Abstract
Lyme borreliosis (LB) is the archetypal emerging zoonosis and is dependent on transmission by ticks in the genus Ixodes. Understanding the origin, maintenance, and spread of these ticks contributes much to our understanding of the spread of LB and other disease agents borne by these ticks. We collected 1232 Ixodes scapularis ticks from 17 east coast sites ranging from New Hampshire to Florida and used mtDNA, three nuclear genetic loci, and incorporated Bayesian analyses to resolve geographically distinct tick populations and compare their demographic histories. A sparse, stable, and genetically diverse population of ticks in the Southeastern US, that is rarely infected with the agent of LB is genetically distinct from an abundant, expanding, and comparatively uniform population in the Northeast, where epidemic LB now constitutes the most important vector borne disease in the United States. The contrasting geography and demography of tick populations, interpreted in the context of the geological history of the region, suggests that during the last glacial period such ticks occupied distinct refugia, with only the northern-most site of refuge giving rise to those ticks and pathogens now fueling the epidemic.
Subject terms: Microbiology, Molecular biology, Zoology
Introduction
Lyme disease is the most commonly reported vector borne disease in North America though its risk varies widely by geographic region1. Ixodes scapularis is the main vector tick of Borrelia burgdorferi, the bacterium that causes Lyme disease. In the United States, most human Lyme disease cases are reported in the Northeast and upper Midwest, while cases in western and southern regions of the country are low2. This contrast is also evident in the entomological record where the frequency of B. burgdorferi is 2–8% in the main vector I. scapularis adult ticks in the South3,4, compared to infection rates averaging 50% in New England5. Environmental factors may affect survival rates of immature I. scapularis and the geographical distribution of Lyme disease6. In addition to the persistent difference in Lyme infection rates, there are major differences of questing behavior, human attacking rate, host preferences, and survival rate between northern and southern populations of I. scapularis ticks7–11. There are also vast differences in abundance, with I. scapularis tick densities in the South being much lower than in the North4,12.
Due to these phenotypic variations, it is not surprising that large genetic differences occur between north and south I. scapularis populations. In 1985, the original description of the north population as a new species I. dammini was driven in part by the report of an abundant I. ricinus-like tick with northern distribution several hundred miles disjunct from that of the southern I. scapularis13. However, the morphological differences between northern and southern populations were cryptic, tending to manifest only during particular life stages. In 1993, Oliver and colleagues suggested that I. dammini should be reduced to a junior synonym of I. scapularis14. Population genetic studies showed no differences in chromosome morphology or allozyme frequencies among populations14,15. The nuclear ribosomal DNA sequences of the ITS-l and ITS-2 regions were also continuously distributed among populations16,17. However, the distribution of mitochondrial haplotypes is distinct and forms two clades: (1) the American clade, found in the northeastern US down to the Carolinas, and (2) the Southern clade, which overlaps in the Carolinas and extends into the southeastern states. More genetic variation and diversity have been observed in ticks collected from southern states18–21 and extremely abundant single nucleotide polymorphisms (SNPs) have been found in I. scapularis22. SNPs analysis for ten ticks showed that samples from New Jersey and Virginia formed a homogeneous group with low genetic diversity, whereas southern ticks from Georgia and Mississippi consisted of two separate groups, each with high genetic diversity. SNPs analysis also revealed a predominantly North to South gene flow23.
While the population genetic structure of blacklegged ticks is a fundamental basis for understanding and predicting Lyme disease epidemiology, relevant studies have mainly utilized phylogenetic methods based on mitochondrial markers from a small number of individuals at limited collection sites. As a result, we still do not know the border between the northern and southern populations. Studies of nuclear, segregating genetic markers that could elucidate the evidence for genetic exchange across the broad geographic range of the east coast of the United States are limited24. Although ticks from northern and southern populations appear to be equally competent B. burgdorferi vectors in laboratory conditions25,26, data on the prevalence of the Lyme spirochete has not been linked to mitochondrial Southern clade ticks. The present study is based on mtDNA and three nuclear genetic loci and incorporated Bayesian analyses to detect evidence of population structure and geographically distinct tick populations. We have also used ecological niche modeling to determine the historical distribution and spread of these ticks. We found that northeastern (northern) and southeastern (southern) I. scapularis tick populations are genetically distinct with different histories and Lyme spirochete infection rates.
Results
Species and PCR amplification
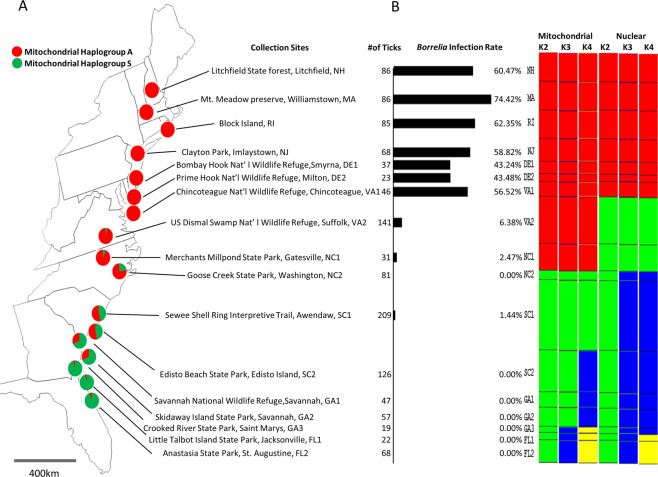
A total of 1236 adult ticks were flagged from vegetation from 17 east coast sites in November to December 2007 (Fig. 1). Four ticks collected at Awendaw, South Carolina were not identified as the species of interest (I. affinis N = 3; and I. brunneus N = 1) based on the mitochondrial 16 S sequences. Fragments of mitochondrial 16 S ribosomal RNA (mt DNA 16 S), nuclear (nuc) Calreticulin (CRT), Glycerol-3-phosphate dehydrogenase (G3PD) and Isocitrate dehydrogenase (IDH) genes were amplified for 1232 adult I. scapularis ticks.
Figure 1.
Collection sites, percentage of mitochondrial Haplogroups, Borrelia infection rates and BAPS population structures. (A) Colored circles are 17 collection sites of 1232 ticks from eastern US. Red and green colors show the percentage of mitochondrial Haplogroup A and S. (B) Total tick numbers in each collection site with Borrelia infection rate. Population structure analysis is based on mitochondrial and 3 nuclear genes using BAPS. Each color represents one population.
Phylogeny and distribution of haplotypes
In agreement with the findings of previous studies18,19, the phylogenetic analysis of 16 S gene revealed two mitochondrial haplogroups (Fig. 2). The proportion and geographic distribution of these two haplogroups clearly shows that one is distributed mainly in the North, but extending to the South, while the second is restricted only to the Southeast locations. From Suffolk, Virginia to Florida the haplotype ratios appear as a latitudinal cline, with the frequency of the southern haplogroup starting at 94.1% in St. Augustine, Florida and declining to 3.5% at Suffolk Virginia, and no individuals within that haplogroup occurring further north (Fig. 1).
Figure 2.
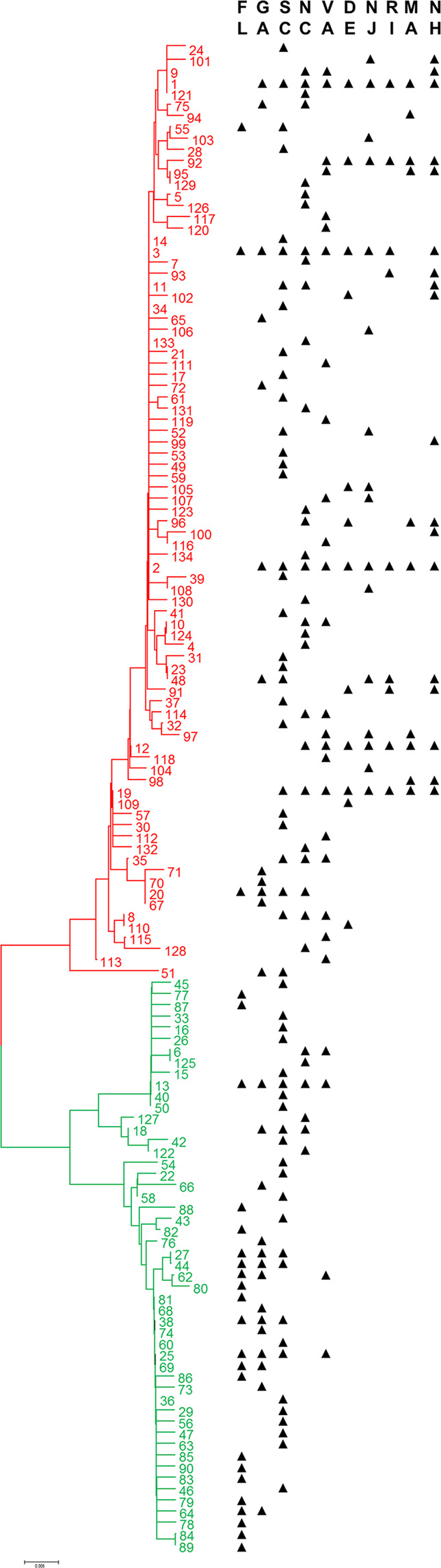
Phylogenetic tree of mtDNA haplotypes. The red color represents Haplogroup A and the green color Haplogroup S. Triangles show geographic distribution of each haplotype (Software used to generate the maximum likelihood (ML) tree was PAUP Version 4.0b10, https://paup.phylosolutions.com).
Analysis of mitochondrial 16 S sequences yielded 134 unique haplotypes. The greater number of haplotypes was detected in the southern locations (Fig. 2) (Supplementary S2 Table 1). The cluster analysis of mitochondrial haplotypes showed two groups. The samples from Florida, Georgia and South Carolina formed one group, and the samples from North Carolina, Virginia, Delaware, New Jersey, Rhode Island, Massachusetts and New Hampshire formed another group. Clearly, the 16 S haplotype distributions are different between the South and the North (Fig. 2).
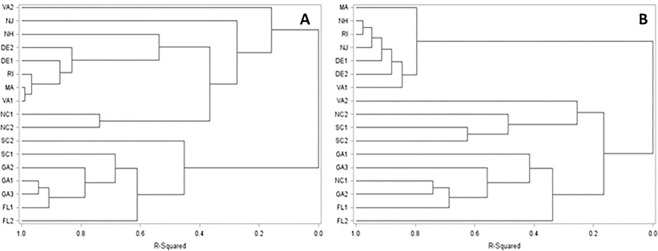
DNA sequences of 3 nuclear genes yielded 342, 201 and 124 unique haplotypes for CRT, IDH and G3PD, respectively. G3PD, CRT and IDH haplotype distributions also showed significant differences between the South and the North (Fig. 3). However, compared to the mitochondrial gene haplotype distribution, the cluster analysis of nuclear gene haplotype distribution suggested the boundary between southern and northern populations to be positioned more to the north, allocating the two Virginia populations to the southern population.
Figure 3.
Cluster Analysis of mtDNA (A) and nucDNA (B) haplotypes distribution among 17 east coast collection sites (New Hampshire: NH; Massachusetts: MA; Rhode Island: RI; New Jersey: NJ; Delaware: DE1 and DE2; Virginia: VA1 and VA2; North Carolina: NC1 and NC2; South Carolina: SC1 and SC2; Georgia: GA1, GA2 and GA3; Florida: FL2 and FL2), (cluster analysis was performed by SAS software Version 9.0, https://www.sas.com).
Population comparison
The mitochondrial 16 S gene, and nuclear genes CRT and IDH supported a separation between northern and southern populations when pairwise differences were used to analyze the population relationships (See Supplementary S1 Fig. A to D). The evaluated Fst values indicated limited gene flow between the southern and the northern populations. However, pairwise comparisons of Fst and Nei’s distance of G3PD gene showed no significant correlations between geographic locations and pairwise differentiation. Strong population structure was detected using AMOVA among populations, both within and between the North and South (Supplementary S2 Table 2).
Demographic history
According to the mismatch distribution analysis of 4 individual genes, the spatial expansion model could not be rejected for all 17 populations. However, the demographic model showed different results (Supplementary S2 Table 3). A mismatch distribution is the frequency distribution of the observed number of differences between pairs of haplotypes. The mitochondrial 16S gene suggested a sudden expansion for northern populations and stable tick populations in Florida, Georgia and South Carolina and North Carolina. The mismatch distribution of CRT and IDH sequences also indicated a different demographic history between southern and northern populations.
Bayesian analysis of population structure
The seventeen populations were clustered using Structure and BAPS software population mixture analysis. Both Structure and BAPS software suggest K = 4 for nuclear genes and K = 2 for mtDNA gene. The mitochondrial and combined nuclear data all suggested two major blacklegged tick population clusters along the eastern U.S. coast, with a single population containing NH, MA, RI, NJ, DE1, DE2 and VA1 and 2–3 possible subpopulations in the Southern area (Fig. 1). The mitochondrial and nuclear genes identified different boundaries of these two populations. Ticks (VA2) from Suffolk, Virginia belonged to the northern population based on mtDNA but to the southern group based on nuclear DNA.
Borrelia infection rate
The Borrelia infection rate ranged from 43.24 to 74.42% in NH, MA, RI, NJ, DE1, DE2 and VA1 tick populations (Fig. 1). The highest infection rate was 74.42% in Massachusetts. The infection rates of the two Virginia populations that belong to the southern population based on nuclear DNA but to the northern population based on mtDNA were very different, with an infection rate of 56.52% in Chincoteague (VA1) population and 6.38% in the Suffolk (VA2) population. In populations allocated to the southern group based on both mtDNA and nuclear DNA the infection rate was very low, ranging from 0 to 2.47%. The Borrelia infection was strictly limited to individuals with mitochondrial Haplogroup A; no Haplogroup S individuals were found positive for Borrelia.
Ecological niche model
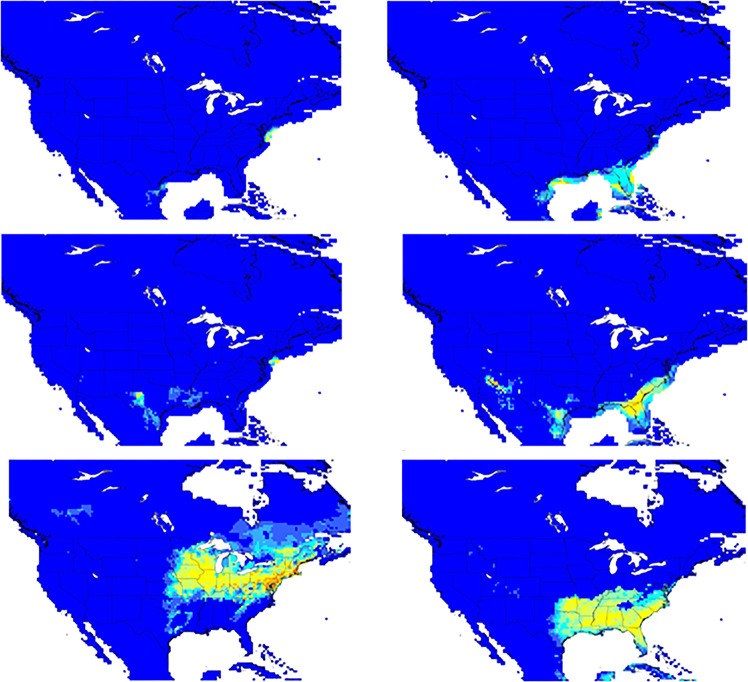
Projection of the ecological niche models (AUC values for the northern and southern population were 0.94 and 0.96) on the two Last Glacial Maximum climate reconstructions (CCSM and MIROC) suggested that the northern tick population was derived from a small refuge located just south of the Pleistocene ice sheet on the Atlantic continental shelf off the current Delmarva Peninsula. The southern I. scapularis population probably originated from a suitable refuge located in the southern coastal plain of today’s Florida, Georgia and South Carolina (Fig. 4).
Figure 4.
The predicted distribution for the northern (left) and southern population (right) of Ixodes scapularis at the Last Glacial Maximum (top), the mid-Holocene (middle) and the present day (bottom), based on ecological niche modeling. The warmer a grid cell’s color, the higher its predicted suitability (Software used to draw the map was Maxent Version 3.3.3k, https://biodiversityinformatics.amnh.org/open_source/maxent/).
Discussion
Based on haplotype distribution, population comparison, phylogenetic analysis, demographic history and Bayesian analysis of population structure, we conclude that there are two distinct blacklegged tick populations (northern and southern) along the US eastern coast, with distinct Borrelia infection rates and post-glacial histories.
In agreement with previous mtDNA studies18–20, this study not only suggested that blacklegged ticks are geographically structured along the east US coast, but also suggested that two distinct clades meet at North Carolina and Virginia. The southern I. scapularis population is characterized by greater heterogeneity and absence of widely distributed haplotypes. In contrast to the results from the ITS-2 gene17, analysis of three nuclear genes confirms the existence of two major structured populations of I. scapularis ticks. Both mitochondrial and nuclear genes are comparatively homogeneous in northern I. scapularis populations, but more diverse in southern I. scapularis populations. We found nuclear genes suggested a boundary between the northern and southern population in Virginia, whereas mtDNA suggested this boundary to be positioned in North Carolina.
The demographic model strongly supports a recent and rapid increase in population size for all Northeastern sites. By contrast, only several southern sites (Florida, Georgia and South Carolina) showed no evidence of changes in historical population size. The ecological niche model suggests that there were two possible distinct refugia in eastern North America during the Last Glacial Maximum. The northern I. scapularis populations are suggested to be derived from a small refugia located to the south of the Pleistocene ice sheet and on the Atlantic continental shelf off the current Delmarva Peninsula. Although this region has been submerged as sea level raised from melted glacial ice, it was a broad coastal plain and well covered with land vegetation during the Last Glacial Maximum27. The southern I. scapularis population may have originated from a suitable refuge that is more to the South, located in the Southern coastal plain. The later expansion of the southern population seemed to occur through much of the Southeastern quadrant of the United States. However, since I. scapularis is expanding its range and has recently shown tolerance for colder conditions in northern US and southern Canada, the current distribution of I. scapularis may not represent the full spectrum of its habitable environment, and the inferred historical range might be underestimated. The New York-Connecticut region20 and the areas further north of Delmarva have been suggested as a likely location of refugia populations of I. scapularis28.
We detected a homogenous northern population and a heterogeneous southern population. The heterogeneity of the southern population is due to introgression from the northern population. In agreement with previous studies18,19, we found that the two major mitochondrial haplogroups co-occur throughout the south. All northern ticks carry only Haplogroup A, while Haplogroups A and S occur mixed throughout the South. This has previously been explained as evidence of continuous gene flow among populations and a northward movement of the southern population3. We, however, found that the relative frequency of A and S haplogroups is a latitudinal cline with S increasing towards the South. The introgression of the northern population mitochondrial DNA might suggest that gene flow between two tick populations has migrated southwards. Positive selection or migrating birds may play an important role for the directional gene flow because they would carry ticks southward more effectively than northward28. However, the expansion of I. scapularis includes southward as well as northward expansion. For example, the recent northward invasion of I. scapularis and B. burgdorferi in Canada has occurred via the introduction by migratory birds29. The environmental conditions and selective pressure from warmer temperatures may change the host-seeking behavior of southern tick population6. Alternatively, the northern mtDNA in the Southeastern population may represent a genetic footprint left after the southern population displaced an expanding northern population30,31. Bayesian Analysis of Population Structure revealed two populations with a possible hybridization zone located in Virginia.
We found that the southern I. scapularis populations appear to be at a demographic equilibrium, while northern populations have gone through a recent demographic expansion, especially after glacial retreat. However, human activities have accelerated the expanding of I. scapularis ticks in the eastern United States and southern Canada during the 20th century32. I. scapularis was first collected on Naushon Island, Massachusetts in 1920. With the return of white-tailed deer to other areas of the Northeast and Midwest, the range of I. scapularis began expanding28. During the 1960s, focal I. scapularis infestations were recognized on Long Island and Nantucket Island in the Northeast and in northwestern Wisconsin. By the mid-1970s, the known range of I. scapularis had expanded, particularly extending in a southerly direction along the middle-Atlantic coast28. Within 100 years, this tick has largely spread throughout most of the eastern United States and into southern Canada32. The expansion of I. scapularis and B. burgdorferi is determined by distinct environmental factors: climate, habitat and host range and abundance29,33,34. In this study, only climatic factors were included in the ecological niche models.
The most interesting finding is that a high infection rate of Borrelia (43–74%) is mostly linked to the northern I. scapularis population and where Borrelia does occur in the southern I. scapularis population (as defined by nuclear DNA), it is always found linked to the northern mtDNA type. The infection rate drops substantially to 0–6% with a change of the nuclear DNA defined population boundary in Virginia. Whether the differences in the infection rate is environmentally determined, is a heritable trait for two populations or is a combination of genetic traits and hosts needs further study. For example, warmer temperatures and a lower density of host population may change the host-seeking behavior of southern tick population and eventually lower the incidence of Lyme disease in areas at low altitudes6. However, environmental factors alone cannot explain the virtual absence of infection rate in I. scapularis. Although different hosts were believed to be the reason for low infection rates in the South, a comparative study between two sites in eastern Virginia35 showed the infection of white-footed mice is 66.5% in the coastal site and 15.5% in the inland sites. Borrelia infection is 35.7% and 0% in the nymphal ticks removed from these mice in the coastal site and in the inland sites, respectively. Recently, the absence of detectable B. burgdorferi infection was found among blacklegged ticks in North Carolina36 and Tennessee37. These may suggest a genetic basis of tick behaviors that influence the epidemiology of vector-borne pathogens. It is very likely that the Northeastern tick population is the principal tick population responsible for transmitting Lyme disease. This same population is solely carrying two zoonotic agents in the Eastern US: Anaplasma phagocytophilum and Babesia microti13.
In this paper, we only sampled adult ticks from eastern coastal sites. Tick-borne disease in the Midwest of the US and Canada is increasing as I. scapularis populations continue to spread and grow29,38. It is worth noting that tick population ecology and spirochete transmission patterns are different between the northeast and northern Midwest. The two foci of B. burgdorferi in the northeastern and midwestern have a shared past and once belonged to an admixed population but are now isolated11,39,40. Transport of B. burgdorferi between northeastern and midwestern I. scapularis populations appears to be relatively infrequent.
The suggestion of a genetic basis to the transmission of Borrelia by Northeastern populations of I. scapularis is of significance for public health. We believe that, currently, the risk of acquiring Lyme disease through I. scapularis population is low in the South. First because they are low in density and they rarely bite humans7–9; more importantly, this population is not likely to be carrying the Lyme spirochete. However, this does not mean that there are no enzootic cycles of spirochetes in the South. Enzootic transmission of Lyme disease spirochetes among rodents and ticks has been documented in Southern and South-central states. Although they rarely bite humans, the sympatric tick species I. affinis, I. minor and I. dentatus may maintain a different enzootic cycle in the South41. In this study, two of three collected I. affinis ticks are Borrelia positive. In North Carolina, Borrelia DNA was detected in 63.2% of 155 I. affinis, but in 0% of 298 I. scapularis36; so-called “Ixodes scapularis” with a 35% Borrelia positive rate was confirmed as a non-accurate identification of I. affinis42. The current and potentially enhanced future role of the southern blacklegged tick population I. scapularis in Lyme epidemiology requires additional studies. Monitoring dynamics of the two populations will help us to predict future changes of tick and tick-borne pathogens.
Materials and Methods
Sampling natural tick populations
Adult ticks were collected from 17 sites in 10 states (New Hampshire: NH; Massachusetts: MA; Rhode Island: RI; New Jersey: NJ; Delaware: DE1 and DE2; Virginia: VA1 and VA2; North Carolina: NC1 and NC2; South Carolina: SC1 and SC2; Georgia: GA1, GA2 and GA3; Florida: FL2 and FL2) throughout the eastern United States by dragging a 1-m2 white flannel flag through vegetation in November - December 2007 (Fig. 1). Ticks were kept alive until we returned to the laboratory, then frozen individually at −80 °C. Total DNA was extracted from individual ticks using the Epicenter Master Complete DNA & RNA Purification Kits (Epicenter Technologies, Madison, WI), following the manufacturer protocols.
Gene markers
We examined multiple genetic loci, including partial sequences of one mitochondrial gene and three nuclear genes. Both mitochondrial and nuclear gene primers were developed from the available sequences in GenBank. Primers TK16S_105F (5′-CGGTCTGAACTCAGATCATGTA-3′) and TK16S_546RC (5′-AATTGCTGTGGTATTTTGACTATAC-3′) were used to amplify a mitochondrial 16 S (441 bp) region. PCR was performed at 95 C 15 sec, 45 C 15 sec and 72 C 60 sec for 40 cycles. Primers CRT_849F (5′-TGGACGAGCCGATGGGTA-3′) and CRT_1657RC (5′-GGGCTGTACTCTGGGTTATCGA-3′), G3PD_3574F (5′-GCGGTGGCACCTTGGTAG-3′) and G3PD_4307RC (5′-CCGTCCTTGTCCCGTTTCT-3′) and IDH_707F (5′-GGTACTTCGTGACCCTCCTGC-3′) and IDH_1436RC (5′-CGACCATAGTTGCGACCAGAC-3′) were designed for genes encoding Calreticulin (CRT, 808 bp), Glycerol-3-phosphate dehydrogenase (G3PD, 733 bp) and Isocitrate dehydrogenase (IDH, 729 bp), respectively. These three nuclear genes were amplified at 95 C 15 sec, 57 C 15 sec and 72 C 60 sec for 40 cycles. The PCR products were treated using Exosap-IT then sequenced with an ABI 3130 sequencer following the manufacturer protocols.
Borrelia burgdorferi real-time PCR Detection
Taqman real-time PCR assays were performed in a duplex format (to detect DNA of tick and B. burgdorferi) with a reaction volume of 20 μl, by using the Brilliant II QPCR Master Mix in a Stratagene MX3000P QPCR System (Agilent, La Jolla, Calif.). The detection of tick DNA served as an internal control with 300 nM of primers Tick16S_F (5′-AATACTCTAGGGATAACAGCGTAATAATTTT -3′) and Tick16S_R (5′-CGGTCTGAACTCAGATCAAGTAGGA-3′), and 125 nM of Tick16S_Probe (FAM-5′- AAATAGTTTGCGACCTCGATGTTGGATTAGGAT 3'- BHQ1). The detection of B. burgdorferi was modified from (Courtney et al. 2004) with 700 nM primers Borrelia23S_F (5′-CGAGTCTTAAAAGGGCGATTTAGT-3′) and Borrelia23S_R (5′-GCTTCAGCCTGGCCATAAATAG-3′), and 300 nM of Borrelia_Probe (HEX-5′- AGATGTGGTAGACCCGAAGCCGAGTG-3′-BHQ1). Cycling conditions included an initial activation of the Taq DNA polymerase at 95 °C for 10 min, followed by 40 cycles of a 15 s denaturation at 95 °C and a 1 min annealing-extension step at 60 °C.
Phylogeny and population structure
DNA sequences were aligned using the ClustalW software. Individual mitochondrial haplotypes were identified and converted to Arlequin format using FaBox43. Phylogenetic analyses were conducted using maximum likelihood (ML) method in PAUP 4.0b1044.
Nuclear haplotypes for heterozygotes were reconstructed using PHASE 2.145 with default parameters. Genetic structure was examined by an analysis of molecular variance (AMOVA), implemented in Arlequin version 3.146. Structure analysis was performed using Structure47 and BAPS version 5.348. Structure analysis was carried out with 10 independent runs per each K value (K = 1–17), with a 100,000 burn-in period and 900,000 Markov chain Monte Carlo iterations. These analyses were performed using a model with admixture, correlated allele frequencies and with no a priori information on the sample location of individuals. The ΔK approach was used to estimate the most likely K value. For BAPS, a spatial clustering algorithm and a mixture analysis of individuals without geographic information was chosen, with 10 replicates from K = 2 to K = 17 ran. When we run clustering of 17 sample populations, K = 4 was calculated based on nuclear genes and K = 2 was justified based on mtDNA. We, therefore, set the maximum number of clusters to 2, 3 and 4 for BAPS.
Locality and climate data used for ecological niche modeling
We composed a data set of 1423 county-level reported occurrences of blacklegged ticks (See Supplementary S3)49,50. These occurrences were separated into two partitions based on genetic results: northern I. scapularis population (n = 825) and southern I. scapularis population (n = 598). For climate layers we used bioclimatic variables for the present, the Mid-Holocene (~6Ka) and the Last Glacial Maximum (~21Ka) available from the WorldClim database 1.4 (http://www.worldclim.org) and resampled these to a 25 arcminute resolution (c. 50 × 50 km). We selected a subset of bioclimatic variables that show a Pearson’s correlation of r < 0.7, and are deemed biologically meaningful based on life history knowledge of the model system, in this case, seasonality: bio2 = mean diurnal temperature range (mean of the monthly difference between the minimum and maximum temperature), bio8 = mean temperature of the wettest quarter, bio9 = mean temperature of the driest quarter, bio16 = precipitation of the wettest quarter, and bio17 = precipitation of the driest quarter51,52 (Supplementary S2 Table 4).
Ecological niche modeling
We created ecological niche models for northern and southern I. scapularis populations with Maxent 3.3.3k53. We restricted the feature type to hinge features as this facilitates extrapolation54. The area between 20 and 55 degrees latitude and -60 and -130 degrees longitude was chosen as environmental background. Ecological niche models were projected on the current and past climate layers. Statistical significance of ecological niche models was confirmed55 by testing their AUC values against a null model based on random localities created with ENMTools 1.356.
Supplementary information
Author contributions
G.X. conceived and conducted the experiments. B.W. analyzed ecological niche modeling data. G.X. wrote the original draft - S.M.R. and B.W. revised and edited. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67259-0.
References
- 1.Bacon RM, Kugeler KJ, Griffith KS, Mead PS. Lyme disease - United States, 2003-2005 (Reprinted from MMWR, vol 56, pg 573-576, 2007) Jama-Journal of the American Medical Association. 2007;298:278–279. doi: 10.1001/jama.298.3.278. [DOI] [Google Scholar]
- 2.Diuk-Wasser MA, et al. Human Risk of Infection with Borrelia burgdorferi, the Lyme Disease Agent, in Eastern United States. Am. J. Trop. Med. Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver JH. Lyme borreliosis in the southern United States: A review. J. Parasitol. 1996;82:926–935. doi: 10.2307/3284201. [DOI] [PubMed] [Google Scholar]
- 4.Diuk-Wasser MA, et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecol. Biogeogr. 2010;19:504–514. [Google Scholar]
- 5.Barbour AG, Fish D. The Biological and Social Phenomenon of Lyme Disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg HS, et al. Environmental Factors Affecting Survival of Immature Ixodes scapularis and Implications for Geographical Distribution of Lyme Disease: The Climate/Behavior Hypothesis. PLoS One. 2017;12:e0168723. doi: 10.1371/journal.pone.0168723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falco RC, Fish D. Ticks Parasitizing Humans in a Lyme-Disease Endemic Area of Southern New-York State. Am. J. Epidemiol. 1988;128:1146–1152. doi: 10.1093/oxfordjournals.aje.a115057. [DOI] [PubMed] [Google Scholar]
- 8.Felz MW, Durden LA, Oliver JH. Ticks parasitizing humans in Georgia and South Carolina. J. Parasitol. 1996;82:505–508. doi: 10.2307/3284095. [DOI] [PubMed] [Google Scholar]
- 9.Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol. 2000;25:102–113. [PubMed] [Google Scholar]
- 10.Arsnoe I, Tsao JI, Hickling GJ. Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 2019;10:553–563. doi: 10.1016/j.ttbdis.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Gatewood AG, et al. Climate and Tick Seasonality Are Predictors of Borrelia burgdorferi Genotype Distribution. Appl. Environ. Microbiol. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stromdahl EY, Hickling GJ. Beyond Lyme: Aetiology of Tick-borne Human Diseases with Emphasis on the South-Eastern United States. Zoonoses Public Hlth. 2012;59:48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 13.Spielman A, Clifford CM, Piesman J, Corwin MD. Human Babesiosis on Nantucket-Island, USA - Description of the Vector, Ixodes (Ixodes) dammini, n sp-Sp (Acarina, Ixodidae) J. Med. Entomol. 1979;15:218–234. doi: 10.1093/jmedent/15.3.218. [DOI] [PubMed] [Google Scholar]
- 14.Oliver JH, et al. Conspecificity of the Ticks Ixodes scapularis and Ixodes dammini (Acari, Ixodidae) J. Med. Entomol. 1993;30:54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 15.Chen CS, Munderloh UG, Kurtti TJ. Cytogenetic Characteristics of Cell-Lines from Ixodes scapularis (Acari, Ixodidae) J. Med. Entomol. 1994;31:425–434. doi: 10.1093/jmedent/31.3.425. [DOI] [PubMed] [Google Scholar]
- 16.Mclain DK, Wesson DM, Oliver JH, Collins FH. Variation in Ribosomal DNA Internal Transcribed Spacers-1 among Eastern Populations of Ixodes scapularis (Acari, Ixodidae) J. Med. Entomol. 1995;32:353–360. doi: 10.1093/jmedent/32.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Wesson DM, Mclain DK, Oliver JH, Piesman J, Collins FH. Investigation of the Validity of Species Status of Ixodes dammini (Acari, Ixodidae) Using rDNA. Proc. Natl. Acad. Sci. USA. 1993;90:10221–10225. doi: 10.1073/pnas.90.21.10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris DE, Klompen JSH, Keirans JE, Black WC. Population genetics of Ixodes scapularis (Acari: Ixodidae) based on mitochondrial 16S and 12S genes. J. Med. Entomol. 1996;33:78–89. doi: 10.1093/jmedent/33.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Rich SM, et al. Distribution of the Ixodes ricinus-Like Ticks of Eastern North-America. Proc. Natl. Acad. Sci. USA. 1995;92:6284–6288. doi: 10.1073/pnas.92.14.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphrey PT, Caporale DA, Brisson D. Uncoordinated Phylogeography of Borrelia burgdorferi and Its Tick Vector, Ixodes scapularis. Evolution. 2010;64:2653–2663. doi: 10.1111/j.1558-5646.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Zee J, et al. High SNP density in the blacklegged tick, Ixodes scapularis, the principal vector of Lyme disease spirochetes. Ticks Tick Borne Dis. 2013;4:63–71. doi: 10.1016/j.ttbdis.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Van Zee, J., Piesman, J. F., Hojgaard, A. & Black, W. C. Nuclear Markers Reveal Predominantly North to South Gene Flow in Ixodes scapularis, the Tick Vector of the Lyme Disease Spirochete. Plos One10 (2015). [DOI] [PMC free article] [PubMed]
- 24.Araya-Anchetta A, Busch JD, Scoles GA, Wagner DM. Thirty years of tick population genetics: A comprehensive review. Infect. Genet. Evol. 2015;29:164–179. doi: 10.1016/j.meegid.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Dolan MC, Maupin GO, Panella NA, Golde WT, Piesman J. Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersoni (Acari: Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J. Med. Entomol. 1997;34:128–135. doi: 10.1093/jmedent/34.2.128. [DOI] [PubMed] [Google Scholar]
- 26.Sanders FH, Oliver JH. Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis (Acari, Ixodidae) from Georgia as Vectors of a Florida Strain of the Lyme-Disease Spirochete, Borrelia burgdorferi. J. Med. Entomol. 1995;32:402–406. doi: 10.1093/jmedent/32.4.402. [DOI] [PubMed] [Google Scholar]
- 27.Whitmore, F. C., Jr., Emery, K. O., Cooke, H. B. & Swift, D. J. Elephant teeth from the atlantic continental shelf. Science 156, 1477–1481, doi:156/3781/1477[pii]10.1126/science.156.3781.1477 (1967). [DOI] [PubMed]
- 28.Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini -Borne Human Babesiosis and Lyme Disease. Annu. Rev. Entomol. 1985;30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 29.Ogden, N. H., Mechai, S. & Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front. Cell Infect. Microbiol 3, doi:ARTN 46 10.3389/fcimb.2013.00046 (2013). [DOI] [PMC free article] [PubMed]
- 30.Currat M, Ruedi M, Petit RJ, Excoffier L. The hidden side of invasions: Massive introgression by local genes. Evolution. 2008;62:1908–1920. doi: 10.1111/j.1558-5646.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 31.Wielstra B. Historical hybrid zone movement: More pervasive than appreciated. J. Biogeogr. 2019;46:1300–1305. doi: 10.1111/jbi.13600. [DOI] [Google Scholar]
- 32.Sonenshine, D. E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Env. Res. Public Health 15, doi:ARTN 478 10.3390/ijerph15030478 (2018). [DOI] [PMC free article] [PubMed]
- 33.Bouchard C, et al. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southeastern Canada: Implications for Borrelia burgdorferi transmission. Ticks Tick Borne Dis. 2011;2:183–190. doi: 10.1016/j.ttbdis.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, Ogden NH. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 2012;49:457–464. doi: 10.1111/j.1365-2664.2012.02112.x. [DOI] [Google Scholar]
- 35.Sonenshine DE, et al. Borrelia burgdorferi in Eastern Virginia - Comparison between a Coastal and Inland Locality. Am. J. Trop. Med. Hyg. 1995;53:123–133. doi: 10.4269/ajtmh.1995.53.123. [DOI] [PubMed] [Google Scholar]
- 36.Maggi RG, Reichelt S, Toliver M, Engber B. Borrelia species in Ixodes affinis and Ixodes scapularis ticks collected from the coastal plain of North Carolina. Ticks Tick Borne Dis. 2010;1:168–171. doi: 10.1016/j.ttbdis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Rosen ME, et al. Borrelia burgdorferi Not Detected in Widespread Ixodes scapularis (Acari: Ixodidae) Collected From White-Tailed Deer in Tennessee. J. Med. Entomol. 2012;49:1473–1480. doi: 10.1603/ME11255. [DOI] [PubMed] [Google Scholar]
- 38.Hamer SA, Hickling GJ, Walker ED, Tsao JI. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the Midwestern United States. Infect. Genet. Evol. 2014;27:531–542. doi: 10.1016/j.meegid.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Hoen AG, et al. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc. Natl. Acad. Sci. USA. 2009;106:15013–15018. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apperson CS, Levine JF, Evans TL, Braswell A, Heller J. Relative Utilization of Reptiles and Rodents as Hosts by Immature Ixodes scapularis (Acari, Ixodidae) in the Coastal Plain of North Carolina, USA. Exp. Appl. Acarol. 1993;17:719–731. doi: 10.1007/BF00051830. [DOI] [PubMed] [Google Scholar]
- 41.Oliver JH, et al. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc. Natl. Acad. Sci. USA. 2003;100:11642–11645. doi: 10.1073/pnas.1434553100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison BA, et al. Recent discovery of widespread Ixodes affinis (Acari: Ixodidae) distribution in North Carolina with implications for Lyme disease studies. J Vector Ecol. 2010;35:174–179. doi: 10.1111/j.1948-7134.2010.00074.x. [DOI] [PubMed] [Google Scholar]
- 43.Villesen P. FaBox: an online toolbox for FASTA sequences. Mol. Ecol. Notes. 2007;7:965–968. doi: 10.1111/j.1471-8286.2007.01821.x. [DOI] [Google Scholar]
- 44.Swofford DL. Paup - a Computer-Program for Phylogenetic Inference Using Maximum Parsimony. J. Gen. Physiol. 1993;102:A9–A9. [Google Scholar]
- 45.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 47.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 48.Corander, J., Marttinen, P., Siren, J. & Tang, J. Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinformatics 9, doi:Artn 539 Doi 10.1186/1471-2105-9-539 (2008). [DOI] [PMC free article] [PubMed]
- 49.Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and in Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 50.Eisen RJ, Eisen L, Beard CB. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 52.Peterson AT. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 2011;38:817–827. doi: 10.1111/j.1365-2699.2010.02456.x. [DOI] [Google Scholar]
- 53.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- 54.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010;1:330–342. doi: 10.1111/j.2041-210X.2010.00036.x. [DOI] [Google Scholar]
- 55.Raes N, ter Steege H. A null-model for significance testing of presence-only species distribution models. Ecography. 2007;30:727–736. doi: 10.1111/j.2007.0906-7590.05041.x. [DOI] [Google Scholar]
- 56.Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607–611. doi: 10.1111/j.1600-0587.2009.06142.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.