Abstract
Pyroptosis is a form of lytic, programmed cell death that functions as an innate immune effector mechanism to facilitate host defense against pathogenic microorganisms, including viruses. This type of proinflammatory cell death is orchestrated by proteolytic activation of human or mouse caspase-1, mouse caspase-11 and human caspase-4 as well as caspase-5 in response to infectious and inflammatory stimuli. Induction of pyroptosis requires either a canonical inflammasome responsible for caspase-1 activation or a noncanonical complex composed of caspase-11 in mice or caspase-4 or caspase-5 in humans. Recent studies have identified the pore-forming protein gasdermin D, a substrate of these inflammatory caspases, as an executioner of pyroptosis. The membrane pores formed by gasdermin D facilitate release of proinflammatory cytokines IL-1β and IL-18 and consequent biologic effects of these cytokines together with other released components. Pyroptosis, like other forms of programmed cell death, helps eliminate infected cells and thereby restricts the replicative niche, undermining survival and proliferation of intracellular pathogens. This includes viruses as well as bacteria where ample evidence supports a critical role for inflammasome effector functions and cell death in host defense. Viruses have evolved their own mechanisms to modulate inflammasome signaling and pyroptosis. Here, we review the current literature regarding the role of pyroptosis in antiviral immune responses.
Keywords: Viruses, Inflammasomes, caspases, cell death, pyroptosis, inflammation, NLRP3, AIM2, Caspase-1, Caspase-11, infection, innate immunity, gasdermin
1. Introduction
Pyroptosis, a form of programmed cell death, has roots that reflect a proinflammatory nature of the pathway. The name pyroptosis, first used to describe a lytic form of caspase-1-dependent cell death, is based on the Greek term ‘pyro’ meaning fire or fever and ‘ptosis’ meaning falling or dropping, distinguishes this pathway from apoptosis and necrosis (Cookson and Brennan 2001). The word ‘inflammation’ itself originates from a Latin term meaning ‘a setting on fire’ and aptly describes cardinal features such as calor (Dondelinger et al. 2015), rubor (redness) and dolor (pain) (Basil and Levy 2016). Inflammatory caspases (human and mouse caspase-1, mouse caspase-11 and human caspase-4 and caspase-5) function as effector caspases to initiate pyroptotic cell death (Jorgensen and Miao 2015; Man et al. 2017). The contribution of these inflammatory caspases distinguishes pyroptosis from either apoptosis or necroptosis, all mechanistically distinct forms of programmed cell death (Man et al. 2017).
Early reports failed to distinguish pyroptosis from apoptosis (Zychlinsky et al. 1992) despite evidence that cell death affecting macrophages in response to infection with Shigella flexineri and Salmonella typhimurium resulted in leakage (Zychlinsky et al. 1992; Chen et al. 1996; Monack et al. 1996). Cell death during infection with these pathogens was later found to be dependent on caspase-1 and/or caspase-11 and to involve morphological changes different from apoptosis (Hilbi et al. 1998; Hersh et al. 1999; Brennan and Cookson 2000; Watson et al. 2000). The initial focus on caspase-1 as a pro-pyroptotic caspase was revised when caspase-1-deficient cells were shown to also lack caspase-11, a closely linked gene that is responsible for pyroptosis induced by the Gram negative bacterial cell wall component, lipopolysacharride (LPS) (Kayagaki et al. 2011). Caspase-11 is now recognized as an interferon-inducable cytosolic sensor required for pyroptosis in response to LPS from Gram negative bacteria (Kayagaki et al. 2013). In contrast, viral infections may trigger caspase-1-dependent pyroptosis. Pyroptosis differs from the inflammatory and lytic necrosis, which occurs due to physical damage, independently of apoptotic caspases (Boise and Collins 2001). Pyroptotic cells are characterized by swelling followed by rupture of the plasma membrane resulting in release of cytoplasmic contents including inflammatory cytokines and alarmins. Dying cells are positive for Annexin V and TUNEL staining, but lack apoptotic markers such as nucleosome laddering and chromatin condensation (Fink and Cookson 2006), features that have been summarized more recently (Jorgensen and Miao 2015). The proinflammatory response initiated by pyroptosis can occur independently of the release of bioactive IL-1β and IL-18 (Man et al. 2017; Monack et al. 2001) due to the release of other immunostimulatory cellular contents (Davis et al. 2019). Unlike the apoptotic signaling cascade, the activation of caspase-1 is mediated by a multiprotein cytosolic complex called an inflammasome (Martinon et al. 2002) and the activation of mouse caspase-11 (human caspase-4 or caspase-5) is mediated directly by cytosolic LPS (Kayagaki et al. 2011; Kayagaki et al. 2013; Hagar et al. 2013; Aachoui et al. 2013). The inflammasome revealed the principle way IL-1 family cytokines become activated and established pyroptosis as an innate immune host defense mechanism acting against a range of pathogens (Man et al. 2017). Here, we provide an overview of the cellular and molecular events leading to activation and execution of pyroptosis and specifically discuss the beneficial and detrimental effects of pyroptosis in antiviral host defense.
2. Inflammasomes: Molecular platforms for activation of inflammatory caspases
Inflammasome assembly is mediated by the nucleotide and oligomerization domain, leucine-rich repeat–containing (NOD) receptor (NLR) family of cytosolic pattern recognition receptors (PRRs) in response to infection or other immunological challenges (Sharma and Kanneganti 2016). Sensors that can initiate inflammasome assembly include the apoptosis-associated speck-like protein containing a CARD domain (ASC)-dependent NLR family member NLRP3 (NOD family, pyrin domain-containing 3), HIN domain-containing family member AIM2 (absent in melanoma 2) and Pyrin and the ASC-independent NLRP1 and NLRC4 (NLR family, CARD domain-containing 4) (Broz et al. 2010; Pelegrin et al. 2008; Sharma and Kanneganti 2016). Apart from these five well-characterized receptors, the NLR family proteins NLRP6, NLRP7 and NLRP12 and certain nucleic acid sensors like retinoic acid inducible gene-1 (RIG-I) and IFNγ-inducible protein 16 (IFI16) are reported to facilitate caspase-1 activation (Broz and Dixit 2016). Inflammasome assembly is initiated upon recognition of pathogen-associated or damage-associated molecular patterns by these PRRs. After sensing the activating ligands or other stimuli, the sensor subsequently recruits and activates caspase-1 in either an ASC-dependent or -independent manner. Once activated, caspase-1 processes pro-IL-1β and pro-IL-18 into their biologically active forms and simultaneously triggers pyroptosis (Man and Kanneganti 2015; Sharma and Kanneganti 2016).
Caspase-11 (orthologous to human caspase-4 and caspase-5) initiates pyroptosis directly by sensing cytosolic LPS, the cell wall component of Gram-negative bacteria (Kayagaki et al. 2011; Kayagaki et al. 2013; Hagar et al. 2013; Aachoui et al. 2013). These caspases do not process pro-IL-1β and pro-IL-18 into their active forms (Shi et al. 2017), although caspase-11-mediated pyroptosis induces changes in intracellular ionic balance that may facilitate assembly of the NLRP3 inflammasome, resulting in caspase-1 activation and processing of pro-IL-1β and pro-IL-18 (Ruhl and Broz 2015).
3. Gasdermins: The executioner of pyroptosis
Although inflammatory caspases mediate pyroptosis, the gasdermin family member gasdermin D is the substrate first identified to execute membrane lysis (Kayagaki et al. 2015; Shi et al. 2015; He et al. 2015). Activated caspases cleave gasdermin D to generate independent N-terminal and C-terminal fragments (Shi et al. 2015). Once free of the autoinhibitory C-terminal fraction, the N-terminus undergoes oligomerization to form membrane pores that drive leakage and lysis of cells (Shi et al. 2015; Kayagaki et al. 2015; Ding et al. 2016; Aglietti et al. 2016; Liu et al. 2016; Sborgi et al. 2016). Thus, the pore-forming function of cleaved gasdermin D carries out execution of pyroptosis and mediates release of IL-β as this cytokine is not secreted. Such proinflammatory cytokines are only released when cells lyse (Kayagaki et al. 2015; Shi et al. 2015). In addition to proteolysis by the inflammatory caspases, gasdermin D is cleaved by apoptotic caspases, caspase-3, caspase-7 and caspase-8, although this does not always lead to activation. Whereas cleavage by caspase-8 liberates the active N-terminal domain to drive pore-forming activity and pyroptosis similar to caspase-11 (Orning et al. 2018; Sarhan et al. 2018), caspase-3 and caspase-7 cleave and inactivate gasdermin D (Rogers et al. 2017; Taabazuing et al. 2017). Although gasdermin D is a key executioner, prolonged inflammasome activation induces pyroptosis in gasdermin D-deficient cells (Shi et al. 2015), consistent with additional pyroptosis executioners (Lamkanfi et al. 2008; Akhter et al. 2009), including additional gasdermin family members. Recently, pyroptosis has extended to include all gasdermin-mediated cell death because all the gasdermin family members except DFNB59 possess pore-forming activity (Shi et al. 2017). So far, only gasdermin E (DFNA5), activated by caspase-3, has been demonstrated to follow a parallel mechanism, in parallel with apoptosis (Rogers et al. 2017; Wang et al. 2017). In addition, gasdermin D drives secondary pyroptosis and IL-1β release even in the absence of caspase 1 (Schneider et al. 2017). Thus, there is still much to be discovered in this arena.
4. Pyroptotic cell death during viral infections: The good and the bad
Programmed cell death pathways remove the replicative niche and restrict the survival and proliferation of obligate intracellular pathogens (Jorgensen and Miao 2015; Dondelinger et al. 2016). Cell death also contributes to the pathogenesis of viral infections (Jorgensen et al. 2017). Moreover, inflammasomes and inflammasome-dependent cytokines contribute to antiviral immunity and resolution of infection (Lupfer et al. 2015; He et al. 2018). Although various DNA and RNA viruses induce pyroptosis via NLRP1, NLRP3 and AIM2 inflammasomes during infection (Lupfer et al. 2015), the overlap between pyroptosis and the release of IL-1β/IL-18 restricts the ability to distinguish the cytokine-independent contributions of pyroptosis in host defense (Jorgensen et al. 2017).
NLRP1 was the first NLR shown to form an inflammasome complex mediating caspase-1 activation (Martinon et al. 2002). NLRP1-mediated pyroptosis of hematopoietic progenitor cells causes prolonged cytopenia, bone marrow hypoplasia and immunosuppression in response to lymphocytic choriomeningitis virus (LCMV) infection (Masters et al. 2012). LCMV directly infects hematopoietic progenitor cells and induces caspase-1-dependent cell death that is enhanced in mice harboring an activating mutation (Nlrp1aQ593P/Q593P). Consistent with this, NLRP1 deficiency in mice limits the induction of pancytopenia and increases virus-specific CD8+ T-cell response with improved recovery from LCMV infection (Masters et al. 2012). This study revealed detrimental effects of pyroptosis on hematopoiesis and generation of an effective immune response.
NLRP3, the most widely studied NLR, is activated during infection with diverse viruses (Lupfer and Kanneganti 2013; Lupfer et al. 2015). The NLRP3 inflammasome is thought to form in response to some change common to disruption of cellular homeostasis and ionic balance rather than any particular microbial or viral ligand (Sharma and Kanneganti 2016; Stewart and Cookson 2016). NLRP3 contributes to immunopathology as well as the quality of innate and adaptive immunity during viral infection above and beyond established microbial settings (Thomas et al. 2009; Tate et al. 2016; Lupfer and Kanneganti 2013). Although the importance of NLRP3 inflammasome-dependent antiviral responses is recognized, most effects have been attributed to the proinflammatory consequences of IL-1β and IL-18. As a result, the relative contribution of NLRP3-mediated pyroptotic cell death and inflammation during virus infection has not yet been defined.
The diverse array of DNA and RNA viruses that activate the NLRP3 inflammasome (Lupfer et al. 2015) include RNA viruses of the families Orthomyxoviridae (influenza virus), Paramyxoviridae (respiratory syncytial virus and measles virus), Rhabdoviridae (vesicular stomatitis virus and rabies virus), Picornaviridae (polio virus, enterovirus and encephalomyocarditis virus) and Flaviviridae (Hepatitis C virus, dengue virus and West Nile virus) as well as DNA viruses in the families Poxviridae (vaccinia virus and myxoma virus), Herpesviridae (herpes simplex 1, cytomegalovirus and varicella zoster) and Adenoviridae (adenovirus 5). DNA viruses such as herpes simplex virus (HSV) 1, murine cytomegalovirus (MCMV) and adenoviruses activate the NLRP3 as well as the AIM2 inflammasome (Lupfer et al. 2015).
Of particular interest is influenza virus, a long-recognized potent activator of the NLRP3 inflammasome (Kuriakose and Kanneganti 2017). The critical role of inflammasome-dependent cytokines and cell death responses have been observed during influenza virus infection in mice. Recent studies have demonstrated parallel and complementary activation of various programmed cell death pathways in response to influenza virus infection (Kuriakose et al. 2016; Kesavardhana et al. 2017; Thapa et al. 2016). Z-nucleic acid binding protein-1 (ZBP1), also known as DAI and DLM1, an IFN-inducible receptor interacting protein (RIP) homotypic interaction motif (RHIM)-containing protein, initiates the NLRP3 inflammasome as well as RIP kinase 3 (RIPK3), inducing combined pyroptosis, apoptosis and necroptosis in influenza virus-infected cells (Kuriakose et al. 2016; Thapa et al. 2016). ZBP1 senses the viral ribonucleoprotein complex of influenza A virus recruiting the RHIM-containing RIPK3 protein to transduce these diverse cell death signaling outcomes (Kesavardhana et al. 2017; Kuriakose et al. 2016). The ZBP1-RIPK3 interaction mediates influenza-induced inflammasome activation and programmed death of infected cells. Both processes are abrogated in ZBP1- and RIPK3- deficient cells (Kuriakose et al. 2016). The distinct contributions of each of these complementary pathways during influenza infection was supported by studies in cells lacking NLRP3, caspase-1, gasdermin D, RIPK3 and combined caspase-8 and RIPK3 (Kuriakose et al. 2016; Thapa et al. 2016; Nogusa et al. 2016). Based on these studies, influenza virus-infected cell death is inhibited in the absence of ZBP1 or when the three programmed cell death pathways and inflammasome activation are blocked or absent. Studies from our group added to prior understanding of innate immune signaling during influenza infection both in vitro and in vivo (Kuriakose and Kanneganti 2017), revealing a specific role for ZBP1 as a PRR that activates assembly of the NLRP3 inflammasome and the execution of pyroptosis in response to influenza infection, but not to other RNA virus infections that have been examined. We propose this unique phenomenon as ‘virus-induced unconventional NLRP3 inflammasome activation’ via ZBP1, which we refer to as the ZBP1-NLRP3 inflammasome. ZBP1 in association with RIPK3 and caspase-8 facilitate activation of the NLRP3 inflammasome that underlies IL-1β and IL-18 release (Kesavardhana et al. 2017; Kuriakose et al. 2016). Whether ZBP1-NLRP3-activated caspase-1 or caspase-8 facilitates gasdermin D cleavage or pyroptosis induction remains to be established.
ZBP1-dependent inflammasome activation and cell death restricts influenza virus replication, but contributes to lung inflammation as well as disease pathogenesis (Kuriakose et al. 2016; Thapa et al. 2016; Nogusa et al. 2016). Dengue virus infection induces activation of combined pyroptotic and apoptotic pathways that control virus replication (Suwanmanee and Luplertlop 2017). Additionally, NLRP3-mediated induction of pyroptosis is observed along with apoptosis during hepatitis C virus (HCV, strain FH1T) infection of cultured HuH-7.5 hepatoma cells, suggesting a role for combined death pathways in HCV-induced liver pathology (Kofahi et al. 2016). Any contribution of ZBP1 as a PRR in dengue or HCV infection remains to be explored.
Inflammatory caspases contribute to central nervous system pathology following infection with an attenuated Evelyn-Rotnycki-Abelseth (ERA) strain of rabies virus (Kip et al. 2017). Morbidity and clinical disease scores are elevated in Casp1−/−Casp11−/− mice compared to either WT or Il1b−/−Il18−/− mice following infection with this strain (Kip et al. 2017). Pyroptosis mediated by inflammatory caspases may therefore contribute to host defense against rabies virus independently of IL-1β and IL-18. However, inflammasome-dependent responses did not modulate disease severity during infection with Challenge Virus Standard strain-11 (CVS-11), a highly virulent neurotropic strain of rabies virus (Kip et al. 2017). NLRP3-driven inflammasome activation was also recently revealed to be a critical component of inflammation during Zika virus (ZIKAV) infection of patients (He et al. 2018).
AIM2-dependent pyroptosis in human monocyte-derived dendritic cells was reported in response to immune-complexed human adenovirus (Eichholz et al. 2016). This demonstrates how humoral immunity engages professional antigen-presenting cells to initiate an innate immune response during persistent or recurring infections. The impact of immune complex-dependent pyroptosis in host defense is unknown although the inflammatory consequences may facilitate the T cell response to adenovirus infection (Eichholz et al. 2016). Induction of AIM2 inflammasome-mediated pyroptosis also occurs during MCMV infection (Rathinam et al. 2010), however this infection induces simultaneous activation of multiple cell death pathways. Apoptosis, necroptosis and pyroptosis were observed in an experimental model of retinitis suggesting involvement of cell death in the development of AIDS-related necrotizing retinitis caused by human CMV infection (Chien and Dix 2012). In addition to these viruses, the role of caspase-1 and caspase-11 was also investigated during infection with encephalomyocarditis virus (EMCV), murine gammaherpesvirus-68, vesicular stomatitis virus (VSV) and West Nile virus (WNV) (Rajan et al. 2011; Cieniewicz et al. 2015; Ramos et al. 2012). However, the individual contributions of pyroptosis and inflammatory cytokines have not been investigated.
Apart from the well-characterized inflammasomes, the DNA sensor IFI16 and RNA sensor RIG-I are also able to complex with ASC and caspase-1 to mediate caspase-1 activation and cytokine secretion (Kerur et al. 2011; Poeck et al. 2010; Pothlichet et al. 2013). RIG-I-dependent, NLRP3-independent caspase-1 activation and IL-1β secretion occur in normal human bronchial epithelial cells infected with influenza virus and in LPS-primed BMDCs infected with VSV (Poeck et al. 2010; Pothlichet et al. 2013). Despite the fact that Kaposi’s sarcoma-associated herpes virus (KSHV) encodes a protein that can inhibit NLRs and block NLRP1-mediated inflammasome activation (Gregory et al. 2011), IFI16 associates with the replicating KSHV DNA in the nucleus of endothelial cells and assembles a complex with ASC to mediate caspase-1 activation (Kerur et al. 2011). IFI16-dependent caspase-1 activation and inflammatory cytokine production also occur in B-cell lines latently infected with KSHV or Epstein Barr virus (EBV) and, possibly, in fibroblasts infected with HSV1 (Ansari et al. 2013; Singh et al. 2013; Johnson et al. 2013), noting that later studies failed to observe similar results with HSV1 (Diner et al. 2015). Although these studies suggest that inflammasome activation occurs downstream of IFI16 and RIG-I, pyroptosis has not been observed.
5. AIDS: When pyroptosis goes off limits
Human immunodeficiency virus (HIV) infection is one of the viral infections where the detrimental effects of pyroptosis are quite apparent (Doitsh and Greene 2016). Infection with HIV leads to a spectrum of diseases resulting in an immunocompromised state referred as acquired immunodeficiency syndrome (AIDS). The progressive depletion of CD4+ T cells is the major pathophysiological feature of AIDS, however, the mechanisms responsible for massive CD4+ T cell death has remained unresolved. Recent studies have identified pyroptosis as the mechanism mediating CD4+ T cell depletion and subsequent progression of AIDS (Doitsh et al. 2014). Interestingly, cell death during HIV infection does not involve virus-infected cells (Finkel et al. 1995). Rather, most of the dying cells in infected patients are bystander CD4+ T cells that acquire HIV by cell-to-cell spread within lymphoid tissues (Finkel et al. 1995; Doitsh et al. 2014; Galloway et al. 2015). The virus undergoes abortive replication in these bystander T cells leading to accumulation of viral DNA, which is sensed by the DNA sensor IFI16 (Monroe et al. 2014) and drives ASC-dependent activation of caspase-1 and induction of pyroptosis independently of NLRP3. The pyroptotic cell death of CD4+ T cells is amplified by the release of proinflammatory mediators including ATP, which subsequently amplifies NLRP3 inflammasome signaling and thereby perpetuates a chronic inflammatory state in infected individuals (Doitsh et al. 2014). Treatment with caspase-1 inhibitors or sh-RNA-mediated knockdown of caspase-1 or ASC prevented HIV-associated CD4+ T cell death in an ex vivo human lymphoid aggregate culture system (Doitsh et al. 2014), suggesting that the host immune response to HIV infection exacerbates pathology. Because pyroptosis contributes to CD4+ T cell depletion as well as chronic inflammation, the two major consequences of HIV infection, combined treatment with inhibitors of pyroptosis and antiretroviral agents is a suggested therapeutic option that may be explored (Doitsh and Greene 2016).
6. Pyroptosis in inflammation: Effector functions by IL-1β and IL-18
The identification of gasdermin D as the main executioner of pyroptosis helps to further confirm the key role of pyroptosis in release of bioactive IL-1β and IL-18. Because these cytokines lack a signal peptide directing them to the secretory pathway, caspase-1-processed forms pass through membrane pores dependent on gasdermin-D (Kayagaki et al. 2015; Shi et al. 2015). In addition to IL-1β and IL-18, many other proinflammatory mediators are released from cells as a consequence of pyroptosis (Man et al. 2017).
IL-1β and IL-18 are known to mediate a wide range of immune and inflammatory responses. During influenza virus infection, IL-1R supports cytotoxic T-cell and antibody responses that promote survival of mice (Pang et al. 2013; Ichinohe et al. 2009). Whereas IL-1β is protective early during infection, this cytokine contributes to immunopathology and disease severity later in infection (Schmitz et al. 2005). Similar to IL-1β, IL-18 enhances immune responses and promotes viral clearance, but also contributes to immunopathology and tissue damage (Denton et al. 2007; Lupfer et al. 2013). The current understanding is that the NLRP3 inflammasome and pyroptosis are both important for antiviral responses to influenza virus. IL-1β is also crucial for control of WNV infection within the CNS such that mice lacking components of the NLRP3 inflammasome or IL-1 signaling show increased susceptibility to WNV infection (Ramos et al. 2012). Elevated levels of IL-1 β in plasma of patients infected with WNV suggests a role for this cytokine in disease pathogenesis (Ramos et al. 2012). Recently, inflammasome-driven IL-1β has also been shown to promote an IRF3-dependent interferon response during WNV infection of myeloid cells, suggesting a tight link between the inflammasome and interferons for the antiviral innate response (Aarreberg et al. 2018). IL-1β and IL-18 similarly contribute to inflammatory responses during dengue virus infection (Tan and Chu 2013; Chirathaworn et al. 2010). Additionally, HCV infection induces production of IL-1β and IL-18 from both circulatory and liver resident macrophages, suggesting a role for these cytokines in hepatic inflammation (Shrivastava et al. 2013). The NLRP3 inflammasome is regarded as a key mediator of neuroinflammation during murine Japanese encephalitis virus infection (Kaushik et al. 2012). IL-18 supports IFNγ production from NK cells and contributes to early control of MCMV infection, further demonstrating the role of inflammasome-dependent cytokines in antiviral immune and inflammatory responses (Rathinam et al. 2010).
7. Interference with pyroptosis: Virus-encoded inhibitors and decoy proteins
Viruses have evolved mechanisms that interfere with inflammasome functions that control inflammatory cytokine and cell death responses (Lamkanfi and Dixit 2011). Inflammasome assembly and signaling as well as caspase-1 enzymatic activity are inhibited by specific viral modulators (Kanneganti 2010; Stewart and Cookson 2016; Lamkanfi and Dixit 2011). The vaccinia virus F1L protein and KSHV ORF63 protein are examples of decoys that inhibit NLRP1 inflammasome assembly, caspase-1 activation and IL-1β secretion (Gregory et al. 2011; Gerlic et al. 2013). Additionally, F1L can inhibit apoptosis by interfering with cytochrome c release (Stewart et al. 2005). ORF63 also interacts and disrupts NLRP3 inflammasome assembly (Gregory et al. 2011). Measles virus V protein interacts with NLRP3 to inhibit IL-1β secretion (Komune et al. 2011). Pyrin-only proteins (POPs) encoded by poxviruses interfere with inflammasome signaling (Kanneganti 2010; Lamkanfi and Dixit 2011). Orthopoxviruses myxomavirus M13L protein and Shope fibroma virus S13L protein also inhibit inflammasome assembly (Johnston et al. 2005; Dorfleutner et al. 2007), possibly by interacting with ASC. Viruses lacking these decoy proteins are attenuated, consistent with a role in viral pathogenesis.
Orthopoxvirus-encoded serpins (serine protease inhibitors or SPIs), including cytokine response modifier A (CrmA, also known as SPI-2) encoded by cowpox and conserved broadly in variola virus, vaccinia virus and ectromelia virus, acts as a pseudo-substrate inhibitor of the proteases caspase-1, caspase-8 and granzyme B, and also inhibits TBK1 (Ray et al. 1992; Quan et al. 1995; Tewari et al. 1995). Consistent with its role in inhibiting inflammasome- and TNF-death receptor-induced caspases, immune cell granzyme and interferon induction, CrmA deficiency dramatically attenuates cowpox virus.
Amongst RNA viruses, the influenza virus NS1 protein is also known to regulate caspase-1 activation as well as IL-1β and IL-18 secretion (Stasakova et al. 2005; Kuriakose and Kanneganti 2017; Chung et al. 2015). However, just like the role of NS1 in controlling type I interferon expression (Rajsbaum et al. 2012), the effect of NS1 on NLRP3 inflammasome activation is strain specific (Park et al. 2018). While the NS1 C-terminus of the A/swine/Saskatchewan/18789/2002/H1N1 strain does not suppress NLRP3 inflammasome activation in primary porcine alveolar macrophages, the NS1 of the pandemic H1N1 strain shows a strong inhibitory effect on NLRP3 inflammasome activation (Park et al. 2018). Meanwhile, pandemic H1N1 NS1 inhibits NLRP3 inflammasome activation by counteracting ASC ubiquitination, but the other strains such as A/PR/8/34 (H1N1), A/WSN/33 (H1N1) and A/Hong Kong/483/1997 (H5N1) disrupt caspase-1 cleavage by interacting with NLRP3 (Chung et al. 2015; Moriyama et al. 2016). It has been reported that the N-terminal RNA-binding domain of NS1 is important in blocking inflammasome activation (Moriyama et al. 2016). The N-terminus of NS1 can block viral RNA through its RNA binding domain and is critical for binding to both TRIM25 and RIG-I in order to suppress antiviral type I IFN responses (Jureka et al. 2015; Gack et al. 2009; Hale et al. 2008) such that NS1 inhibition of inflammasome responses may be a consequence of antagonizing type I IFN production (Kuriakose et al. 2016; Kochs et al. 2007). In addition to targeting the inflammasome components, viruses have also evolved to inhibit pyroptosis by directly processing the executioner gasdermin D. For example, the viral protease 3C of enterovirus 71 is able to cleave in the N-terminus to inactivate gasdermin D, which is critical for virus replication (Lei et al. 2017).
8. Conclusions and future perspectives
Programed cell death during viral infections is well established as an antiviral host defense mechanism to curtail survival and replication of these intracellular pathogens. Because of its proinflammatory nature, pyroptosis triggers activation of additional inflammatory cascades and immune surveillance systems to facilitate viral clearance and recovery from infection. Whereas the antiviral functions of inflammasomes and inflammasome-dependent cytokines are investigated in detail, only limited studies have explored the importance of pyroptotic cell death in antiviral host defense. Like any other inflammatory response, pyroptosis also can be a double-edged sword with both beneficial and detrimental effects. Recent studies have uncovered the molecular and biochemical mechanisms governing activation and execution of pyroptosis and discovered additional molecules in the cascade leading to pyroptosis. However, the functional relevance of many of these components including caspase-11 and gasdermin D has not been investigated in the context of viral infections. The activation of complementary cell death pathways and overlapping effects of cell death and inflammatory cytokine secretion complicates our understanding of pyroptosis in antiviral responses. Although gasdermin D is highly expressed in the epithelial cells of the gastrointestinal tract, the induction or effects of pyroptosis during viral infections in the gastrointestinal tract is not known. The newly generated gasdermin D-deficient mice in parallel with Casp1−/−, Casp11−/−, Il-1b−/− and Il18−/− mice provide an unprecedented opportunity to dissect the unique contributions of pyroptosis during viral infections. A better understanding of the mechanisms and functions of pyroptosis during viral infections may provide therapeutic options targeted either to harness the beneficial effects or to block the harmful effects of pyroptosis. Therefore, ‘Pathfinders’ are needed to further probe the role of pyroptosis in antiviral immunity.
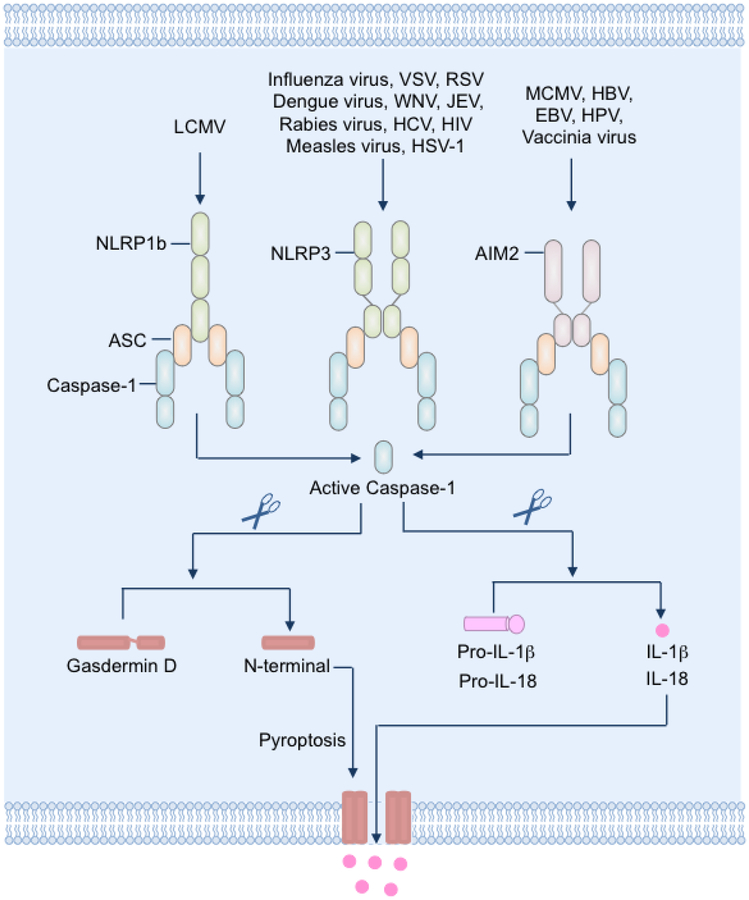
Figure 1: Induction of pyroptosis during viral infections.
The cytosolic pattern recognition receptors NLRP1, NLRP3 and AIM2 sense cytosolic pathogen-associated or danger-associated molecular patterns in virus-infected cells and trigger the formation of inflammasome complexes with the adaptor ASC and the cysteine protease caspase-1. Assembly of the inflammasome complex facilitates proteolytic activation and cleavage of the caspase-1 zymogen. Active caspase-1 in turn cleaves the inactive pro-IL-1β and pro-IL-18 into bioactive cytokines. Caspase-1 also cleaves the membrane pore-forming protein gasdermin D into N-terminal and C-terminal fragments. The N-terminal fragment of gasdermin D binds to lipids in the plasma membrane and oligomerizes to form pores. The formation of pores results in loss of cell membrane integrity and lysis of the cell. The membrane pores formed by gasdermin D also facilitate release of bioactive IL-1β and IL-18.
Acknowledgements
Research studies from our laboratory are supported by the US National Institutes of Health (AI101935, AI124346, AR056296 and CA163507 to T.D.K.) and the American Lebanese Syrian Associated Charities (to T.D.K.).
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA (2013) Caspase-11 protects against bacteria that escape the vacuole. Science 339(6122):975–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarreberg LD, Wilkins C, Ramos HJ, Green R, Davis MA, Chow K, Gale M Jr. (2018) Interleukin-1beta Signaling in Dendritic Cells Induces Antiviral Interferon Responses. mBio 9(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC (2016) GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A 113(28):7858–7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, Marsh CB, Wewers MD, Tridandapani S, Kanneganti TD, Amer AO (2009) Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5(4):e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B (2013) Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol 87(15):8606–8623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basil MC, Levy BD (2016) Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16(1):51–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise LH, Collins CM (2001) Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol 9(2):64–67 [DOI] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT (2000) Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 38(1):31–40 [DOI] [PubMed] [Google Scholar]
- Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16(7):407–420 [DOI] [PubMed] [Google Scholar]
- Broz P, von Moltke J, Jones JW, Vance RE, Monack DM (2010) Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8(6):471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kaniga K, Galan JE (1996) Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol 21(5):1101–1115 [DOI] [PubMed] [Google Scholar]
- Chien H, Dix RD (2012) Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: apoptosis, necroptosis, and pyroptosis. J Virol 86(20):10961–10978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirathaworn C, Rianthavorn P, Wuttirattanakowit N, Poovorawan Y (2010) Serum IL-18 and IL-18BP levels in patients with Chikungunya virus infection. Viral Immunol 23(1):113–117 [DOI] [PubMed] [Google Scholar]
- Chung WC, Kang HR, Yoon H, Kang SJ, Ting JP, Song MJ (2015) Influenza A Virus NS1 Protein Inhibits the NLRP3 Inflammasome. PLoS One 10(5):e0126456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieniewicz B, Dong Q, Li G, Forrest JC, Mounce BC, Tarakanova VL, van der Velden A, Krug LT (2015) Murine Gammaherpesvirus 68 Pathogenesis Is Independent of Caspase-1 and Caspase-11 in Mice and Impairs Interleukin-1beta Production upon Extrinsic Stimulation in Culture. J Virol 89(13):6562–6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA (2001) Pro-inflammatory programmed cell death. Trends Microbiol 9(3):113–114 [DOI] [PubMed] [Google Scholar]
- Davis MA, Fairgrieve MR, Den Hartigh A, Yakovenko O, Duvvuri B, Lood C, Thomas WE, Fink SL, Gale M Jr. (2019) Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc Natl Acad Sci U S A 116(11):5061–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AE, Doherty PC, Turner SJ, La Gruta NL (2007) IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol 37(2):368–375 [DOI] [PubMed] [Google Scholar]
- Diner BA, Lum KK, Cristea IM (2015) The emerging role of nuclear viral DNA sensors. J Biol Chem 290(44):26412–26421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535(7610):111–116 [DOI] [PubMed] [Google Scholar]
- Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505(7484):509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Greene WC (2016) Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 19(3):280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P (2016) An evolutionary perspective on the necroptotic pathway. Trends Cell Biol 26(10):721–732 [DOI] [PubMed] [Google Scholar]
- Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ (2015) NF-kappaB-Independent Role of IKKalpha/IKKbeta in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol Cell 60(1):63–76 [DOI] [PubMed] [Google Scholar]
- Dorfleutner A, Talbott SJ, Bryan NB, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C (2007) A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35(3):685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholz K, Bru T, Tran TT, Fernandes P, Welles H, Mennechet FJ, Manel N, Alves P, Perreau M, Kremer EJ (2016) Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog 12(9):e1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8(11):1812–1825 [DOI] [PubMed] [Google Scholar]
- Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A (1995) Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med 1(2):129–134 [DOI] [PubMed] [Google Scholar]
- Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A (2009) Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5(5):439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC (2015) Cell-to-Cell Transmission of HIV-1 Is Required to Trigger Pyroptotic Death of Lymphoid-Tissue-Derived CD4 T Cells. Cell Rep 12(10):1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlic M, Faustin B, Postigo A, Yu EC, Proell M, Gombosuren N, Krajewska M, Flynn R, Croft M, Way M, Satterthwait A, Liddington RC, Salek-Ardakani S, Matsuzawa S, Reed JC (2013) Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc Natl Acad Sci U S A 110(19):7808–7813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B (2011) Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331(6015):330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341(6151):1250–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale BG, Randall RE, Ortin J, Jackson D (2008) The multifunctional NS1 protein of influenza A viruses. J Gen Virol 89(Pt 10):2359–2376 [DOI] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25(12):1285–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Chen J, Zhu X, An S, Dong X, Yu J, Zhang S, Wu Y, Li G, Zhang Y, Wu J, Li M (2018) NLRP3 Inflammasome Activation Mediates Zika Virus-Associated Inflammation. J Infect Dis 217(12):1942–1951 [DOI] [PubMed] [Google Scholar]
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A 96(5):2396–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A (1998) Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 273(49):32895–32900 [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206(1):79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Chikoti L, Chandran B (2013) Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87(9):5005–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, McFadden G (2005) A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23(6):587–598 [DOI] [PubMed] [Google Scholar]
- Jorgensen I, Miao EA (2015) Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 265(1):130-–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Rayamajhi M, Miao EA (2017) Programmed cell death as a defence against infection. Nat Rev Immunol 17(3):151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jureka AS, Kleinpeter AB, Cornilescu G, Cornilescu CC, Petit CM (2015) Structural basis for a novel interaction between the NS1 protein derived from the 1918 Influenza virus and RIG-I. Structure 23(11):2001–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD (2010) Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol 10(10):688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Gupta M, Kumawat KL, Basu A (2012) NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS One 7(2):e32270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015) Caspase-11 cleaves gasdermin D for noncanonical inflammasome signalling. Nature 526(7575):666–671 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM (2011) Noncanonical inflammasome activation targets caspase-11. Nature 479(7371):117–121 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341(6151):1246–1249 [DOI] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9(5):363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti TD (2017) ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med 214(8):2217–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kip E, Naze F, Suin V, Vanden Berghe T, Francart A, Lamoral S, Vandenabeele P, Beyaert R, Van Gucht S, Kalai M (2017) Impact of caspase-1/11, −3, −7, or IL-1beta/IL-18 deficiency on rabies virus-induced macrophage cell death and onset of disease. Cell Death Discov 3:17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochs G, Garcia-Sastre A, Martinez-Sobrido L (2007) Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol 81(13):7011–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS (2016) Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep 6:37433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komune N, Ichinohe T, Ito M, Yanagi Y (2011) Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1beta secretion. J Virol 85(24):13019–13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Kanneganti TD (2017) Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol 86:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD (2016) ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM (2011) Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 187(2):597–602 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G (2008) Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7(12):2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J (2017) Enterovirus 71 Inhibits Pyroptosis through Cleavage of Gasdermin D. J Virol 91(18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610):153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupfer C, Kanneganti TD (2013) The expanding role of NLRs in antiviral immunity. Immunol Rev 255(1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupfer C, Malik A, Kanneganti TD (2015) Inflammasome control of viral infection. Curr Opin Virol 12:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, Huang G, Green M, Kundu M, Chi H, Xavier RJ, Green DR, Lamkanfi M, Dinarello CA, Doherty PC, Kanneganti TD (2013) Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol 14(5):480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Kanneganti TD (2015) Regulation of inflammasome activation. Immunol Rev 265(1):6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426 [DOI] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, Cengia LH, Henley KJ, Collinge JE, Kastner DL, Feigenbaum L, Hilton DJ, Alexander WS, Kile BT, Croker BA (2012) NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37(6):1009–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Navarre WW, Falkow S (2001) Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect 3(14–15):1201–1212 [DOI] [PubMed] [Google Scholar]
- Monack DM, Raupach B, Hromockyj AE, Falkow S (1996) Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A 93(18):9833–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC (2014) IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343(6169):428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Chen IY, Kawaguchi A, Koshiba T, Nagata K, Takeyama H, Hasegawa H, Ichinohe T (2016) The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1beta Secretion. J Virol 90(8):4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, Verbist K, Gough PJ, Bertin J, Hartmann BM, Sealfon SC, Kaiser WJ, Mocarski ES, Lopez CB, Thomas PG, Oberst A, Green DR, Balachandran S (2016) RIPK3 activates parallel pathways of MLKL-criven necroptosis and FADD-mediated apoptosis to protect against Influenza A virus. Cell Host Microbe 20(1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E (2018) Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362(6418):1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IK, Ichinohe T, Iwasaki A (2013) IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol 14(3):246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Liu G, Thulasi Raman SN, Landreth SL, Liu Q, Zhou Y (2018) NS1 Protein of 2009 Pandemic Influenza A Virus Inhibits Porcine NLRP3 Inflammasome-Mediated Interleukin-1 Beta Production by Suppressing ASC Ubiquitination. J Virol 92(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Barroso-Gutierrez C, Surprenant A (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol 180(11):7147–7157 [DOI] [PubMed] [Google Scholar]
- Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J (2010) Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol 11(1):63–69 [DOI] [PubMed] [Google Scholar]
- Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, Vidal SM (2013) Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog 9(4):e1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan LT, Caputo A, Bleackley RC, Pickup DJ, Salvesen GS (1995) Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem 270(18):10377–10379 [DOI] [PubMed] [Google Scholar]
- Rajan JV, Rodriguez D, Miao EA, Aderem A (2011) The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J Virol 85(9):4167–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU (2012) Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog 8(11):e1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M Jr. (2012) IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 8(11):e1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11(5):395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ (1992) Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69(4):597–604 [DOI] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017) Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8:14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Broz P (2015) Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol 45(10):2927–2936 [DOI] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A 115(46):E10888–E10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35(16):1766–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Kurrer M, Bachmann MF, Kopf M (2005) Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79(10):6441–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KS, Gross CJ, Dreier RF, Saller BS, Mishra R, Gorka O, Heilig R, Meunier E, Dick MS, Cikovic T, Sodenkamp J, Medard G, Naumann R, Ruland J, Kuster B, Broz P, Gross O (2017) The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep 21(13):3846–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kanneganti TD (2016) The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol 213(6):617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665 [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Mukherjee A, Ray R, Ray RB (2013) Hepatitis C virus induces interleukin-1beta (IL-1beta)/IL-18 in circulatory and resident liver macrophages. J Virol 87(22):12284–12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B (2013) Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol 87(8):4417–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasakova J, Ferko B, Kittel C, Sereinig S, Romanova J, Katinger H, Egorov A (2005) Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J Gen Virol 86(Pt 1):185–195 [DOI] [PubMed] [Google Scholar]
- Stewart MK, Cookson BT (2016) Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses. Nat Rev Microbiol 14(6):346–359 [DOI] [PubMed] [Google Scholar]
- Stewart TL, Wasilenko ST, Barry M (2005) Vaccinia virus F1L protein is a tail-anchored protein that functions at the mitochondria to inhibit apoptosis. J Virol 79(2):1084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanmanee S, Luplertlop N (2017) Immunopathogenesis of dengue virus-induced redundant cell death: apoptosis and pyroptosis. Viral Immunol 30(1):13–19 [DOI] [PubMed] [Google Scholar]
- Taabazuing CYO, Okondo MC, Bachovchin DA(2017) Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24(4):507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TY, Chu JJ (2013) Dengue virus-infected human monocytes trigger late activation of caspase-1, which mediates pro-inflammatory IL-1beta secretion and pyroptosis. J Gen Virol 94(Pt 10):2215–2220 [DOI] [PubMed] [Google Scholar]
- Tate MD, Ong JD, Dowling JK, McAuley JL, Robertson AB, Latz E, Drummond GR, Cooper MA, Hertzog PJ, Mansell A (2016) Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep 6:27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Telford WG, Miller RA, Dixit VM (1995) CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem 270(39):22705–22708 [DOI] [PubMed] [Google Scholar]
- Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, tenOever BR, Thomas PG, Upton JW, Balachandran S (2016) DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20(5):674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Dash P, Aldridge JR Jr., Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30(4):566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG W; Shi X; Ding J; Liu W; He H; Wang K; Shao F (2017) Chemotherapy drugs induce pyroptosis through caspse-3 cleavage of a gasdermin. Nature 547(7661):99–103 [DOI] [PubMed] [Google Scholar]
- Watson PR, Gautier AV, Paulin SM, Bland AP, Jones PW, Wallis TS (2000) Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun 68(6):3744–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature 358(6382):167–169 [DOI] [PubMed] [Google Scholar]