Abstract
The mechanism for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection requires the binding of the virus to the angiotensin-converting enzyme 2 (ACE2) receptor, well-known for its role in counteracting ACE. ACE2 is involved in modulating blood pressure and establishing blood pressure homeostasis. Recently, a critical debatable question has arisen whether using antihypertensive medications will have a favorable impact on people infected with SARS-CoV-2 or a deleterious one, mainly because angiotensin-converting enzyme inhibitor (ACEI) and angiotensin-receptor blocker (ARB) therapy can modulate the expression of ACE2 protein. The concern is that the use of ACEIs and ARBs will increase the expression of ACE2 and increase patient susceptibility to viral host cell entry and propagation. On the other hand, several genetic association studies have examined the relationship between ACE2 genetic variants and the risk of developing hypertension in different ethnic populations. In this review, we discuss the ongoing arguments in the literature about ACE2’s role in mortality rate among coronavirus disease 2019 (COVID-19) patients comorbid with hypertension and critically evaluate the current debate about the usage or discontinuation of ACEI/ARB antihypertensive drugs. Moreover, we explore the two opposing roles that ACE2 genetic variants might be playing in COVID-19 by reducing ACE2 receptor effectiveness and mitigating SARS-CoV-2 infectivity.
Keywords: ACE2, hypertension, genetic variants, angiotensin-renin system, SARS-CoV-2, ACEI, ARBs
Graphical Abstract
Bosso et al. discuss the ongoing arguments about ACE2’s role in the mortality associated with COVID-19 cases and weigh on the current debate about the usage or discontinuation of ACEI/ARB anti-hypertensive medication. They also explore the two opposing roles that ACE2 genetic variants might be playing in COVID-19.
Main Text
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic of acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The mortality rates, as well as the infectious capacity of the virus, ranging from 1% to >5%, have raised a major concern worldwide. Older people with comorbid conditions, such as pulmonary disease, cardiac disease, kidney disease, diabetes, and hypertension, are associated with higher mortality rates.1 According to recent literature reports, it is now well accepted that hypertension is associated with increased mortality rates in COVID-19 patients. For example, Wu et al.2 found hypertension to have a hazard ratio of 1.70 for death and 1.82 for acute respiratory distress syndrome (ARDS) in 201 patients with COVID-19. Zhou et al.3 also found hypertension to have a hazard ratio of 3.05 for in-hospital mortality in 191 patients with COVID-19.
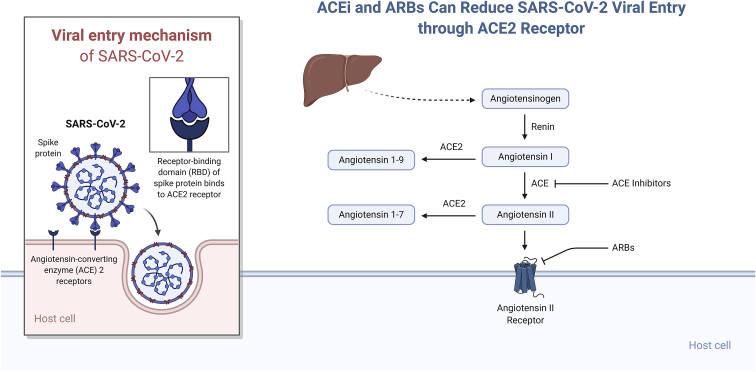
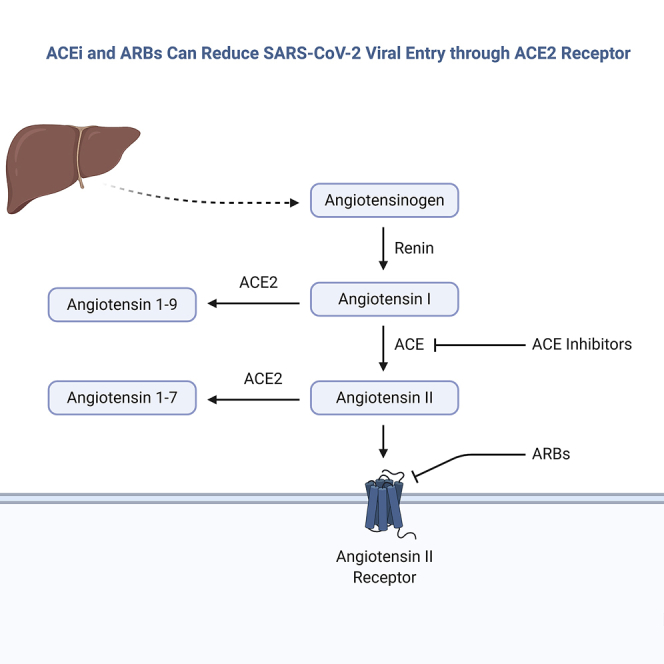
The mechanism for SARS-CoV-2 infection necessitates the binding of the virus to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) receptor and internalization of the complex by the host cell (Figure 1). Apart from its role as a receptor for SARS-CoV-2 (and for both the SARS-CoV and the related human respiratory coronavirus NL63), ACE2 is well-known for its role in hypertension. ACE2 modulates blood pressure and maintains blood pressure homeostasis through negatively regulating the renin-angiotensin system (RAS).4,5 ACE and its homolog ACE2 are two key enzymes involved in the synthesis of bioactive components of the RAS.6 ACE2 exerts its functions through cleaving either angiotensin I (Ang I) or Ang II into the inactive peptides Ang (1–9) and Ang (1–7), respectively (Figure 1). Ang (1–9) gets further metabolized into Ang (1–7). Ang (1–7) is a vasodilator, hence ACE2 counteracts the vasoconstrictor effects of the ACE-Ang II axis. The mechanism by which ACE2 antagonizes the effects of Ang II is either by cleaving the precursor Ang I, which reduces Ang II synthesis in tissues, or by directly hydrolyzing Ang II and reducing its levels in plasma.
Figure 1.
ACE2 in the Entry of SARS-CoV-2 into the Host Cell
Illustration of the two key arms in the renin-angiotensin system.
Both ACE and ACE2 are endothelium-bound carboxypeptidases that can be cleaved by different metalloproteases located on the cell surface and released in a soluble form. Contrary to ACE, which is widely expressed in many tissues and organs, ACE2’s high expression is confined to the endothelial cells of the arteries, arterioles, and venules of the heart and kidney.4 Therefore, ACE2 has been a potential therapeutic target in treating hypertension and cardiac dysfunctions. Several animal studies, carried out on diet-induced hypertension rat models, have established a link between increased blood pressure and reduced mRNA expression and protein levels of ACE2. Data showed that low levels of ACE2 can lead to elevated levels of Ang II and consequently hypertension.4 Animal studies have suggested that ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may upregulate ACE2 expression, thus increasing the availability of target molecules for SARS-CoV-2.
The Leeds Family Study by Rice et al.7 is one of the first studies to demonstrate the link of ACE2 polymorphisms and inheritance of hypertension and levels of circulating ACE2. Several genetic association studies have examined the relationship between ACE2 genetic variants and the risk of developing hypertension in different ethnic populations (Table 1).8 One of the highly reported variants is rs2285666; AG genotype at this variant is protective because it poses a lower risk to develop hypertension in females.9,10 In contrast, AA genotype, as opposed to AG+GG at the same single-nucleotide polymorphism (SNP) rs2285666, has been reported to be associated with a higher risk of hypertension in different ethnic populations.11, 12, 13 Other highly reported variants known to be associated with high risk to develop hypertension are rs2106809 with TT genotype and rs879922 with C allele.10,14, 15, 16, 17, 18 However, the European population study revealed the absence of an association between rs879922 and high blood pressure.19 Further, although there are several genetic studies on ACE2’s role in hypertension using different continental populations (such as China, Europe, Australia, and India), ethnic populations such as from the Middle East are not included in these global studies. Thus, genetic association data involving ACE2 with hypertension have been debatable in terms of transferability across different ethnicities.
Table 1.
Reported ACE2 Polymorphisms Associated with the Risk of Hypertension and/or Cardiovascular Disease in Different Populations/Ethnicities
| Polymorphism ID | Allele and/or Genotype | Ethnicity/Population Size | Associated Predisposition to Hypertension | Publications |
|---|---|---|---|---|
| rs4646188 | T | China | high | 17,18 |
| rs2074192 | T | China | high | 17,18,32 |
| rs4646155 | T, TT | China | high | 10,17,18 |
| rs4240157 | C | China | high | 17,18 |
| rs4830542 | C | China | high | 17,18 |
| rs879922 | C | China | high | 10,17,18 |
| rs2106809 | TT, T | China, India | high | 14, 15, 16, 17, 18,32 |
| rs2285666 | A, TC, G, AA | Europe metaanalysis (five cohorts: ATBC, FINRISK, Northern Sweden, PRIME/Belfast, and PRIME/France), China | low (preventive) | 9,10 |
| rs2285666 | GG, AA | Europe, meta-analysis (China, Anglo Celtic Australian) | high | 11, 12, 13 |
| rs1514283 | CC | China | high | 10 |
| rs4646176 | GG | China | high | 10 |
| A1075G (rs1978124) | GG | China, Europe, meta-analysis (China, Anglo Celtic Australian) | high | 12,40,41 |
| rs6632677 | C | China | high | 42 |
Importantly, ACE2 is currently at the center of an intense debate among cardiologists; there are concerns on whether medical management of hypertension involving the use of inhibitors of the renin-angiotensin-aldosterone system (RAAS) (such as ACEIs and ARBs) have a favorable impact on people infected with SARS-CoV-2 or a deleterious one mainly because ACEI and ARB therapies can modulate the expression of ACE2. The concern is that the use of ACEIs and ARBs will increase the expression of ACE2 and increase patient susceptibility to viral host cell entry and propagation. As a result, there has been a call for the discontinuation of ACEI/ARB usage prophylactically and in the context of suspected COVID-19 cases.
In this review, we shed light on the current debate about ACE2’s role in the mortality associated with COVID-19 cases with hypertension and weigh on the current argument about the usage or discontinuation of ACEI/ARB hypertensive medication. Finally, we explore the role of ACE2 genetic variants in the predisposition for hypertension and the response to hypertension treatments.
ACE2 and Hypertension Medication
Several epidemiological studies have established the augmented mortality in COVID-19 patients with hypertension. For instance, Wu et al.2 and Zhou et al.3 had found hypertension to have a hazard ratio of 1.70 and 3.05 for death in 201 and 191 patients with COVID-19, respectively. However, a critical question that remains unanswered is whether this association is solely attributed to the pathogenesis of hypertension or to the associated comorbidity or therapy. In this section, we review recent findings regarding the role of antihypertensive medications, which have been at the center of a considerable debate, namely, ACEIs and ARBs. The main question has been whether they have a favorable impact on the people infected with SARS-CoV-2 or a deleterious one mainly because ACEI and ARB therapies can modulate the expression of ACE2, which has been identified as a receptor for SARS-COV2. The concern is that the use of ACEIs and ARBs will increase the expression of ACE2 and increase patient susceptibility to viral host cell entry and propagation. As a result, there has been a call for the discontinuation of ACEIs/ARBs usage prophylactically and in the context of suspected COVID-19.
However, other groups have been suggesting the opposite, where increased ACE2 can act as a vasodilator, antioxidant, and anti-inflammatory. This is because ACE2 primarily acts to counterpoise the effect of ACE. Because ACE produces Ang II from Ang I, ACE2 generates Ang (1–9) from Ang I or Ang (1–7) from Ang II, respectively. Ang (1–7) possesses vasodilator, antioxidant, and anti-inflammatory properties that, upon binding to the Mas receptor broadly, shifts the balance from vasoconstriction with Ang II to vasodilation with Mas receptor activation in the affected vascular bed.20,21 The role this vasodilatory effect has in the pathogenesis of SARS-CoV-2 is unclear, but some animal data suggest a connection. ACE2 and Ang (1–7) are protective in several different lung injury models.
Despite the lack of evidence, there have been advocates for both the use and the cessation of ACEIs, ARBs, or both during the treatment for COVID-19 in patients with hypertension.5 Given the common use of ACEIs and ARBs worldwide, guidance based on experimental evidence on the use of these drugs in patients with COVID-19 is immensely needed. A few studies have emerged that tackled this question, providing initial data to answer this question. In one retrospective single-center case series study of 1,178 COVID-19 cases, Li et al.4 examined the association between the severity and mortality of COVID-19 and antihypertensive medications ACEIs or ARBs. A total of 362 patients were hypertensive and 115 patients were treated with ACEIs or ARBs. The authors observed no difference in the severity of COVID-19, as well as mortality rates between the people taking ACEIs or ARBs or not. Similarly, there was no difference in severity or mortality rates between patients taking ACEIs and those taking ARBs.4 Vaduganathan et al.22 in a recent paper explicitly support the continuation of RAAS inhibitors in patients in otherwise stable clinical condition who are at risk for COVID-19 or have COVID-19.
Similarly, Reynolds et al.5 have recently investigated the likelihood of testing positive or increased COVID-19 severity in people in New York who are taking various antihypertensive therapies and diuretics among 5,894 COVID-19 patients, of whom 2,573 had hypertension. They showed that none of these medications (ACEIs, ARBs, beta-blockers, calcium-channel blockers, or thiazide diuretics) were associated with increased infectivity to SARS-CoV-2 or increased COVID-19 severity. The study predefined a 10% difference as a substantial difference, even though the study was powered to detect as low as a 6% difference. It is worth mentioning that the data pointed to a modest decrease in the likelihood of testing positive for SARS-CoV-2, but not the severity in patients taking beta-blockers, that was slightly significant in an analysis that included all matched patients. One caveat of both of the studies (namely, that of Li et al.4 and Reynolds et al.5) is the lack of adjustment for potential confounders, such as socioeconomic status, insurance, or health care access in propensity scores.23 Nonetheless, both studies highlighted that ACEIs and ARBs, as well as other antihypertensive drugs, were safe to use.23,24
In contrast, Zhang et al. showed in a retrospective multi-center study, which included 1,128 COVID-19 patients with hypertension, that mortality was lower in people taking ACEIs and ARBs.25 The study compared 188 patients taking ACEIs/ARBs with 940 patients not using these medications from nine hospitals in Hubei Province, China, from December 31, 2019, to February 20, 2020. The mortality rate in patients taking either ACEIs or ARBs was 3.7% versus 9.8% in those not taking ACEIs/ARBs. This lowered mortality risk was persistent even after adjusting for confounders in a propensity-score-matched analysis.25
Along the same lines, Bean et al.26 assessed 53 patients out of 205 COVID-19 patients who took ACEIs within 7 days before symptom onset or during hospitalization (notably, this study has not yet been peer-reviewed and hence should be viewed tentatively). Their data showed that treatment with ACEIs was associated with a reduced risk of rapidly deteriorating severe disease. There was a lesser rate of death or transfer to the ICU within 7 days in patients on an ACEI; an odds ratio of 0.29 (confidence interval [CI]: 0.10–0.75; p < 0.01) was observed after adjusting for age, gender, and several comorbidities, such as hypertension, diabetes mellitus, ischemic heart disease, and heart failure. They concluded that among hospitalized COVID-19 patients with hypertension, inpatient usage of ACEIs/ARBs was associated with a lesser risk of all-cause mortality compared with those not using ACEIs/ARBs.26
Interestingly, binding of the SARS-CoV-2 spike protein (S-protein) to ACE2 has been proposed to induce ACE2 shedding from the cell surface, which might, in turn, reduce ACE2 surface expression.27 Such a concept is becoming an attractive research field to ameliorate the response and prognosis in hypertensive patients infected by SARS-CoV-2.8,22,28,29 Additionally, some investigators proposed the refurbishment of ACE2 by the administration of recombinant ACE2 (rhACE2) to converse the lung-damage process during viral infections.30 These effects are being studied in a current randomized, open-label, controlled clinical study (ClinicalTrials.gov: NCT04287686), together with the use of losartan as the first therapy for COVID-19 in hospitalized (ClinicalTrials.gov: NCT04312009) or not hospitalized patients (ClinicalTrials.gov: NCT04311177).
Is There a Common Genetic Basis Linking ACE2 with Both Hypertension and SARS-CoV-2 Infection?
The points presented so far propose that the link, involving ACE2, between the pre-existent condition of hypertension and an elevated risk of SARS-CoV-2 infection (and the resultant mortality) is mostly due to the regulation of ACE2 expression, which is mediated by the use of hypertensive medication. In this section, we evaluate the following: (1) the expression quantitative trait locus (eQTL) variants that regulate the expression of ACE2 gene, (2) ACE2 polymorphisms altering the efficacy of hypertensive medication can have an impact on SARS-CoV-2 infection, and (3) ACE2 polymorphisms that predispose to hypertension can also alter the SARS-COV-2 infection rates.
-
1.
eQTL variants regulating the ACE2 gene: Higher levels of ACE2 expression are supposed to lead to high levels of coronavirus infection. The Genotype-Tissue Expression (GTEx) database revealed 15 eQTL variants that regulate the expression of ACE2 in human (Table 2). Whereas two of the variants, namely, rs112171234 and rs75979613, downregulate the expression of ACE2, the others upregulate. The eQTL variants are from the following genes: the intergenic region of (ACE2, BMX), FANCB, the intergenic region of (MOSPD2, ASB9), ACE2, the intergenic region of (CA5BP1, CA5B), and CLTRN. Almost all of these variants are “common” variants (minor allele frequency ≥ 5%) in one or the other population, raising the possibility that genetic regulation of ACE2 expression can be more widespread. However, none of these variants are associated with hypertension in any of the global studies, ruling out the possibility of a common genetic mechanism for the onset of hypertension and SARS-CoV-2 infection. Even at the level of genes, which harbor these eQTL variants, none other than ACE2 is known to be directly involved in the onset of hypertension and COVID-19; however, it requires further studies to examine whether these genes are contributing to more extensive pathways regulating hypertension or COVID-19.
-
2.
ACE2 polymorphisms altering the efficacy of hypertensive medication: A recent review by Oliveira-Paula et al.31 on pharmacogenomics of ACEIs and ARBs anti-hypertensive medications lists a number of insertions/deletions in ACE2 gene and a number of SNPs from a wide spectrum of genes (ACE, CYP11B2, NOS3, BDKRB2, BDKRB2/PRKCA/NOS3, PRKCA, VEGFA, CAMK1D, SCNN1G, GPR83, FUT4, Nphs1). However, none of these genes are known to be involved in the onset of SARS-CoV-2 infection or replication. As regards polymorphisms in ACE2 modulating the efficacy of ACEI, we can cite ACE2 rs2106809; this has been reported as an important predictive factor of the response to antihypertensive treatment with ACEIs in Chinese female hypertensive patients.32 The T allele leads to reduced response to ACEIs in women.33 However, it is not known whether the alteration of responsiveness is due to altered expression of ACE2, although it is known that the rs2106809 (and rs2074192) can be the determinant of circulating Ang (1–7) levels in hypertensive females.32 Considering that this variant is intronic, it is not expected to have an effect on the protein sequence of ACE2 and thereby on the binding efficiency of SARS-CoV-2 S-protein and ACE2 receptor. However, functional studies are needed to confirm the impact of this variant on the expression of ACE2 receptor and its availability for SARS-COV-2 infection.
-
3.
ACE2 polymorphisms that predispose to hypertension can also alter the SARS-COV-2 infection rates: Several variants from ACE2 have been associated with high or low risk for hypertension by global studies on various populations (Table 1).8 The fact that all of these variants are intronic does not rule out their possible impacts on the expression and structure of ACE2 receptor gene and its associated genes, especially if they are placed in an enhancer or in intronic splice-site junction positions. In a study by Luo et al.,18 one of these variants, namely, rs2285666, was associated with hypertension in Chinese, Finland, Northern Sweden, France, and Belfast;9, 10, 11 Asselta et al.,35 studied the association between the three genotypes at ACE2 rs2285666 and ACE2 protein level as measured by ELISA, and identified A/A genotype as having an expression level almost 50% lower than the G/G genotype.35 Considering the position of the variant, at nucleotide +4 in the donor splice site of intron 3 (c.439+4G>A), they calculated the predicted impact on splicing and the substitution of G with an A, and their results were in agreement with the higher level of ACE2 protein in serum.10 Such data suggest that the predisposition to hypertension through genetic variants of ACE2 has the potential to alter the expression levels of ACE2, and thereby can account for different infection rates for the virus in people with hypertension compared with people without hypertension. Other variants of ACE2, although they do not alter the expression of the ACE2 gene, can be deleterious in terms of rendering the expressed protein dysfunctional.
Table 2.
eQTL Variants that Regulate the Expression of ACE2
| Variant ID | Gene | Tissue | GTEx, p Value | GTEx, NES | Minor Allele Frequency |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| KWT | IRN | QAR | EUR | EAS | AFR | |||||
| rs112171234a | ACE2a, BMXa | adipose: visceral (omentum) | 4.1E−5 | −0.85 | 0.00 | 0.00 | 0.06 | 6.4E−4 | 0.00 | 0.20 |
| rs75979613 | FANCB | breast: mammary tissue | 3.3E−5 | −0.44 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 1.7E−3 |
| rs12010448 | MOSPD2, ASB9 | muscle: skeletal | 9.8E−5 | 0.43 | 0.00 | 0.00 | 0.03 | 1.9E−4 | 0.00 | 0.11 |
| rs4646127a | ACE2a | nerve: tibial | 7.5E−9 | 0.2 | 0.00 | 0.00 | 0.58 | 0.62 | 1.00 | 0.78 |
| rs5936029 | CA5BP1-CA5B | brain: nucleus accumbens (basal ganglia) | 9.6E−16 | 0.6 | 0.00 | 0.00 | 0.49 | 0.48 | 1.00 | 0.87 |
| rs6632704 | CLTRN | nerve: tibial | 4.6E−16 | 0.26 | 0.00 | 0.00 | 0.41 | 0.48 | 0.95 | 0.50 |
| rs1996225 | CA5BP1-CA5B | nerve: tibial | 3.3E−17 | 0.27 | 0.00 | 0.42 | 0.39 | 0.36 | 0.82 | 0.60 |
| rs6629110a | ACE2a | nerve: tibial | 3.4E−15 | 0.25 | 0.00 | 0.00 | 0.41 | 0.47 | 0.96 | 0.43 |
| rs2158082a | ACE2a | nerve:tibial | 1.0E−16 | 0.28 | 0.51b | 0.00 | 0.55 | 0.48 | 1.00 | 0.93 |
| rs4830974 | CLTRN | brain: frontal cortex (BA9) | 2.6E−17 | 0.60 | 0.47b | 0.00 | 0.48 | 0.48 | 0.95 | 0.72 |
| rs5936011 | CLTRN | brain: nucleus accumbens (basal ganglia) | 3.2E−16 | 0.62 | 0.00 | 0.00 | 0.50 | 0.48 | 1.00 | 0.86 |
| rs4060 | CA5BP1-CA5B | brain: frontal cortex (BA9) | 2.6E−17 | 0.60 | 0.04 | 0.50 | 0.44 | 0.48 | 0.95 | 0.65 |
| rs4830983 | CA5BP1-CA5B | nerve: tibial | 3.0E−16 | 0.28 | 0.00 | 0.00 | 0.51 | 0.48 | 1.00 | 0.93 |
NES, normalized effect size. With the exceptions noted below, all variants are located within genes other than ACE2 and can affect the expression of ACE2.
Variants are located within ACE2 and can affect ACE2 gene expression.
The two minor allele frequencies that are found in reference to the Kuwaiti population.
A recent pioneering report on the genetics of ACE2 in the context of SARS-CoV-2 infection by Cao et al.36 reported a systematic allele frequency analysis of coding region variants in the ACE2 gene variants and eQTL variants that affect expression levels of ACE2 among Chinese and other continental populations. They found differences in allele frequencies at coding variants in different populations. Further, they found higher allele frequencies at ACE2-upregulating eQTL variants in East Asian populations. They further proposed that different populations may exhibit a different response to SARS-CoV-2 because of such genetic differences.
In a recent paper, Al-Mulla et al.37 showed that K26R and N720D were the most frequent ACE2 missense variants in the global datasets. Al-Mulla et al.37 suggested that the ACE2 receptor N720D variant may enhance transmembrane protease, serine 2 (TMPRSS2) activation and subsequent viral entry. In agreement with their analysis, structural predictions by Stawiski et al.38 revealed that the K26R missense variant enhanced the affinity of ACE2 for SARS-CoV-2, whereas N720D had little involvement in the SARS-CoV-2 S-protein interaction. Interestingly, the prevalence of the most ACE2 activating variant N720D was much higher among Europeans (2.5%) and Iranians (0.6%) when compared with Kuwaitis (0.3%), Qataris (0.2%), and other global populations (0.4%), and minor allele frequency (MAF) of these variants significantly correlated with mortality rates in the corresponding countries. Furthermore, their data also showed two important missense variants that have been identified in the Irani population, rs769062069 (R708Q, p.Arg708Gln), and in Kuwaiti and Irani populations, rs776995986 (R708W, p.Arg708Trp), with positive functional risk prediction scores. However, these two variants were not detected in Europe or Qatar populations. Both variants lie in ACE2’s TMPRSS2-cleavage site. The cleavage of ACE2 by TMPRSS2 can enhance SARS-CoV-2 viral entry into the cell. Thus, damaging variants in the TMPRSS2-cleavage site within ACE2 would be protective against SARS-CoV2 infection. Specifically, mutating variants of arginine and lysine residues in the amino acid sequences that correspond to R697-R716 in the TMPRSS2-cleavage region in the ACE2 would inhibit processing of ACE2 by TMPRSS2 enzyme.39 These variants are suggested to be protective in the Kuwaiti population and could be another explanation for the low infectivity rate in the Kuwaiti population.
Nonetheless, Al-Mulla et al.37 highlighted the need for further functional assessments to confirm their predictions. Furthermore, based on eQTL variants expression, the authors showed that people from an Arab background had a very low frequency of upregulating variants and higher downregulating variants compared with Europeans. The resultant lower levels of ACE2 in this population, although leading to a high prevalence of hypertension, can possibly lead to lower availability of receptor for the entry of SARS-COV-2 in this population and lower infectivity/mortality.
Conclusions
Taken together, various scientific evidence provides tentative reassurance that, at least, ACEIs and ARBs are not harmful in COVID-19 patients. Whether they are truly beneficial is now being studied prospectively. In agreement with our suggestion, Vaduganathan et al.22 propose that both ACE2 expression and activity could be beneficial in patients with lung injury because of their anti-inflammatory effect through the Ang (1–7) axis. In addition, Vaduganathan et al.22 disagree with the discontinuation of ACEIs in patients otherwise with stable condition and who are infected with SARS-CoV-2 or susceptible to COVID-19.
In several trials, COVID-19 patients who already were taking these drugs are being randomized to continue or to stop them. Furthermore, we must consider the higher rate of cardiac injury and adverse outcomes in hypertensive patients during the COVID-19 pandemic. Undeniably, the loss of ACEIs/ARBs cardiopulmonary protective effects could be damaging. Additionally, based on genetic analysis, the eQTL variants regulating the expression of ACE2 gene are not so far shown to be associated with hypertension; however, the ACE2 polymorphisms associated with hypertension or with the efficacy of ACEI or ARB could have the potential to alter the binding of SARS-COV-2 S-protein with ACE2 receptor. Moreover, it requires further studies involving large cohorts to evaluate the genetic association of the above-mentioned eQTL variants and the gene loci with hypertension traits, and to evaluate the involvement of the above-mentioned gene loci that affect the efficacy of ACEI and ARB in SARS-CoV-2 infection and proliferation. Hence ACEIs/ARBs chronic therapy should not be discontinued in hypertensive patients with COVID-19. Moreover, in the absence of adequate follow-up visits, changing ACEIs/ARBs with another anti-hypertensive therapy could lead to inadequate control of blood pressure. Thus, as recommended by several medical associations, and in light of more scientific evidence supporting their beneficial/non-harmful impact, ACEIs/ARBs should be continued in COVID-19 patients.
Author Contributions
M.B., M.A.-F., T.A.T., M.A., F.A.-M., and J.A. all contributed to the design, writing, and planning of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The study was supported by Kuwait Foundation for the Advancement of Sciences (KFAS) COVID-19 resilience grant.
Contributor Information
Jehad Abubaker, Email: jehad.abubakr@dasmaninstitute.org.
Fahd Al-Mulla, Email: fahd.almulla@dasmaninstitute.org.
References
- 1.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., China Medical Treatment Expert Group for COVID-19 Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Published online March 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaddam R.R., Chambers S., Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm. Allergy Drug Targets. 2014;13:224–234. doi: 10.2174/1871528113666140713164506. [DOI] [PubMed] [Google Scholar]
- 7.Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 8.Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vangjeli C., Dicker P., Tregouet D.A., Shields D.C., Evans A., Stanton A.V., MORGAM project A polymorphism in ACE2 is associated with a lower risk for fatal cardiovascular events in females: the MORGAM project. J. Renin Angiotensin Aldosterone Syst. 2011;12:504–509. doi: 10.1177/1470320311405557. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q., Cong M., Wang N., Li X., Zhang H., Zhang K., Jin M., Wu N., Qiu C., Li J. Association of angiotensin-converting enzyme 2 gene polymorphism and enzymatic activity with essential hypertension in different gender: A case-control study. Medicine (Baltimore) 2018;97:e12917. doi: 10.1097/MD.0000000000012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong J., Yan Z., Liu D., Ni Y., Zhao Z., Zhu S., Tepel M., Zhu Z. Association of angiotensin-converting enzyme 2 gene A/G polymorphism and elevated blood pressure in Chinese patients with metabolic syndrome. J. Lab. Clin. Med. 2006;147:91–95. doi: 10.1016/j.lab.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu W., Qi Y., Hou S., Zhou W., Qiu C. Correlation of angiotensin-converting enzyme 2 gene polymorphisms with stage 2 hypertension in Han Chinese. Transl. Res. 2007;150:374–380. doi: 10.1016/j.trsl.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Lu N., Yang Y., Wang Y., Liu Y., Fu G., Chen D., Dai H., Fan X., Hui R., Zheng Y. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol. Biol. Rep. 2012;39:6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu D., Chen Y., Zhang P., Zhong J., Jin L., Zhang C., Lin S., Wu S., Yu H. Association between circulating levels of ACE2-Ang-(1-7)-MAS axis and ACE2 gene polymorphisms in hypertensive patients. Medicine (Baltimore) 2016;95:e3876. doi: 10.1097/MD.0000000000003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patnaik M., Pati P., Swain S.N., Mohapatra M.K., Dwibedi B., Kar S.K., Ranjit M. Association of angiotensin-converting enzyme and angiotensin-converting enzyme-2 gene polymorphisms with essential hypertension in the population of Odisha, India. Ann. Hum. Biol. 2014;41:145–152. doi: 10.3109/03014460.2013.837195. [DOI] [PubMed] [Google Scholar]
- 16.Wang S.X., Fu C.Y., Zou Y.B., Wang H., Shi Y., Xu X.Q., Chen J.Z., Song X.D., Huan T.J., Hui R.T. Polymorphisms of angiotensin-converting enzyme 2 gene associated with magnitude of left ventricular hypertrophy in male patients with hypertrophic cardiomyopathy. Chin. Med. J. (Engl.) 2008;121:27–31. [PubMed] [Google Scholar]
- 17.Pan Y., Wang T., Li Y., Guan T., Lai Y., Shen Y., Zeyaweiding A., Maimaiti T., Li F., Zhao H., Liu C. Association of ACE2 polymorphisms with susceptibility to essential hypertension and dyslipidemia in Xinjiang, China. Lipids Health Dis. 2018;17:241. doi: 10.1186/s12944-018-0890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y., Liu C., Guan T., Li Y., Lai Y., Li F., Zhao H., Maimaiti T., Zeyaweiding A. Association of ACE2 genetic polymorphisms with hypertension-related target organ damages in south Xinjiang. Hypertens. Res. 2019;42:681–689. doi: 10.1038/s41440-018-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieb W., Graf J., Götz A., König I.R., Mayer B., Fischer M., Stritzke J., Hengstenberg C., Holmer S.R., Döring A. Association of angiotensin-converting enzyme 2 (ACE2) gene polymorphisms with parameters of left ventricular hypertrophy in men. Results of the MONICA Augsburg echocardiographic substudy. J. Mol. Med. (Berl.) 2006;84:88–96. doi: 10.1007/s00109-005-0718-5. [DOI] [PubMed] [Google Scholar]
- 20.Santos R.A. Angiotensin-(1-7) Hypertension. 2014;63:1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 21.El-Hashim A.Z., Renno W.M., Raghupathy R., Abduo H.T., Akhtar S., Benter I.F. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-κB-dependent pathways. Br. J. Pharmacol. 2012;166:1964–1976. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yagil Y., Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension. 2003;41:871–873. doi: 10.1161/01.HYP.0000063886.71596.C8. [DOI] [PubMed] [Google Scholar]
- 24.Bitker L., Burrell L.M. Classic and Nonclassic Renin-Angiotensin Systems in the Critically Ill. Crit. Care Clin. 2019;35:213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crackower M.A., Sarao R., Oliveira-dos-Santos A.J., Da Costa J., Zhang L. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 26.Bean D., Kraljevic Z., Searle T., Bendayan R., Pickles A., Folarin A., Roguski L., Noor K., Shek A., O’Gallagher K. Treatment with ACE-inhibitors is not associated with early severe SARS-Covid-19 infection in a multi-site UK acute Hospital Trust. ResearchGate. 2020 doi: 10.13140/RG.2.2.34883.14889/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020;16:305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Hao G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa093. Published online April 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira-Paula G.H., Pereira S.C., Tanus-Santos J.E., Lacchini R. Pharmacogenomics and hypertension: Current insights. Pharm. Genomics Pers. Med. 2019;12:341–359. doi: 10.2147/PGPM.S230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y.Y., Zhang P., Zhou X.M., Liu D., Zhong J.C., Zhang C.J., Jin L.J., Yu H.M. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J. Clin. Pharm. Ther. 2018;43:189–195. doi: 10.1111/jcpt.12625. [DOI] [PubMed] [Google Scholar]
- 33.Fan X., Wang Y., Sun K., Zhang W., Yang X., Wang S., Zhen Y., Wang J., Li W., Han Y., Study Group for Pharmacogenomic Based Antihypertensive Drugs Selection, Effects and Side Effects, in Rural Area Chinese Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin. Pharmacol. Ther. 2007;82:187–196. doi: 10.1038/sj.clpt.6100214. [DOI] [PubMed] [Google Scholar]
- 35.Asselta R., Paraboschi E.-M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020;12:10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-mulla F., Mohammad A., Al Madhoun A., Haddad D., Ali H., Eaaswarkhanth M., John S.E., Nizam R., Channanath A., Abu-Farha M. A comprehensive germline variant and expression analyses of ACE2, TMPRSS2 and SARS-CoV-2 activator FURIN genes from the Middle East: Combating SARS-CoV-2 with precision medicine. bioRxiv. 2020 doi: 10.1101/2020.05.16.099176. [DOI] [Google Scholar]
- 38.Stawiski E.W., Diwanji D., Suryamohan K., Gupta R., Fellouse F.A., Sathirapongsasuti J.F., Liu J., Jiang Y.-P., Ratan A., Mis M. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. bioRxiv. 2020 doi: 10.1038/s42003-021-02030-3. 2020.04.07.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjafield A.V., Wang W.Y.S., Morris B.J. No association of angiotensin-converting enzyme 2 gene (ACE2) polymorphisms with essential hypertension. Am. J. Hypertens. 2004;17:624–628. doi: 10.1016/j.amjhyper.2004.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer B.R., Jarvis M.D., Pilbrow A.P., Ellis K.L., Frampton C.M., Skelton L., Yandle T.G., Doughty R.N., Whalley G.A., Ellis C.J. Angiotensin-converting enzyme 2 A1075G polymorphism is associated with survival in an acute coronary syndromes cohort. Am. Heart J. 2008;156:752–758. doi: 10.1016/j.ahj.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Wang S., Fu C., Zou Y., Wang H., Shi Y., Xu X., Chen J., Song X., Huan T., Hui R. Polymorphisms of angiotensin-converting enzyme 2 gene associated with magnitude of left ventricular hypertrophy in male patients with hypertrophic cardiomyopathy. Chin. Med. J. (Engl). 2008;121:27–31. [PubMed] [Google Scholar]