Abstract
Introduction
There are limited data on the association of kidney dysfunction with prognosis in coronavirus disease 2019 (COVID-19), and the extent to which acute kidney injury (AKI) predisposes patients to severe illness and inferior outcomes is unclear. We aim to assess the incidence of AKI among patients with COVID-19 and examine their associations with patient outcomes as reported in the available literature thus far.
Methods
We systematically searched MEDLINE, EMBASE, SCOPUS, and MedRxiv databases for full-text articles available in English published from December 1, 2019 to May 24, 2020. Clinical information was extracted and examined from 20 cohorts that met inclusion criteria, covering 13,137 mostly hospitalized patients confirmed to have COVID-19. Two authors independently extracted study characteristics, results, outcomes, study-level risk of bias, and strength of evidence across studies. Neither reviewer was blind to journal titles, study authors, or institutions.
Results
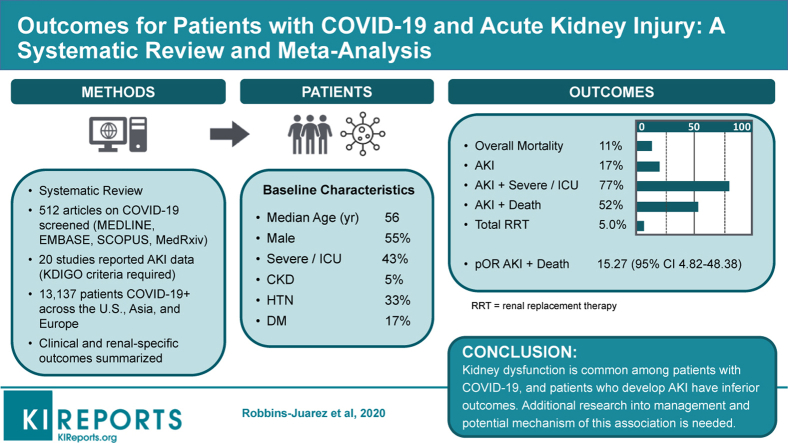
Median age was 56 years, with 55% male patients. Approximately 43% of patients had severe COVID-19 infection, and approximately 11% died. Prevalence of AKI was 17%; 77% of patients with AKI experienced severe COVID-19 infection, and 52% died. AKI was associated with increased odds of death among COVID-19 patients (pooled odds ratio, 15.27; 95% CI 4.82-48.36), although there was considerable heterogeneity across studies and among different regions in the world. Approximately 5% of all patients required use of renal replacement therapy (RRT).
Conclusions
Kidney dysfunction is common among patients with COVID-19, and patients who develop AKI have inferior outcomes. Additional research into management and potential mechanisms of this association is needed.
Keywords: acute kidney injury, COVID-19, meta-analysis, mortality, renal replacement therapy, systematic review
Graphical abstract
After the emergence of a cluster of infections causing respiratory failure in Wuhan, Hubei Province, China in December 2019, researchers identified severe acute respiratory syndrome coronavirus 2 as the causative pathogen for the respiratory disease later named COVID-19 by the World Health Organization.1 Morbidity and mortality from COVID-19 have been primarily attributed to respiratory failure and acute respiratory distress syndrome, often in the setting of multiorgan failure.2, 3, 4
The incidence of AKI and impact on the patient outcomes with COVID-19 remains unclear but is of particular interest given the need to plan for deploying limited RRT options in an acute clinical setting and questions about prognosis. Reports from previous outbreaks of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus described notable rates of acute renal failure, ranging from 5% to 15% of total cases, and utilization of continuous RRT for 5% to 58% of critically ill patients.5, 6, 7, 8 The extent to which pre-existing chronic kidney disease (CKD), including end-stage kidney disease, or the development of AKI is associated with severe COVID-19 and inferior outcomes is also not clear given their association with relative immune dysregulation and exaggerated inflammatory responses.9 AKI can lead to impaired acid-base, fluid, and electrolyte homeostasis, all of which may contribute to worse outcomes for patients with COVID-19. Given the high prevalence of kidney disease in the United States, which currently has more cases of COVID-19 than any other nation, an improved understanding of the associated risks and the need for the resources and the impact on outcomes are needed to inform clinical management and resource planning during the pandemic. Given the relative paucity of reports focused on kidney-related outcomes, in this systematic review we assess the incidence of AKI among patients with COVID-19 and examine their associations with patient outcomes as reported in the available literature thus far.
Methods
Data Source and Searches
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplementary Figure S1). Literature for this review was identified by searching MEDLINE, SCOPUS, EMBASE, and medRxiv databases. The following search terms were used: (“COVID” OR “SARS-COV-2”) AND (“outcomes” OR “clinical features” OR “clinical characteristics”) AND (“acute kidney injury” OR “acute renal injury” OR “acute kidney failure” OR “acute renal failure” OR “chronic kidney disease” OR “chronic renal disease” OR “chronic kidney insufficiency” OR “chronic renal insufficiency”). The search was limited to original research published between December 1, 2019 and May 24, 2020 with full text available in English.
Study Selection
Articles were initially screened by title and abstract to assess for relevance; those with specialized populations such as pediatric, pregnant, transplant, end-stage kidney disease, or cancer patients were excluded, as were reviews, case reports, cross-sectional studies, and randomized controlled trials for drug therapies. The full texts of the remaining studies were then assessed for the following inclusion criteria: retrospective and prospective cohort studies and case series with more than 20 patients, with extractable quantitative data on patient demographics as well as data on AKI, CKD, interventions used, and outcomes. We stringently excluded all studies that did not specify use of the Kidney Disease: Improving Global Outcomes criteria to define AKI.10 When multiple studies were published from the same institution with data from similar time periods with likely overlapping cohorts, we selected 1 study for inclusion in the meta-analysis, based on the following criteria in order of priority: the most general population, most detailed extractable kidney-related data, and largest number of patients included. Two authors independently screened all retrieved studies and assessed full-text articles for inclusion; disagreements were resolved through consensus. Neither reviewer was blind to journal titles, study authors, or institutions.
Data Extraction and Quality Assessment
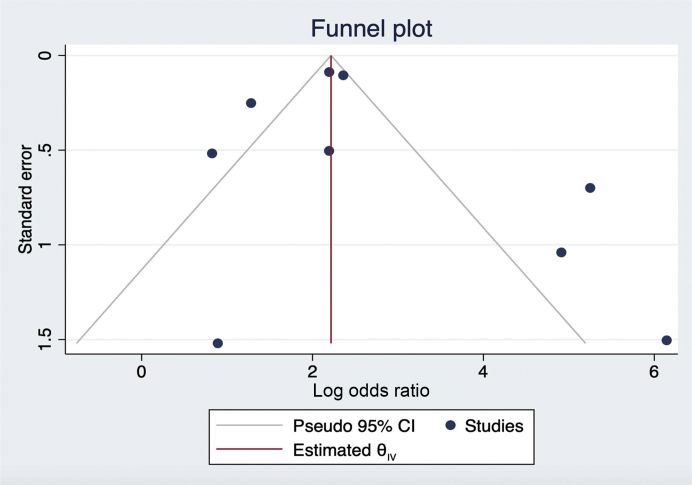
Clinical information including cohort demographics and prevalence of comorbidities including CKD were extracted. Similarly, information on all kidney-related complications and outcomes including incidence of AKI, need for RRT, and mortality were identified and extracted. The primary outcomes of interest were mortality and severe illness, each modeled as a binary outcome. Definitions of severe COVID-19 infection were inconsistent across studies, with European and U.S. studies using admission to an intensive care unit (ICU) to stratify patients, whereas most studies from China used the National Health Commission of the People’s Republic of China Clinical Severity Definitions.11 We defined “severe illness” as either admitted to an ICU or categorized as severe or critical based on the National Health Commission of the People’s Republic of China criteria. The quality of individual studies was assessed based on the National Heart, Lung, and Blood Institute Study Quality Assessment Tool for Case Series Studies (Supplementary Table S1). Small-study effects and publication bias were assessed visually using a random-effects model funnel plot.
Data Synthesis and Analysis
Medians, interquartile ranges, and overall ranges were calculated for continuous demographic and clinical variables from all reported study-level values among the 20 studies meeting inclusion criteria. Random-effects meta-analyses were performed to obtain pooled prevalence, pooled odds ratio (OR) estimates, and 95% confidence intervals (CIs) for categorical variables and for severe illness and mortality outcomes using the meta and metaprop commands. After the initial analysis, heterogeneity in mortality outcomes was investigated by excluding cohorts with particularly high mortality and by conducting stratified analyses. We stratified studies based on preprint versus peer-reviewed status, geographic location (Europe, Asia, or United States), and illness severity (<50% or ≥50% of the cohort severely ill). All analyses were performed using Stata 16 (College Station, TX), and an alpha of 0.05 determined statistical significance.
Results
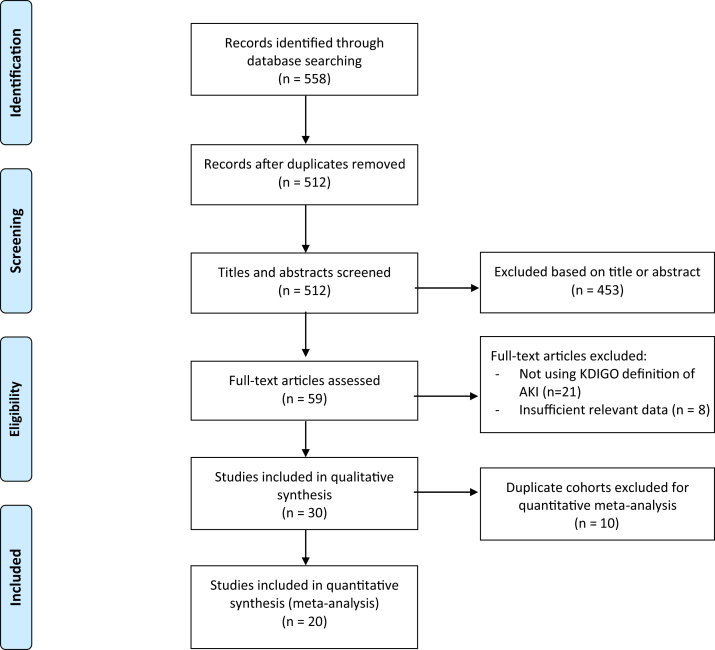
We screened a total of 512 articles, of which 59 full-text articles were assessed for eligibility and 30 met our inclusion criteria (Figure 1).3,4,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 The included studies encompassed 21,591 patients from hospitals in Asia, Europe, and the United States and took place from December 11, 2019 through May 24, 2020. All studies included only hospitalized patients, except for Guan et al.,4 which included both outpatients and inpatients. Four studies were limited to patients admitted to the ICU only.12,26,34,37 Five studies included only deceased and/or discharged patients.16,28,30,38,39 After accounting for potential overlapping patient cohorts in these smaller studies, we identified 20 unique cohorts encompassing 13,137 patients with available kidney disease–related information.4,12, 13, 14, 15,19, 20, 21, 22, 23, 24,26,30,32,33,35, 36, 37, 38, 39 Of these 20 cohorts included in the statistical analysis, 13 were from China, 1 was from Korea, 3 were from the United States, and 3 were from Europe. The quality of peer-reviewed studies and preprint studies were similar (Supplementary Table S1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses search flowsheet. AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes.
Across the 20 cohorts, the median age was 56 years (range, 43–72 years) with 55% male patients (Table 1).3,4,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Forty-three percent of patients (range, 13.3%–100%) either required an ICU admission or were reported to have severe infection, and 11% of patients (range, 0–52.4%) died. Only 5% of all patients (range, 0.6%–57.1%) were reported to have evidence of CKD at baseline; however, 1 ICU cohort from the United States reported a CKD prevalence of 57.1%.12 The prevalence of diabetes was 17% (range, 6%–33.3%) and of hypertension was 33% (range, 11.5%–64.7%). Only 1 study, by Pei et al.,22 reported presence of proteinuria (43.9%) or hematuria (26.7%) on admission. Four studies reported the prevalence of the use of renin-angiotensin-aldosterone system inhibition before admission (24%; range, 11.5%–32%).19,22,24,26
Table 1.
Demographic characteristics of cohorts selected for quantitative or qualitative analysis
| Cohort/study | Hospital and location | Population | No. of cases | Demographics |
ICU or severe coronavirus (%) | Creatinine |
Comorbidities |
Other |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median age, yr | Sex (% male) | All | ICU or severe coronavirus | Chronic kidney disease (%) | Hypertension (%) | Diabetes mellitus (%) | Proteinuria (%) | Hematuria (%) | Home renin-angiotensin-aldosterone system inhibitor (%) | |||||
| Cohorts selected for quantitative analysis | ||||||||||||||
| Arentz et al.12 | Evergreen, Washington, USA | ICU | 21 | 70 | 52 | 21 (100)a | 1.45 | 1.45 | 12 (57.1) | — | 7 (33.3) | — | — | — |
| Brill et al.13 | Barnett Hospital, London, UK | Hospitalized | 450 | 72 | 60.4 | — | — | — | 43 (195) | 30 (134) | — | — | — | |
| Cai et al.14 | Third People’s, Shenzhen, China | Hospitalized | 298 | 47 | 49 | 58 (20)b | 0.71 | 0.81 | — | 41 (15.8) | 18 (6) | — | — | — |
| Chan et al.15 | Mt. Sinai, New York, USA | Hospitalized | 3235 | 66.5 | 57.7 | 815 (25.2)a | 0.95 | — | 10 (323) | 36.9 (1193) | 24.7 (800) | — | — | — |
| Guan et al.4 | 522 hospitals, China | All | 1099 | 47 | 58.1 | 173 (15.7)c | — | — | 8 (0.7) | 165 (14.9) | 81 (7.4) | — | — | — |
| Hirsch et al.19 | 13 hospitals, New York, USA | Severe + critical | 5449 | 64 | 60.9 | 1395 (25.6)a | 1.01 | — | — | 3037 (55.7) | 1797 (33) | — | — | 1556 (28.6) |
| Hong et al.20 | Yeungnam, Daegu, Korea | Hospitalized + ICU | 98 | 55.4 | 38.8 | 13 (13.3)a | 0.9 | 1.0 | — | 30 (30.6) | 9 (9.2) | — | — | — |
| Jiang et al.21 | Wuxi 5th People’s Hospital, Jiangsu, China | Hospitalized | 55 | 45 | 49.1 | 8 (14.5)d | 0.63 | 0.75 | 1.8 (1) | 30.9 (17) | 16.4 (9) | — | — | — |
| Pei et al.22 | Tongji (Sino-French), Wuhan, China | Hospitalized, chronic kidney disease excluded | 333 | 56.3 | 54.7 | 189 (56.8)d | 0.80 | 0.88 | — | 107 (32.2) | 76 (22.9) | 219 (65.8) | 139 (41.7) | 37 of 321 (11.5) |
| Qiu et al.23 | 2 hospitals, Hunan, China | Hospitalized | 104 | 43 | 47.1 | 16 (15.4)d | 0.75 | — | — | 11.5 (12) | 14.4 (15) | — | — | — |
| Regina et al.24 | Lausanne University Hospital, Switzerland | Hospitalized | 200 | 70 | 60 | — | 1.01 | — | 14 (28) | 43.5 (87) | 21.5 (43) | — | — | 51 (26.2) |
| Rubin et al.26 | University Hospital of Bordeaux, France | ICU | 71 | 61.2 | 77 | 71 (100)a | 1.31 | 1.31 | 6 (4) | 61 (43) | 30 (21) | — | — | 23 (32) |
| Wang et al.30 | Zhongnan and Xishui, Wuhan, China | Deceased + discharged | 107 | 51 | 53.3 | — | 0.81 | — | 3 (2.8) | 26 (24.3) | 11 (10.3) | — | — | — |
| Xiao et al.32 | Hankou Hospital, Wuhan, China | Hospitalized | 287 | 62 | 55.7 | 124 (43)e | — | — | 1.7 (5) | 30.3 (87) | 15.7 (45) | — | — | — |
| Yan et al.33 | Multiple hospitals, Hainan, China | Hospitalized | 168 | 51 | 48.2 | 36 (21.4)d | 0.7 | 0.65 | 0.6 (1) | 14.3 (24) | 7.1 (12) | — | — | — |
| Zhang et al.35 | Renmin, Wuhan, China | Hospitalized | 663 | 55.6 | 48.4 | 409 (61.7)d | — | — | — | — | — | — | — | — |
| Zhao et al.36 | You’an Hospital, Beijing, China | Hospitalized | 77 | 52 | 44.2 | 20 (26)d | 0.72 | 0.77 | 6.5 (5) | 20.8 (16) | 7.8 (6) | — | — | — |
| Zheng et al.37 | 1st Aff. Hosp. of Zhejiang U. Coll. of Medicine, Hangzhou, China | ICU | 34 | 66 | 67.6 | 34 (100)a | 0.95 | 0.95 | 5.9 (2) | 64.7 (22) | 23.5 (8) | — | — | — |
| Zhou et al.38 | Jinyintan, Wuhan, China | Deceased + discharged | 191 | 56 | 62 | 119 (62.3)d | — | — | 2 (1) | 58 (30.4) | 36 (18.8) | — | — | — |
| Zhou et al.39 | 2 Hospitals, Yichang, China | Discharged | 197 | 56 | 50.3 | 56 (28.4)a | 1.21 | 1.5 (3) | — | 9.1 (18) | — | — | — | |
| Cohorts for qualitative analysis only | ||||||||||||||
| Chen et al.16 | Tongji, Wuhan, China | Deceased + discharged | 274 | 62 | 62 | — | 0.86 | — | 4 (1) | 93 (34) | 47 (17) | — | — | — |
| Chen et al.17 | Tongji, Wuhan, China | Hospitalized | 21 | 56 | 81 | 11 (52.4)d | 0.92 | 0.93 | — | 5 (23.8) | 3 (14.3) | — | — | — |
| Cheng et al.18 | Tongji and Huazhong, Wuhan, China | Hospitalized | 701 | 63 | 52.4 | 297 (42.4)d | 0.88 | — | 101 (14.4) | 233 (33.4) | 100 (14.3) | 194 of 442 (43.9) | 118 of 442 (26.7) | 33 (4.7) |
| Huang et al.3 | Jinyintan, Wuhan China | Hospitalized | 41 | 49 | 73 | 13 (31.7)a | 0.84 | 0.9 | 4 (9.8) | 6 (14.6) | 8 (19.5) | — | — | — |
| Richardson et al.25 | 12 hospitals, New York, USA | Hospitalized | 5700 | 63 | 60.3 | 1281 (22.5)a | — | — | 454 (8.0) | 3026 (56.6) | 1808 (33.8) | — | — | 456 of 2411 (18.9) |
| Shi et al.27 | Renmin, Wuhan, China | Hospitalized | 416 | 64 | 49.3 | — | 0.67 | — | 14 (3.4) | 127 (30.5) | 60 (14.4) | — | — | — |
| Shi et al.28 | Renmin Hospital, Wuhan, China | Deceased | 101 | 71 | 59.4 | — | 0.9 | — | 9.9 (10) | 58.4 (59) | 20.8 (21) | — | — | — |
| Wang et al.31 | Renmin, Wuhan, China | Hospitalized + age > 60 yr | 339 | 69 | 49 | 239 (70.5)d | — | — | 13 (3.9) | 138 (40.8) | 54 (16) | — | — | — |
| Wang et al.29 | Zhongnan, Wuhan, China | Hospitalized | 138 | 56 | 54.3 | 36 (26.1)a | 0.82 | 0.91 | 4 (2.9) | 43 (31.1) | 14 (10.1) | — | — | — |
| Yang et al.34 | Jinyintan and Wuhan Pulmonary, Wuhan, China | ICU | 52 | 59.7 | 67 | 52 (100)a | 0.79 | 0.79 | — | — | 9 (17) | — | — | — |
ATS CAP, American Thoracic Society community-acquired pneumonia; COVID-19, coronavirus disease 2019; ICU, intensive care unit; NHC, National Health Commission.
Some studies separated cohorts into ICU or non-ICU hospitalized populations, whereas other studies separated cohorts based on the National Health Commission of the People’s Republic of China COVID-19 clinical guidelines, where severe disease was defined as any 1 of the following: tachypnea with respiratory rate ≥ 30, Spo2 ≤ 93% at rest, or Pao2/Fio2 ≤ 300 mm Hg. Cai et al. used imaging criteria to define severity, whereas Guan et al., used the ATS CAP guidelines.
ICU.
Imaging.
ATS CAP guidelines.
NHC.
Severity criteria not specified.
The prevalence of AKI across the 20 cohorts was 17%, with a range of 0.5% to 80.3% (Table 2).3,4,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Six studies provided a breakdown of the severity of AKI using Kidney Disease: Improving Global Outcomes staging (15% stage 1, 7% stage 2, 11% stage 3), with Xiao et al.32 not differentiating between stage 2 and stage 3 (data were only used for stage 1).15,19,22,24,26 Approximately 77% of patients (range, 39.3%–100%) with AKI either had evidence of severe infection or needed ICU level of care according to the 14 studies that reported on the association of AKI and severity of illness. Among the 8 studies that reported use of RRT for any indication, 5% of total patients (range, 0.8%–14.7%) required RRT.
Table 2.
Outcomes of cohorts selected for quantitative or qualitative analysis
| Cohort/study | Population | No. of cases | Mortality (%) | AKI |
CKD |
Renal replacement therapy used (% total number of cases) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (%) | ICU or severe coronavirus (% total AKI) | Death (% total AKI) | Total (%) | ICU or severe coronavirus (% total CKD) | Death (% total CKD) | |||||
| Cohorts selected for quantitative analysis | ||||||||||
| Arentz et al.12 | ICU | 21 | 11 (52.4) | 4 (19) | 4 (100) | — | 12 (57.1) | 12 (100) | — | — |
| Brill et al.13 | Hospitalized | 450 | 173 (38.4) | 85 (19) | — | 54 (53) | — | — | — | — |
| Cai et al.14 | Hospitalized | 298 | 3 (1) | 17 (5.7) | 13 (22.4) | — | — | — | — | 4 (1.3) |
| Chan et al.15 | Hospitalized | 3235 | 771 (23.8) | 1406 (44) | 39.3 | 638 (45) | 323 (10) | — | — | 280 (8.7) |
| Stage 1: 492 (15) | ||||||||||
| Stage 2: 281 (9) | ||||||||||
| Stage 3: 633 (20) | ||||||||||
| Guan et al.4 | All | 1099 | 15 (1.4) | 6 (0.5) | 5 (83.3) | — | 8 (0.7) | 3 (37.5) | — | 9 |
| Hirsch et al.19 | Severe + critical | 5449 | 888 (16.3) | 1993 (37) | 1060 (53.2) | 694 (34.8) | — | — | — | 285 (5.2) |
| Stage 1: 927 (17) | ||||||||||
| Stage 2: 447 (8) | ||||||||||
| Stage 3: 619 (11) | ||||||||||
| Hong et al.20 | Hospitalized + ICU | 98 | 5 (5.1) | 9 (9.2) | 8 (88.9) | — | — | — | — | 3 (3.1) |
| Jiang et al.21 | Hospitalized | 55 | 0 (0) | 3 (69.5) | 66.7 | — | 1 (1.8) | — | — | — |
| Pei et al.22 | Hospitalized, CKD excluded | 333 | 29 (8.7) | 22 (6.6) | — | 3 (13.6) | — | — | — | — |
| Stage 1: 4 (1.2) | ||||||||||
| Stage 2: 7 (2.1) | ||||||||||
| Stage 3: 11 (3.3) | ||||||||||
| Qiu et al.23 | Hospitalized | 104 | 1 (1) | 2 (1.9) | — | — | — | — | — | — |
| Regina et al.24 | Hospitalized | 200 | 25 (12.5) | 48 (24) | — | — | 28 (14) | — | — | — |
| Stage 1: 41 (21) | ||||||||||
| Stage 2: 4 (2) | ||||||||||
| Stage 3: 3 (1.5) | ||||||||||
| Rubin et al.26 | ICU | 71 | 4 (6) | 57 (80) | 100 | 4 (7) | 4 (6) | 4 (100) | — | 10 (14) |
| Stage 1: 20 (28) | ||||||||||
| Stage 2: 20 (28) | ||||||||||
| Stage 3: 17 (24) | ||||||||||
| Wang et al.30 | Deceased + discharged | 107 | 19 (17.8) | 14 (13.1) | — | 14 (100) | 3 (2.8) | — | 1 (33.3) | — |
| Xiao et al.32 | Hospitalized | 287 | 19 (6.6) | 55 (19) | 61.8 | 12 (22) | 5 (1.7) | — | — | — |
| Stage 1: 41 (14) | ||||||||||
| Stages 2 and 3: 14 (5) | ||||||||||
| Yan et al.33 | Hospitalized | 168 | 6 (3.6) | 6 (3.6) | 50 | — | 1 (0.6) | 1 (100) | — | — |
| Zhang et al.35 | Hospitalized | 663 | 25 (3.8) | 68 (10) | 56 (82.3) | 5 (7.4) | — | — | — | — |
| Zhao et al.39 | Hospitalized | 77 | 5 (6.5) | 2 (2.6) | 50 | — | 5 (6.5) | — | — | — |
| Zheng et al.37 | ICU | 34 | 0 (0) | 7 (20.6) | 100 | — | 2 (5.9) | 2 (100) | — | 5 (14.7) |
| Zhou et al.38 | Deceased + discharged | 191 | 54 (28.3) | 28 (14.7) | 28 (100) | 27 (96.4) | 2 (1) | 2 (100) | 2 (100) | 10 |
| Zhou et al.39 | Discharged | 197 | 28 (14.2) | 16 (8.1) | — | — | 3 (1.5) | — | — | — |
| Cohorts for qualitative analysis only | ||||||||||
| Chen et al.16 | Deceased + discharged | 274 | 113 (14) | 29 (11) | — | 28 (96.6) | 4 (1) | — | — | — |
| Chen et al.17 | Hospitalized | 21 | 4 (19) | 2 (9.5) | 2 (100) | — | — | — | — | — |
| Cheng et al.18 | Hospitalized | 701 | 113 (16.1) | 36 (5.1) | — | — | 101 (14.4) | — | — | — |
| Stage 1: 13 (1.9) | ||||||||||
| Stage 2: 9 (1.3) | ||||||||||
| Stage 3: 14 (2) | ||||||||||
| Huang et al.3 | Hospitalized | 41 | 6 (14.6) | 3 (7.3) | 3 (100) | — | 4 (9.8) | — | — | 3 (7.3) |
| Richardson et al.25 | Hospitalized | 5700 | 553 (9.7) | 1370 (24) | — | — | 454 (8.0) | — | — | 225 (3.9) |
| Shi et al.27 | Hospitalized | 416 | 57 (13.7) | 8 (1.9) | — | — | 14 (3.4) | — | — | 2 (0.5) |
| Shi et al.28 | Deceased | 101 | 101 (100) | 24 (23.8) | 100 | — | 10 (9.9) | — | — | 5 (5) |
| Wang et al.31 | Hospitalized + age > 60 yr | 339 | 65 (19.2) | 27 (8.1) | — | 17 | 13 (3.9) | — | 4 (30.8) | — |
| Wang et al.29 | Hospitalized | 138 | 6 (4.3) | 5 (3.6) | 3 (60) | — | 4 (2.9) | 2 (50) | — | 2 |
| Yang et al.34 | ICU | 52 | 32 (61.5) | 15 (28.8) | 15 (100) | 12 (80) | — | — | — | 9 |
AKI, acute kidney injury; CKD, chronic kidney disease; ICU, intensive care unit.
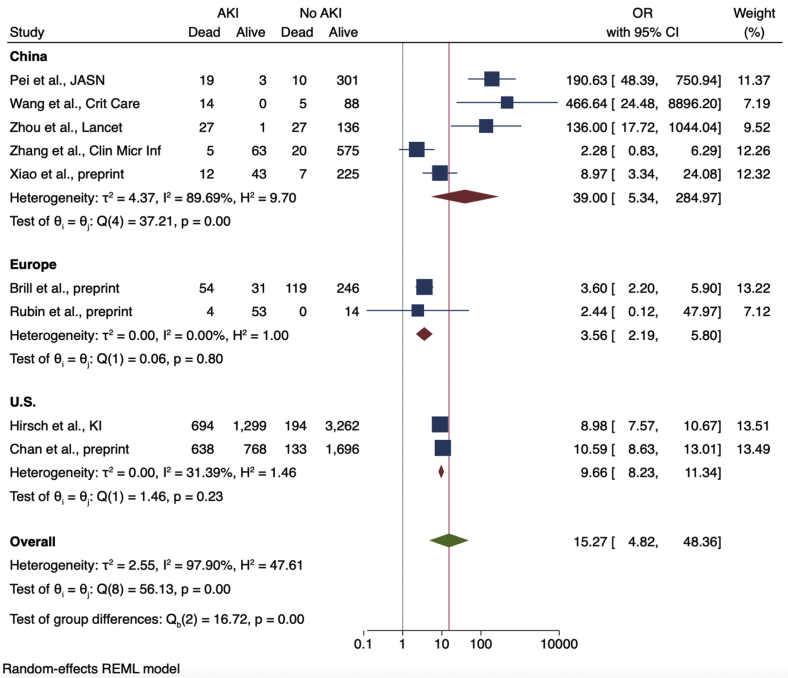
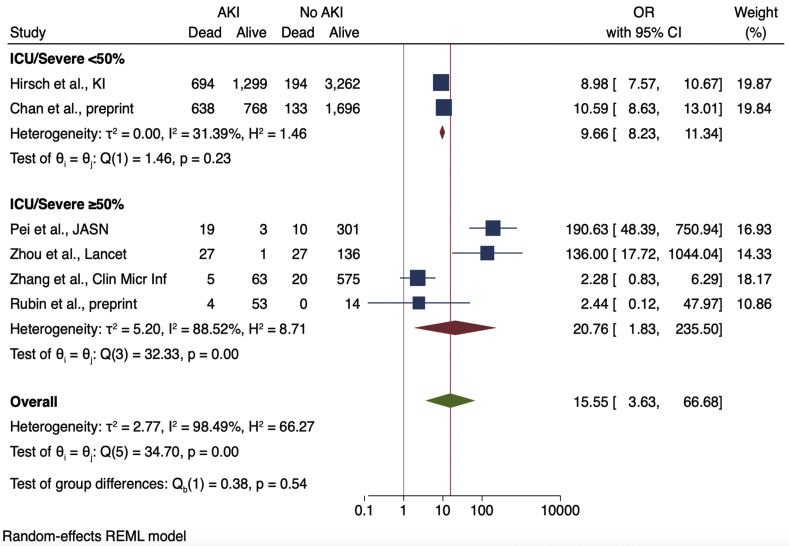
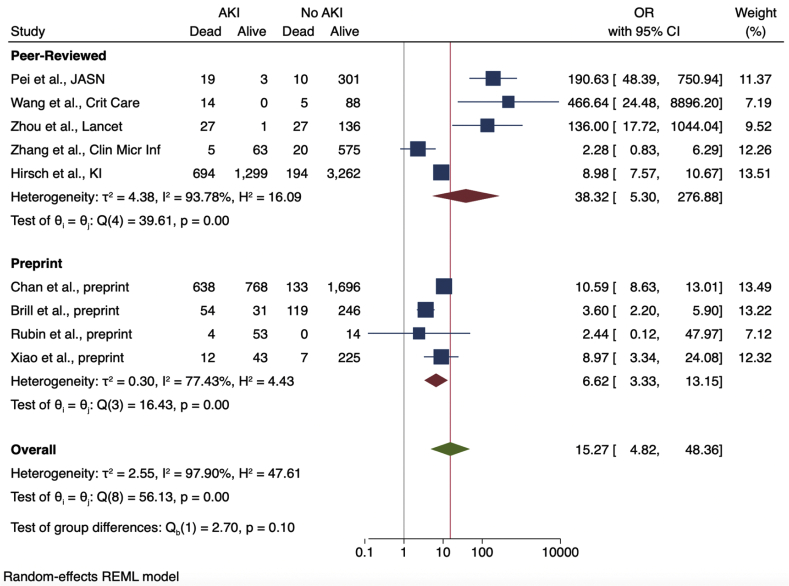
Nine studies provided enough details in their reported outcomes to determine the association of AKI with subsequent mortality (Figure 2). The mortality rate of patients with AKI was 52% (range, 7%–100%). Across the 9 studies, AKI was associated with significantly higher mortality among COVID-19 patients, with a pooled OR of 15.27 (95% CI, 4.82–48.36) compared with patients without AKI. A sensitivity analysis that excluded data from 3 cohorts with particularly high mortality (among 64 patients with AKI from the Pei et al.,22 Wang et al.,30 and Zhou et al.38 cohorts, only 4 survived) showed a higher mortality associated with AKI, albeit with a lower OR of 6.20 (95% CI, 3.63–10.59). Heterogeneity was high across studies (I2 = 97.9%) but was lower after stratifying based on the proportion of severely ill patients (Figure 3). Cohorts with ≥50% severely or critically ill patients had a higher pooled OR (14.18; 95% CI, 1.91–105.44) with more heterogeneity (I2 = 87.7) compared with cohorts with <50% severely or critically ill patients (pooled OR, 9.66; 95% CI, 8.23–11.34; I2 = 31.4%).
Figure 2.
Odds ratios (ORs) of acute kidney injury (AKI) and death, stratified by geographic location of patient cohorts. CI, confidence interval.
Figure 3.
Odds ratios (ORs) of acute kidney injury (AKI) and death, stratified by proportion of patients with severe disease or intensive care unit admission. CI, confidence interval.
Heterogeneity was also reduced after stratifying studies by region (Figure 2). Studies conducted in China had very high heterogeneity (I2 = 89.7%) compared with those from Europe or the United States (I2 = 0% and 31.4%, respectively). Studies from China had a high pooled OR (39.0; 95% CI, 5.34–284.97) and heterogeneity (I2 = 89.7%), whereas those from Europe (3.56; 95% CI, 2.19–5.8; I2 = 0.0%) or the United States (9.66; 95% CI, 8.23–11.34; I2 = 31.4%) were considerably lower. Subgroup analysis conducted using only preprinted studies found a similar OR of 6.62 (95% CI, 3.33–13.15) with less heterogeneity compared with peer-reviewed studies only (I2 = 77.4% vs. 93.8%, respectively; Figure 4). Figure 5 shows a funnel plot including all 9 studies used to calculate the OR of death among patients with AKI.
Figure 4.
Odds ratios (ORs) of acute kidney injury (AKI) and death, stratified by preprint versus peer-reviewed studies. CI, confidence interval.
Figure 5.
Funnel plot of studies reporting mortality associated with acute kidney injury.
Discussion
Across 20 cohorts encompassing 13,137 patients with confirmed COVID-19 infection from Asia, Europe, and the United States, we identified a wide range of AKI prevalence and associated mortality. AKI prevalence was 17% but ranged from 0.5% to 80.3%, perhaps reflecting varied disease severity thresholds for hospitalization across the globe and potential differences in clinical practices in monitoring for renal dysfunction. Nine cohorts reported data on mortality and AKI, with a pooled OR of 15.27 (95% CI, 4.82–48.36) for death compared with those without AKI. Although heterogeneity across all studies was extremely high, it was reduced after excluding cohorts with particularly high mortality, and the association between patient mortality and AKI persisted even after elimination of these studies. Although the development of AKI among patients with COVID-19 portended a worse prognosis across all the cohorts, the extent to which this represented an increased risk for mortality was somewhat variable. This variability may result from differences in the severity of the AKI observed as well as differences in the availability of RRT resources for those with the most severe forms of AKI.
Differences in COVID-19 severity likely also contribute to the observed heterogeneity. We found a stronger association between AKI and death among cohorts reporting a higher proportion of severely ill patients, suggesting that AKI may have a more pronounced adverse effect for patients with more severe pulmonary disease as opposed to patients who are not critically ill. However, it is worth noting that what defines “severe” disease varied across studies, with most studies from China classifying severity by the National Health Commission of the People’s Republic of China Clinical Severity Definitions, seventh edition, and others using the American Thoracic Society Guidelines for Community-Acquired Pneumonia definitions, or ICU admission itself; some studies did not specify what “severe” meant. Such inconsistent definitions of severe disease likely contributed to the high heterogeneity that persisted in the subgroup of cohorts with ≥50% severe or critical patients. The absence of adequately granular details on several aspects of the AKI that patients experienced including information on severity, treatment choices, and temporal relationship to pulmonary disease limited our ability to draw further conclusions about the prognostic implication of varying degrees of AKI.
We also observed a substantial need for RRT among hospitalized patients with COVID-19. Among the 8 cohorts that reported use of RRT, 5% of all patients required RRT. However, it is unclear from these studies whether RRT was used to treat AKI alone or for other indications such as volume overload or end-stage kidney disease, as demonstrated by the study by Guan et al.4 where the number of patients who needed RRT exceeded the combined number of patients with CKD or AKI. Additionally, it is unclear how utilization rates for RRT were influenced by local resource availability or local clinical practices such as the thresholds at which RRT is initiated (or not) for patients with AKI. The high proportion of patients with AKI requiring RRT across the cohorts underscores the need for resource planning to focus on the ability to provide adequate renal support as well during the pandemic, particularly given the grim prognosis associated with AKI among patients with COVID-19.
Further investigation is needed to elucidate the risk conferred by AKI among patients with specific comorbidities of interest, such as diabetes or hypertension. Among the cohorts we identified, such data were extremely limited. Data for proteinuria, hematuria, or home use of renin-angiotensin-aldosterone system inhibitors were also extremely limited, despite current clinical interest and their potential to affect clinical outcomes. In addition, we were only able to study the association between renal disease and severe illness or death; other pertinent clinical questions, including the apparent temporal association of AKI with intubation or the association of AKI with time to extubation, hospitalization time, and overall disease-related morbidity, could not be examined using the currently available data. Absent data about renal recovery from the cohorts prevented any estimation of the extent of renal recovery among patients who survived to discharge.
Our study has several limitations. Although our inclusion criteria requiring explicit compliance with the Kidney Disease: Improving Global Outcomes definition of AKI enabled us to compute meaningful analyses across cohorts, this also resulted in excluding studies that did not specify using the Kidney Disease: Improving Global Outcomes definition from our final analysis. In addition, given the rapid continuous expansion of the COVID-19 literature, many cohorts had relatively short follow-up periods and limitations in their description of details, and there are new cohorts being reported continuously. Furthermore, as with any review, despite a detailed, comprehensive search strategy by 2 independent reviewers, it remains possible that some studies were missed. Finally, we allowed non–peer-reviewed literature to be more inclusive. However, the lack of peer review of these analyses may adversely impact the stability of our estimates. Nevertheless, analysis based on the National Heart, Lung, and Blood Institute Study Quality Assessment Tool showed similar quality among the preprint and peer-reviewed studies, and heterogeneity among the 4 preprint studies used for meta-analysis was actually lower than that of the peer-reviewed studies.
In conclusion, there is a growing body of evidence that AKI occurs in a substantial number of COVID-19 cases and that developing AKI is associated with significantly worse outcomes for patients with COVID-19. Given the extent of the adverse impact of AKI, it is imperative that future studies provide more detailed information on the extent and severity of the renal injury as well as the need for RRT to allow for a more nuanced understanding of the prognosis for patients with COVID-19 and appropriate resource planning in this pandemic.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Acknowledgments
SAH is supported by the National Center for Translational Sciences (KL2 TR001874). SM is supported by National Institutes of Health grants R01-DK114893, U01-DK116066, and R01-MD014161. The funders had no role in study design, data collection, analysis, or reporting; or the decision to submit for publication.
Author Contributions
SYR-J, SAH, JSS, and SM conceived the study. SYR-J and LQ collected the data. SYR-J, LQ, KLK, JSS, SAH, JR, and SM interpreted the data. SYR-J, LQ, KLK, JSS, SAH, JR, and SM prepared the manuscript and approved the submitted version of the manuscript.
Footnotes
Figure S1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Checklist.
Table S1. Quality assessment of included studies based on the National Heart, Lung, and Blood Institute Case Series Quality Assessment Tool.
Supplementary Material
References
- 1.Jiang S., Shi Z., Shu Y. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arabi Y.M., Al-Omari A., Mandourah Y. Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 6.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 8.Naicker S., Yang C.-W., Hwang S.-J. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 11.He Y. Translation: diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Infectious Microbe Diseases. 2020;2:48–54. [Google Scholar]
- 12.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brill S.E., Jarvis H., Ozcan E. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. medRxiv. 2020 doi: 10.1101/2020.05.11.20097790. Accessed May 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q., Huang D., Ou P. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752 doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 15.Chan L., Chaudhary K., Saha A. Acute kidney injury in hospitalized patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.04.20090944. Accessed May 24, 2020. [DOI] [Google Scholar]
- 16.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y., Luo R., Wang K. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong K.S., Lee K.H., Chung J.H. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X., Tao J., Wu H. Clinical features and management of severe COVID-19: a retrospective study in Wuxi, Jiangsu Province, China. medRxiv. 2020 doi: 10.1101/2020.04.10.20060335. Accessed May 24, 2020. [DOI] [Google Scholar]
- 22.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165 doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C., Deng Z., Xiao Q. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China [e-pub ahead of print] J Med Virol. Accessed May 24, 2020. [DOI] [PMC free article] [PubMed]
- 24.Regina J., Papadimitriou-Olivgeris M., Burger R. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: an observational retrospective study. medRxiv. 2020 doi: 10.1101/2020.05.11.20097741. Accessed May 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin S., Orieux A., Prevel R. Characterisation of acute kidney injury in critically ill patients with severe coronavirus disease-2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.05.06.20069872. Accessed May 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q., Zhao K., Yu J. Clinical characteristics of 101 COVID-19 nonsurvivors in Wuhan, China: a retrospective study. medRxiv. 2020 doi: 10.1101/2020.03.04.20031039. Accessed May 24, 2020. [DOI] [Google Scholar]
- 29.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D., Yin Y., Hu C. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao G., Hu H., Wu F. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv. 2020 doi: 10.1101/2020.04.06.20055194. Accessed May 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan S., Song X., Lin F. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20038539. Accessed May 24, 2020. [DOI] [Google Scholar]
- 34.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J., Wang X., Jia X. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767–772 doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W., Yu S., Zha X. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.03.13.20035436. Accessed May 24, 2020. [DOI] [Google Scholar]
- 37.Zheng Y., Sun L., Xu M. Clinical characteristics of 34 COVID-19 patients admitted to ICU in Hangzhou, China. J Zhejiang Univ Science B. 2020;21:378–387 doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F., Yu X., Tong X., Zhang R. Clinical features and outcomes of 197 adult discharged patients with COVID-19 in Yichang, Hubei. medRxiv. 2020 doi: 10.1101/2020.03.26.20041426. Accessed May 24, 2020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.