Abstract
The Culex pipiens complex encompasses five species and subspecies of the genus Culex. Over time, a multitude of morphologically indistinguishable species has been assigned to this complex with several species being classified as important vectors for different diseases. Some species of this complex hibernate in subterranean habitats, and it has been proven that viruses can survive this phase of hibernation. However, studies focusing on the environmental requirements, ecology and spatial and temporal distribution patterns of mosquitos in underground habitats are sparse. Here, we investigate the main environmental factors and dependencies of Culex, considering the number of individuals and survival probabilities in underground habitats during the winter months. Methods. Since the State of Hesse, Germany harbors about 3500 to 4000 subterranean shelters ample availability of subterranean habitats there provides a good opportunity to conduct detailed investigations of the Culex pipiens complex. In this study, we identified a sample of 727 specimens of overwintering females within the Culex pipiens complex from 52 different underground sites collected over a period of 23 years using qPCR. A complete data set of samplings of hibernating mosquitos from 698 subterranean habitats in Central Germany over the same period was available to study the spatial and temporal patterns and the effect of temperature and precipitation conditions on these hibernating populations using a generalized linear model (GLM). Results. Our qPCR-results show, similar to aboveground studies of mosquitos, that Culex pipiens pipiens and Culex torrentium occur sympatrically. On the other hand, Culex pipiens molestus occurred very rarely. The GLM revealed no shifts in species composition over time, but different preferences for subterranean hibernacula, chemical effects on overwintering populations as well as effects of annual and seasonal mean temperature and precipitation during the active phase from March to November. Cx. p. pipiens and Cx. torrentium are the most common species within Hessian caves and other underground habitats during winter. They co-occur with different frequency without any patterns in species composition. Weather conditions influence the number of overwintering mosquitos during the activity phase. Depending on cave parameters, the number of mosquitos decreases during the winter months.
Subject terms: Ecology, Biodiversity, Biogeography, Ecological modelling, Population dynamics
Introduction
Culicidae members belonging to the Culex pipiens complex are difficult to distinguish morphologically. The most characteristic features include the male genitalia and the larval siphon1. According to Vinogradova1 the complex encompasses Culex pipiens pipiens var. pipiens2, its biotype Culex pipiens pipiens var. molestus3, Culex torrentium4, Culex pipiens quinquefasciatus5, Culex pipiens pallens6, and Culex vagans7. The first three are abundant in Germany. While this complex is comprised largely of mosquitos inhabiting urban areas in temperate climates8, Cx. pipiens, including its subspecies and biotypes, may be the most abundant mosquito species worldwide9.
Like many other mosquito species, members of the Culex pipiens complex transmit different arthropod-borne viruses (arboviruses). Notable is the West Nile Virus (WNV), which has triggered fatal infections and epidemics in Eastern and Central Europe10–12 and is also known in Asia, Australia, Africa, the Caribbean and North America12. Several studies show that Culex species are competent vectors13–15. This may also be true for Cx. torrentium which is widely distributed in Central Europe13,14. Since Cx. p. pipiens is ornithophilic15,16, it plays a major role in the transmission of WNV within wild bird populations, except in the northern Central and Mid-Atlantic United States, where it shows higher than usual affinity for humans and becomes a bridge vector17. Andreadis17 attributes this alteration of host preference to potential genetic ancestry with Cx. p. molestus and is considered analogous to the assumed hybridization18. Cx. p. molestus is known to be mammophilic19 and found to have a very different behavior compared to Cx. p. pipiens. However, Cx. p. molestus has shown no difference in feeding behavior compared to Cx. p. pipiens16 in a survey area in western Portugal. Today, it is commonly accepted that Cx. p. molestus is adapted to a subterranean environment20–23 and is autogenous (requiring no blood meal prior to its first oviposition, due to a higher nutrient supply during the larval stage), stenogamous (mating in confined spaces) and homodynamous (non-diapausing). On the other hand, Cx. p. pipiens is anautogenous (requiring a blood meal prior to its first oviposition), eurygamous (mating in open spaces) and heterodynamous (diapausing)1,20,21. The general autogeny and stenogamy of Cx. p. molestus and the anautogamy and eurygamy of Cx. p. pipiens respectively has been demonstrated in a breeding experiment23. Cx. p. pipiens is often seen as a subterranean form of Cx. pipiens19,22,24,25 and could therefore be much more abundant in caves than in contrasting epigean habitats. Hybrids of Cx. p. pipiens and Cx. p. molestus were proposed to exist and could serve as bridge vectors for arboviruses from birds to humans since they would show a feeding strategy including mammals and birds26. Hybrids of Cx. p. molestus and Cx. p. pipiens may therefore, play a key role in the distribution of certain zoonotic diseases such as WNV.
Depending on the species, mosquitos can survive winter in all three life stages27. Either eggs survive the cold season on dry ground, usually in floodplains, and hatch as soon as temperatures rise and a sufficient amount of water is available, or they overwinter as hatched larvae under the ice cover of low waters. Diapausing or hibernating females in underground systems such as caves or mines is the third option.
It is generally assumed that inseminated female Cx. p. pipiens hibernate25,28 while Cx. p. molestus does not need to29–31. Depending on the environmental conditions, the lack of diapause of Cx. p. molestus may occur either as expressed or as suppressed homodynamy32. According to Kjærandsen33, Cx. pipiens hibernates in caves and cave-like environments, however, the author did not distinguish between Cx. p. pipiens and Cx. p. molestus in his study. Caves are considered thermally insulated systems34, a frequent and established point of view, corroborating Barr35 in that the temperature in a cave is constant and close to the average annual temperature of the surrounding region. Caves are divided into three ecological zones: the entrance zone, twilight zone and depth zone36. Caves and other subterranean habitats not only have a relatively constant temperature mostly fluctuating in the entrance region, but also have a generally constant humidity gradient. There are several different categories of caves, ranging from caves that have running water to almost completely dry ones37. According to Buffington38, cave humidity is not a determining factor for choosing a site for diapausing. However, extensively tested reactions of Cx. fatigans to different temperatures and humidity levels could prove the avoidance of subterranean habitats with greater than 95% and below 40% relative humidity39.
Considering the still unresolved structure of the Culex pipiens complex as well as the variability in their biological interactions and lack of knowledge within Germany, this is the first study to include hibernating mosquitos on a larger scale. Hesse is particularly suited to study the population structure and hibernation preferences of Culex due to its many subterranean habitats, the wide-ranging distribution of various Culex species and the temperate Central European climate in this region of Germany.
We examined the co-occurrence of the three Culex pipiens complex species present in Germany and tested whether spatial patterns within the study area occur. Furthermore, we examined if temperature and precipitation conditions in the preceding activity phases influence the number of mosquitos found during winter. We additionally investigated whether the abundance within the subterranean shelters decreases over the winter months and if this temporal pattern is dependent on certain subterranean parameters.
Material and methods
Sample material
A data set consisting of 1827 samples from 698 underground sites served as the basis for our investigations. A total of 8750 mosquitos from the Culex pipiens complex were collected from walls and ceilings of subterranean shelters by the Hesse Federation for Cave and Karst Research. Samples were collected during all months throughout the years 1991 to 2014 with a strong focus on winter months in caves, tunnels, cellars and other subterranean shelters. Collection was implemented during the regular inventories of Hessian underground structures. All samples were stored in small, 100% ethanol-filled vials at room temperature until further examination. Specimens collected the same day in the same subterranean shelter were stored together in one vial and labelled as one sample accordingly. We genetically examined a subsample of 727 mosquitos from 52 of the 698 available sites (Table 1) and employed a modified version of the real-time qPCR (Table 2) developed by Rudolf et al.40 to gain comparable results. Regarding temporal and spatial patterns of the species’ distribution, the whole dataset of 1827 samplings and species counts from 698 subterranean shelters was used. The spatial distribution patterns of the species compositions are shown in a Gis map (Fig. 1). The temperature and precipitation ratios of the years 2001 to 2014 are shown in comparison with the abundance distributions of the respective years (Fig. 2).
Table 1.
Spatial pattern analysis (BB = brick-built, BMC = bridge maintenance chamber) of subterranean sites.
| Nr | Shelter Type | Humidity | Dec. N | Dec. E | Sampling years |
|---|---|---|---|---|---|
| 1 | Concrete tunnel | Moist | 50.7967 | 9.5485 | 2007, 2013 |
| 2 | BB tunnel | Very dry | 51.1948 | 9.0862 | 2011 |
| 3 | BMC | Dry | 50.2387 | 9.5996 | 2011, 2013 |
| 4 | Rock cellar | Moist | 50.7540 | 9.2631 | 2008 |
| 5 | Natural cave | Medium | 50.4896 | 8.0363 | 2010 |
| 6 | Mine shaft | Wet | 51.2896 | 8.6955 | 2006, 2008 |
| 7 | Touristic mine | Moist | 51.3750 | 8.8005 | 2004, 2006 |
| 8 | Rock cellar | Moist | 51.0945 | 8.6287 | 2004 |
| 9 | BMC | Moist | 50.9221 | 9.9102 | 2003, 2006 |
| 10 | Bunker in quarry | Dry | 51.1585 | 9.4466 | 2006 |
| 11 | Bunker complex | Moist | 51.5189 | 9.3776 | 2006, |
| 12 | Rock cellar | Dry | 50.6820 | 9.3776 | 2009 |
| 13 | BB cellar | Medium | 50.6987 | 9.7299 | 2003 |
| 14 | Mine shaft | Medium | 50.2439 | 8.1010 | 2008, 2011 |
| 15 | Rock cellar | Medium | 51.0323 | 8.9745 | 2001, 2005 |
| 16 | Rock cellar | Wet | 50.6401 | 9.4005 | 2007, 2008 |
| 17 | Rock cellar | Wet | 50.5010 | 9.1237 | 2008, 2010 |
| 18 | Mine shaft | MEDIUM | 50.1673 | 9.3542 | 2005, 2006 |
| 19 | Rock cellar | Wet | 51.1297 | 8.7965 | 2001, 2014 |
| 20 | Rock cellar | MOIST | 50.4867 | 9.8731 | 2003, 2004, 2005, 2014 |
| 21 | Mine shaft | Dry | 51.0362 | 9.9002 | 2002, 2004, 2005, 2006, 2013 |
| 22 | Rock cellar | Medium | 50.5900 | 9.9984 | from 2003 to 2014 |
| 23 | BB cellar | Dry | 50.4286 | 9.7630 | 2010 |
| 24 | Natural cave | Medium | 50.1705 | 9.4033 | 2001, 2007, 2010, 2011 |
| 25 | Mine shaft | Medium | 50.8330 | 8.5444 | 2003, 2009 |
| 26 | Mine shaft | Medium | 50.6164 | 8.3905 | 2005 |
| 27 | Rock cellar | Medium | 50.1720 | 8.4602 | 2011 |
| 28 | Mine shaft | Moist | 51.2733 | 9.8713 | 1994, 2004, 2005, 2007, 2009, 2011, 2012, 2013 |
| 29 | Sand mine | Medium | 51.1166 | 10.1677 | 1996, 2000, 2002, 2004, 2006, 2008, 2011, 2012, 2013 |
| 30 | Natural cave | Medium | 50.6852 | 8.2132 | 1995, 1997, 2005, 2007 |
| 31 | Sand mine | Medium | 51.2148 | 10.0778 | 2003, 2004, 2009, 2011, 2013 |
| 32 | Mine shaft | Dry | 50.1016 | 7.9153 | 2011 |
| 33 | Mine shaft | Medium | 50.3864 | 8.0706 | 2013 |
| 34 | Mine shaft | Medium | 50.5896 | 8.6391 | 2008 |
| 35 | BB tunnel | Dry | 50.3302 | 9.6025 | 2014 |
| 36 | Mine shaft | Wet | 50.8598 | 9.7555 | 2001, 2003, 2007, 2011 |
| 37 | Sand mine | Medium | 51.2150 | 10.0790 | 1994, 2003, 2004, 2009, 2011, 2013 |
| 38 | Mine shaft | Moist | 50.0609 | 7.7813 | 2002, 2005 |
| 39 | Mine shaft | Wet | 51.1151 | 9.0086 | 2001, 2004, 2005, 2008, 2013, 2014 |
| 40 | Mine shaft | Moist | 51.3177 | 9.3952 | 2003, 2006, 2012 |
| 41 | Natural cave | Moist | 51.2325 | 8.9012 | 2003, 2004, 2005, 2007, 2008, 2010, 2013, 2014 |
| 42 | Mine shaft | Moist | 50.3665 | 8.6317 | 2011 |
| 43 | Mine shaft | Wet | 50.5168 | 9.5344 | 2001, 2003, 2004, 2005, 2007, 2008, 2010, 2011, 2013, 2014 |
| 44 | Mine shaft | Moist | 50.9963 | 8.5831 | 2003, 2004, 2005, 2008, 2011, 2012, 2013, 2014 |
| 45 | Mine shaft | Medium | 50.1555 | 8.0817 | 2011 |
| 46 | Sand mine | Dry | 51.3843 | 8.9929 | 2001 |
| 47 | Mine shaft | Moist | 50.2259 | 8.2693 | 2005 |
| 48 | BB tunnel | Dry | 50.9569 | 9.8046 | 2000, 2002, 2005, 2008, 2009, 2011, 2012, 2013 |
| 49 | Natural cave | Medium | 50.5162 | 8.3752 | 2001, 2002, 2004 |
| 50 | Mine shaft | Moist | 49.6714 | 8.8525 | 2005 |
| 51 | Mine shaft | Wet | 50.0075 | 7.9601 | 2007 |
| 52 | Natural cave | Moist | 51.3209 | 9.8542 | 2007 |
Table 2.
Primers and probes used (modified after Rudolf et al. 2013).
| Name of primer | Sequence |
|---|---|
| PipF | 5′-GCGGCCAAATATTGAGACTT-3’ |
| PipR | 5′-CGTCCTCAAACATCCAGACA-3’ |
| TorrF | 5′-GACACAGGACGACAGAAA-3’ |
| TorrR | 5′-GCCTACGCAACTACTAAA-3’ |
| Name of Probe | Sequence |
|---|---|
| PipPipProbe | 5′-GCTTCGGTGAAGGTTTGTGT-3’ |
| PipMolProbe | 5′-TGAACCCTCCAGTAAGGTATCAACTAC-3’ |
| TorrProbe | 5′-CGATGATGCCTGTGCTACCA-3’ |
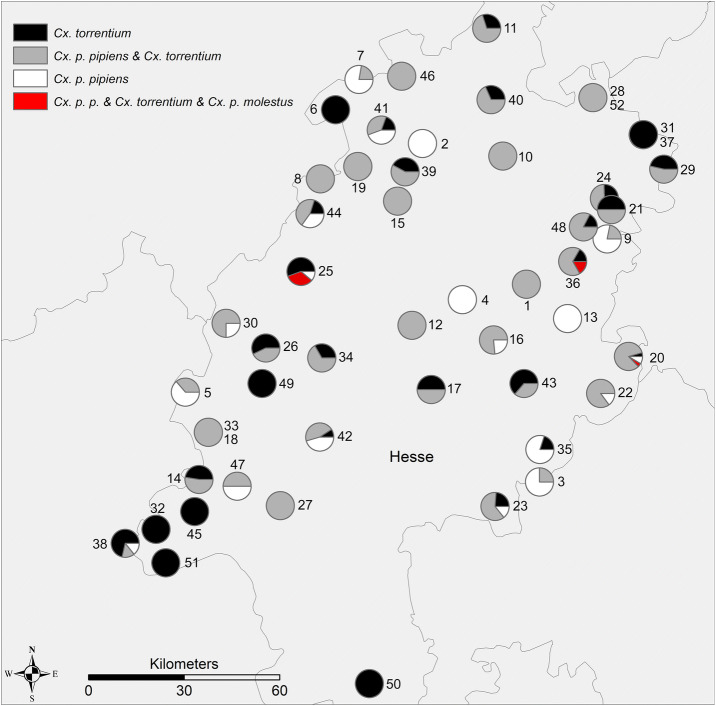
Figure 1.
Overview of genetically assessed sample material. Numbers refer to the shelter numbers shown in Table 1. Figure created with ArcGIS Version 10.746.
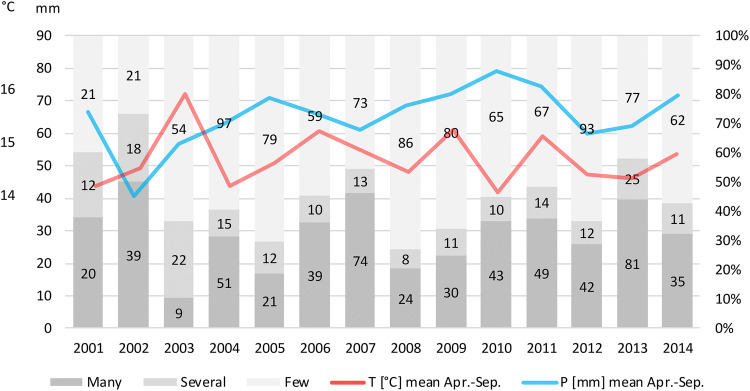
Figure 2.
Abundance of hibernating mosquitos in comparison with the climate conditions during the previous activity phase. X-Axis: Year, left Y-Axis: temperature and precipitation, right Y-Axis: composition of categories within the sampling. The numbers in the bar graphs show the absolute frequency of categories in the respective years. Categories: many: > 20 individuals, several: 10 to 20 individuals, few: < 10 individuals found within the subterranean shelter.
Effects of precipitation and temperature
In order to investigate effects of temperature and precipitation conditions on these hibernating populations we performed a generalized linear model (GLM). Since the exact numbers of observed individuals were counted only up to 20 mosquitos in the original data collection we considered the number of hibernating mosquitos as a categorical variable of three classes: f = few for counts between 1 and 10 individuals found within the subterranean shelter; s = several for counts between 11 and 20 individuals; and m = many for counts more than 20 individuals. For this analysis we only considered hibernating mosquitoes found in the winter months (December to February).
To refine data quality and to reduce spatial autocorrelation we removed repeated samples from the same or nearby underground sites (within a radius of 600 m). Among all data from sampling sites within a certain area and of the whole time period from 1991 to 2014 we chose only one sample at random but favoring a sampling date early in winter (i.e. December > January > February) in order to minimize the effect of potential die-off of the mosquitoes during winter. This procedure resulted in 390 samples when only taking one sample per cave into account and finally 271 samples when removing additional caves within a radius of 600 m.
As explaining variables, we considered temperature and precipitation during spring (i.e. March, April, May), Summer (i.e. June, July, August) and Fall (i.e. September, October, November). These variables were only little intercorrelated (see Table 1 in the supplementary). Additionally, we accounted for the sampling month during winter (coded as December = 1, January = 2 and February = 3) and altitude. The altitude was taken into account as we used the regional averages of temperature and precipitation recorded for the State of Hesse by the Deutscher Wetterdienst (DWD = German weather service) as explaining variables in the GLM. Thus altitude was included in our model to account for the effect of decreasing temperature in higher altitudes. We assume that weather could have a different effect on mosquito abundance in different elevation (e.g. an extremely warm summer may have a positive effect in higher altitudes, while the differing temperature in lower altitudes is detrimental to mosquitoes). The inclusion of the sampling month was carried out due to the assumption that the number of mosquitoes in the caves tends to decrease over the winter months. Whether the number of mosquitoes in the caves decreases over the winter months will be examined below. The analysis was performed in R41 with package VGLM42,43.
Effects of surroundings
Since it is assumed that the number of mosquitos decreases over winter, the mosquito abundance was compared between the winter months as well. We tested for significant differences in the mosquito abundance frequencies between winter sampling month by means of a chi squared test.
Since we assume that a decrease in mosquito abundance may be influenced by cave parameters, we perform the test separately for source material and cave moisture. The main rock type of the shelter or of the walls and ceilings was recorded and shelters were divided into two categories, acidic or alkaline, according to the surrounding rock types and their influence on the pH of water. During site visits, the underground sites were also characterized by moisture level (very dry, dry, humid, wet, constant flow). Due to the paucity of extremes, the categories very dry/dry and constant flow/wet were combined. With a Chi square test, we tested for significance between the frequencies of recorded abundances of hibernating mosquitoes (few, several, many) and winter months (December to February, with GraphpadPrism 8.0244) in the respective parameters and presented them as stacked bar charts.
Additionally, we performed further analysis on the surroundings of sampling sites. Different cave zones were also recorded whereby each mosquito specimen, as well as the counting or frequency estimation was attributed to a zone. Caves with several zones inhabited by mosquitos were also included in the calculation in the corresponding categories.
The surrounding environment of the caves was characterized to reveal potential associations with the number of mosquitos (categories based on numbers of individuals found and their frequency in the data set). For each cave, the percentages of different land cover types (using the Corine Land Cover data45) were calculated (using ArcGIS Version 10.746) within the surrounding 200 m, 400 m, 800 m and 1600 m. The Land Cover data was compiled into four groups (coniferous forest (CLC category 312), broadleaf forest (CLC 311), anthropogenic (CLC categories 111, 112, 121, 122, 142), agriculture (CLC categories 211, 222, 231, 242, 243). Data that could not be effectively assigned to any category (less than 50% cover of a category) was omitted. These datasets were also tested for significance with a Chi squared test (GraphpadPrism 8.0244) in the respective parameters and presented as stacked bar charts.
Results
Genetic species composition
The molecular species identification shows a sympatric occurrence of Cx. p. pipiens and Cx. torrentium in most of the cavernous habitats while there were no clear differences in species composition when comparing different sampling years (results not shown). Shown are the species proportions of the samples over the years (min years of sampling per cave = 1, max sampling per cave = 13) (Fig. 1). Numbers of Cx. p. pipiens and Cx. torrentium caught were of similar size and distribution while the subspecies Cx. p. molestus occurred very rarely in our sampling (Pools with: only Cx. torrentium: 183 (25%), only Cx. p. pipiens: 135 (19%), both: 397 (55%), both and Cx. p. molestus: 8 (1%)). Our records also confirmed other mosquito species in Hessian caves: Aedes cinereus/geminus (1 female), Aedes rossicus (67 female,1 male), Anopheles maculipennis s.l. (3 female), Anopheles marteri (5 female), Culiseta annulata (204 female, 2 male).
Effects of precipitation and temperature
We first displayed temperature and precipitation conditions (yearly mean temperature and mean precipitation during the active phase from April through September) together with the observed mosquito abundances in the caves during winter months (Fig. 2). In comparatively hot and dry years such as 2003, a low percentage of caves with high abundances can be observed, whereas high abundances could be observed in comparatively cool, humid years such as 2010 or 2007. This is not the case in 2008, for example. Therefore, in the GLM we do not consider the temperature and precipitation ratios averaged over the whole activity phase but by quarters.
The GLM revealed that the abundance of hibernating mosquitoes is significantly affected by temperature in summer and fall as well as by precipitation of all three considered quarters (Supplementary). We illustrated the positive and negative effects of these variables in Fig. 3. Temperature in the spring months (March, April, May—T spring) had no significant effect while there was a significant increase during Summer (June, July, August) and Fall (September, October, November). Higher precipitation (P) had a significant negative effect during Spring and a significantly positive effect during Summer and Fall. A higher Altitude had a significant positive effect on the abundance as well (Supplementary model 1).
Figure 3.
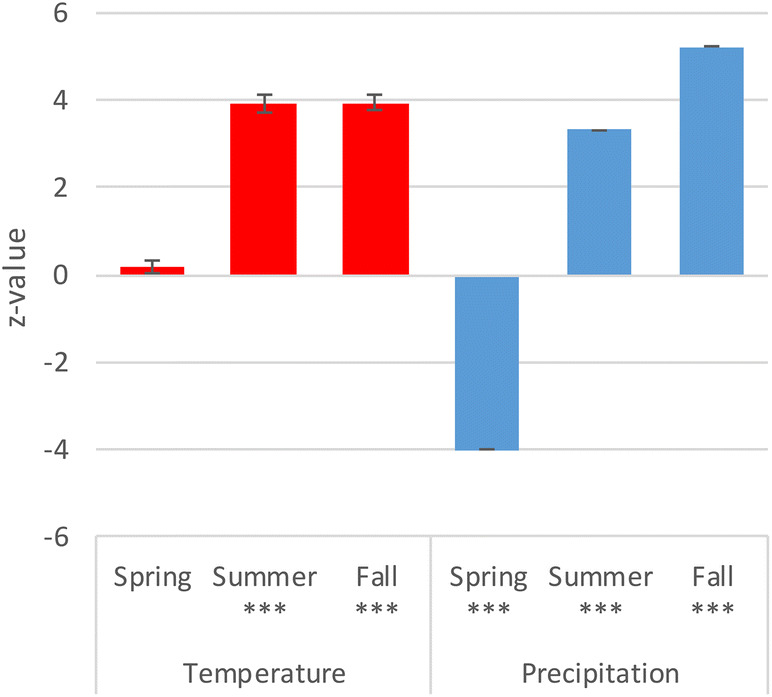
Effects of higher temperature and precipitation during activity and transition phases (March to November) on mosquito density in hibernacula during the hibernation phase (December through February). Depicted are the z-values of the GLM and their standard errors (p-values: *** < 0.001).
Effects of surroundings
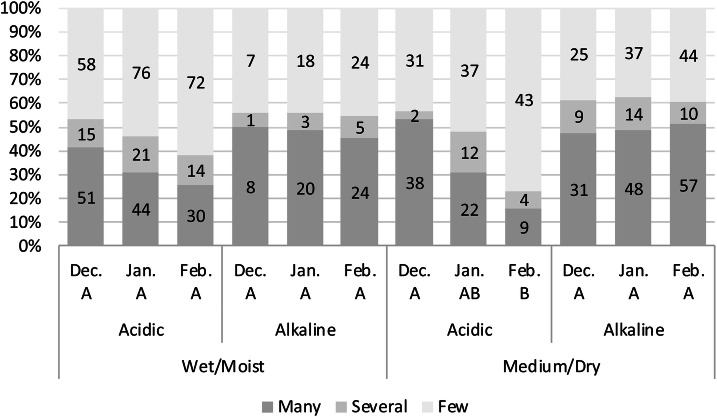
There was a significant decrease in mosquito abundance during the winter months in subterranean environments surrounded by acidic rock composition in both, wet and dry underground shelters. On the other hand, the density of mosquitos within hibernacula classified as alkaline did not change during the winter months. The difference was strongest within the group of medium and dry underground shelters (Fig. 4).
Figure 4.
Comparison of mosquito abundance within hibernacles of alkaline and acidic surrounding rock combined with humidity levels. Statistical significance is symbolized with A, B and C, where non-matching letters are significantly different (Chi Square: 61.9, 22 df, p = 0.0002; corrected min./max. value A against B: p = 0.0004/0.0016). Y-Axis: composition of categories within the sampling. The numbers in the bar graphs show the absolute frequency of categories in the respective years. Categories: many: > 20 individuals, several: 10–20 individuals, few: 1–10 individuals found within the subterranean shelter.
A relationship between the abundance of mosquitos and the stated moisture levels of the underground habitats could not be established.
There is a significant difference between the number of mosquitos in the entrance and twilight zones compared to the dark zone (Fig. 5) in subterranean shelters. Dark zones seem to be favored be hibernating mosquitoes.
Figure 5.
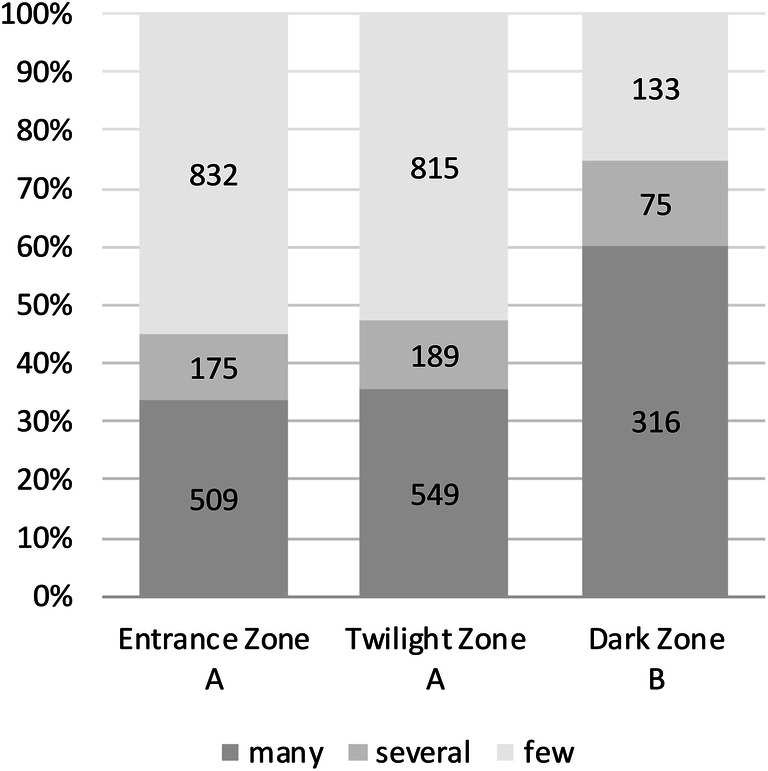
Comparison of mosquito abundance of the different depth zones within cavernous habitats during the hibernation period. Statistical significance is symbolized with A and B, where non-matching letters are significantly different (Chi Square: 154.3, 4 df, p < 0.0001; corrected value A against B: p < 0.0001). Y-Axis: composition of categories within the sampling. The numbers in the bar graphs show the absolute frequency of categories in the respective years. Categories: many: > 20 individuals, several: 10–20 individuals, few: 1–10 individuals found within the cave.
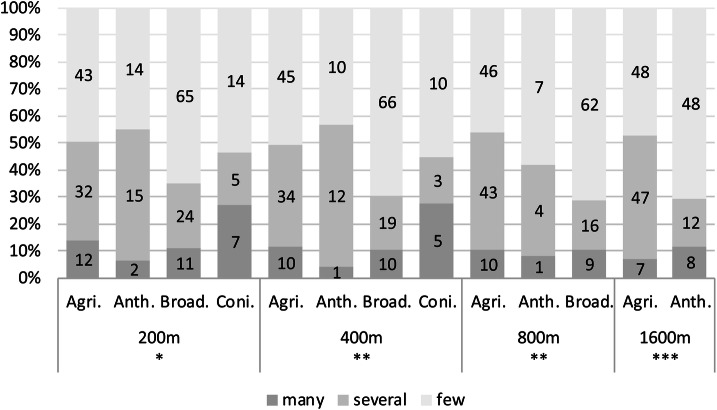
In addition, we considered the land cover characteristics of the surroundings of the underground sites. We found significant differences of the abundances of overwintering mosquitos between subterranean shelters surrounded by Broadleaf Forest and Conifer Forest and agricultural areas and Anthropogenic-purposed land (i.e. urbanization) (Fig. 6).
Figure 6.
Comparison of mosquito abundance and different main land cover categories surrounding hibernacles in four different radiuses during the hibernation period. Abbreviations: Agri., agriculture and pastures; Anth., anthropogenic and urban; Broad., broadleaved forest; Coni., coniferous forest. p-values and Chi square statistics: 200 m: 0.02, 14.67, 6 df, Anth. against Coni.: 0.03, Anth. against Broad.: 0.03; 400 m: 0.004, 18.99, 6 df, Anth against Coni: 0.02, Anth. against Broad.: 0.007, Agri. against Broad.: 0.01; 800 m: 0.007, 14.8, 4 df, Agri. against Broad.: 0.007; 1600 m: 0.0007, 14.61, 2 df, Agri against Anth.: 0.0007. Omitted data from adjusted sample set of 263 sampling points: 200 m: 7%, 400 m: 14%, 800 m: 25%, 1600 m: 35%. Y-Axis: composition of categories within the sampling. The numbers in the bar graphs show the absolute frequency of categories in the respective years. Categories: many: > 20 individuals, several: 10–20 individuals, few: 1–10 individuals found within the subterranean shelter.
Discussion
Spatial patterns
No spatial pattern of species composition was detected in the subterranean shelters located in Hesse. However, the co-occurrence of the two species Cx. p. pipiens and Cx. torrentium was confirmed (Fig. 1). Similar results were shown by Rudolf et al.40 with a sample set of mosquitos collected in Germany above ground. For the State of Hesse, they found more Cx. torrentium than Cx. p. pipiens and no samples of Cx. p. molestus. Werblow et al.47 detected a general pattern of fewer Cx. torrentium than Cx. pipiens. According to Hesson et al.14, Cx. torrentium and Cx. pipiens occur sympatrically with more Cx. torrentium north of the Alps. In Central Europe and Austria, distributional patterns of both species are very similar48–50. Overall, it seems that Cx. torrentium and Cx. p. pipiens occur equally abundant in central Germany.
We could detect one hybridization event in our sampling. One pool of two collected specimens were molecularly flagged for all three subspecies. Although several authors already suggested hybridization between Cx. p. pipiens and Cx. p. molestus51–53, we were not able to determine which two out of the three sampled species hybridized. Since their genetic differences are overall very small22,24, it is questionable whether these two species should be distinguished as different subspecies. The authors suggested that the verification of gene flow between both forms allows for two different interpretations: They could be two genetically distinct forms, which converge and hybridize where their distribution areas are overlapping. Otherwise, Cx. p. molestus might just be a biotype that originated from Cx. p. pipiens, and the two of them are not yet reproductively isolated whereby similarities in behavior and physiology suggest hybridization. This suggests that Cx. p. molestus is a biotype of Cx. pipiens and, at least in Germany, relatively rare. Amara Korba et al.23 could prove the stenogamy and autogamy of Cx. p. molestus in 78.6% of their cases albeit this result does not account for all of the individuals. Another study revealed no prey choice preferences amongst birds and mammals regarding Cx. p. pipiens and Cx. p. molestus16. Overall, our findings corroborate those of other studies and point not to a picture of Cx. p. molestus as a subterranean subspecies that feeds on cave-dwelling mammals, but rather, to it being a biotype of Cx. pipiens that potentially has a shifted niche towards smaller enclosures like tree cavities or burrows of different animals that can also adapt to a surface habitat. If the two forms were only ecological varieties of one species, mixed forms should not be classified as hybrids.
The complete dataset contains 8750 Culex mosquitos, all collected within subterranean habitats. Of these samples, only 22 were male Culex mosquitos (collected in the months from May to December), of which 12 samples were classified as Cx. torrentium and 10 as Cx. p. pipiens, a finding which is supported by another study54. In light of the very low numbers of males and the lack of Cx. p. molestus or hybrid males, and since hibernating females of Cx. p. molestus collected in the subterranean shelters matched the percentage of those found by aboveground sampling, the surveyed Hessian Cx. p. molestus show no sign of homodynamy. Similar results confirm our assumption that Cx. p. molestus is not an underground biotype of Cx. p. pipiens or that subterranean objects are particularly suitable for hybridization54.
All other species, except two, were collected between April and September within the subterranean shelters, and therefore, show no evidence of hibernation within subterranean shelters. Only Culiseta annulata and one specimen of Anopheles maculipennis were collected during the winter months, possibly using caves for hibernation. Compared to the other rarer mosquitos, higher individual numbers of Cu. annulata underscore this consideration.
Effects of precipitation and temperature
We provide evidence that weather conditions during the previous activity phase as well as the time of sampling influence the abundance of species within the hibernacles in the following winter. Temperature in the Spring (March, April and May) had no significant effect. This can be explained by a previous finding that higher temperatures in March probably cause the mosquitos to exit their wintering grounds prematurely55, resulting in a negative effect that is cancelled out by the following two months.
Climate effects on mosquitos were studied from early May to mid-September in a north-western province in Italy56. Although the climate of northern Italy is not completely comparable with that of Hesse, partially similar patterns were observed. These results are somewhat different from our calculations whereby a higher temperature in Summer (June, July and August) as well as in the Fall (September, October and November) correlated with a significant increase of mosquitos within the hibernacles. Lack of information concerning Italian populations after September precludes further comparison. Higher temperatures in September potentially affect the behavior of mosquitos, which generally start migrating into shelters for the winter from the beginning of October. Higher temperatures in October and November could enable more mosquitos to find suitable shelter for the winter, which results in a positive effect for our “Fall” category. Our results show a negative effect in Spring and a positive one in Summer and Fall, corroborating a preference for higher precipitation that was reported before56,57. Another study detected a direct correlation between higher temperature and the number of mosquitos two weeks later58. Our calculation revealed a significant effect of sampling months on the number of mosquitos found inside the underground structure, i.e. the later the sampling in the winter, the fewer mosquitos were found inside the cave, which is corroborated by the study of Zittra et al.54.
Effects of surroundings
A decrease of mosquitos in January and February was detected. Smaller numbers are most prominent in underground habitats classified as dry/medium and acidic (Fig. 4). A similar, although not significant pattern is visible in wet/humid, acidic habitats. An attenuation of the impact on mosquito numbers with regard to increased humidity can be explained by the fact that contact more water weakens the pH-lowering effect of the surrounding material. More acidic environments may simply not be conducive for mosquito hibernation whereby the mechanisms involved could merit further investigation.
Distribution within caves and the higher number of mosquitos in the deeper parts of the underground habitats was unexpected. It was previously assumed that mosquitos would hibernate mainly in the entrance and twilight zones36. There might be a possible tradeoff reflected in the results in that overwintering in the deeper zones might guarantee better shelter against freezing outside temperatures and higher survival rates.
The effective flight distance for Culex mosquitos throughout their life span is between 600 to 2000 meters59,60. For our analysis, we therefore set four different radiuses of 200, 400, 800 and 1600 m around the hibernation site to analyze land cover more closely. Overall, anthropogenic and agricultural surroundings have a positive effect on mosquito density within the hibernacles when compared to forests. Although coniferous forests in Germany are often monocultures for timber production that provide a suitable habitat for only a limited number of vertebrate species61–63, we could not detect a significant difference between the two forest types. The increased proportion of the “many” and “several” categories in agricultural and anthropogenic areas might be explained by the fact that fields and pastures are frequently used by large grazing animals that serve as hosts for adult mosquitos. Water troughs as well as car tires, commonly used as weights on tarpaulins, are ideal breeding grounds for mosquito larvae. Most settlements within the flight radius of the surveyed hibernacles are small clusters of single-family houses with large gardens, which often also contain many small collections of water in rainwater barrels, plant pots or buckets. These breeding options as well as proximity to humans, pets and birds provide excellent living conditions for mosquitos. Our findings of greater abundancies of species in hibernacles surrounded by anthropogenic and agriculturally influenced terrains is a common pattern found in urban habitats15,48,50,57.
Conclusion
Germany and the State of Hesse lie in the temperate climate zone, where caves and other underground shelters offer an advantage for Cx. pipiens and Cx. torrentium, if not a necessity. Our study complements existing knowledge about the ecological requirements of this species complex. By using information about climate conditions and mosquito densities within caves the following winters, it might be possible to estimate which years witness a large mosquito density and thus create temporal pattern forecasts. The sites, their characteristics and surroundings are important for the occurrence of the species and create spatial patterns. Spatial and temporal patterns are particularly important for vector species as they allow the necessary precautions to be assessed and applied more quickly.
In summary, based on results of previous studies, we expected to find significantly higher proportions of Cx. p. molestus inside the caves, but our results indicate a similar species composition of the Culex pipiens complex as that found outside the caves. We did not find any male specimens of Cx. p. molestus, a fact that suggests that at least in our study area, Cx. p. molestus lacks permanent underground populations and does not reproduce in subterranean environments. Our results also show that for Hesse, the previous theory that mosquitos hibernate primarily in the entrance zones of caves should be re-evaluated. We assume that the frequency of mosquitos within the caves is determined by the frequency of mosquitos on the surface. This assumption is supported by our results that the climate conditions during the activity phase have a significant effect on the frequency of hibernating mosquitos. Therefore, we argue that the number of hibernating mosquitos could be taken as a proxy for the overall density of the mosquito population but would require further investigation. The availability of suitable hibernation sites ensures the continuation of the species in the following year. Dependent on cave parameters, we could detect a decrease in the abundance of overwintering mosquitos during the winter months.
Supplementary information
Acknowledgements
This research was funded by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant numbers 2819104415 and 2819105115 as well as by the Uniscientia Foundation (P 121–2017). We thank all members of the Hesse Federation for Cave and Karst Research for sample collection as well as Dr. Adrienne Jochum and Dr. Judith Kochmann for proofreading the manuscript.
Author contributions
Dorian D. Dörge designed and conceptionalized the study, wrote the main manuscript text, executed the statistical analysis, interpreted the data and prepared all figures. Sarah Cunze wrote the main manuscript text, executed the statistical analysis, interpreted the data and prepared all figures. Henrik Schleifenbaum executed the genetic data analysis and prepared Fig. 1. Stefan Zaenker acquired the samples and designed the study. Sven Klimpel designed and conceptionalized the study and wrote the main manuscript text. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67422-7.
References
- 1.Vinogradova EB. Culex pipiens pipiens mosquitoes. Taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Sofia: Pensoft; 2000. [Google Scholar]
- 2.Linnaeus C. Systema naturae Vol. 1. No. part 1. Stockholm: Laurentii-Salvii; 1758. [Google Scholar]
- 3.Forskål, P. Flora Ægyptiaco-Arabica sive descriptiones plantarum quas per ægytum inferiorem et arabiam felicem detexit, illustravit Petrus Forskål. Post mortem auctoris edidit Carsten Niebuhr (1775).
- 4.Martini R. Zwei bemerkenswerte Culiciden von einem eigenartigen Biotop. Int Rev Hydrobiol. 1925;12:333–337. doi: 10.1002/iroh.19250120503. [DOI] [Google Scholar]
- 5.Say T. Descriptions of dipterous insects of the United States. J. Acad. Nat. Sci. Philadelphia. 1823;3:9–54. [Google Scholar]
- 6.Coquillett DW. Report on a collection of Japanese Diptera, presented to the U.S. national museum by the Imperial University of Tokyo. Proc. US Natl. Museum. 1898;21:301–340. doi: 10.5479/si.00963801.21-1146.301. [DOI] [Google Scholar]
- 7.Meigen, J. W. & Wiedemann, C. R. W. Aussereuropäische Zweiflügelige Insekten / beschrieben von Christ. Rud. Wilh. Wiedemann ; als Fortsetzung des Meigenischen Werkes.; 10.5962/bhl.title.14603 (1828).
- 8.Barr AR. Occurrence and distribution of the Culex pipiens complex. Bull. World Health Organ. 1967;37:293–296. [PMC free article] [PubMed] [Google Scholar]
- 9.Hubálek Z, Halouzka J. West Nile Fever–a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundström JO. Mosquito-borne viruses in western Europe: a review. J. Vector Ecol. . 1999;24:1–39. [PubMed] [Google Scholar]
- 11.Hayes CG. West Nile virus: Uganda, 1937, to New York City, 1999. Ann. N. Y. Acad. Sci. 2001;951:25–37. doi: 10.1111/j.1749-6632.2001.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 12.Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103:29–43. doi: 10.1007/s00436-008-1064-7. [DOI] [PubMed] [Google Scholar]
- 13.Werblow A, Bolius S, Dorresteijn AWC, Melaun C, Klimpel S. Diversity of Culex torrentium Martini, 1925—a potential vector of arboviruses and filaria in Europe. Parasitol Res. 2013;112:2495–2501. doi: 10.1007/s00436-013-3418-z. [DOI] [PubMed] [Google Scholar]
- 14.Hesson JC, et al. The arbovirus vector Culex torrentium is more prevalent than Culex pipiens in northern and central Europe. Med. Vet. Entomol. 2014;28:179–186. doi: 10.1111/mve.12024. [DOI] [PubMed] [Google Scholar]
- 15.Becker N, et al. Mosquitoes and Their Control. 2. Heidelberg: Springer; 2010. [Google Scholar]
- 16.Gomes B, et al. Feeding patterns of molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in a region of high hybridization. Parasites Vectors. 2013;6:93. doi: 10.1186/1756-3305-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreadis TG. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Ame. Mosquito Control Assoc. 2012;28:137–151. doi: 10.2987/8756-971X-28.4s.137. [DOI] [PubMed] [Google Scholar]
- 18.Lõhmus M, Lindström A, Björklund M. How often do they meet? Genetic similarity between European populations of a potential disease vector Culex pipiens. Infect. Ecol. Epidemiol. 2012;2:12001. doi: 10.3402/iee.v2i0.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash. 1984;86:521–542. [Google Scholar]
- 20.Knight KL. A Review of the Culex pipiens complex in the Mediterranean Subregion (Diptera, Culicidae) Trans. R. Entomol. Soc. Lond. 1951;102:354–364. doi: 10.1111/j.1365-2311.1951.tb00754.x. [DOI] [Google Scholar]
- 21.Kamura T, Bekku H. Studies on the Culex pipiens group of Japan. IV. Ecological studies on the Nagasaki molestus. Endemic Dis. Bull. Nagasaki Univ. 1959;1(1):51–59. [Google Scholar]
- 22.Kent RJ, Harrington LC, Norris DE. Genetic Differences Between Culex pipiens f. molestus and Culex pipiens pipiens (Diptera: Culicidae) in New York. J. Med. Entomol. 2007;44:50–59. doi: 10.1603/0022-2585(2007)44[50:gdbcpf]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amara Korba R, et al. Ecological differentiation of members of the Culex pipiens complex, potential vectors of West Nile virus and Rift Valley fever virus in Algeria. Parasites Vectors. 2016;9:1405. doi: 10.1186/s13071-016-1725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinogradova EB, Shaikevich EV. Morphometric, physiological and molecular characteristics of underground populations of the urban mosquito Culex pipiens Linnaeus f. molestus Forskål (Diptera: Culicidae) from several areas of Russia. Eur Mosq Bull. 2007;22:17–24. [Google Scholar]
- 25.Sulaiman S, Service MW. Studies on hibernating populations of the mosquito Culex pipiens L. in southern and northern England. J. Nat. History. 1983;17:849–857. doi: 10.1080/00222938300770661. [DOI] [Google Scholar]
- 26.Huang S, et al. Genetic Variation Associated with Mammalian Feeding in Culex pipiens from a West Nile Virus Epidemic Region in Chicago Illinois. Vector-Borne Zoonotic Dis. 2009;9:637–642. doi: 10.1089/vbz.2008.0146. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger DL, Armbruster PA. Mosquito diapause. Annu. Rev. Entomol. 2014;59:73–93. doi: 10.1146/annurev-ento-011613-162023. [DOI] [PubMed] [Google Scholar]
- 28.Onyeka JOA, Boreham PFL. Population studies, physiological state and mortality factors of overwintering adult populations of females of Culex pipiens L. (Diptera: Culicidae) BER. 1987;77:99. doi: 10.1017/S0007485300011585. [DOI] [Google Scholar]
- 29.Spielman A. Studies on Autogeny in Culex pipiens Populations in Nature. I. Reproductive isolation between autogenous and anautogenous populations. Am. J. Hygiene. 1964;80:175–183. doi: 10.1093/oxfordjournals.aje.a120466. [DOI] [PubMed] [Google Scholar]
- 30.Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation and taxonomic status. Proc Entomol. 1984;86:521–542. [Google Scholar]
- 31.Harbach RE, Dahl C, White GB. Culex (Culex) pipiens Linnaeus (Diptera: Culicidae): Concepts, type designations, and description. Proc. Entomol. Soc. Wash. 1985;87:24. [Google Scholar]
- 32.Merdić E, Vujičić-Karlo S. Two types of Hibernation of Culex pipiens complex (Diptera: Culicidae) in Croatia. Entomol. Croatia. 2005;9:71–76. [Google Scholar]
- 33.Kjærandsen J. Diptera in mines and other cave systems in southern Norway. Entomologica Fennica. 1993;4:151–160. doi: 10.33338/ef.83761. [DOI] [Google Scholar]
- 34.Badino G. Cave temperatures and global climatic change. IJS. 2004;33:103–113. doi: 10.5038/1827-806X.33.1.10. [DOI] [Google Scholar]
- 35.Barr RA. Ocurrence and distribution of the Culex pipiens Complex. Bull. World Health Organ. 1967;37:293–296. [PMC free article] [PubMed] [Google Scholar]
- 36.Gunn J, editor. Encyclopedia of caves and karst science. New York: Fitzroy Dearborn; 2004. [Google Scholar]
- 37.Höhlen. Verborgene Welten. 1st ed. (Primus-Verl., Darmstadt, 2008).
- 38.Buffington JD. Hibernaculum choice in Culex Pipiens. J. Med. Entomol. 1972;9:128–132. doi: 10.1093/jmedent/9.2.128. [DOI] [PubMed] [Google Scholar]
- 39.Thomson RCM. The reactions of mosquitoes to temperature and humidity. Bull. Entomol. Res. 1938;29:125. doi: 10.1017/S0007485300026158. [DOI] [Google Scholar]
- 40.Rudolf M, et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE. 2013;8:e71832. doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Core Team . R. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 42.Yee TW, Wild CJ. Vector generalized additive models. J. R. Stat. Soc. Ser. B (Methodol.) 1996;58:481–493. [Google Scholar]
- 43.Yee TW. Vector Generalized Linear and Additive Models: with an Implementation in R. Berlin: Springer; 2015. [Google Scholar]
- 44.GraphPad Software. GraphPad Prism (La Jolla California USA).
- 45.Copernicus Land Monitoring Service. Corine Land Cover Data (European Environment Agency (EEA), 2019).
- 46.Systems E. ArcGIS Desktop. CA: Redlands; 2019. [Google Scholar]
- 47.Werblow A, et al. Population structure and distribution patterns of the sibling mosquito species Culex pipiens and Culex torrentium (Diptera: Culicidae) reveal different evolutionary paths. PLoS ONE. 2014;9:e102158. doi: 10.1371/journal.pone.0102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weitzel T, Jawień P, Rydzanicz K, Lonc E, Becker N. Culex pipiens s.l. and Culex torrentium (Culicidae) in Wrocław area (Poland): occurrence and breeding site preferences of mosquito vectors. Parasitol Res. 2015;114:289–295. doi: 10.1007/s00436-014-4193-1. [DOI] [PubMed] [Google Scholar]
- 49.Lühken R, et al. Physico-chemical characteristics of Culex pipiens sensu lato and Culex torrentium (Diptera: Culicidae) breeding sites in Germany. J. Med. Entomol. 2015;52:932–936. doi: 10.1093/jme/tjv070. [DOI] [PubMed] [Google Scholar]
- 50.Zittra C, et al. Ecological characterization and molecular differentiation of Culex pipiens complex taxa and Culex torrentium in eastern Austria. Parasites Vectors. 2016;9:197. doi: 10.1186/s13071-016-1495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fonseca DM, et al. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- 52.Gomes B, et al. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol. Biol. 2009;9:262. doi: 10.1186/1471-2148-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreadis TG, Huang S, Molaei G. Reexamination of Culex pipiens hybridization zone in the Eastern United States by Ribosomal DNA-based single nucleotide polymorphism markers. Am. J. Trop. Med. Hyg. 2011;85:434–441. doi: 10.4269/ajtmh.2011.10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zittra C, Moog O, Christian E, Fuehrer H-P. DNA-aided identification of Culex mosquitoes (Diptera: Culicidae) reveals unexpected diversity in underground cavities in Austria. Parasitol. Res. 2019;118:1385–1391. doi: 10.1007/s00436-019-06277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The Effect of Temperature on Life History Traits of Culex Mosquitoes. J. Med. Entomol. 2014;51:55–62. doi: 10.1603/ME13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosà R, et al. Early warning of West Nile virus mosquito vector: climate and land use models successfully explain phenology and abundance of Culex pipiens mosquitoes in north-western Italy. Parasites Vectors. 2014;7:269. doi: 10.1186/1756-3305-7-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zittra C, et al. Landscape structure affects distribution of potential disease vectors (Diptera: Culicidae) Parasites Vectors. 2017;10:205. doi: 10.1186/s13071-017-2140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paz S, Albersheim I. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile fever outbreaks (Israeli Case Study: 2001–2005) EcoHealth. 2008;5:40–48. doi: 10.1007/s10393-007-0150-0. [DOI] [PubMed] [Google Scholar]
- 59.Verdonschot PFM, Besse-Lototskaya AA. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. doi: 10.1016/j.limno.2013.11.002. [DOI] [Google Scholar]
- 60.Ciota AT, et al. Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J. Med. Entomol. 2012;49:35–42. doi: 10.1603/me11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otto H-J. Waldökologie. Stuttgart: E. Ulmer; 1994. [Google Scholar]
- 62.Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J. Plantation forests and biodiversity: oxymoron or opportunity? Biodivers. Conserv. 2008;17:925–951. doi: 10.1007/s10531-008-9380-x. [DOI] [Google Scholar]
- 63.Ellenberg H, Leuschner C, Dierschke H. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. 6. Stuttgart: E. Ulmer; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.