Abstract
Hospital-associated respiratory virus infections (HARVI) are an underappreciated source of morbidity and mortality. We examined HARVI incidence and clinical respiratory virus testing practices in a cohort of hospitalized patients with acute respiratory illness. HARVI were identified in patients of all ages, both during and outside of the influenza season.
Keywords: diagnostic testing, hospital-associated infection, incidence, infection prevention, respiratory viruses
Hospital-associated respiratory virus infections (HARVI) are an underappreciated source of morbidity and mortality. We examined HARVI incidence in a cohort of hospitalized patients. HARVIs were identified in patients of all ages, both during and outside of the influenza season.
Hospitalized patients are at increased risk for severe outcomes associated with respiratory virus infections because this population is more likely to be at extremes of age and have more comorbidities than the general population [1, 2]. Substantial efforts are made to identify and prevent hospital-acquired influenza and respiratory syncytial virus (RSV) infections. Although other respiratory viruses have been implicated in severe illness, relatively less attention has been devoted to identifying their potential for transmission in hospitalized patients and associated outcomes [3]. Underdiagnosis of hospital-associated respiratory virus infections (HARVI) leads to missed opportunities for intervention, prevention, and treatment. An improved understanding of the burden of respiratory viruses in the hospital setting is needed to inform optimal infection control practices and sick leave policies [4].
METHODS
We examined HARVI incidence and respiratory virus testing practices in a cohort of hospitalized patients with acute respiratory illness (ARI).
Study Population
The cohort included patients admitted to a University of Michigan adult or pediatric hospital between July 1, 2016 and June 30, 2017 with an ARI hospitalization, defined as having an International Classification of Diseases, Tenth Revision diagnosis code assigned at discharge consistent with ARI (Supplemental Table 1). Analyses were performed at the level of inpatient encounter; individual patients could have more than 1 hospitalization during the study period. Patient characteristics, including age, sex, race/ethnicity, timing of hospital admission within or outside of the influenza season (December through April), and adult and pediatric-specific chronic condition scores [5, 6], were determined from the electronic medical record. Two outcomes were defined: (1) respiratory virus testing status and (2) whether HARVI was identified. Hospital-associated respiratory virus infections was defined as a respiratory virus initially identified in a test ordered >72 hours after hospital admission. The comparison group of patients without HARVI included both those who were and were not tested. The study was approved by the University of Michigan Medical School Institutional Review Board.
Laboratory Data
Respiratory virus test results were retrieved from the electronic medical record. Specific assays included (1) influenza and RSV reverse-transcription polymerase chain reaction and (2) molecular respiratory virus panel (FilmArray Respiratory Panel) testing for adenovirus, coronaviruses (229E, HKU1, NL63, and OC43), human metapneumovirus, human rhinovirus-enterovirus, influenza (A[H1N1pdm09], A[H3N2], and B), parainfluenza (1, 2, 3, and 4), and RSV.
Statistical Analysis
Cumulative incidence was calculated as the number of HARVI cases per 10 000 ARI hospitalizations. Adult-specific (≥18 years) and child-specific (<18 years) multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) comparing testing and HARVI status by the following a priori selected variables: age category (<5 and 5–17 years in child models; 18–64 and ≥65 years in adult models), race (non-Hispanic white, non-Hispanic black, other), sex, timing of admission relative to the influenza season, and chronic condition score (0, 1–2, ≥3). For adults, chronic condition scores correspond to Charlson comorbidity index scores of 0, 1–2, and >3 [5]. For children, categories correspond to Pediatric Complex Chronic Condition counts of 0, 1–2, and >3 [6].
RESULTS
During the 1-year study period, 16 217 individuals had 22 936 ARI hospitalizations (Figure 1); 44% of hospitalizations occurred during the influenza season. Patients of all ages were included (median, 58 years; interquartile range [IQR], 34–70); however, only 15% were children, over half of whom were <5 years old. At least 1 chronic condition was noted for 89% of adults and 60% of children. Distributions of demographic characteristics were similar at the unique patient and overall hospitalization levels (Supplemental Table 2).
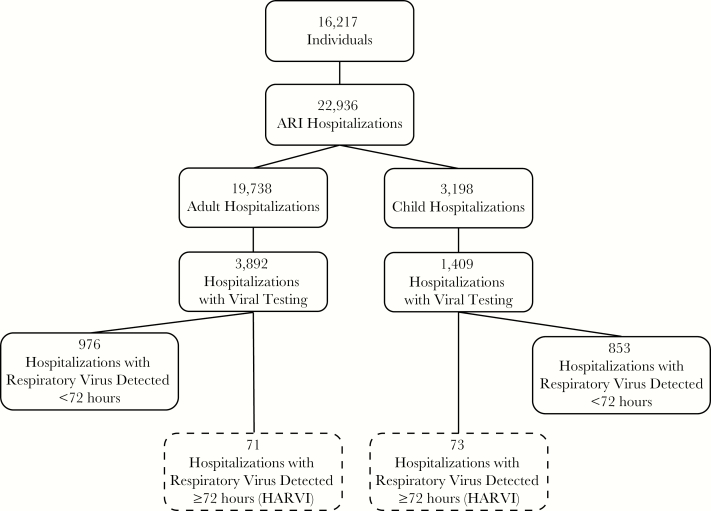
Figure 1.
Flow diagram of study population, respiratory virus testing, and respiratory virus detection. HARVI, hospital-associated respiratory virus infection.
Predictors of Respiratory Virus Testing
A total of 6036 respiratory virus tests were performed during 5301 (23%) of the 22 936 hospitalizations. The median time from admission to first test order was 3.5 hours (IQR, 1–11); 950 (16%) tests were ordered >72 hours after admission. Children were more likely to be tested than adults (44% vs 20%), and younger age was significantly associated with increased testing within both children (<18 years) and adults (≥18 years) (Supplemental Table 3). Testing was more likely to be performed during the influenza season than outside of the season for both children (OR, 1.59; 95% CI, 1.37–1.84) and adults (OR, 2.05; 95% CI, 1.91–2.20). Children with more chronic conditions were more likely to be tested, but adults with more chronic conditions were less likely to be tested (Supplemental Table 3).
Predictors of Hospital-Associated Respiratory Virus Infections
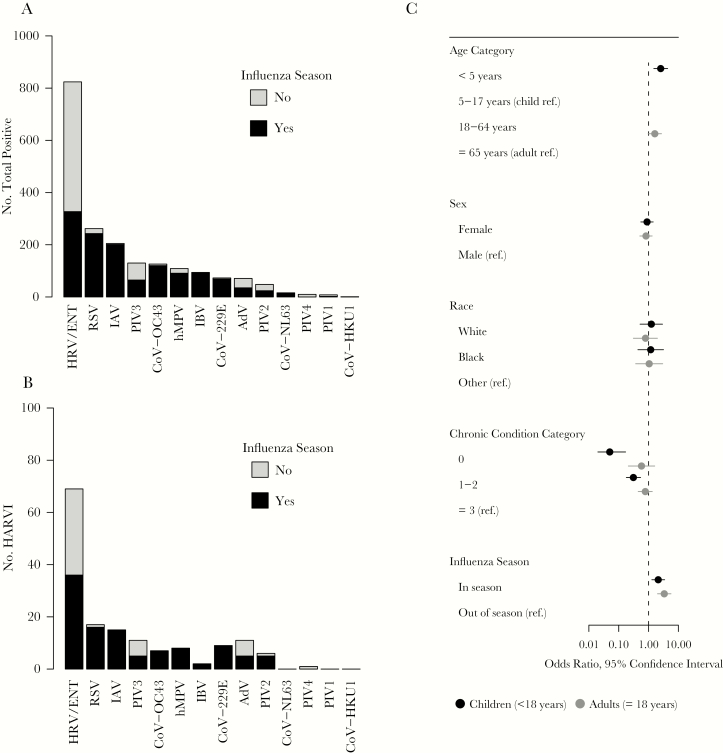
Respiratory viruses were identified during 1829 ARI hospitalizations; 1685 were identified within the first 72 hours after admission, and 144 were detected ≥72 hours after admission and categorized as HARVI (Figure 1). Using the total number of ARI hospitalizations as a denominator, the 144 respiratory viruses identified after 72 hours translates to an overall HARVI incidence of 63 per 10 000 ARI hospitalizations. Approximately 28% of HARVI cases were identified 3–4 days after admission, 24% were identified 5–7 days after admission, 19% were identified 8–13 days after admission, and 28% were identified 14 or more days after admission. Thirty-seven (26%) HARVI cases had prior respiratory virus testing with a negative result during their admission. The most common pathogens among the 144 HARVIs were rhinovirus (n = 69, 48%), RSV (n = 17, 12%), and influenza A (n = 15, 10%), similar to the distribution of all respiratory viruses diagnosed in the hospital during the same time period (Figure 2A and B; Supplemental Table 4). Rhinovirus, parainfluenza, and adenovirus were the viruses most likely to be identified outside of the influenza season (Figure 2A and B).
Figure 2.
(A) Total and (B) hospital-associated respiratory infections (HARVI) by virus type and occurrence during or outside of the influenza season, and (C) associations between patient characteristics and odds of HARVI. Hospital-associated respiratory virus infection was defined by positive result for any respiratory virus in a clinical test ordered ≥72 hours after hospital admission. AdV, adenovirus; CoV-229E, coronavirus 229E; CoV-HKU1, coronavirus HKU1; CoV-NL63, coronavirus NL63; CoV-OC43, coronavirus OC43; hMPV, human metapneumovirus; HRV/ENT, human rhinovirus/enterovirus; IAV, influenza A virus; IBV, influenza B virus; No., number; PIV1, parainfluenza virus 1; PIV2, parainfluenza virus 2; PIV3, parainfluenza virus 3; PIV4, parainfluenza virus 4; ref., reference; RSV, respiratory syncytial virus.
Half of all HARVI were identified in adults, but incidence was higher in children (36 vs 228 per 10 000 ARI hospitalizations, P < .001). Children <5 years of age were 2 times more likely to be HARVI cases than older children (OR, 2.55; 95% CI, 1.50–4.36) (Supplemental Table 5). Hospital-associated respiratory virus infections were significantly more likely to occur during the influenza season for both children and adults, but 45 (31%) of the 144 total cases occurred outside of the influenza season. Hospital-associated respiratory virus infections were much more likely to occur in children with chronic conditions (Figure 2C; Supplemental Table 5). In contrast, there was no significant association between chronic conditions and HARVI in adults. A sensitivity analysis limited to only individuals who were tested produced similar results and did not change conclusions (Supplemental Table 6).
DISCUSSION
We identified 144 HARVIs, approximately 8% of all hospital-based respiratory virus identifications for the year. Of note, 76% of HARVIs were associated with viruses other than influenza or RSV, and approximately one third occurred outside of the influenza season. Similar to previous studies, HARVI incidence was more than 5 times higher in children than adults [7–9]. However, a similar overall number of HARVIs were identified among adults because of the larger population of hospitalized adults. We also found that pediatric patients were more likely to be tested than adults with ARI, and testing was most common during the influenza season independent of age. In addition, children with more chronic conditions were more likely to be tested and to have HARVI identified compared to those without chronic conditions. These factors could inform efforts to increase testing and identification of HARVI that have been associated with severe morbidity and mortality in previous studies [9–11].
Recommendations for the prevention of HARVI include pathogen-specific contact and/or droplet precautions, use of personal protective equipment by healthcare workers, healthcare worker vaccination and sick leave policies, patient placement, hand hygiene, and visitor management [12–14]. Respiratory virus panel testing, when available, is recommended for hospitalized patients who are immunocompromised and is an option for other hospitalized patients if testing might affect care or infection control [15]. Considerable variability in the operationalization of these prevention and diagnostic recommendations suggests that there is room for improvement [16, 17]. The results of our study suggest that respiratory viruses are a significant problem worthy of continued work to improve best practices for their prevention.
The generalizability of this single-year study conducted in a single hospital might be limited because the incidence of respiratory viruses varies year to year, and testing and infection control policies vary substantially from hospital to hospital. Although institutional policies are consistent with recommendations to use respiratory virus panel testing for immunocompromised patients and in situations when results might impact clinical care, University of Michigan Hospitals have a relatively high utilization of respiratory virus panel testing. The choice between influenza and RSV-specific testing and the full respiratory virus panel is typically a matter of the preference of the ordering clinician. Institutional policies also recommend private rooms for individuals with ARI or cohorting based on identification of the same virus if private rooms are unavailable. In addition, the number of HARVI may have been underestimated due to missed cases among patients who were not tested. In contrast, HARVI incidence may have been overestimated because viral shedding can persist for 1 week or more [18]. However, we expect that relatively few of our HARVI cases were actually delayed diagnosis of community infections, because the median time from admission to specimen collection for a HARVI case was more than 7 days, and, presumably, new symptoms occurring more than 3 days after admission prompted testing.
CONCLUSIONS
In conclusion, HARVI is an important and underrecognized phenomenon, due to a variety of different viruses. Improved understanding of the epidemiology of HARVI is necessary to optimize safety of both patients and healthcare workers.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases (K01AI141579; to J. G. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: American Society for Microbiology Microbe, June 10, 2018, Atlanta, GA.
References
- 1. Boyce TG, Mellen BG, Mitchel EF Jr, et al. . Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000; 137:865–70. [DOI] [PubMed] [Google Scholar]
- 2. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 3. Gilca R, Amini R, Douville-Fradet M, et al. . Other respiratory viruses are important contributors to adult respiratory hospitalizations and mortality even during peak weeks of the influenza season. Open Forum Infect Dis 2014; 1:ofu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow EJ, Mermel LA. More than a cold: hospital-acquired respiratory viral infections, sick leave policy, and a need for culture change. Infect Control Hosp Epidemiol 2018; 39:861–2. [DOI] [PubMed] [Google Scholar]
- 5. Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 6. Feudtner C, Feinstein JA, Zhong W, et al. . Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valenti WM, Menegus MA, Hall CB, et al. . Nosocomial viral infections: I. Epidemiology and significance. Infect Control 1980; 1:33–7. [DOI] [PubMed] [Google Scholar]
- 8. Choi HS, Kim MN, Sung H, et al. . Laboratory-based surveillance of hospital-acquired respiratory virus infection in a tertiary care hospital. Am J Infect Control 2017; 45:e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow EJ, Mermel LA. Hospital-acquired respiratory viral infections: incidence, morbidity, and mortality in pediatric and adult patients. Open Forum Infect Dis 2017; 4:ofx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong HL, Hong SB, Ko GB, et al. . Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One 2014; 9:e95865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zinna S, Lakshmanan A, Tan S, et al. . Outcomes of nosocomial viral respiratory infections in high-risk neonates. Pediatrics 2016; 138:e20161675. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention (CDC). Prevention Strategies for Seasonal Influenza in Healthcare Settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed 28 January 2020. [Google Scholar]
- 13. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(10 Suppl 2):S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dare RK, Talbot TR. Health care-acquired viral respiratory diseases. Infect Dis Clin North Am 2016; 30:1053–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uyeki TM, Bernstein HH, Bradley JS, et al. . Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis 2019; 68:e1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubin LG, Kohn N, Nullet S, Hill M. Reduction in rate of nosocomial respiratory virus infections in a children’s hospital associated with enhanced isolation precautions. Infect Control Hosp Epidemiol 2018; 39:152–6. [DOI] [PubMed] [Google Scholar]
- 17. Forkpa H, Rupp AH, Shulman ST, et al. . Association between children’s hospital visitor restrictions and healthcare-associated viral respiratory infections: a quasi-experimental study. J Pediatric Infect Dis Soc 2020; 9:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peltola V, Waris M, Kainulainen L, et al. . Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin Microbiol Infect 2013; 19:E322–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.