significant changes, supporting the value of studying advanced models of tissue water diffusion in addition to traditional DTI.
Previous DTI-based studies assessing the relationship between WM structure and cerebral amyloid load measured with PiB (Chao et al., 2013; Racine et al., 2014; Wolf et al., 2015) or CSF Aβ levels (Pietroboni et al., 2017), observed changes in parts of the cingulum, corona radiata, and corpus callosum. However, the reported directions of changes in DTI metrics are not well agreed upon. On one hand, two studies spanning subjects who were cognitively normal or mild cognitively impaired, found that Aβ positivity was associated with reduced FA in the fornix and splenium of the corpus callosum (Chao et al., 2013; Pietroboni et al., 2017), or an increased MD in WM lesions (Pietroboni et al., 2017). Yet another study found increased FA and lower MD with cerebral amyloid deposition in cognitively healthy subjects separated into Aβ–, Aβi, and Aβ+ groups defined based on specific PiB threshold uptake (Racine et al., 2014). This study’s finding between Aβ− and Aβi is concordant with our results, but they did not report divergent changes observed in the current study between Aβi and Aβ+. A recent DTI study of ADNI data reported a non-linear association between global WM diffusion metrics and PiB amyloid deposition (Wolf et al., 2015) which is also consistent with the current study. Our study expands on these previous studies by including higher order diffusion metrics (i.e., DKI parameters and AWF) in addition to DTI, and by identifying the specific WM tracts involved in early AD, among three categorizable levels of amyloid burden (low, intermediate, high) in cognitively healthy controls and MCI/AD. While DKI metrics are sensitive but not specific to features of microstructure, the AWF derived from the WMTI model provides a specific measure of the relative size of the intra- vs. the extra-axonal compartments, weighted by their respective T2 values. Our findings were not altered when including clinical status (determined by the GDS) as a covariate, suggesting that they cannot be explained in terms of cognitive impairment but rather are directly related to Aβ burden, which is in agreement with previous studies in either cognitively healthy controls (Racine et al., 2014; Wolf et al., 2015) or mixed cohorts with status being accounted for (Chao et al., 2013; Pietroboni et al., 2017). Because the relationship of WM changes with respect to Aβ burden was not altered by the inclusion of GDS as a covariate, this suggests there may be a mechanistic relationship between amyloid burden and white matter degeneration that may not directly link to or potentially precede cognitive decline.
Mechanistic Insights
Inflammatory processes such as microglial activation and reactive astrocytes have been observed in both aging and AD brains as seen histologically in both mice and human WM (Raj et al., 2017). The observed lower RD, and increased FA, RK, and AWF of global WM tracts, particularly the genu of corpus callosum and fornix, in the Aβi group, could potentially be explained as a result of these underlying pathological events (Brun and Englund, 1986). In a validation study of diffusion MRI versus histology in cuprizone-fed mice, a similar association of lower RD, and increased FA, RK and AWF was observed in the corpus callosum during the acute inflammatory demyelinating phase characterized by extensive infiltration and proliferation of microglia (Guglielmetti et al., 2016), where these changes were explained due to increased cellularity and membrane barriers resulting in increased restriction and microscopic complexity. Another possible explanation would be that, in the initial demyelination and inflammation stages, iron release results in shortened T2 for the extra-axonal space, and thus seemingly increased AWF. When the neurodegeneration stage is reached, AWF decreases because the extra-axonal space is relatively expanded due to the loss of myelin and of axons. This is consistent with the myelin model introduced by Bartzokis et al. (Bartzokis, 2004; Bartzokis, 2011) suggesting that myelin breakdown releases iron, which promotes the development of amyloid plaques, which in turn destroys more myelin until the neurodegenerative stage. Multi-modal approaches, combining diffusion with myelin estimates from relaxometry for example (Bouhrara et al., 2018) could provide more complete in-vivo assessment of microstructural changes in the AD cascade.
The nonmonotonic trend reported here for DKI and WMTI metrics in WM matches trends reported for other AD biomarkers in gray matter. In particular, a cross-sectional study reported cortical thickening in Aβ+/p-tau- groups (Fortea et al., 2014a) and in groups with transitional CSF Aβ levels (Fortea et al., 2011), while a longitudinal study showed reduced rates of cortical atrophy in Aβi compared to normal aging from Aβ− (Pegueroles, J. et al., 2017). Furthermore, a biphasic trajectory has been observed for cortical MD, similar to the one observed in the WM in current study, in a cross-sectional cohort consisting of healthy controls, MCI and AD subjects (Montal et al., 2018). Interestingly, here we further observed a trend towards larger hippocampal volume in the Aβi group and lower in the Aβ+ group, which could be interpreted as swelling in the Aβi group (due to increased vascular permeability and/or inflammation), and atrophy in the Aβ+ group. In addition to these observations of non-monotonic (micro)structural changes, cerebral blood flow (CBF) and hippocampal activation also have been shown to be higher in MCI than in AD and normal aging (Dickerson et al., 2005; Sierra-Marcos, 2017). While there is no data correlating diffusion with perfusion and fMRI in AD, it is possible that gray matter hyper-perfusion and hyperconnectivity in early stages of the disease, which is believed to be part of an initial compensatory mechanism also including pathological elevation of neural activity and release of inflammatory molecules, could parallel the early changes in the white matter that cause an inflammatory response.
Altogether, these findings suggest that the opposing WM diffusion changes that are observed with increasing amyloid burden may be from the fact that inflammation is an early event in AD, and that neurodegeneration increases with disease duration, and dominates WM diffusion changes later in the disease course. Inflammation may stop or its signature/influence on diffusion MRI parameters may eventually be superseded by the effect of neurodegeneration, including demyelination and axonal loss. Indeed, along with Aβ deposition, AD is also characterized by tau protein misfolding and neurodegenerative processes, including cell death, demyelination, and axonal loss in WM (Brun and Englund, 1986; Englund et al., 1988), which microscopically results in a less restricted and/or hindered space, and thereby higher RD and lower FA, RK and AWF in the Aβ+ group. Macroscopically, the classical findings of neurodegeneration in AD are cortical and hippocampal atrophy. In agreement, our cohort also showed lower hippocampal volume in the Aβ+ group as compared to the Aβi (and Aβ−) group. Interestingly, in the Aβi as compared to the Aβ-group, we observed a trend towards larger hippocampal volume (p = 0.06), and corresponding significant WM differences indicative of higher restriction to diffusion. Similar to findings of biphasic changes in both MD in gray matter and cortical thickness in preclinical AD (Montal et al., 2018), our data support a close relationship between the microstructural WM changes measured with diffusion MRI and macrostructural gray matter changes measured with conventional anatomical MRI. Furthermore, as our results did not change when accounting for cognitive status, these findings point to mechanistic changes that are relatively independent of cognitive status.
Limitations and Future Directions
This study may have some limitations that needs to be addressed in future research. First, this is a cross-sectional study, and it cannot be assumed that a subject with a certain level of Aβ is temporally similar in clinical manifestation to another subject with the same level of Aβ. Future longitudinal studies combining diffusion MRI with amyloid or tau imaging or CSF markers in individual subjects should better elucidate how WM microstructure changes over time and the underlying pathogenesis. Studying tau would be particularly valuable as this protein has been proposed as the confounding factor explaining the non-linear trajectory of changes in cortical gray matter (Fortea et al., 2014b).
Second, cardiovascular disease may have influenced the diffusion metrics. As cardiovascular diseases are neither static nor binary entities (e.g. diabetes, hypertension, and other cardiovascular diseases range widely in severity, and can fluctuate over time), we chose to quantitatively measure leukoaraiosis (i.e. periventricular and subcortical white matter FLAIR hyperintensity) to characterize cerebral effects from cardiovascular disease burden, and found no effect at the group level. APOE4 status is an independent risk factor for cerebral amyloid deposition and has been associated with the progression of white matter hyperintensities in AD. Unfortunately, no APOE genotype data were available in our cohort.
Third, our limited sample size necessitates to some extent the quantitative categorization of the cerebral amyloid burden, which is a continuous variable by nature, though continuous sampling was not achieved in our sample size (shown in Figure 1). While statistically significant changes were observed, the relatively small sample size of our study may limit the generalizability of our results.
Lastly, our study includes subjects from three different global stages of cognition (as per the GDS scale), which may complicate the interpretation of the relationship between WM integrity and Aβ burden, because subjects with different cognitive status might have different neurodegeneration and neuropathological burden such as tau. Due to our sample size, limiting the analysis to subjects with the same level of cognition would have resulted in underpowering the study, as discussed in the previous limitation. We emphasize however that our findings were not altered when including GDS as a covariate in the analyses, which means GDS did not have a notable influence over the relationship between WM integrity and amyloid burden.
CONCLUSION
White matter diffusion-derived kurtosis and white matter tract integrity parameters change in opposite directions between Aβ low and Aβ intermediate, and between Aβ intermediate and Aβ high cohorts, respectively, suggesting that different mechanisms affect WM microstructure during different stages of AD. For low Aβ deposition, mechanisms including microglial activation may restrict diffusion, while later on, neurodegenerative effects such as demyelination and axonal loss may dominate and result in less restricted diffusion. The study results emphasize that WM injury occurs in the preclinical or early clinical stages of AD, and that diffusion-derived kurtosis and white matter tract integrity parameters may provide useful quantitative biomarkers of early AD.
Supplementary Material
Supplementary Figure 1. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβ− group (67 y/o female).
Supplementary Figure 2. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβi group (65 y/o female).
Supplementary Figure 3. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβ+ group (65 y/o female).
Supplementary Figure 4: Uncorrected TBSS Results: Axial and mid-sagittal views show significant differences between Aβ−/Aβi groups and Aβi/Aβ+ groups for FA (row 1), RD (row 2), RK (row 3), and AWF (row 4). Clusters of higher (red/orange) and lower (blue/purple) are overlaid on the FA template, together with the mean skeleton (green). The observed difference between Aβ−/Aβi comparison are in the opposite direction of those in Aβi/Aβ+ comparison. TBSS revealed significant differences involving the genu of the corpus callosum and the anterior corona radiata. Directions of changes are consistent as those observed in the ROI analysis
HIGHLIGHTS.
White matter (WM) changes are a less recognized feature of early AD pathogenesis
We assess WM microstructural integrity with respect to Aβ levels using PET-MRI
Increased diffusion restriction suggestive of white matter inflammation with lower Aβ load
Less diffusion restriction suggestive of neurodegeneration with higher Aβ load
ACKNOWLEDGMENTS
The authors thank Emma Ben-Avi, Sonja Blum, MD, Tracy Butler, MD, Stephanie Chrisphonte, MD, Patrick Harvey, Thet Oo MBBS, Matthew Lustberg, Martin Sadowski, MD, PhD, Jacqueline Smith, Alok Vedvyas, and Thomas M Wisniewski, MD for help with subject recruitment, and Kimberly Jackson for technical assistance with data acquisition.
FUNDING
The radiotracer Amyvid (florbetapir) was provided to the study by Avid Radiopharmaceuticals Inc., Philadelphia, PA, USA. This work was supported by the Alzheimer Drug Discovery Foundation (E.F.), NIH NIA 1K23AG048622–01 (T.M.S), NIH NINDS R01NS088040 (E.F. and D.S.N.), NIH NIA R01AG040211 (J.E.G.), R01AG056531 (E.F. and R.S. O.) and R01AG056031 (E.F. and R.S. O.). This work was also supported in part by the Center for Advanced Imaging Innovation and Research, a NIH NIBIB Biomedical Technology Resource Center (P41EB017183) and also benefitted greatly from the Alzheimer’s disease center (ADC) program grant (P30AG0851). There are no potential conflicts related to the presented work.
Footnotes
FINANCIAL DISCLOSURES
Els Fieremans, Dmitry S. Novikov are co-inventors and New York University School of Medicine (NYUSoM) is owner in the denoising technology used in this manuscript as part of the routine data image processing; a patent application has been filed and is pending. E.F., T.M.S. and D.S.N, and NYUSoM are shareholders and have advisory roles at Microstructure Imaging, Inc. E.F. is inventor on a patent application for real-time diffusional kurtosis imaging parameters, which is related to (but not exactly the same) the offline image postprocessing used in this study. Other authors do not have any potential conflict to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aasheim LB, Karlberg A, Goa PE, Haberg A, Sorhaug S, Fagerli UM, Eikenes L, 2015. PET/MR brain imaging: evaluation of clinical UTE-based attenuation correction. Eur J Nucl Med Mol Imaging 42(9), 1439–1446. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M, 2011. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 258(3), 853–863. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3), 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S, 2007. Non-linear registration aka Spatial normalisation: FMRIB Technical Report TR07JA2.
- Andersson JLR, Sotiropoulos SN, 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, 2004. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging 25(1), 5–18. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, 2011. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 32(8), 1341–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C, 1996. Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. Journal of Magnetic Resonance, Series B 111(3), 209–219. [DOI] [PubMed] [Google Scholar]
- Benitez A, Fieremans E, Jensen JH, Falangola MF, Tabesh A, Ferris SH, Helpern JA, 2013. White matter tract integrity metrics reflect the vulnerability of late-myelinating tracts in Alzheimer’s disease. Neuroimage. Clinical 4, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez A, Jensen JH, Falangola MF, Nietert PJ, Helpern JA, 2018. Modeling white matter tract integrity in aging with diffusional kurtosis imaging. Neurobiology of Aging 70, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello M, Doshi J, Nabavizadeh SA, Toledo JB, Erus G, Xie SX, Trojanowski JQ, Han X, Davatzikos C, 2015. Correlating Cognitive Decline with White Matter Lesion and Brain Atrophy Magnetic Resonance Imaging Measurements in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 48(4), 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M, An Y, Helphrey J, Elkins W, Gomez G, Wong DF, Davatzikos C, Ferrucci L, Resnick SM, 2018. Effects of amyloid pathology and neurodegeneration on cognitive change in cognitively normal adults. Brain : a journal of neurology 141(8), 2475–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, Spencer RG, 2018. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 14(8), 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E, 1986. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Annals of neurology 19(3), 253–262. [DOI] [PubMed] [Google Scholar]
- Chang YL, Chen TF, Shih YC, Chiu MJ, Yan SH, Tseng WY, 2015. Regional cingulum disruption, not gray matter atrophy, detects cognitive changes in amnestic mild cognitive impairment subtypes. Journal of Alzheimer’s disease : JAD 44(1), 125–138. [DOI] [PubMed] [Google Scholar]
- Chao LL, DeCarli C, Kriger S, Truran D, Zhang Y, Laxamana J, Villeneuve S, Jagust WJ, Sanossian N, Mack WJ, Chui HC, Weiner MW, 2013. Associations between White Matter Hyperintensities and P Amyloid on Integrity of Projection, Association, and Limbic Fiber Tracts Measured with Diffusion Tensor MRI. PloS one 8(6), e65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM, 2012. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. The Lancet Neurology 11(8), 669–678. [DOI] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Reiman PEM, Zehntner SP, Skovronsky DM, Group AAS, 2011. Use of florbetapir-PET for imaging beta-amyloid pathology. Jama 305(3), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, Beach TG, Duara R, Fleisher AS, Frey KA, Walker Z, Hunjan A, Holmes C, Escovar YM, Vera CX, Agronin ME, Ross J, Bozoki A, Akinola M, Shi J, Vandenberghe R, Ikonomovic MD, Sherwin PF, Grachev ID, Farrar G, Smith APL, Buckley CJ, McLain R, Salloway S, 2015. Phase 3 Trial of Flutemetamol Labeled With Radioactive Fluorine 18 Imaging and Neuritic Plaque Density. JAMA neurology 72(3), 287–294. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Phillips O, Orfei MD, Piras F, Cacciari C, Caltagirone C, Spalletta G, 2015. Corpus Callosum Structure is Topographically Correlated with the Early Course of Cognition and Depression in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 45(4), 1097–1108. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA, 2005. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65(3), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C, 1988. White matter changes in dementia of Alzheimer’s type. Biochemical and neuropathological correlates. Brain: A Journal of Neurology 111 ( Pt 6), 1425–1439. [DOI] [PubMed] [Google Scholar]
- Fieremans E, Benitez A, Jensen JH, Falangola MF, Tabesh A, Deardorff RL, Spampinato MV, Babb JS, Novikov DS, Ferris SH, Helpern JA, 2013. Novel white matter tract integrity metrics sensitive to Alzheimer disease progression. AJNR. American journal of neuroradiology 34(11), 2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E, Jensen JH, Helpern JA, 2011. White matter characterization with diffusional kurtosis imaging. Neuroimage 58(1), 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E, Jensen JH, Helpern JA, 2011. White matter characterization with diffusional kurtosis imaging. NeuroImage 58(1), 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher As CKLX, et al. , 2011. USing positron emission tomography and florbetapir f 18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to alzheimer disease. Arch. Neurol 68(11), 1404–1411. [DOI] [PubMed] [Google Scholar]
- Fortea J, Sala-Llonch R, Bartrés-Faz D, Lladó A, Solé-Padullés C, Bosch B, Antonell A, Olives J, Sanchez-Valle R, Molinuevo JL, Rami L, 2011. Cognitively Preserved Subjects with Transitional Cerebrospinal Fluid β-Amyloid 1–42 Values Have Thicker Cortex in Alzheimer’s Disease Vulnerable Areas. Biological Psychiatry 70(2), 183–190. [DOI] [PubMed] [Google Scholar]
- Fortea J, Vilaplana E, Alcolea D, Carmona-Iragui M, Sánchez-Saudinos M-B, Sala I, Antón-Aguirre, González S, Medrano S, Pegueroles J, Morenas E, Clarimón J, Blesa R, Lleó A, for the Alzheimer’s Disease Neuroimaging, I., 2014a. Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Annals of neurology 76(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Fortea J, Vilaplana E, Alcolea D, Carmona-Iragui M, Sánchez-Saudinos M-B, Sala I, Antón-Aguirre, González S, Medrano S, Pegueroles J, Morenas E, Clarimón J, Blesa R, Lleó A, Initiative, f.t.A.s.D.N., 2014b. Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann. Neurol 76(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Karlsson I, Svennerholm L, 1996. Membrane components separate early-onset Alzheimer’s disease from senile dementia of the Alzheimer type. International psychogeriatrics 8(3), 365–372. [DOI] [PubMed] [Google Scholar]
- Guglielmetti C, Veraart J, Roelant E, Mai Z, Daans J, Van Audekerke J, Naeyaert M, Vanhoutte G, Delgado y Palacios R, Praet J, Fieremans E, Ponsaerts P, Sijbers J, Van der Linden A, Verhoye M, 2016. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage 125, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology 12(2), 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelescu IO, Veraart J, Adisetiyo V, Milla SS, Novikov DS, Fieremans E, 2015. One diffusion acquisition and different white matter models: How does microstructure change in human early development based on WMTI and NODDI? NeuroImage 107, 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelescu IO, Veraart J, Fieremans E, Novikov DS, 2016a. Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue. NMR in biomedicine 29(1), 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelescu IO, Zurek M, Winters KV, Veraart J, Rajaratnam A, Kim NS, Babb JS, Shepherd TM, Novikov DS, Kim SG, Fieremans E, 2016b. In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. NeuroImage 132, 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. NeuroImage 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, 2010. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in biomedicine 23(7), 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM, 2012. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 53(3), 378–384. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Schwarz CG, Reid RI, et al. , 2014. White matter integrity determined with diffusion tensor imaging in older adults without dementia: Influence of amyloid load and neurodegeneration. JAMA neurology 71(12), 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner E, Dhital B, Kiselev VG, Reisert M, 2016. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 76(5), 1574–1581. [DOI] [PubMed] [Google Scholar]
- Kemper TL, 1994. Neuroanatomical and neuropathological changes during aging and dementia, Clinical neurology of aging, 2nd ed. Oxford University Press, New York, NY, US, pp. 3–67. [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y, 2002. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathology and applied neurobiology 28(3), 238–251. [DOI] [PubMed] [Google Scholar]
- Kunz N, da Silva AR, Jelescu IO, 2018. Intra- and extra-axonal axial diffusivities in the white matter: Which one is faster? Neuroimage 181, 314–322. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW, 2009. Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychol Rev 19(4), 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone MJ, Szoke MC, 1985. Neurochemical changes in white matter. Aged human brain and Alzheimer’s disease. Arch Neurol 42(11), 1063–1066. [DOI] [PubMed] [Google Scholar]
- Mayo CD, Mazerolle EL, Ritchie L, Fisk JD, Gawryluk JR, 2017. Longitudinal changes in microstructural white matter metrics in Alzheimer’s disease. NeuroImage: Clinical 13, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon ET, Helpern JA, Jensen JH, 2018. Modeling white matter microstructure with fiber ball imaging. NeuroImage 176, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Kozauer NA, Chan KC, George M, Toroney J, Zerrate M, Bandeen-Roche K, Wang MC, Vanzijl P, Pekar JJ, Mori S, Lyketsos CG, Albert M, 2009. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. NeuroImage 46(1), 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG, 2012. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s & Dementia 8(2), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H, 2008. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J. Magn. Reson. Imaging 27(6), 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A, 2018. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain : a journal of neurology 141(3), 888–902. [DOI] [PubMed] [Google Scholar]
- Montal V, Vilaplana E, Alcolea D, Pegueroles J, Pasternak O, Gonzalez-Ortiz S, Clarimon J, Carmona- Iragui M, Illan-Gala I, Morenas-Rodriguez E, Ribosa-Nogue R, Sala I, Sanchez-Saudinos MB, Garcia-Sebastian M, Villanua J, Izagirre A, Estanga A, Ecay-Torres M, Iriondo A, Clerigue M, Tainta M, Pozueta A, Gonzalez A, Martinez-Heras E, Llufriu S, Blesa R, Sanchez-Juan P, Martinez-Lage P, Lleo A, Fortea J, 2018. Cortical microstructural changes along the Alzheimer’s disease continuum. Alzheimers Dement 14(3), 340–351. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40(2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA, 2006. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord 20(4), 210–216. [DOI] [PubMed] [Google Scholar]
- Novikov DS, Fieremans E, Jespersen SN, Kiselev VG, 2019. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. NMR Biomed. 32(4), e3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov DS, Veraart J, Jelescu IO, Fieremans E, 2018. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage 174, 518–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Lyketsos CG, 2014. Alzheimer’s disease and the fornix. Front Aging Neurosci 6, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegueroles J, Vilaplana E, Montal V, Sampedro F, Alcolea D, Carmona-Iragui M, Clarimon J, Blesa R, Lleo A, Fortea J, 2017. Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 13(5), 499–509. [DOI] [PubMed] [Google Scholar]
- Pegueroles J, Vilaplana E, Montal V, Sampedro F, Alcolea D, Carmona-Iragui M, Clarimon J, Blesa R, Lleo A, Fortea J, 2017. Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13(5), 499–509. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B, 2001. Current Concepts in Mild Cognitive Impairment. Arch. Neurol 58(12), 1985–1992. [DOI] [PubMed] [Google Scholar]
- Pietroboni AM, Scarioni M, Carandini T, Basilico P, Cadioli M, Giulietti G, Arighi A, Caprioli M, Serra L, Sina C, Fenoglio C, Ghezzi L, Fumagalli GG, De Riz MA, Calvi A, Triulzi F, Bozzali M, Scarpini E, Galimberti D, 2017. CSF P-amyloid and white matter damage: a new perspective on Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. [DOI] [PubMed] [Google Scholar]
- Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC, 2014. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: A multimodal imaging investigation. Neuroimage. Clinical 4, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, Olah M, Mantingh-Otter IJ, Van Dam D, De Deyn PP, den Dunnen W, Eggen BJL, Amor S, Boddeke E, 2017. Increased White Matter Inflammation in Aging- and Alzheimer’s Disease Brain. Front Mol Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T, 1982. The Global Deterioration Scale for assessment of primary degenerative dementia. The American journal of psychiatry 139(9), 1136–1139. [DOI] [PubMed] [Google Scholar]
- Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T, 2002. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’s disease. Biochemistry 41(37), 11080–11090. [DOI] [PubMed] [Google Scholar]
- Sabri O, Seibyl J, Rowe C, Barthel H, 2015. Beta-amyloid imaging with florbetaben. Clin Transl Imaging 3(1), 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Marcos A, 2017. Regional Cerebral Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease Measured with Arterial Spin Labeling Magnetic Resonance Imaging. Int J Alzheimers Dis 2017, 5479597–5479597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ, 2006. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH, 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7(3), 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L, Gottfries CG, 1994. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). Journal of neurochemistry 62(3), 1039–1047. [DOI] [PubMed] [Google Scholar]
- Veraart J, Fieremans E, Novikov DS, 2016a. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med, n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E, 2016b. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B, 2013. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. NeuroImage 81, 335–346. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC, 2009. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis. Assoc. Disord 23(2), 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. NeuroImage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Fischer FU, Scheurich A, Fellgiebel A, 2015. Non-Linear Association between Cerebral Amyloid Deposition and White Matter Microstructure in Cognitively Healthy Older Adults. Journal of Alzheimer’s disease : JAD 47(1), 117–127. [DOI] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ, 2010. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). Journal of nuclear medicine : official publication, Society of Nuclear Medicine 51(6), 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman R, 2015. Biomarkers in the diagnosis and management of Alzheimer’s disease. Metabolism: clinical and experimental 64(3 Suppl 1), S47–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβ− group (67 y/o female).
Supplementary Figure 2. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβi group (65 y/o female).
Supplementary Figure 3. Parametric maps of FA, RD, RK and AWF in a representative subject from the Aβ+ group (65 y/o female).
Supplementary Figure 4: Uncorrected TBSS Results: Axial and mid-sagittal views show significant differences between Aβ−/Aβi groups and Aβi/Aβ+ groups for FA (row 1), RD (row 2), RK (row 3), and AWF (row 4). Clusters of higher (red/orange) and lower (blue/purple) are overlaid on the FA template, together with the mean skeleton (green). The observed difference between Aβ−/Aβi comparison are in the opposite direction of those in Aβi/Aβ+ comparison. TBSS revealed significant differences involving the genu of the corpus callosum and the anterior corona radiata. Directions of changes are consistent as those observed in the ROI analysis