Abstract
Tumour-Node-Metastasis (TNM) staging of colorectal cancer (CRC) needs further classification for better treatment because of disease heterogeneity. Although molecular classifications which are expensive and laborious are under study, cost and labour efficient subtyping is desirable. We assessed the combinations of preoperative tumour marker (TM) elevation and tumour lymphovascular invasion (LVI) as a solution. We used the pooled data of 7151 colon cancer (CC) patients and 4620 rectal cancer (RC) patients who received curative surgery between 2004 and 2008 in Japan. The best-matched subtyping for predicting relapse-free survival (RFS) was statistically selected using the c-index and Akaike’s information criterion. This subtyping (TM-LVI), which consisted of three categories by TM elevation status and severity of LVI status, was an independent prognostic factor for RFS of CC (stage IIa, IIIb, and IIIc) and RC (stage I, IIa, IIb, IIIa, and IIIb) and also for disease specific survival of CC (stage IIa, IIb, IIIb, and IIIc) and RC (all stage except for IIc). Although TM-LVI classified CRC patients into low and high recurrence risk groups, the application of adjuvant therapy was not accordance with the TM-LVI status. TM-LVI may be a cost and labour efficient subtyping of colorectal cancer for better treatment strategy.
Subject terms: Translational research, Surgical oncology, Cancer therapy, Colorectal cancer, Predictive markers, Prognostic markers
Introduction
The treatments of colorectal cancer (CRC) patients after curative surgery are based on clinical staging. CRC patients with high risk stages (i.e., stages II and III) are recommended for adjuvant therapy1–5. However, the heterogeneous characteristics of CRC results in different prognoses among CRC patients within the same clinical stage. As a result, both the American Joint Committee on Cancer/Union for International Cancer Control staging system (Tumour-Node-Metastasis [TNM] staging system) and the Japanese Society for Cancer of the Colon and Rectum (JSCCR) staging system have developed several editions to improve the accuracy of predicting prognosis6–8. These staging systems are composed of tumour depth, nodal status, and metastatic status, although there are differences among the versions even in the same staging system. Therefore, further subtyping in each clinical stage is indispensable for better treatment strategy.
Recently, classifications of CRC using genetic background have been proposed as alternative or additional tools for staging. Microsatellite instability (MSI) status indicated good prognosis in patients with stage II/III right-sided colon cancer (CC)9. Consensus molecular subtypes consisting of four groups (MSI immune, canonical, metabolic, and mesenchymal) have been presented as powerful tools for CRC biology and treatment10–13. These classifications based on genetic analysis have been expected to replace TNM staging in the future, although benefit for treatment decision has not been validated.
However, high risk patients for recurrence have been selected using clinicopathological features. Many articles have demonstrated that elevations in tumour markers (TMs, e.g., carcinoembryonic antigen [CEA] or cancer antigen 19-9 [CA19-9]) have been associated with poor prognosis in CRC14–18. Lymphovascular invasion (LVI) was also indicated to be a prognostic factor for CRC19–22. However, these factors have not been included in the TNM staging systems and have not been assessed in combination. We considered that improvement of classification by combination of these features was necessary before applying novel classifications that require more labour and cost.
Thus, we statistically selected the most suitable subtyping combined the influence of TM elevation and LVI on relapse-free survival (RFS) using the pooled data collected by the Japanese Study Group for Postoperative Follow-up of CRC (JFUP-CRC), which is one of the largest data collections in Japan23,24. We evaluated this classification (so called TM-LVI) as a prognostic factor for RFS and disease specific survival (DSS) in each TNM staging. We also assessed the association between application of adjuvant therapy and TM-LVI status.
Results
Clinicopathological characteristics of the patients
Clinicopathological characteristics were compared between CC and rectal cancer (RC) patients. There were significant differences between CC and RC patients in age (P < 0.0001), sex (P < 0.0001), histological type (P < 0.0001), the ratio of CEA elevation (P = 0.008), the degree of LVI (P < 0.0001), dissected lymph node number (12 ≤ or not), TNM stage (P < 0.0001), and the application of adjuvant therapy (P < 0.0001) but not in the ratio of CA19-9 elevation (Supplementary Table 1).
Adjuvant therapy consisted of chemotherapy except for two cases of radiotherapy and seven cases of chemoradiotherapy in RC. Most patients (94.6%) received 5-fluorouracil based chemotherapy. A total of 1.6% and 1.1% of the patients received oxaliplatin-based and irinotecan-based chemotherapy, respectively (Supplementary Table 1).
Thus, further analysis was performed by CC and RC.
Selection of the most suitable subtyping
Among six candidate subtypes (Table 1), ABC1 was the most statistically suitable subtype with the lowest Akaike’s information criterion (AIC, Supplementary Table 2) and the highest Harrell’s concordance index (c-index, Supplementary Table 3) according to the models including TNM staging. Then, we called ABC1 as TM-LVI. Both TNM and TM-LVI were significant in the Cox model for RFS in CC and RC patients. The interaction term between TNM and TM-LVI was significant in RC but not CC. Thus, in ranking the incidence rate of RFS, the main effect model with TNM and TM-LVI was applied to CC, and the interaction model was employed for RC. When RFS was ranked from 1st to 21st by TNM staging and TM-LVI, RFS was not ordered by TNM staging. Stage IIIa was a low recurrence risk group compared to most of stage II (Supplementary Table 4). Category C by TM-LVI belonged to the highest recurrence risk group in each TNM stage.
Table 1.
Candidate subtypes were determined depending on the tumour marker elevation status and lymphovascular invasion status.
| Subtypes | Tumour marker elevation | Lymphovascular invasion | |||
|---|---|---|---|---|---|
| None | Slight | Mild | Severe | ||
| ABC1 | Both CEA and CA19-9 | B | C | C | C |
| Either CEA or CA19-9 | A | B | C | C | |
| None | A | A | B | C | |
| ABC2 | Both CEA and CA19-9 | B | B | C | C |
| Either CEA or CA19-9 | A | B | C | C | |
| None | A | A | B | C | |
| ABC3 | Both CEA and CA19-9 | A | B | C | C |
| Either CEA or CA19-9 | A | B | C | C | |
| None | A | B | B | C | |
| ABC4 | Both CEA and CA19-9 | A | B | C | C |
| Either CEA or CA19-9 | A | B | C | C | |
| None | A | A | B | C | |
| ABC5 | Both CEA and CA19-9 | B | B | C | C |
| Either CEA or CA19-9 | B | B | C | C | |
| None | A | B | B | C | |
| AB | Both CEA and CA19-9 | A | A | B | B |
| Either CEA or CA19-9 | A | A | B | B | |
| None | A | A | A | B | |
CA19-9, Cancer antigen 19-9; CEA, Carcinoembryonic antigen.
Validation of TM-LVI for RFS and DSS
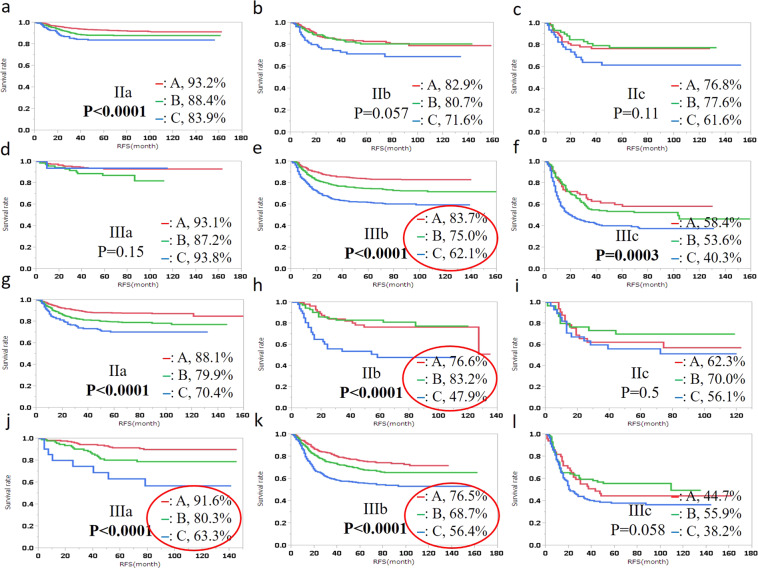
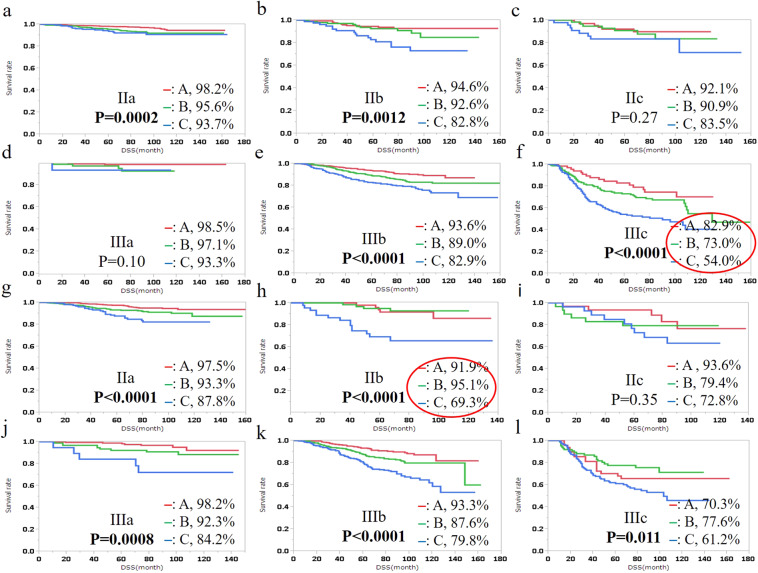
Log-rank test demonstrated that RFS was significantly different by TM-LVI status in CC (stage IIa, IIIb, and IIIc) and RC (stage I, IIa, IIb, IIIa, IIIb) (Fig. 1). In particular, the 5-year RFS differed more than 20% by TM-LVI status between A and C (83.7% and 62.1% in stage IIIb of CC, 76.6% and 47.9% in stage IIb of RC, 91.6% and 63.3% in stage IIIa of RC, and 76.5% and 56.4% in stage IIIb of RC). Log-rank test also demonstrated that DSS was significantly different by TM-LVI in CC (stage IIa, IIb, IIIb, and IIIc) and RC (except for IIc) (Fig. 2). The 5-year DSS differed more than 20% by TM-LVI status between A and C (82.9% and 54.3% in stage IIIc of CC and 91.9% and 69.3% in stage IIb of RC).
Figure 1.
Relapse-free survival (RFS) of colon cancer (a–f) and rectal cancer patients (g-l) by TM-LVI status is shown for each TNM stage. The 5-year RFS rate is described on the right of the TM-LVI status. Bold type, P < 0.05; Red circle, difference in the RFS rate among TM-LVI statuses > 20%.
Figure 2.
Disease free survival (DSS) of colon cancer (a–f) and rectal cancer (g–l) patients by TM-LVI status is shown for each TNM stage. The 5-year DSS rate is described on the right of the TM-LVI status. Bold type, P < 0.05; Red circle, difference of DSS rate among TM-LVI statuses > 20%.
We assessed the factors associated with RFS and DSS by univariate and multivariate analysis. TM-LVI was an independent prognostic factor for RFS of CC (stage IIa, IIIb, and IIIc, Tables 1,2) and RC (stage I, IIa, IIb, IIIa, and IIIb, Table 3) and also for DSS of CC (stage IIa, IIb, IIIb, and IIIc, Table 4) and RC (all stage except for IIc, Table 5).
Table 2.
Univariate and multivariate analysis for relapse-free survival of colon cancer was performed in each clinical stage.
| Stage | I | IIa | IIb | IIc | IIIa | IIIb | IIIc | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Univariate analysis | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | |
| Age | ||||||||||||||||||||
| 75≥ /<75 | 0.97. | 0.94 | 1.22 | 0.21 | — | — | 1.3 | 0.29 | 1.63 | 0.10 | 1.02 | 0.97 | 1.12 | 0.30 | — | — | 1.12 | 0.50 | ||
| Gender | ||||||||||||||||||||
| M/F | 1.53 | 0.20 | 1.25 | 0.14 | — | — | 0.96 | 0.84 | 1.04 | 0.88 | 0.93 | 0.86 | 1.29 | 0.01 | 1.29 | 0.01 | 1.21 | 0.17 | 1.21 | 0.16 |
| Histology | ||||||||||||||||||||
| Diff/Undiff | 0.94 | 0.95 | 6.26 | 0.0003 | 6.11 | 0.0004 | 1.51 | 0.34 | 0.88 | 0.78 | 0.61 | 0.53 | 1.11 | 0.60 | — | — | 0.95 | 0.76 | ||
| Dissected LN | ||||||||||||||||||||
| 12 > /12≤ | 0.89 | 0.71 | 1.54 | 0.01 | 1.42 | 0.11 | 1.37 | 0.27 | 2.13 | 0.03 | 0.99 | 0.97 | 1.41 | 0.008 | 1.36 | 0.06 | 1.30 | 0.30 | ||
| Adjuvant | ||||||||||||||||||||
| Yes/No | 6.25 | 0.002 | 1.01 | 0.98 | — | — | 1.39 | 0.19 | 0.80 | 0.53 | 0.82 | 0.62 | 0.94 | 0.55 | — | — | 0.92 | 0.57 | ||
| TM-LVI | ||||||||||||||||||||
| C/A | 1.9×10−9 | 0.42 | 2.31 | <0.0001 | 2.32 | 0.0001 | 1.80 | 0.08 | 1.79 | 0.14 | 0.98 | 0.19 | 2.84 | <0.0001 | 2.84 | <0.001 | 1.91 | 0.0004 | 1.94 | 0.0004 |
| B/A | 0.96 | 1.60 | 1.54 | 1.03 | 0.91 | 2.17 | 1.70 | 1.69 | 1.19 | 1.21 | ||||||||||
| C/B | 2.1×10-9 | 1.44 | 1.50 | 1.75 | 1.95 | 0.45 | 1.66 | 1.68 | 1.61 | 1.60 | ||||||||||
TM-LVI was an independent prognostic factor for relapse-free survival of colon cancer in stage IIa, IIIb, and IIIc.
M/F, Male/Female; Diff/Undiff, Differentiated/Undifferentiated; LN, Lymph node; RR, Risk ratio; Bold type, P < 0.05.
Table 3.
Univariate and multivariate analysis for relapse-free survival of rectal cancer was performed in each clinical stage.
| Stage | I | IIa | IIb | IIc | IIIa | IIIb | IIIc | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | ||||||||||||
| RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | |
| Age | ||||||||||||||||||||||||
| 75≥/<75 | 1.66 | 0.048 | 1.52 | 0.10 | 1.16 | 0.43 | — | — | 0.92 | 0.83 | 0.86 | 0.77 | 2.36 | 0.028 | 1.74 | 0.16 | 1.09 | 0.55 | — | — | 0.95 | 0.85 | ||
| Gender | ||||||||||||||||||||||||
| M/F | 0.84 | 0.38 | — | — | 1.27 | 0.12 | — | — | 0.90 | 0.71 | 1.37 | 0.35 | 1.79 | 0.07 | 1.27 | 0.03 | 1.24 | 0.054 | 1.15 | 0.39 | ||||
| Histology | ||||||||||||||||||||||||
| Diff/Undiff | 1.04 | 0.96 | — | — | 0.97 | 0.94 | — | — | 0.51 | 0.19 | 0.45 | 0.14 | 0.43 | 0.12 | 0.31 | 0.056 | 1.06 | 0.83 | — | — | 0.64 | 0.049 | ||
| Dissected LN | ||||||||||||||||||||||||
| 12 > /12≤ | 1.38 | 0.13 | — | — | 1.11 | 0.55 | — | — | 1.54 | 0.2. | 1.86 | 0.24 | 0.98 | 0.95 | 1.60 | 0.0007 | 1.54 | 0.007 | 0.85 | 0.90 | ||||
| Adjuvant | ||||||||||||||||||||||||
| Yes/No | 1.99 | 0.089 | — | — | 1.46 | 0.029 | 1.36 | 0.07 | 1.27 | 0.44 | 1.00 | 1.00 | 0.42 | 0.006 | 0.53 | 0.053 | 0.75 | 0.014 | 0.76 | 0.02 | 0.90 | 0.56 | ||
| TM-LVI | ||||||||||||||||||||||||
| C/A | 3.40 | <0.0001 | 3.24 | 0.0002 | 2.66 | <0.0001 | 2.59 | <0.0001 | 2.98 | 0.0004 | 3.25 | 0.0003 | 1.22 | 0.50 | 5.61 | 0.0005 | 4.15 | 0.002 | 2.13 | <0.0001 | 2.07 | <0.0001 | 1.30 | 0.06 |
| B/A | 2.29 | 2.24 | 1.80 | 1.77 | 0.88 | 0.98 | 0.74 | 2.33 | 2.42 | 1.37 | 1.32 | 0.84 | ||||||||||||
| C/B | 1.49 | 1.45 | 1.48 | 1.47 | 3.38 | 3.30 | 1.65 | 2.41 | 1.71 | 1.56 | 1.56 | 1.54 | ||||||||||||
TM-LVI was an independent prognostic factor for relapse-free survival of rectal cancer in stage I, IIa, IIb, IIIa, and IIIb.
M/F, Male/Female; Diff/Undiff, Differentiated/Undifferentiated; LN, Lymph node; RR, Risk ratio; Bold type, P < 0.05.
Table 4.
Univariate and multivariate analysis for disease specific survival of colon cancer was performed in each clinical stage.
| Stage | I | IIa | IIb | IIc | IIIa | IIIb | IIIc | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||
| RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | |
| Age | ||||||||||||||||||||||
| 75≥/<75 | 1.15 | 0.83 | 2.21 | 0.0006 | 2.01 | 0.0025 | 1.97 | 0.051 | 1.78 | 0.10 | 1.79 | 0.2 | 1.49 | 0.64 | 1.35 | 0.052 | 1.30 | 0.20 | ||||
| Gender | ||||||||||||||||||||||
| M/F | 1.58 | 0.44 | 1.39 | 0.14 | 0.80 | 0.49 | 1.00 | 1.0 | 0.88 | 0.85 | 1.42 | 0.01 | 1.41 | 0.02 | 1.10 | 0.57 | ||||||
| Histology | ||||||||||||||||||||||
| Diff/Undiff | 6.8×108 | 0.44 | 5.23 | 0.026 | 4.67 | 0.04 | 2.00 | 0.29 | 1.45 | 0.6 | 1.9×109 | 0.37 | 0.75 | 0.26 | 0.61 | 0.02 | 0.63 | 0.03 | ||||
| Dissected LN | ||||||||||||||||||||||
| 12 > /12≤ | 0.65 | 0.64 | 2.64 | 0.0002 | 2.21 | 0.004 | 1.41 | 0.64 | 1.26 | 0.68 | ND | 0.88 | 1.49 | 0.049 | 1.41 | 0.1 | 1.54 | 0.15 | ||||
| Adjuvant | ||||||||||||||||||||||
| Yes/No | 1.5×10−8 | 0.43 | 0.62 | 0.19 | 1.47 | 0.28 | 0.33 | 0.09 | 0.86 | 0.84 | 0.90 | 0.46 | 0.92 | 0.64 | ||||||||
| TM-LVI | ||||||||||||||||||||||
| C/A | 1.6×10−9 | 0.13 | 2.85 | 0.0003 | 2.80 | 0.0009 | 3.68 | 0.003 | 3.47 | 0.006 | 2.29 | 0.3 | 4.61 | 0.14 | 2.64 | <0.0001 | 2.64 | <0.0001 | 2.65 | <0.0001 | 2.63 | <0.0001 |
| B/A | 1.6×10−9 | 2.25 | 2.04 | 1.53 | 1.51 | 1.38 | 4.05 | 1.68 | 1.67 | 1.53 | 1.53 | |||||||||||
| C/B | 1.0 | 1.27 | 1.37 | 2.41 | 2.30 | 1.66 | 1.14 | 1.57 | 1.58 | 1.73 | 1.72 | |||||||||||
TM-LVI was an independent prognostic factor for relapse-free survival of colon cancer in stage IIa, IIb, IIIb, and IIIc.
M/F, Male/Female; Diff/Undiff, Differentiated/Undifferentiated; LN, Lymph node; RR, Risk ratio; Bold type, P < 0.05.
Table 5.
Univariate and multivariate analysis for disease specific survival of rectal cancer was performed in each clinical stage.
| Stage | I | IIa | IIb | IIc | IIIa | IIIb | IIIc | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||||
| RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | |
| Age | ||||||||||||||||||||||||||
| 75 ≥ /<75 | 3.02 | 0.007 | 2.68 | 0.02 | 2.52 | 0.0007 | 2.55 | 0.0006 | 1.14 | 0.81 | 0.78 | 0.73 | 1.90 | 0.28 | 1.69 | 0.01 | 1.30 | 0.23 | 1.59 | 0.13 | 1.28 | 0.43 | ||||
| Gender | ||||||||||||||||||||||||||
| M/F | 1.61 | 0.19 | 1.50 | 0.10 | 0.56 | 0.17 | 1.74 | 0.23 | 3.17 | 0.02 | 2.57 | 0.07 | 1.09 | 0.58 | 1.19 | 0.39 | ||||||||||
| Histology | ||||||||||||||||||||||||||
| Diff/Undiff | 1.8×109 | 0.21 | 0.88 | 0.86 | 0.62 | 0.55 | 0.32 | 0.07 | 0.18 | 0.03 | 0.22 | 0.047 | 1.16 | 0.67 | 0.67 | 0.17 | 0.69 | 0.22 | ||||||||
| Dissected LN | ||||||||||||||||||||||||||
| 12 > /12≤ | 2.27 | 0.04 | 2.05 | 0.07 | 1.49 | 0.15 | 2.86 | 0.044 | 2.73 | 0.06 | 3.07 | 0.08 | 1.15 | 0.86 | 1.83 | 0.006 | 1.67 | 0.03 | 1.24 | 0.54 | ||||||
| Adjuvant | ||||||||||||||||||||||||||
| Yes/No | 1.21 | 0.80 | 1.48 | 0.16 | 1.36 | 0.49 | 2.02 | 0.12 | 0.39 | 0.04 | 0.45 | 0.1 | 0.63 | 0.005 | 0.71 | 0.06 | 0.68 | 0.1 | 0.70 | 0.13 | ||||||
| TM-LVI | ||||||||||||||||||||||||||
| C/A | 2.34 | 0.004 | 1.94 | 0.01 | 3.69 | <0.0001 | 3.75 | <0.0001 | 4.55 | 0.0003 | 4.32 | 0.0004 | 2.20 | 0.35 | 7.25 | 0.007 | 5.10 | 0.037 | 3.26 | <0.0001 | 3.16 | <0.0001 | 1.52 | 0.01 | 1.52 | 0.01 |
| B/A | 3.40 | 3.12 | 1.94 | 1.92 | 0.69 | 0.67 | 1.49 | 2.39 | 2.00 | 1.75 | 1.72 | 0.73 | 0.74 | |||||||||||||
| C/B | 0.69 | 0.62 | 1.90 | 1.95 | 6.57 | 6.46 | 1.47 | 3.04 | 2.54 | 1.86 | 1.84 | 2.07 | 2.06 | |||||||||||||
TM-LVI was an independent prognostic factor for relapse-free survival of rectal cancer in stage I, IIa, IIb, IIIa, IIIb, and IIIc.
M/F, Male/Female; Diff/Undiff, Differentiated/Undifferentiated; LN, Lymph node; RR, Risk ratio; Bold type, P < 0.05.
Association between the adjuvant therapy and TM-LVI status
The application of adjuvant therapy significantly differed by TM-LVI in stage I, IIa, IIIa, and IIIc CC and stage IIIa RC (Table 6). However, the application of adjuvant therapy was not irrelevant with the recurrence risk evaluated by TM-LVI except for stage IIa CC. The application of adjuvant therapy did not differ by TM-LVI status (stage IIIb CC and in stage I, IIa, IIb, and IIIb RC) or adversely decreased in spite of the increased recurrence (stage IIIc CC and stage IIIa RC), although TM-LVI status was an independent prognostic factor for both RFS and DSS in these stages. These results suggested that TM-LVI, which represents tumour marker elevation and lymphovascular invasion, was not used for determining the use of adjuvant treatment.
Table 6.
Association between the adjuvant therapy and TM-LVI status was assessed in each clinical stage.
| Location | TNM stage | TM-LVI | P | ||
|---|---|---|---|---|---|
| A Number (%) | B Number (%) | C Number (%) | |||
| Colon | I | 35(2.1%) | 5(2.0%) | 4(8.5%) | 0.013 |
| IIa | 148(11.3%) | 85(14.1%) | 44(18.5%) | 0.0054 | |
| IIb | 44(23.4%) | 33(21.3%) | 17(19.8%) | 0.77 | |
| IIc | 15(19.5%) | 14(21.2%) | 11(22.9%) | 0.90 | |
| IIIa | 145(67.1%) | 46(62.2%) | 5(31.3%) | 0.014 | |
| IIIb | 398(61.1%) | 401(66.7%) | 261(62.3%) | 0.10 | |
| IIIc | 58(80.6%) | 112(70.9%) | 130(63.4%) | 0.0003 | |
| Rectum | I | 45(3.9%) | 14(6.3%) | 2(4.0%) | 0.25 |
| IIa | 86(14.8%) | 85(19.4%) | 37(21.1%) | 0.059 | |
| IIb | 12(20.7%) | 26(34.2%) | 11(22.5%) | 0.16 | |
| IIc | 9(26.5%) | 8(26.7%) | 13(44.8%) | 0.22 | |
| IIIa | 151(77.8%) | 72(73.5%) | 11(52.4%) | 0.036 | |
| IIIb | 272(73.5%) | 293(72.7%) | 225(68.8%) | 0.34 | |
| IIIc | 34(66.7%) | 68(75.6%) | 117(75.0%) | 0.45 | |
The application of adjuvant therapy was not related to the risk of recurrence as estimated by TM-LVI status except for stage IIa CC. Red circles indicated that TM-LVI was an independent prognostic factor for both relapse-free survival and disease specific survival. Adjuvant therapy may be recommended according to TM-LVI status in these stages.
Bold type, P < 0.05.
Discussion
Due to the heterogeneity of the disease, further classification beyond TNM-based clinical staging has been considered indispensable for determining the treatment strategy of CRC. Despite continued effort, novel modalities are still under development10–13. We combined TM elevation and LVI for subtyping of the TNM staging system because of their potential as prognostic factors and ready-to-use availability. Among candidate classifications, we selected the most statistically suitable classification and named TM-LVI.
Our data demonstrated that TM-LVI was useful for subtyping and prognosis for not only RFS but also DSS, although we picked up TM-LVI depending on RFS. This may be consistent with the fact that TM elevation and LVI have been considered prognostic factors for RFS and overall survival, respectively16–18,21,22.
Our data indicated that adjuvant treatment was not considered in accordance with the recurrence risk determined by TM-LVI status. Thus, TM-LVI may be useful for considering adjuvant therapy after curative surgery when TM-LVI is an independent prognostic factor for both RFS and DSS (stage IIa, IIIb, and IIIc CC and stage I, IIa, IIb, IIIa, and IIIb RC).
We evaluated LVI by scoring both lymphatic invasion and venous invasion, although LVI is usually discussed as positive or negative. This may be because pathological assessments differ among pathologists regarding LVI status. In our massive dataset, the influence of pathologists may be reduced compared to data from single institute.
Our study has several limitations. First, the pathological results of LVI were not discussed among the pathologists to standardize the evaluation of LVI. Second, in this retrospective study, the treatment of the patients may vary depending on the clinicians and the hospitals. Third, genetic information was not collected. MSI status, which is associated with the prognosis of CRC patients, is not routinely assessed in most Japanese hospitals25.
In conclusion, we present a cost and labour efficient subtyping method (TM-LVI) for CRC patients using clinicopathological features routinely assessed in the clinic all over the world. The usefulness of TM-LVI should be validated in the future by randomized clinical trials regarding adjuvant treatment after curative surgery for patients with poor prognosis as estimated by TM-LVI.
Methods
Patients and data collection
The JFUP-CRC contains data from twenty-three institutes in Japan (Sapporo Medical University Hospital, Hirosaki University Hospital, Niigata University Hospital, Niigata Cancer Center Hospital, National Defence Medical College Hospital, Tochigi Cancer Center Hospital, Tokyo University Hospital, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, National Cancer Center Hospital, Tokyo Women’s Medical University Hospital, National Center for Global Health and Medicine Hospital, Tokyo Medical and Dental University Hospital, Keio University Hospital, Teikyo University Hospital, Kyorin University Hospital, Kitasato University Hospital, Fujita Health University Hospital, Aichi Cancer Center Hospital, Kyoto University Hospital, Osaka International Cancer Institute Hospital, Osaka Rosai Hospital, Hyogo College of Medicine Hospital, and Kurume University Hospital). Each hospital retrospectively collected the clinical data of patients with CRC who underwent curative surgery. This study was approved by the institutional review board or ethics committee at all 23 hospitals above and was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Clinical Research. The patients provided written informed consent, and patients had the option to opt-out if there was any disagreement with this study. The JFUP-CRC office pooled and organized the data for this study. Among the patients whose data are contained in the database, we assessed 11771 patients, consisting of 7151 CC patients and 4620 RC patients, who received curative surgery between 2004 and 2008. We classified these patients by the 8th edition TNM staging system6,7. A higher level of CEA or CA19-9 than the upper limit in each hospital was determined to indicate TM elevation. TM elevation was classified into three categories: both CEA and CA19-9 elevation, either CEA or CA19-9 elevation, or no elevation. Lymphatic or venous invasion was evaluated as 0 (no invasion), 1 (minimal invasion), 2 (moderate invasion), or 3 (severe invasion) by pathologists in each hospital according to the classification by the JSCCR8. We summed the evaluation in both lymphatic invasion and venous invasion as LVI, which was categorized as none (0), slight (1-2), mild (3-4), or severe (5-6).
Selection of the most suitable subtyping
To select the most suitable subtyping using both TM elevation and LVI, we assessed six candidate classifications (ABC1-ABC5, AB), which simplified 12 categories determined by TM elevation (both, either, or none) and LVI (none, slight, mild, or severe) into three (A, B, and C; ABC1-ABC5) or two (A, B; AB) subtypes (Table 1). Then, Akaike’s information criterion (AIC) and Harrell’s concordance index (c-index) were derived from the Cox proportional hazard model with TNM and each subtype to explore the most suitable (lower AIC and/or higher c-index) subtyping for RFS. If the interaction term between TNM and the candidate classification was significant, the term was included in the Cox model for ranking the incidence rates of RFS within each subtype. We did not exclude the patients who received adjuvant therapy, because we explored the subtyping available in all patients who received curative surgery.
Validation of TM-LVI for RFS and DSS
Univariate analysis was performed using Cox proportional hazard model, along with age (75 ≤ or not), sex, histological type (differentiated type or not), number of dissected lymph nodes (12 ≤ or not), and adjuvant therapy. Multivariate analysis was also performed using Cox proportional hazard model with the factors that showed significant differences (p < 0.05) in the univariate analysis. When TM-LVI was the only significant factor in the univariate analysis, multivariate analysis was performed using Cox proportional hazard model using the factors with p < 0.2. The influence of TM-LVI on RFS and DSS in each TNM stage was also assessed by Kaplan-Meier curve and evaluated by the Log-rank test.
Data analysis
The comparisons of the clinicopathological characteristics between CC and RC patients were assessed by the chi-squared test or t-test. The influence of the clinicopathological features on RFS and DSS was evaluated by a Cox proportional hazard model. RFS and DSS was calculated by the Kaplan-Meier method and compared by the log-rank test. Differences in the application of adjuvant therapy by combined subtyping were evaluated by the chi-squared test. Multivariate analysis for RFS and DSS was performed using a Cox proportional hazard model. A P value of <0.05 was considered significant for all analyses. All statistical analyses were performed using SAS software (SAS Institute, Cary, NC, USA).
Supplementary information
Acknowledgements
We appreciate the following members of the JFUP-CRC who provided the data: I. Takemasa (Sapporo Medical University), K. Hakamada (Hirosaki University), H. Kameyama (Niigata University), Y. Takii (Niigata Cancer Center Hospital), K. Hase (National Defence Medical College), H. Ozawa (Tochigi Cancer Center), H. Nozawa (Tokyo University), K. Takahashi (Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital), Y. Kanemitsu (National Cancer Center Hospital), M. Itabashi (Tokyo Women’s Medical University), H. Yano (National Center for Global Health and Medicine), Y. Kinugasa (Tokyo Medical and Dental University), H. Hasegawa (Keio University), Y. Hashiguchi (Teikyo University), T. Masaki (Kyorin University), M. Watanabe (Kitasato University), T. Hanai (Fujita Health University), K. Komori (Aichi Cancer Center Hospital), Y. Sakai (Kyoto University), M. Ohue (Osaka International Cancer Institute), S. Noura (Osaka Rosai Hospital), and Y. Akagi (Kurume University). We also appreciate American Journal Experts for editing our manuscript. Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
T.Y.: Conception, study design, data analysis, acquisition of data, and manuscript writing; S. Y.: Data analysis and acquisition of data, M.I.: Statistical data analysis and manuscript writing, Y.T., J.S., K.K., M.Y., A.B., K.K., N.B., M.I., N.T. and K.S.: Data analysis and acquisition of data, All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66652-z.
References
- 1.André T, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl 6):vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. (NCCN Guidelines) version1. 2019 colon cancer. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 4.NCCN. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. (NCCN Guidelines) version1. 2019 rectal cancer. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 5.Iveson TJ, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562–578. doi: 10.1016/S1470-2045(18)30093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley, J. D., Gospodarowicz, M. K., Wittekind, C. TNM classification of malignant tumours. 8th ed. (JOHN WILEY & SONS, LTD. 2017).
- 7.Amin, M. B. et al. AJCC Cancer Staging Manual, 8th Edition. (Springer International Publishing, 2017).
- 8.Japanese Classification of Colorectal Carcinoma by Japanese Society for Cancer of the Colon and rectum, Second English edition. Tokyo (Kanehara, 2009).
- 9.Sinicrope FA, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 2013;31:3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guinney J, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dienstmann R, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, et al. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2019;55:37–52. doi: 10.1016/j.semcancer.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana E, Eason K, Cervantes A, Salazar R, Sadanandam A. Context matters-consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann. Oncol. 2019;30:520–527. doi: 10.1093/annonc/mdz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann. Surg. Oncol. 2009;16:3087–3093. doi: 10.1245/s10434-009-0625-z. [DOI] [PubMed] [Google Scholar]
- 15.Lin PC, et al. Carbohydrate antigen 19-9 is a valuable prognostic factor in colorectal cancer patients with normal levels of carcinoembryonic antigen and may help predict lung metastasis. Int. J. Colorectal Dis. 2012;27:1333–1338. doi: 10.1007/s00384-012-1447-1. [DOI] [PubMed] [Google Scholar]
- 16.Shibutani M, et al. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer. Res. 2014;34:3753–3758. [PubMed] [Google Scholar]
- 17.Kim CG, et al. Preoperative Serum Carcinoembryonic Antigen Level as a Prognostic Factor for Recurrence and Survival After Curative Resection Followed by Adjuvant Chemotherapy in Stage III Colon Cancer. Ann. Surg. Oncol. 2017;24:227–235. doi: 10.1245/s10434-016-5613-5. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, Chen Z, Wu J, Li Z, Wu Y. Prognostic value of pretreatment serum carbohydrate antigen 19-9 level in patients with colorectal cancer: A meta-analysis. PLoS One. 2017;12:e0188139. doi: 10.1371/journal.pone.0188139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huh JW, Lee JH, Kim HR, Kim YJ. Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am. J. Surg. 2013;206:758–763. doi: 10.1016/j.amjsurg.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Nikberg M, et al. Lymphovascular and perineural invasion in stage II rectal cancer: a report from the Swedish colorectal cancer registry. Acta Oncol. 2016;55:1418–1424. doi: 10.1080/0284186X.2016.1230274. [DOI] [PubMed] [Google Scholar]
- 21.Yuan H, et al. Lymphovascular invasion is a high risk factor for stage I/II colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:46565–46579. doi: 10.18632/oncotarget.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang HH, et al. Prognostic significance of lymphovascular invasion in colorectal cancer and its association with genomic alterations. World J. Gastroenterol. 2019;25:2489–2502. doi: 10.3748/wjg.v25.i20.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamano T, et al. Evaluation of appropriate follow-up after curative surgery for patients with colorectal cancer using time to recurrence and survival after recurrence: a retrospective multicenter study. Oncotarget. 2018;9:25474–25490. doi: 10.18632/oncotarget.25312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamano T, et al. Influence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: A retrospective multicentre study. Eur. J. Cancer. 2017;81:90–101. doi: 10.1016/j.ejca.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Yamano T, et al. Management strategies in Lynch syndrome and familial adenomatous polyposis: a national healthcare survey in Japan. Cancer Sci. 2017;108:243–249. doi: 10.1111/cas.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.