Abstract
Acute Lymphoblastic Leukemia (ALL) is the most common cancer in children. Differences are found among ethnic groups in the results of the treatment of pediatric ALL. In general, children with a high level of native American ancestry tend to respond less positively to ALL treatments, which may be related to specific genomic variants found in native American groups. Despite the evidence, few data are available on the distribution of the pharmacogenomic variants relevant to the treatment of ALL in traditional Amerindian populations, such the those of the Amazon region. Given this, the present study investigated 27 molecular markers related to the treatment of ALL in Amerindians from Brazilian Amazonia and compared the frequencies with those recorded previously on five continents, that are available in the 1,000 Genomes database. The variation in the genotype frequencies among populations was evaluated using Fisher’s exact test. The False Discovery Rate method was used to correct the results of the multiple analyses. Significant differences were found in the frequencies of the majority of markers between the Amerindian populations and those of other regions around the world. These findings highlight the unique genetic profile of the indigenous population of Brazilian Amazonia, which may reflect a distinct therapeutic profile for the treatment of ALL in these populations.
Subject terms: Cancer, Genetics, Molecular biology
Introduction
Acute Lymphoblastic Leukemia (ALL) is the most common cancer subtype found in children, accounting for almost 80% of cases1,2. The treatment of ALL is based on the application of a combination of chemotherapeutic agents. Two drugs, 6-mercaptopurine (6-MP) and Methotrexate (MTX), are the principal types of medication used during the consolidation and maintenance phases of the treatment of ALL. Survival rates are relatively high, at approximately 80%, although around 20% of the children treated present serious toxicological complications that frequently lead to the interruption of the treatment. The variation in the toxicological response of patients to the treatment of ALL may be determined by different polymorphic variants of the genes involved in the pharmacokinetics and/or pharmacodynamics of these drugs3,4. Ethnic differences have been recognized in many clinical studies of the treatment of ALL5–10. In general, Hispanic children present worse results in the treatment of ALL than European children5–7,9. This difference may be related to an increase in the frequency of variants of the germinative lineage associated with native American ancestry in Hispanic patients, which have a negative influence on the treatment of ALL9.
Despite the apparent influence of genetic variants linked to native American ancestry on the treatment of ALL in children, few data are available on the frequency of these variants in traditional Amerindian populations, such as those from the Amazon region11. The vast majority of the available studies have focused on European or American populations, which emphasizes the need to identify the genetic profile of Amerindian populations, in particular the variants that are significantly more common in these populations, as well as compiling a database for the development of clinical applications appropriate for this ethnic group and for populations admixed with this group, such as the general Brazilian population11.
In this context, the present study investigated 27 molecular markers related to the treatment of ALL in a combined Amerindian population from Brazilian Amazonia. These findings were compared with the data available on representative populations from five continents, that are available in the 1,000 Genomes database.
Material and methods
Study populations
The present study was approved by the Brazilian National Committee for Ethics in Research (CONEP), through protocol 961,451. The informed consent was obtained from each study participant and all research methods in this study were performed in accordance with approved guidelines and regulations. The study sample consisted of 203 healthy individuals from three Amerindian groups resident in the Brazilian Amazon region: 25 individuals from the Asurini do Koatinemo group, 84 individuals from the Asurini do Trocará group, and 94 from the Kayapó-Xicrin group. These individuals were combined in a single sample, denominated NAT (or Native), for the statistical analyses. For the comparisons with populations from other continents, data were obtained from the database of the 1,000 Genomes project, available at https://www.1000genomes.org. This sample (Supplementary Figure 1) included 661 individuals from Africa (AFR), 503 from Europe (EUR), 347 from the Americas (AMR), 504 from East Asia (EAS), and 489 from South Asia (SAS).
Patients
Markers that showed a significantly different allelic distribution between Amerindian and of 1,000 Genomes populations were investigated in a sample of 42 patients diagnosed with B-cell ALL, from the Brazilian Amazon region and who had a high contribution of Amerindian genetic ancestry (see Supplementary Figure 2). For statistical analysis, these individuals were grouped as ALL_NAT. Diagnosis was confirmed based on the criteria of the French-American-British (FAB) classification system between the years of 2006 and 2018 in two reference public hospitals in the treatment of childhood cancer (Hospital Ophir Loyola and Hospital Oncológico Infantil Octavio Lobo, Belém-PA, Brazil), and peripheral blood samples were collected at the time of diagnosis. Clinical and demographic data of these patients are described in Supplementary Table 1.
Selection of the genes and polymorphisms
A total of 27 genetic markers (Supplementary Table 2) were selected for the present study. All these markers (from the genes ABCC1, ABCC2, ABCC3, AMPD1, ATIC, CCND1, GGH, ITPA, MTHFD1, MTHFR, MTRR, NALCN, NOS3, SHMT1, SLCO1B1, TLR4, TNFAIP3, and TPMT) participate in the pharmacokinetics and/or pharmacodynamics of one or both of the drugs (6-MP or MTX) used to treat ALL. The polymorphisms were selected based on the information available in the PharmGKB, NCBI, and Ensembl databases, and through a literature search. The polymorphisms selected for the present study have potentially functional effects, including alterations of amino acids and the alternative splicing of promoter regions or are present in regions linked to transcription factors, as well as markers included in previous studies of ALL or the drugs used to treat this cancer.
Genotyping of the polymorphisms and quality control
The genetic material was extracted from samples of peripheral blood, collected from the participants, using the commercial Biopur Mini Spin Plus–250 extraction kit (Biopur, Brazil), following the manufacturer’s instructions, and quantified using a NanoDrop 1,000 spectrophotometer (Termo Scientific NanoDrop 1,000; NanoDrop Technologies,Wilmington, DE). The polymorphisms were genotyped using the TaqMan OpenArray Genotyping technology (Applied Biosystems, Life Technologies, Carlsbad, CA) in a QuantStudio 12 K Flex Real-Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA), following the protocol published by Applied Biosystems. The Taqman Genotyper software was used to analyze the data from the plates and the precision of the genotype reads, as well as to control the quality of the genotyping.
As fixed mutations are uninformative, the polymorphisms with Minor Allele Frequencies (MAFs) of less than 1% were omitted from the analyses, as were polymorphisms with no genotyping and absent genotyping rates of 20% or more. This left 12 of the 27 polymorphisms investigated originally: rs2372536 (ATIC), rs4673993 (ATIC), rs9344 (CCND1), rs1800909 (GGH), rs3758149 (GGH), rs2236225 (MTHFD1), rs1801133 (MTHFR), rs1801394 (MTRR), rs2306283 (SLCO1B1), rs4149056 (SLCO1B1), rs1142345 (TPMT), and rs1800460 (TPMT).
Ethnic classification of Amerindian populations
The Amerindian ancestry of the individuals included in the present study was confirmed using the set of 61 Ancestry-Informative Markers (AIMs) described by Santos et al.12 and Ramos et al.13. The proportion of ancestry was estimated in STRUCTURE v2.3.3, assuming the contribution of three parental populations, African, European, and Native American. Amerindians were defined as having at least 95% native American ancestry.
All the individuals included in the present study had more than 95% native American ancestry. The overall mean genomic ancestry of the study population was 0.022 European, 0.014 African, and 0.964 native American (see supplementary Figure 3).
Statistical analysis
The statistical analyses were run in the R v.3.4.0 program (R Foundation for Statistical Computing). The inter-population variability of the polymorphisms analyzed in the present study was evaluated using Wright’s Fixation Index (FST). The FST values were used to run a multidimensional scaling analysis to plot the genetic differentiation of the Amerindian population with those from the five continental populations being included for comparison. The differences between the genotype frequencies of the Amerindian populations and those from the 1,000 Genomes database were evaluated using Fisher’s exact test. The False Discovery Rate (FDR) was used to correct the multiple analyses14. A p ≤ 0.05 significance was considered for all analyses.
Results
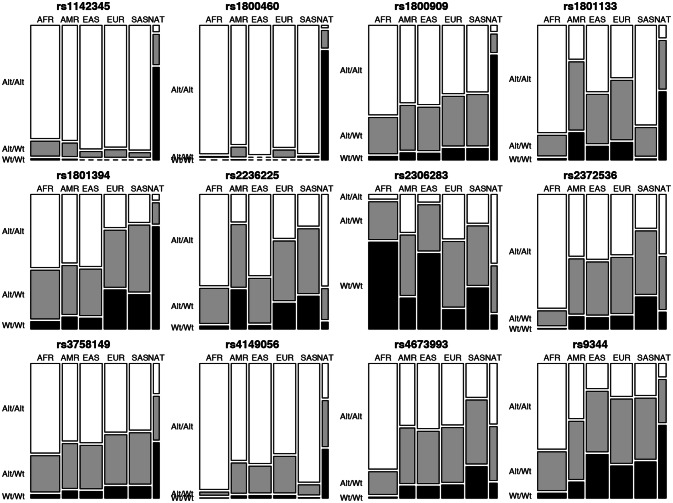
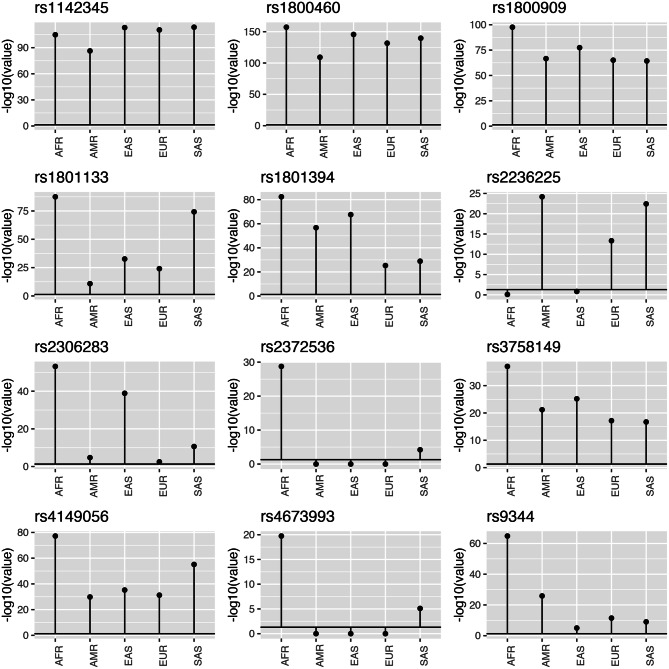
The allele frequencies recorded for the 12 markers investigated in the NAT population and the continental populations (AFR, AMR, EAS, EUR, and SAS) are shown in Table 1, and the distribution of the genotypes is plotted in Fig. 1. Nine of the 12 markers investigated in the present study vary significantly in the NAT population in comparison with all five continental populations. These polymorphisms are rs9344_CCND1, rs1800909_GGH, rs3758149_GGH, rs1801133 _MTHFR, rs1801394_MTRR, rs2306283_SLCO1B1, rs4149056_SLCO1B1, rs1142345_TPMT and rs1800460_TPMT (Fig. 2, Table 2). The other three markers presented significant differences between the NAT population and only some of the continental populations. In the case of the rs2236225 polymorphism of the MTHFD1 gene, for example, the NAT population was not significantly different (P > 0.05) from either the AFR or the EAS population, while rs23725336 (ATIC gene) was only significantly different from the AFR population, and rs4673993 (ATIC), only from the AFR and SAS populations.
Table 1.
Allele frequencies of the markers investigated in the different study populations (AFR, AMR, EAS, EUR, SAS, and NAT).
| Gene | SNP, ID | Reference allele | Frequency in population: | |||||
|---|---|---|---|---|---|---|---|---|
| NAT | AFR | AMR | EAS | EUR | SAS | |||
| TPMT | rs1142345 | T | 0.873 | 0.933 | 0.942 | 0.978 | 0.971 | 0.982 |
| TPMT | rs1800460 | C | 0.946 | 0.997 | 0.959 | 1 | 0.972 | 0.995 |
| MTHFR | rs1801133 | G | 0.712 | 0.909 | 0.525 | 0.704 | 0.635 | 0.881 |
| MTRR | rs1801394 | A | 0.903 | 0.754 | 0.719 | 0.737 | 0.477 | 0.475 |
| MTHFD1 | rs2236225 | G | 0.174 | 0.842 | 0.455 | 0.801 | 0.570 | 0.495 |
| GGH | rs1800909 | A | 0.883 | 0.833 | 0.772 | 0.780 | 0.720 | 0.713 |
| GGH | rs3758149 | G | 0.581 | 0.833 | 0.772 | 0.780 | 0.721 | 0.713 |
| ATIC | rs4673993 | T | 0.323 | 0.905 | 0.693 | 0.706 | 0.686 | 0.509 |
| ATIC | rs2372536 | C | 0.336 | 0.934 | 0.691 | 0.707 | 0.683 | 0.507 |
| SLCO1B1 | rs2306283 | A | 0.290 | 0.182 | 0.527 | 0.238 | 0.597 | 0.452 |
| SLCO1B1 | rs4149056 | T | 0.548 | 0.986 | 0.865 | 0.876 | 0.838 | 0.957 |
| CCND1 | rs9344 | G | 0.735 | 0.812 | 0.651 | 0.428 | 0.502 | 0.483 |
Figure 1.
Mosaic plots of the distribution of the genotypes of the 12 markers investigated in the six study populations (AFR, AMR, EAS, EUR, SAS, and NAT). Genotypes: Alt/Alt = mutant homozygote, Alt/Wt = heterozygote, and Wt/Wt = wild homozygote.
Figure 2.
Plots of the adjusted p values (FDR) for the comparison of the 12 markers investigated in the present study between the NAT population and the five continental populations (AFR, AMR, EAS, EUR, SAS), based on Fisher’s exact test. The significance threshold (p = 0.05) is represented by the bold horizontal line.
Table 2.
Adjusted p values (FDR) for the comparison of the 12 markers investigated in the present study between the NAT population and the five continental populations (AFR, AMR, EAS, EUR, SAS), based on Fisher’s exact test.
| Gene | SNP, ID | Reference allele | Adjusted p value (FDR) for the NAT population versus: | ||||
|---|---|---|---|---|---|---|---|
| AFR | AMR | EAS | EUR | SAS | |||
| TPMT | rs1142345 | T | 1.48e−105 | 5.44e−87 | 8.46e−114 | 3.82e−111 | 3.07e−114 |
| TPMT | rs1800460 | C | 6.47e−158 | 5.74e−110 | 2.64e−146 | 2.24e−132 | 2.24e−140 |
| MTHFR | rs1801133 | G | 3.17e−88 | 1.58e−11 | 2.31e−33 | 8.71e−25 | 5.72e−75 |
| MTRR | rs1801394 | A | 4.35e−83 | 2.04e−57 | 2.69e−68 | 4.89e−26 | 1.22e−29 |
| MTHFD1 | rs2236225 | G | 8.00e−01 | 6.73e−25 | 1.45e−01 | 4.58e−14 | 3.78e−23 |
| GGH | rs1800909 | A | 2.69e−98 | 2.59e−67 | 3.86e−78 | 8.70e−66 | 5.40e−65 |
| GGH | rs3758149 | G | 9.35e−38 | 6.70e−22 | 6.57e−26 | 6.77e−18 | 1.96e−17 |
| ATIC | rs4673993 | T | 1.85e−20 | 1.00e+00 | 1.00e+00 | 1.00e+00 | 7.89e−06 |
| ATIC | rs2372536 | C | 1.80e−29 | 1.00e+00 | 1.00e+00 | 1.00e+00 | 6.31e−05 |
| SLCO1B1 | rs2306283 | A | 7.09e−54 | 1.89e−05 | 1.33e−39 | 3.26e−03 | 2.37e−11 |
| SLCO1B1 | rs4149056 | T | 6.09e−78 | 1.48e−30 | 5.85e−36 | 5.73e−32 | 7.22e−56 |
| CCND1 | rs9344 | G | 1.49e−65 | 1.39e−26 | 1.04e−05 | 3.65e−12 | 1.02e−09 |
The plot of the Multidimensional Scaling (MDS) analysis of the FST values of the pairwise comparisons of the polymorphisms is presented in Fig. 3. The distribution of the points reveals that both the Amerindian (NAT) and the African (AFR) populations have distinct genetic profiles from those of the other populations (AMR, EAS, EUR, and SAS) included in the analysis, given their isolation in the extremities of the plot. The genetic profile of the Amerindian population is most similar to those of the American (AMR) and South Asian (SAS) populations.
Figure 3.
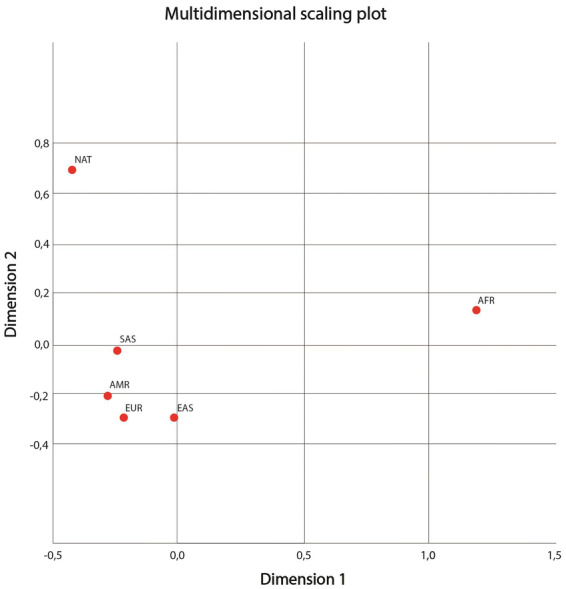
Plot of the results of the multidimensional spacing analysis of the genetic profiles of the six study populations investigated in the present study (AFR, AMR, EAS, EUR, SAS, and NAT), based on the 12 polymorphisms selected for analysis.
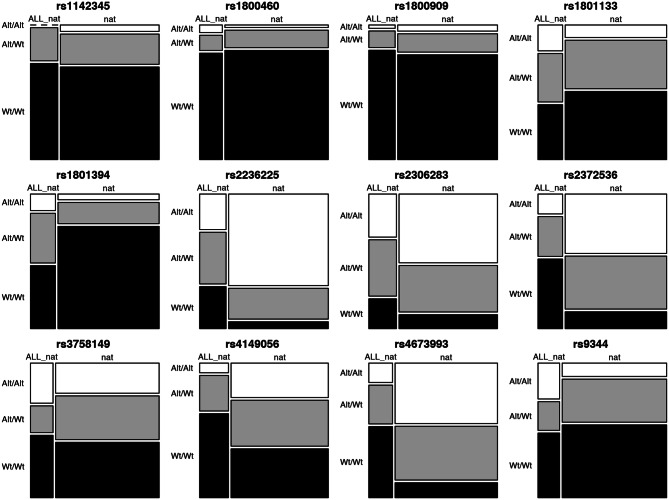
An additional analysis was performed to show whether the allelic variations of the 12 markers investigated in traditional Amerindian populations would show the same genetic profile in ALL patients with a high Amerindian genetic contribution. Thus, the genotypic distribution of these markers was compared between the Amerindian populations investigated in this study (NAT) and leukemic patients (ALL_NAT) with an average of 50% of Amerindian genetic ancestry (see Supplementary Table 1). Differences between genotypic frequencies were observed using Fisher's exact test and FDR was used to correct multiple analyses. The results are shown in Fig. 4. Of the 12 markers, eight of them (rs1142345, rs1800460, rs1800909, rs1801133, rs2306283, rs3758149, rs4149056 and rs9344) did not show a statistically significant difference, which indicates that the two populations are genetically similar.
Figure 4.
Mosaic plots of the genotype distribution of the 12 markers investigated in Amerindian populations (NAT) and ALL patients (ALL_NAT). Genotypes: Alt/Alt = mutant homozygote, Alt/Wt = heterozygote, and Wt/Wt = wild homozygote. The p values from right to left are: 1; 1; 1; 0.292; 0.07; ≤ 0.001; 0.154; ≤ 0.001; 0.986; 0.051; ≤ 0.001; 0.112.
Discussion
Disparities among ethnic groups in the treatment of ALL have been reported widely5–10, and the evidence indicates, in particular, that Hispanic children have the worst response to the therapeutic treatment of this cancer5–7,9. While this tendency has been associated partially with the contribution of native American ancestry in admixed Hispanic populations9,15–17, no previous study had focused specifically on the genetic markers associated with the treatment of ALL in Brazilian Amerindian populations. This reinforces the need for the investigation of the molecular variants in Amerindian populations, not only for a better understanding of the genetic variability of this population, but also to provide important empirical insights for the development of personalized protocols for the treatment of ALL in this ethnic group, as well as in other groups with a high level of Amerindian admixture. The findings of the present study should thus contribute to the improvement of the efficacy and security of the medication used to treat ALL in the Amerindian populations of the Amazon region.
Here, the distribution of the allele and genotype frequencies of 12 important pharmacogenomic markers related to 6-MP and MTX, the principal drugs used in the treatment of ALL, was compared between Amerindian (NAT) populations and five representative populations from the 1,000 Genomes database, using Fisher’s exact test with the standard statistical correction (FDR) being used to avoid misleading correlations.
The frequencies of all the pharmacogenomic markers analyzed in the present study were significantly different in the NAT population in comparison with all (in nine cases) or most of the continental populations (AFR, AMR, EAS, EUR, and SAS). The least differentiated marker was rs23725336 (ATIC), which varied significantly only between the NAT and AFR populations, while the frequency of rs4673993 (ATIC) of the NAT population was significantly different only from those of the AFR and SAS populations. The third marker, rs2236225 (MTHFD1), was significantly different in the AMR, EUR, and SAS populations.
The MDS analysis based on the FST values indicated that the Amerindian (NAT) and African (AFR) populations were the most genetically distinct. This analysis also found that the genetic profile of the Amerindian (NAT) population is most similar to those of the American (AMR) and South Asian (SAS) populations, which is consistent with their closer links through the history of human migrations18,19.
The 6-MP drug is an anti-metabolic purine analog that interferes with the biosynthesis of nucleic acids and is a key medication for the successful treatment of children with ALL. This drug is the only anti-leukemia medication used in the treatment of ALL for which the FDA (Food and Drug Administration) recommends the prior determination of the patient’s genetic profile prior to the administration of the drug, to allow for the adjustment of the treatment. A number of studies have shown an association between the rs1142345 and rs1800460 markers of the TPMT gene and an increased susceptibility to grave toxicity during the treatment of ALL with 6-MP20–22.
The MTX is a folate inhibitor that is an important component of virtually all the pediatric ALL treatment protocols. The association between the MTHFR, MTHFD1, and MTRR genes and the treatment of ALL with MTX has been the focus of a number of studies. In particular, the rs1801133 (MTHFR)23,24, rs2236225 (MTHFD1)25,26, and rs1801394 (MTRR)27,28 polymorphisms have all been associated with relapses and toxicity in MTX-based treatments.
In a Genome-Wide Association (GWA) study, Trevino et al.29 analyzed a number of polymorphisms of the SLCO1B gene, including rs4149056, and found a strong association with toxicity to MTX. Other studies have also recorded an association between the rs2306283 polymorphism of the SLCO1B gene and toxicity in MTX treatment30–32.
A number of other studies have found an association between variants of other genes involved the metabolic pathways of MTX and toxicity in treatments based on this drug. These variants include the rs2372536 and rs4673993 of the ATIC gene, which is involved in the synthesis of purines33–35 and polymorphisms of genes, such as CCND1 (rs9344), involved in the regulation of the cell cycle and the enzymes targeted by MTX36,37. In addition, the rs1800909 and rs3758149 polymorphisms of the GGH gene, which catalyzes the degradation of the active polyglutamates of the natural folate and the MTX, have also been identified as predictors of toxicity in MTX38,39.
Our work aimed to provide information on the distribution of pharmacogenomic variants relevant to the treatment of ALL in traditional Amerindian populations. It would be interesting to demonstrate whether the results observed for the markers investigated here would show the same profile in leukemic patients of the same ethnic group. However, samples from indigenous patients with ALL are difficult to obtain, given the rarity of the disease and due to these populations living in remote rural areas that are difficult to access, which reflects a difficulty in care and clinical monitoring, coupled with the cultural factor, in which the treatment is, mostly, based on traditional indigenous medicine. To get around this issue, we used, for statistical comparison purposes, sample data from 42 patients with ALL, from the Brazilian Amazon metropolitan region, who had a high Amerindian genetic contribution. Of the 12 markers investigated, eight of them (rs1142345, rs1800460, rs1800909, rs1801133, rs2306283, rs3758149, rs4149056 and rs9344) did not show statistically significant differences between traditional Amerindian populations (NAT) and patients with ALL (ALL_NAT). This outcome may allow us to suggest a genetic similarity between the two groups. These markers were investigated for the risk of developing severe toxicity in a study previously carried out by our research group involving an admixed population of the Brazilian Amazon region10. In this study, the G allele of the rs2306283 variant of the SLCO1B1 gene was associated with an almost three-fold increase in the risk of developing severe central nervous system toxicity during the consolidation phase of ALL treatment. In addition, a strong, but not significant, association was identified between the rs1801133 variant of MTHFR gene and the risk of developing severe toxicity in the disease consolidation phase.
In the current study, four markers were statistically different between Amerindian populations and samples of ALL patients (rs1801394, rs2236225, rs2372535 and rs4673993). The statistical difference observed for these markers could be due to their strong relationship with the susceptibility to ALL development. These polymorphisms were investigated in a study developed by our research group40 involving miscegenated populations from the Amazon. The rs2236225 variant of MTHFD1 gene was related to a protective effect against the development of ALL, while the rs4673993 variant of ATIC gene was associated with a risk effect of developing the disease. In a meta-analysis developed by Fang et al.41, the GG genotype of the rs1801394 variant of MTRR gene was associated with a reduced risk of developing ALL in a Caucasian population.
The results of the present study indicate that the genotypic distribution of the markers analysed here in the Amerindian population is distinct from that of the other populations included here for comparison, from Africa, Europe, the Americas, and southern and eastern Asia. These findings highlight the unique nature of the genetic profile of the indigenous population of the Brazilian Amazon region, which also implies a distinct profile of efficacy and toxicity in the treatment of ALL in these populations, as well as other populations in Brazil formed through a high degree of admixture with this indigenous group, such as the Brazilian Amazonian populations.
Supplementary information
Acknowledgements
We acknowledge funding from CNPq (Conselho Nacional de Desenvolvimento Científico and Tecnológico), UFPA (Universidade Federal do Pará), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). These funding organizations played no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. We also thank Giovanna Cavalcante and Fernando Mello for technical assistance.
Author contributions
D.C.C. and A.V.W. conducted the survey and data analysis, and wrote the text; A.M.R.S., F.C.M., R.A.S., M.R.F., A.C.M., T.P.S., A.C.-P., L.P.C.L. and J.C.G.R. analyzed the data, ran the statistical analyses, and revised the manuscript; A.L.C.S., J.F.G., S.S., A.S.K., P.P.A. and N.P.C.S. reviewed the statistical analyses, and edited and approved the manuscript. All the authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67312-y.
References
- 1.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J. Clin. Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescente cancer statistics. CA Cancer J. Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Hareedy MS, et al. Genetic variants in 6-mercaptopurine pathway as potential factors of hematological toxicity in acute lymphoblastic leukemia patients. Pharmacogenomics. 2015;16:1119–1134. doi: 10.2217/PGS.15.62. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Lopez E, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genom. 2013;23:53–61. doi: 10.1097/FPC.0b013e32835c3b24. [DOI] [PubMed] [Google Scholar]
- 5.Pollock BH, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J. Clin. Oncol. 2000;18:813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2000;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 8.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Yang JJ, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat. Genet. 2011;43:237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Carvalho DC, et al. Pharmacogenomics and variations in the risk of toxicity during the consolidation/maintenance phases of the treatment of pediatric B-cell leukemia patients from an admixed population in the Brazilian Amazon. Leuk. Res. 2018;74:10–13. doi: 10.1016/j.leukres.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues JCG, et al. Polymorphisms of ADME-related genes and their implications for drug safety and efficacy in Amazonian Amerindians. Sci. Rep. 2019;9:7201. doi: 10.1038/s41598-019-43610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos NP, et al. Assessing individual interethnic admixture and population substructure using a 48-insertion-deletion (INSEL) ancestry-informative marker (AIM) panel. Hum. Mutat. 2010;31:184–190. doi: 10.1002/humu.21159. [DOI] [PubMed] [Google Scholar]
- 13.Ramos BR, et al. Neither self-reported ethnicity nor declared family origin are reliable indicators of genomic ancestry. Genetica. 2016;44:259–265. doi: 10.1007/s10709-016-9894-1. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 15.Sherborne AL, et al. Variation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia risk. Nat. Genet. 2010;42:492–494. doi: 10.1038/ng.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijayakrishnan J, Sherborne AL, Sawangpanich R, Hongeng S, Houlston RS, Pakakasama S. Variation at 7p12.2 and 10q21.2 influences childhood acute lymphoblastic leukemia risk in the Thai population and may contribute to racial differences in leukemia incidence. Leuk. Lymphoma. 2010;51:1870–1874. doi: 10.3109/10428194.2010.511356. [DOI] [PubMed] [Google Scholar]
- 17.Walsh KM, et al. Associations between genome-wide Native American ancestry, known risk alleles and B-cell ALL risk in Hispanic children. Leukemia. 2013;27:2416–2419. doi: 10.1038/leu.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoglund P, Reich D. A genomic view of the peopling of the Americas. Curr Opin Genet Dev. 2016;41:27–35. doi: 10.1016/j.gde.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffecker JF, Elias SA, O’Rourke DH, Scott GR, Bigelow NH. Beringia and the global dispersal of modern humans. Evol. Anthropol. 2016;25:64–78. doi: 10.1002/evan.21478. [DOI] [PubMed] [Google Scholar]
- 20.Carleton BC, et al. Genetic markers of cisplatin-induced hearing loss in children. Clin. Pharmacol. Ther. 2014;96:296–298. doi: 10.1038/clpt.2014.92. [DOI] [PubMed] [Google Scholar]
- 21.Zgheib NK, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leucemia at the Children's Cancer Center of Lebanon. Pediatr. Blood Cancer. 2017;64:146–150. doi: 10.1002/pbc.26189. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, et al. Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. 2018;18:516. doi: 10.1186/s12885-018-4398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vagace JM, de la Maya MD, Caceres-Marzal C, Gonzalez de Murillo S, Gervasini G. Central nervous system chemotoxicity during treatment of pediatric acute lymphoblastic leukemia/lymphoma. Crit. Rev. Oncol. Hematol. 2012;84:274–286. doi: 10.1016/j.critrevonc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Zgheib NK, et al. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenet Genom. 2014;24:387–396. doi: 10.1097/FPC.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 25.Krajinovic M, Moghrabi A. Pharmacogenetics of methotrexate. Pharmacogenomics. 2004;5:819–834. doi: 10.1517/14622416.5.7.819. [DOI] [PubMed] [Google Scholar]
- 26.Erčulj N, Kotnik BF, Debeljak M, Jazbec J, Dolžan V. Influence of folate pathway polymorphisms on high-dose methotrexate-related toxicity and survival in childhood acute lymphoblastic leukemia. Leuk. Lymphoma. 2012;53:1096–1104. doi: 10.3109/10428194.2011.639880. [DOI] [PubMed] [Google Scholar]
- 27.Jonge R, et al. Effect of polymorphisms in folate-related genes on in vitro methotrexate sensitivity in pediatric acute lymphoblastic leukemia. Blood. 2005;106:717–720. doi: 10.1182/blood-2004-12-4941. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Tissing WJ, de Jonge R, van Zelst BD, Pieters R. Polymorphisms in folate-related genes: association with side effects of high-dose methotrexate in childhood acute lymphoblastic leukemia. Leukemia. 2008;22:1798–1800. doi: 10.1038/leu.2008.66. [DOI] [PubMed] [Google Scholar]
- 29.Treviño LR, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J. Clin. Oncol. 2009;27:5972–5978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey LB, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey LB, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radtke S, et al. Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood. 2013;121:5145–5153. doi: 10.1182/blood-2013-01-480335. [DOI] [PubMed] [Google Scholar]
- 33.James HM, et al. Common polymorphisms in the folate pathway predict efficacy of combination regimens containing methotrexate and sulfasalazine in early rheumatoid arthritis. J. Rheumatol. 2008;35:562–571. [PubMed] [Google Scholar]
- 34.Warren RB, et al. Outcomes of methotrexate therapy for psoriasis and relationship to genetic polymorphisms. Br. J. Dermatol. 2009;160:438–441. doi: 10.1111/j.1365-2133.2008.08898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen SA, Hider SL, Martin P, Bruce IN, Barton A, Thomson W. Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. Pharmacogenom. J. 2013;13:227–234. doi: 10.1038/tpj.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costea I, Moghrabi A, Laverdiere C, Graziani A, Krajinovic M. Folate cycle gene variants and chemotherapy toxicity in pediatric patients with acute lymphoblastic leukemia. Haematologica. 2006;91:1113–1116. [PubMed] [Google Scholar]
- 37.Jekic B, et al. Association of the TYMS 3G/3G genotype with poor response and GGH 354GG genotype with the bone marrow toxicity of the methotrexate in RA patients. Eur. J. Clin. Pharmacol. 2013;69:377–383. doi: 10.1007/s00228-012-1341-3. [DOI] [PubMed] [Google Scholar]
- 38.Yanagimachi M, et al. Influence of polymorphisms within the methotrexate pathway genes on the toxicity and efficacy of methotrexate in patients with juvenile idiopathic arthritis. Br J. Clin. Pharmacol. 2011;71:237–243. doi: 10.1111/j.1365-2125.2010.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SM, Sun LL, Zeng WX, Wu WS, Zhang GL. Influence of genetic polymorphisms of FPGS, GGH, and MTHFR on serum methotrexate levels in Chinese children with acute lymphoblastic leukemia. Cancer Chemother. Pharmacol. 2014;74:283–289. doi: 10.1007/s00280-014-2507-8. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho DC, et al. Association of genes ARID5B, CEBPE and folate pathway with acute lymphoblastic leukemia in a population from the Brazilian Amazon region. Leuk. Res. Rep. 2019;27:100188. doi: 10.1016/j.lrr.2019.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang DH, Ji Q, Fan CH, An Q, Li J, et al. Methionine synthase reductase A66G polymorphism and leukemia risk: evidence from published studies. Leuk. Lymphoma. 2014;55:1910–1914. doi: 10.3109/10428194.2013.867492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.