Abstract
Bacterial promoters are usually formed by multiple cis-regulatory elements recognized by a plethora of transcriptional factors (TFs). From those, global regulators are key elements since these TFs are responsible for the regulation of hundreds of genes in the bacterial genome. For instance, Fis and IHF are global regulators that play a major role in gene expression control in Escherichia coli, and usually, multiple cis-regulatory elements for these proteins are present at target promoters. Here, we investigated the relationship between the architecture of the cis-regulatory elements for Fis and IHF in E. coli. For this, we analyze 42 synthetic promoter variants harboring consensus cis-elements for Fis and IHF at different distances from the core −35/−10 region and in various numbers and combinations. We first demonstrated that although Fis preferentially recognizes its consensus cis-element, it can also recognize, to some extent, the consensus-binding site for IHF, and the same was true for IHF, which was also able to recognize Fis binding sites. However, changing the arrangement of the cis-elements (i.e., the position or number of sites) can completely abolish the non-specific binding of both TFs. More remarkably, we demonstrated that combining cis-elements for both TFs could result in Fis and IHF repressed or activated promoters depending on the final architecture of the promoters in an unpredictable way. Taken together, the data presented here demonstrate how small changes in the architecture of bacterial promoters could result in drastic changes in the final regulatory logic of the system, with important implications for the understanding of natural complex promoters in bacteria and their engineering for novel applications.
Keywords: regulatory network, cis-regulatory elements, complex promoters, global regulators, transcriptional crosstalk, fine-tuning
Introduction
Bacteria have evolved complex gene regulatory networks to coordinate the expression level of each gene in response to changing environmental conditions. In this aspect, a typical bacterium such as Escherichia coli uses around 300 different transcriptional factors (TFs) to control the expression of more than 5,000 genes, and gene regulation in bacteria has been extensively investigated in the last six decades (Lozada-Chavez, 2006). Among the known TFs from E. coli, global regulators are able to control the highest percentage of transcriptional units in response to significant physiological or environmental signals, such as the metabolic state of the cell, the availability of carbon sources, and the presence of oxygen (Martínez-Antonio et al., 2003; Ishihama, 2010), while local regulators are responsible for gene regulation in response to specific signals (such as sugars and metals) (Ishihama, 2010; Browning and Busby, 2016). Most TFs control gene expression through their interaction with specific DNA sequences located near the promoter region, the cis-regulatory element, or transcriptional factor binding site (Browning and Busby, 2004, 2016). Over the decades, many cis-regulatory elements for many TFs from E. coli have been experimentally characterized, mapped, and compiled in databases such as RegulonDB and EcoCyc (Gama-Castro et al., 2016; Keseler et al., 2017). Analysis of these datasets demonstrates that TFs usually act in a combinatorial way to control gene expression, where multiple cis-regulatory elements for different TFs are located in the upstream region of the target genes (Guazzaroni and Silva-Rocha, 2014; Rydenfelt et al., 2014; Gama-Castro et al., 2016). Therefore, the arrangement of cis-regulatory elements at the target promoters is crucial to determine which TFs will be able to control the target gene and how these regulators interact with each other once bound to the DNA (Collado-Vides et al., 1991; Ishihama, 2010).
Several studies have explored the relationship between the architecture of cis-regulatory elements and the final logic of the target promoters, and initial attempts have focused on the mutation of cis-regulatory elements from natural promoters to investigate how these elements specify the promoter activity dynamics (Sawers, 1993; Darwin and Stewart, 1995; Izu et al., 2002; Setty et al., 2003). More recently, synthetic biology approaches have been used to construct artificial promoters through the combination of several cis-regulatory elements, and these have been characterized to decipher their architecture/dynamics relationship (Cox et al., 2007; Isalan et al., 2008; Kinkhabwala and Guet, 2008; Shis et al., 2014). However, while most synthetic biology approaches have focused on cis-elements for local regulators (which do not commonly regulate gene expression in a combinatorial manner), we recently investigated this combinatorial regulation problem with global regulators (Guazzaroni and Silva-Rocha, 2014; Amores et al., 2015; Monteiro et al., 2018). This is important because global regulators (such as IHF, Fis, and CRP) have numerous binding sites along the E. coli genome and frequently co-occur at target promoters (Guazzaroni and Silva-Rocha, 2014). Thus, Fis and IHF are two global regulators that play a critical role in coordinating gene expression in E. coli as well as in mediating DNA condensation in the cell (Azam and Ishihama, 1999; Browning and Busby, 2004; Browning et al., 2010; Ishihama, 2010). Fis, an abundant nucleoid-associated protein (NAP), is related to gene expression regulation in fast-growing cells, varying its function (as a repressor or activator transcriptional factor) according to its biding site position related to the core promoter (Hirvonen et al., 2001), while IHF is a NAP, which activity relates to changes in gene expression in cells during the transition from exponential to stationary phase (Azam and Ishihama, 1999; Azam et al., 2000; Browning et al., 2010). Moreover, IHF binds to AT-rich DNA motifs with well-defined sequence preferences, while Fis also prefers AT-rich regions with a more degenerate sequence preference (Déthiollaz et al., 1996; Ussery et al., 2001; Dorman and Deighan, 2003; Aeling et al., 2006). Additionally, cross-regulation between Fis and IHF has been demonstrated for several systems, and how specific vs. promiscuous DNA recognition can be achieved for these two global regulators is not fully understood (Browning et al., 2010; Ishihama, 2010; Rossiter et al., 2015).
We previously explored how complex synthetic promoters harboring cis-regulatory elements for CRP and IHF can generate diverse regulatory logic depending on the final architecture of synthetic promoters, demonstrating that it is not possible to predict the regulatory logic of complex multiple promoters from the known dynamics of their simple versions (Monteiro et al., 2018). Here, we further explore this approach to investigate the relationship between cis-regulatory elements for Fis and IHF. Using consensus binding sites for these 2 TFs at different promoter positions and in different numbers, we first demonstrated that while some promiscuous interactions occur between the TFs and the binding sites, some specific cis-regulatory architectures can completely abolish non-specific interactions. Additionally, complex promoters constructed by the combination of cis-elements for Fis and IHF can generate many completely different outputs, such as Fis-repressed promoters, IHF-repressed promoters, and systems where Fis and IHF act as activators. As these changes in promoter logic result from changes in promoter architecture only (and not on the affinity of the transcriptional factor to each individual cis-element), the data presented here reinforce the notion that complex bacterial promoters can display emergent properties, where their final behavior cannot be defined from the characterization of the individual component. Taken together, our findings present a comprehensive strategy for fine-tuning gene circuits to perform optimally in a given context (e.g., engineering of synthetic promoters) as well as provide insights for the understanding of natural complex promoters controlled by global regulators.
Results and Discussion
Generation of Complex Promoters for Fis and IHF
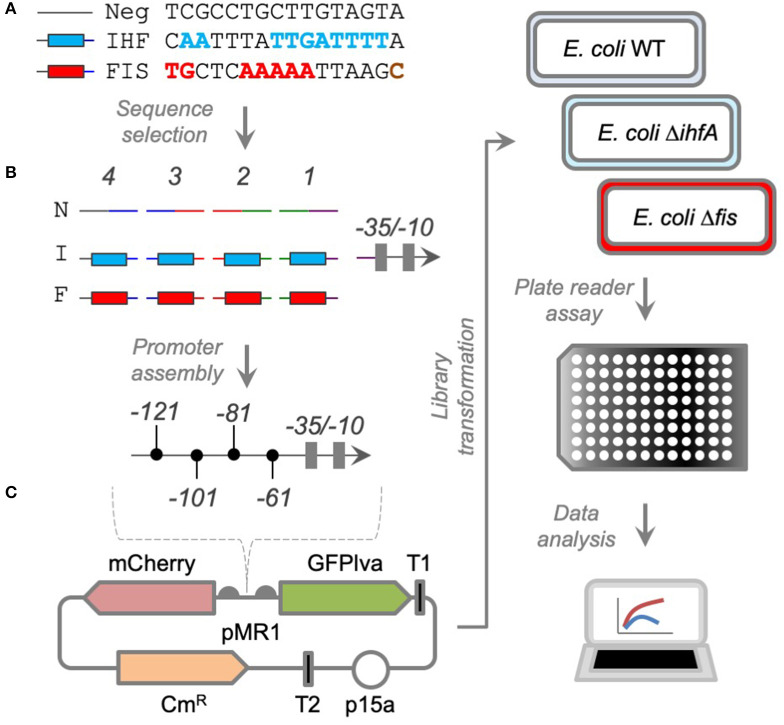
In order to investigate the effect of promoter architecture in the regulation by Fis and IHF, we evaluated the effect of 12 complex promoters constructed in early work (Monteiro et al., 2018) and we constructed 30 new combinatorial promoters with consensus DNA sequences for Fis (Fis-BS) and IHF (IHF-BS) binding sites positioned upstream of a weak core promoter (−35/−10 region) at specific positions (1–4) centered at the −61, −81, −101, and −121 regions related to the transcriptional start site (TSS) (Figure 1). For that, we generated double-strand DNA sequences for Fis-BS, IHF-BS, and a neutral sequence (Neg) with no related transcriptional binding site, which were combined for the generation of a library of synthetic promoters, merging the transcriptional binding sites for Fis, IHF, and/or neutral sequence for each position (Table 1). The complex promoters were assembled by DNA ligation and cloned into pMR1, a mid-copy number vector harboring mCherry and GFPlva as reporter fluorescent proteins (Figure 1). The resulting reporter plasmids (with each promoter controlling only by GFPlva expression) were used to transform competent E. coli wild-type strain (BW25113—WT) and/or E. coli mutants for ihfA (ΔihfA) and fis (Δfis) (from Keio collection) (Baba et al., 2006). Using these constructs, we assayed promoter activity for 8 h in minimal media (M9 complete), measuring the relative GFP expression (GFP/OD) in all strains in the plate reader fluorimeter Victor X3 (PerkinElmer). As a negative control, we used the Neg sequence occupying the 4 possible positions before the core promoter. All data presented in this work are referred to 4 h of cell growth. In the next sections, we present the results of the promoter analysis per category to uncover the cis-regulatory logic for each variant.
Figure 1.
Strategies to construct synthetic complex promoters. (A) DNA sequences harboring the consensus sequence for IHF or Fis binding were selected, along with a control sequence that cannot be recognized by any TF. (B) Double-stranded DNA fragments were produced with cohesive ends specific for each promoter position (numbered from 1 to 4) and assembled together with a weak core promoter harboring the −35/−10 boxes for RNAP recognition (Guazzaroni and Silva-Rocha, 2014). (C) The fragments were cloned into a promoter probe vector (pMR1) harboring resistance to chloramphenicol (CmR), a medium-copy number origin of replication (p15a), and two reporter genes (mCherry and GFPlva). The libraries were introduced into wild-type and mutant strains of E. coli from the KEIO collection (Baba et al., 2006). The resultant strains were analyzed at the population level in a plate reader and the data processed using script in R.
Table 1.
Strains, plasmids, and primers used in this study.
| Strains, plasmids, and primers | Description | References |
|---|---|---|
| Strains | ||
| E. coli DH10B | F− endA1 deoR+ recA1 galE15 galK16 nupG rpsL Δ(lac)X74 ϕ80lacZΔM15 araD139 Δ(ara,leu)7697 mcrA Δ(mrr-hsdRMS-mcrBC) StrR λ− | Sambrook et al., 1989 |
| E. coli BW25113 | lacI+rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78 rph-1 Δ(araB–D)567 Δ(rhaD–B)568 ΔlacZ4787(::rrnB-3) hsdR514 rph-1 | Datsenko and Wanner, 2000 |
| E. coli JW1702 | E. coli BW25113 with ΔihfA mutation | Baba et al., 2006 |
| E. coli JW3229 | E. coli BW25113 with Δfis mutation | Baba et al., 2006 |
| Plasmids | ||
| pMR1 | CmR; orip15a; Promoter probe vector with mCherry and GFPlva reporters | Guazzaroni and Silva-Rocha, 2014 |
| pMR1-NNNN | pMR1 with a reference promoter with 4 non-regulatory sequences | Monteiro et al., 2018 |
| pMR1-FNNN | pMR1 with a synthetic promoter with a Fis cis-elements at position 4 | This study |
| pMR1-NFNN | pMR1 with a synthetic promoter with a Fis cis-elements at position 3 | This study |
| pMR1-NNFN | pMR1 with a synthetic promoter with a Fis cis-elements at position 2 | This study |
| pMR1-NNNF | pMR1 with a synthetic promoter with a Fis cis-elements at position 1 | This study |
| pMR1-NNFF | pMR1 with a synthetic promoter with Fis cis-elements at positions 2 and 1 | This study |
| pMR1-FNNF | pMR1 with a synthetic promoter with Fis cis-elements at positions 4 and 1 | This study |
| pMR1-FFNN | pMR1 with a synthetic promoter with Fis cis-elements at positions 4 and 3 | This study |
| pMR1-NFFN | pMR1 with a synthetic promoter with Fis cis-elements at positions 3 and 2 | This study |
| pMR1-NFNF | pMR1 with a synthetic promoter with Fis cis-elements at positions 3 and 1 | This study |
| pMR1-FNFN | pMR1 with a synthetic promoter with Fis cis-elements at positions 4 and 2 | This study |
| pMR1-FFNF | pMR1 with a synthetic promoter with Fis cis-elements at positions 4, 3 and 1 | This study |
| pMR1-FNFF | pMR1 with a synthetic promoter with Fis cis-elements at positions 4, 2 and 1 | This study |
| pMR1-NFFF | pMR1 with a synthetic promoter with Fis cis-elements at positions 3, 2 and 1 | This study |
| pMR1-FFFN | pMR1 with a synthetic promoter with Fis cis-elements at positions 4, 3 and 2 | This study |
| pMR1-FFFF | pMR1 with a synthetic promoter with Fis cis-elements at positions 4, 3, 2 and 1 | This study |
| pMR1-INNN | pMR1 with a synthetic promoter with a IHF cis-element at position 4 | Monteiro et al., 2018 |
| pMR1-NINN | pMR1 with a synthetic promoter with a IHF cis-element at position 3 | Monteiro et al., 2018 |
| pMR1-NNIN | pMR1 with a synthetic promoter with a IHF cis-element at position 2 | Monteiro et al., 2018 |
| pMR1-NNNI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 | Monteiro et al., 2018 |
| pMR1-IINN | pMR1 with a synthetic promoter with IHF cis-elements at positions 4 and 3 | Monteiro et al., 2018 |
| pMR1-NIIN | pMR1 with a synthetic promoter with IHF cis-elements at positions 3 and 2 | Monteiro et al., 2018 |
| pMR1-NNII | pMR1 with a synthetic promoter with IHF cis-elements at positions 2 and 1 | Monteiro et al., 2018 |
| pMR1-ININ | pMR1 with a synthetic promoter with IHF cis-elements at positions 4 and 2 | Monteiro et al., 2018 |
| pMR1-NINI | pMR1 with a synthetic promoter with IHF cis-elements at positions 3 and 1 | Monteiro et al., 2018 |
| pMR1-INNI | pMR1 with a synthetic promoter with IHF cis-elements at positions 4 and 1 | Monteiro et al., 2018 |
| pMR1-IIII | pMR1 with a synthetic promoter with IHF cis-elements at positions 4, 3, 2 and 1 | Monteiro et al., 2018 |
| pMR1-FNNI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 and Fis cis- element at position 4 | This study |
| pMR1-NFNI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 and Fis cis- element at position 3 | This study |
| pMR1-NNFI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 and Fis cis- element at position 2 | This study |
| pMR1-NFFI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 and Fis cis- elements at positions 3 and 2 | This study |
| pMR1-FFNI | pMR1 with a synthetic promoter with a IHF cis-element at position 1 and Fis cis- elements at positions 4 and 3 | This study |
| pMR1-IFNN | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- element at position 3 | This study |
| pMR1-INFN | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- element at position 2 | This study |
| pMR1-INNF | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- element at position 1 | This study |
| pMR1-IFFN | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- elements at positions 3 and 2 | This study |
| pMR1-IFNF | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- elements at positions 3 and 1 | This study |
| pMR1-INFF | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- elements at positions 2 and 1 | This study |
| pMR1-IFFF | pMR1 with a synthetic promoter with a IHF cis-element at position 4 and Fis cis- elements at positions 3, 2 and 1 | This study |
| pMR1-IFFI | pMR1 with a synthetic promoter with a IHF cis-elements at positions 4 and 1. Fis cis- elements at positions 3 and 2 | This study |
| pMR1-IFNI | pMR1 with a synthetic promoter with a IHF cis-elements at positions 4 and 1. Fis cis- element at position 3 | This study |
| pMR1-INFI | pMR1 with a synthetic promoter with a IHF cis-elements at positions 4 and 1. Fis cis- element at position 2 | This study |
| Primers | ||
| P1-N5 | AATTCTCGCCTGCTTGTAGTA* | Monteiro et al., 2018 |
| P1-N3 | CGCCTACTACAAGCAGGCGAG | Monteiro et al., 2018 |
| P2-N5 | GGCGTCGCCTGCTTGTAGTA | Monteiro et al., 2018 |
| P2-N3 | GCGGTACTACAAGCAGGCGA | Monteiro et al., 2018 |
| P3-N5 | CCGCTCGCCTGCTTGTAGTA | Monteiro et al., 2018 |
| P3-N3 | CCAATACTACAAGCAGGCGA | Monteiro et al., 2018 |
| P4-N5 | TTGGTCGCCTGCTTGTAGTA | Monteiro et al., 2018 |
| P4-N3 | CAAGTACTACAAGCAGGCGA | Monteiro et al., 2018 |
| P1-I5 | AATTCCAATTTATTGATTTTA* | Monteiro et al., 2018 |
| P1-I3 | CGCCTAAAATCAATAAATTGG | Monteiro et al., 2018 |
| P4-I5 | TTGGCAATTTATTGATTTTA | Monteiro et al., 2018 |
| P4-I3 | CAAGTAAAATCAATAAATTG | Monteiro et al., 2018 |
| P1-F5 | AATTCTGCTCAAAAATTAAGC* | This study |
| P1-F3 | CGCCGCTTAATTTTTGAGCAG | This study |
| P2-F5 | GGCGTGCTCAAAAATTAAGC | This study |
| P2-F3 | GCGGGCTTAATTTTTGAGCA | This study |
| P3-F5 | CCGCTGCTCAAAAATTAAGC | This study |
| P3-F3 | CCAAGCTTAATTTTTGAGCA | This study |
| P4-F5 | TTGGTGCTCAAAAATTAAGC | This study |
| P4-F3 | CAAGGCTTAATTTTTGAGCA | This study |
| CoreP-5 | CTTGAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAG | Monteiro et al., 2018 |
| CoreP-3 | GATCCTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCT* | Monteiro et al., 2018 |
| pMR1-F | CTCGCCCTTGCTCACC | Monteiro et al., 2018 |
| pMR1-R | ACAAGAATTGGGACAACTCC | Monteiro et al., 2018 |
Restriction sites are underlined in the primer sequences.
Changing the Fis Binding Site Architecture Modulates Fis and IHF Binding Specificity
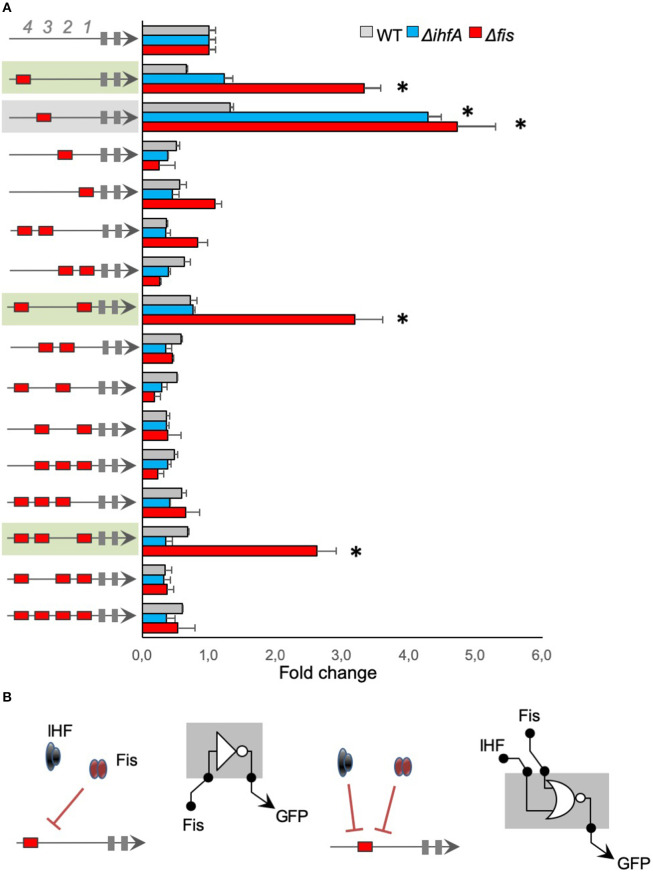
We analyzed the architecture effect for Fis cis-regulatory elements by evaluating the influence of position and sequence combination for Fis-BS. For that, we used promoters merging Fis-BS and Neg sequences to measure relative GFP expression (GFP/OD) levels after 4 h of cell growth in wild-type, Δfis, and ΔihfA E. coli strains, and normalized the results to our negative control (top bars in Figure 2). The results displayed in Figure 2 show that most of the promoters harboring Fis-BS exhibit low activity in wild-type E. coli, comparable to the negative control. However, when these promoters were assayed in E. coli Δfis strain (red bars), 4 of them displayed a significant increase in activity compared to the wild-type strain (green and gray in Figure 2). Particularly, in the presence of Fis protein, Fis could occupy Fis-BS and act as a repressor of promoter activity. However, not all architectures with Fis-BS at the 4th or 3rd positions display this promoter behavior. This phenomenon only occurs in two other cases with more than 1 Fis-BS combination (promoters shaded in green in Figure 2). This reveals a complex association between promoter architecture and expression profile, which seems to be dependent on the Fis-BS position and arrangement.
Figure 2.
The activity of promoters harboring Fis-binding sites (A) The architecture of the synthetic promoter is shown on the left (red boxes represent Fis-BS). Promoter activities are shown in bars and normalized based on the activity of the reference promoter (i.e., a promoter with 4 neutral sequences). Promoter analyses were performed for 4 h of growth, three genetic backgrounds of E. coli (wild type—gray bars, Δfis—red bars, and ΔihfA—blue bars). Promoters that displayed a significant increase in activity compared to the wild-type strain were shaded in green or gray for easy viewing. Statistical differences between synthetic promoters and their control (wild type condition) are highlighted by (*) as analyzed using Student's t-test with p < 0.05. (B) Summary of most significant changes in promoter architecture leading to changes in promoter logic.
We also assayed Fis-BS promoters in the E. coli ΔihfA strain (blue bars) to evaluate the specificity of Fis for Fis-BS. Strikingly, despite most promoters display similar activity levels in the ΔihfA strain as in the wild-type, 1 single promoter variant harboring Fis-BS at the 3rd position (−101 relative to the TSS) displayed a substantial increase in activity in the ihfA mutant relative to the wild-type strain (promoter shaded in gray in Figure 2). This result indicates that IHF also acts as a repressor of this promoter variant. Although it was restricted to a single promoter variant, these results suggest that non-specific IHF binding to the Fis-BS exists, suggesting that promiscuous regulatory interaction could occur and seems to be dependent on promoter architecture, since this phenomenon is detected only for Fis-BS at the 3rd position. Altogether, these results suggest a complex interplay between the position and combination of Fis-BS and the regulation of gene expression.
IHF Binding Sites Can Be Recognized by the Fis Regulator in an Architecture-Dependent Manner
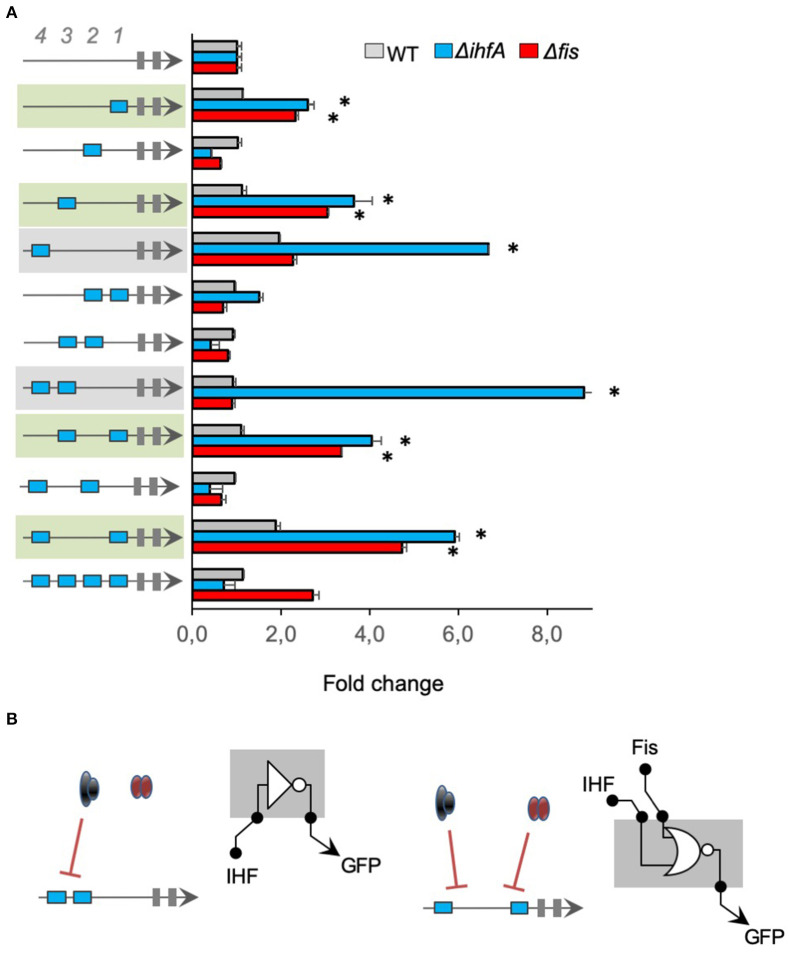
Using the same strategy as in Figure 2, we investigated the regulatory logic of promoters harboring multiple cis-regulatory elements for IHF, merging IHF-BS, and Neg sequences. Figure 3 shows that most promoters displayed low activity in the wild type strain of E. coli and higher activity in E. coli ΔihfA (blue bars), in agreement with previous data on complex IHF promoters (green and gray shaded) (Monteiro et al., 2018). However, when these promoters were assayed in E. coli Δfis strain (red bars), we observed that 4 promoter architectures also displayed higher activity in this mutant (promoters shaded in green in the figure), indicating that Fis was also able to repress these promoter variants, highlighting a possible crosstalk (Cepeda-Humerez et al., 2015; Friedlander et al., 2016) between these 2 TFs, which should be further investigated in the future. However, it is worth noticing that the promoter variants harboring cis-regulatory elements for IHF at 4th or 3rd and 4th positions (−101 and −121 relative to the TSS) displayed both a strong repression by IHF but no modulation by Fis (promoter shaded in gray in Figure 3). Again, these results reinforce that the gene expression pattern and the promiscuous or specific binding to transcriptional factors allows for the fine-tuning of promoter activities based on their architectures.
Figure 3.
The activity of promoters harboring IHF-binding sites. (A) The architecture of the synthetic promoter is shown on the left (blue boxes represent IHF-BS). Promoter activities are shown in bars and normalized based on the activity of the reference promoter (i.e., a promoter with 4 neutral sequences). Eleven promoter variants previously described (Monteiro et al., 2018) were analyzed in wild type (gray bars) Δfis (red bars), and ΔihfA (blue bars) mutant strains of E. coli. Promoter architectures that displayed higher activity in E. coli Δfis and ΔihfA are shaded in green, indicating that Fis was also able to repress these promoter variants, highlighting a possible crosstalk. Promoter that displayed a strong repression by IHF but no modulation by Fis are shaded in gray, reinforce that the gene expression pattern and the promiscuous or specific binding to transcriptional factors allows for the fine-tuning of promoter activities based on their architectures. Statistical differences between synthetic promoters and their control (wild type condition) are highlighted by (*) as analyzed using Student's t-test with p < 0.05. (B) Summary of most significant changes in promoter architecture leading to changes in promoter logic. Statistical differences between synthetic promoters and their control are highlighted by (*) as analyzed using Student's t-test with p < 0.05.
Merging IHF-BS and Fis-BS Leads to an Unpredictable Expression Pattern
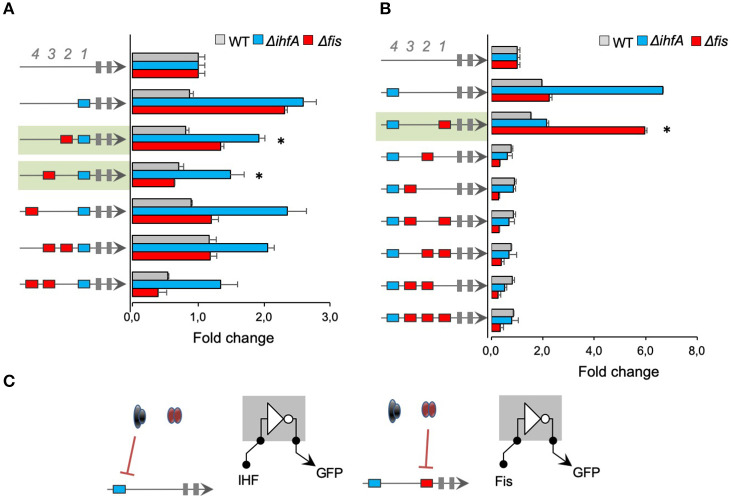
After we investigated the regulatory interactions for promoters harboring cis-regulatory elements for a single transcriptional factor (IHF or Fis), we constructed promoters combining binding sites for both TFs and Neg sequences. In order to systematically investigate the effect of combined transcriptional factor-binding sites on promoter logic, we first fixed 1 IHF-BS at the 1st position (−61) and varied Fis-BS for the 2nd, 3rd, and 4th positions. As shown in Figure 4A, 1 promoter harboring 1 single IHF-BS at the 1st position showed no activity in the wild-type E. coli strain but increased activity in the fis and ihfA mutant strains. However, adding Fis-BS at the 2nd or 3rd position resulted in promoters with reduced activity in the ihfA and fis mutant strains, compared to IHF-BS at the 1st position (promoters shaded in green in Figure 4A). Comparison of these green shaded promoters to promoters with 1 single Fis-BS at the 2nd or 3rd positions in Figure 2, we cannot observe any patterns between the merging of binding sites for these transcriptional factors, that is, the activity of promoters consisting of both Fis-BS and IHF-BS is not the sum of behaviors from Fis-BS and IHF-BS individually. When 1 single IHF-BS was fixed at the 4th position (−121), the resulting promoter displayed strong activity in ΔihfA strains (Figure 4B). However, when 1 single Fis-BS was added at the 1st position (−61), the resulting promoter displayed increased activity in the E. coli Δfis strain, while it showed no activity in the wild type and ΔihfA strains. Therefore, this promoter architecture may be being repressed, especially by Fis regulator (shaded in green). However, for promoters with Fis-BS fixed at the 1st position (Figure 2), we observed a reduction in the promoter activity in the Δfis strain, demonstrating that the presence of IHF in this specific position may influence a positive expression in the absence of Fis. Finally, the addition of 1 single or multiple Fis-BS at different positions completely blocked promoter activity, and this was not relieved in either Δfis or ΔihfA strains, showing that transcriptional factors and binding site sequences of IHF and Fis contribute to promoter complexity. A mutant for ihfA and fis should be a compelling model to completely understand this promoter logic, but a mutant for both TFs has proven to be difficult to construct. It is important to note that IHF and Fis, which are transcriptional factors, are also NAPs, so the gene expression identified here could be related to possible changes in the DNA geometry (Déthiollaz et al., 1996). Taken together, these results also suggest that Fis and IHF proteins and their binding sites exert complex regulatory patterns, hampering promoter behavior predictions.
Figure 4.
The activity of promoters with combined IHF- and Fis-binding sites. The architecture of the synthetic promoter is shown on the left (blue boxes represent IHF-BS and red boxes represent Fis-BS). Promoter activities are shown in bars and normalized based on the activity of the reference promoter (i.e., a promoter with 4 neutral sequences). Promoter variants were analyzed in wild type (gray bars) Δfis (red bars), and ΔihfA (blue bars) mutant strains of E. coli. (A) Characterization of promoters with a single IHF-BS fixed at position 1 (−61) and varying Fis-BS. Promoters shaded in green present reduced activity in the ihfA and fis mutant strains, compared to IHF-BS at the 1st position. Statistical differences between synthetic promoters and their control (PNNNI) are highlighted by (*) as analyzed using Student's t-test with p < 0.05. (B) Characterization of promoters with a single IHF-BS fixed position 4 (−121) and varying Fis-BS. Promoter shaded in green displayed increased activity in the E. coli Δfis strain, while it showed no activity in the wild type and ΔihfA strains. Statistical differences between this promoter in Δfis condition and wild type and ΔihfA are highlighted by (*) as analyzed using Student's t-test with p < 0.05. (C) Summary of most significant changes in promoter architecture leading to changes in promoter logic. Statistical differences between synthetic promoters and their control are highlighted by (*) as analyzed using Student's t-test with p < 0.05.
Combination of Fis and IHF Binding Sites Generates Strong Fis and IHF Activated Promoters
In all promoters presented until this point, while the combination of different cis-regulatory genes was able to determine the regulatory logic displayed by IHF and Fis, the 2 TFs acted as repressors of promoter activity (Figures 2–4). However, this behavior shifted when we constructed promoter versions harboring IHF-BS at the 1st and 4th positions and varying sites for Fis-BS (Figure 5). As shown in this figure, when 1 single Fis-BS was added at the 2nd position (−81), the resulting promoter displayed a strong activity in the wild-type strain of E. coli, when compared to the version lacking this element (promoter in the green shaded region in Figure 5). Furthermore, when these promoters were assayed in E. coli Δfis or ΔihfA, we observed a substantial reduction in their activity, indicating that both TFs acted as activators of the combinatorial promoter. The same behavior was also observed for a promoter harboring 2 IHF-BS (at the 1st and 4th positions) and 2 Fis-BS (2nd and 3rd positions), where reduction in the gene expression was even more evident. The same does not occur for a promoter harboring the 2 sites of IHF-BS and Fis-BS at the 3rd position, indicating the dependence and complexity of the relationship between promoter architecture and gene expression. These results highlight the rise of emergent properties in complex promoters for global regulators (Monteiro et al., 2018), as increasing the number of cis-regulatory elements can drastically shift the final regulatory logic of the system.
Figure 5.
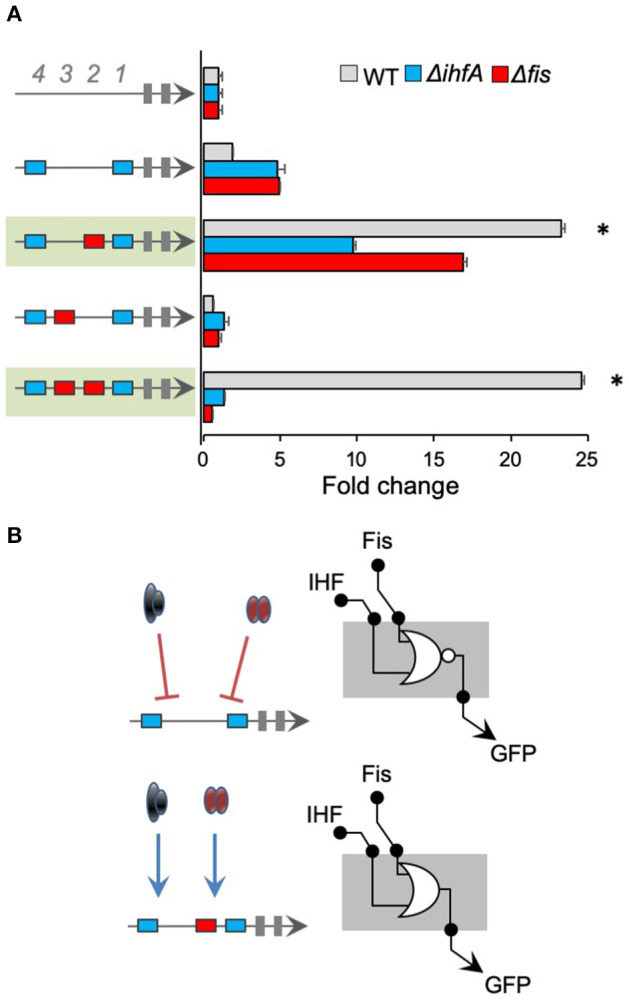
Analysis of promoters with 2 fixed IHF-binding sites. (A) The architecture of the synthetic promoter is shown on the left (blue boxes represent IHF-BS and red boxes represent Fis-BS). Promoter activities are shown in bars and normalized based on the activity of the reference promoter (i.e., a promoter with 4 neutral sequences). Promoter variants were analyzed in wild type (gray bars) Δfis (red bars), and ΔihfA (blue bars) mutant strains of E. coli. For this analysis, IHF cis-regulatory elements were placed at positions 1 and 4, and additional Fis-BS were introduced into the promoters. Promoter that displayed a strong activity in the wild-type when compared to the Δfis or ΔihfA mutant of E. coli are shaded in green. Statistical differences between synthetic promoters in wild type condition and Δfis and ΔihfA condition are highlighted by (*) as analyzed using Student's t-test with p < 0.05. (B) Summary of most significant changes in promoter architecture leading to changes in promoter logic. Statistical differences between synthetic promoters and their control are highlighted by (*) as analyzed using Student's t-test with p < 0.05.
Conclusions
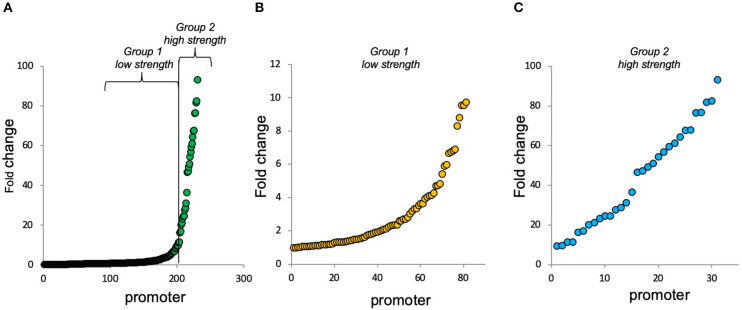
Bacteria are naturally endowed with complex promoters harboring multiple binding sites for several TFs. While several works based on mathematical modeling have argued that combinatorial regulation can be predicted from the characterization of individual promoter elements (Yuh, 1998; Bintu et al., 2005; Hermsen et al., 2006; Zong et al., 2018), along with the previous report (Monteiro et al., 2018) and here we provide growing evidence that small changes in the architecture of cis-regulatory elements can drastically change the final response of the system (Kreamer et al., 2016). The unpredictable behaviors observed in these studies might also depict a deeper evolutionary trend in gene regulation that has selected molecular systems/mechanisms capable of promoting both evolvability and robustness of gene expression levels through non-linear gene regulation (Steinacher et al., 2016). Thus, understanding the way the architecture of cis-regulatory elements determines gene expression behavior is pivotal not only to understand natural bacterial systems but also to provide novel conceptual frameworks for the construction of synthetic promoters for biotechnological applications (Monteiro et al., 2019b). Frequently, in genetic bioengineering applications, it is also necessary to fine-tune and balance specific gene expression due to the complexity of regulatory networks (Boyle and Silver, 2012; Scalcinati et al., 2012; Steinacher et al., 2016). Several recent studies have focused on the improvement of this strategy for diverse purposes (Egbert and Klavins, 2012; Siegl et al., 2013; Hwang et al., 2018). The present adjusting approach could be used as a strategy for the fine-tuning of genetic circuits to perform optimally in a given context. Our approach provided a library (from this study and from our previous work (Monteiro et al., 2018) of 74 promoter architectures characterized in different strains and conditions for in total of 230 outputs (different promoters in different strains and growth conditions) (Figure 6 and Table S1). Promoters from our synthetic promoter library with small adaptations could be used for diverse purposes in the biotechnological and bacterial network gene regulation fields.
Figure 6.
Fold change of complex synthetic promoters at 4 h of growth. Complex promoters' approach could be used as a strategy for the fine-tuning of genetic circuits to perform optimally in a given context. A total of 230 promoter characterizations (74 architectures characterized in different strains and conditions). All promoter details are shown in Table S1. (A) All 230 promoter characterizations (from the lowest to the highest fold change) that were divided into 2 groups of interests. (B) Promoters with fold change between 1 and 10 (group 1: low strength). (C) Promoters with fold change up to 10 (group 2—high strength).
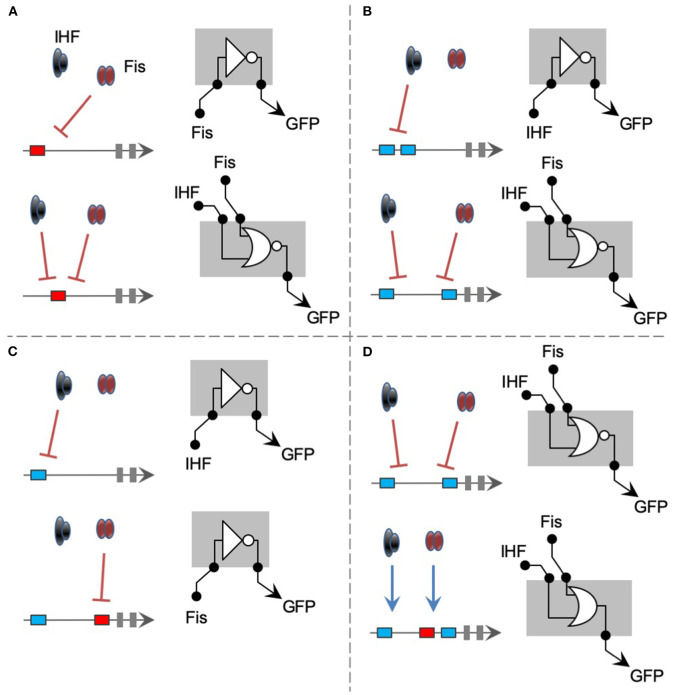
Abstracting all the gene regulations investigated in this work, we are able to provide a visual summary of the findings reported here from a Boolean logic perspective (Figure 7). As shown in Figure 7A, changing a perfect Fis binding in 20 bp (from position –121 to –101) can turn a specific Fis-repressed promoter into a system repressed by both Fis and IHF. Using a more formal logic gate definition (Amores et al., 2015), this modification can turn a promoter with a NOT logic into one with an NOR logic. However, a promoter harboring 2 IHF-binding sites at positions –121 and –101 displayed specific IHF-repression, while changing the second binding site to position –61 resulted in a promoter repressed by both IHF and Fis (Figure 7B). In terms of promoter logic, this change in cis-element architecture also turns a promoter with NOT logic into one with an NOR logic. When a single IHF-binding site was presented at position –121, the final promoter was only repressed by IHF (Figure 7C). Yet, introducing an additional Fis-binding site at position –61 of this promoter turned it into a system exclusively repressed by Fis. This change maintained the NOT logic of the promoter but changed the TF able to repress the activity. Finally, and more remarkably, while a promoter with 2 IHF-BS (at positions –121 and –61) was repressed by both Fis and IHF, adding a third binding site for Fis at position –81 resulted in a promoter strongly activated by both TFs (Figure 7D). Therefore, this single-change cis-element architecture turned a promoter with NOR logic into an entirely OR promoter responsive to the same TFs. This remarkable regulatory versatility and unpredictability unveiled by synthetic combinatorial promoter shows that we only start to understand the complexity of gene regulation in bacteria. While the work presented here covers two of the main global regulators of E. coli, further studies are still necessary to uncover the hidden complexity of combinatorial gene regulation in this bacterium.
Figure 7.
Summary of most significant changes in promoter architecture leading to changes in promoter logic. The figures are represented using logic gate representation for gene regulation, even. (A) The change of a single Fis-BS from position −121 to −101 turns a NOT gate for Fis into an NOR gate for Fis and IHF. (B) Two IHF-BSs (at positions −121 and −101) worked as a NOT gate for IHF, while changing 1 site to position −61 generates an NOR gate for both IHF and Fis. (C) While a single IHF-binding site at position −121 results into a NOT gate for IHF, adding a Fis-binding site to the position −61 creates a NOT gate exclusively dependent on Fis. (D) Finally, adding a Fis-BS (position −81) to the NOR gate promoter presented in B drastically changes its logic to an OR gate, where the 2 TFs act as activators.
Materials and Methods
Plasmids, Bacterial Strains, and Growth Conditions
E. coli DH10B was used for cloning procedures, while E. coli BW25113 was used as the wild-type strain (WT); E. coli JW1702-1 was used as a mutant for the IHF transcription factor (TF), and E. coli JW3229 was used as a mutant for the Fis TF. All strains were obtained from the Keio collection. For the procedures and analyses, E. coli strains were grown in M9 minimal media (6.4 g/L, Na2HPO4•7H2O, 1.5 g/L KH2PO4, 0.25 g/L NaCl, 0.5 g/L NH4Cl) supplemented with chloramphenicol at 34 μg/mL, 2 mM MgSO4, 0.1 mM casamino acids, and 1% glycerol as the sole carbon source (Complete M9) at 37°C. Plasmids, bacterial strains, and primers used in this study are listed in Table 1.
Design of Synthetic Promoter Scaffolds and Ligation Reactions
The construction of synthetic promoters was performed by the ligation reaction of 5′–end phosphorylated oligonucleotides acquired from Sigma Aldrich (Table 1). The design of all single strands was projected to carry a 16 bp sequence containing the Fis binding site (F), IHF binding site (I), or a neutral motif (N), which is a sequence where any TF is able to bind (Figure 1A). These locations were identified as positions 1, 2, 3, and 4, respectively (Figure 1B) and to be located at −61, −81, −101, or −121 bp upstream of the core promoter (Figure 1C). In addition to the 16 bp oligonucleotides, all single strands were designed to contain 3 base pairs overhang for its corrected insertion on the promoter (Figure 1C). Additionally, a core promoter based on the lac promoter, which is a weak promoter and therefore requires activation. The design of the synthetic promoters and the positions of the cis-elements were made based on strategies already performed by our group (Monteiro et al., 2018), aiming to arrange the cis-elements aligned to the transcription initiation site, considering the DNA curvature. To assemble the synthetic promoters, the 5′ and 3′ strands corresponding to each position were mixed at equimolar concentrations and annealed by heating at 95°C for 5 min, followed by gradual cooling to room temperature (25°C) for 5 min, and finally maintained at 0°C for 5 min. The external overhangs of the cis-element at position 4 and the core promoter were designed to carry EcoRI and BamHI digested sites. In this way, it was allowed to ligate to a previously digested EcoRI/BamHI pMR1 plasmid. All five fragments (4 cis-elements positions plus core promoter) were mixed equally in a pool with the final concentration of 5′ phosphate termini fixed at 15 μM. For the ligase reaction, 1 μL of the fragment pool was added to 50 ng EcoRI/BamHI pMR1 digested plasmid in the presence of ligase buffer and ligase enzyme to a final volume of 10 μL. Ligation was performed for 1 h at 16°C, after which the ligase reaction was inactivated for 15 min at 65°C. Two μL of the ligation was used to electroporate 50 μL of E. coli DH10B competent cells. After 1-h regenerating in 1 mL LB media, the total volume was plated in LB solid dishes supplemented with chloramphenicol at 34 μg/mL. Clones were confirmed by colony PCR with primers pMR1-F and pMR1-R (Table 1) using pMR1 empty plasmid PCR reaction as further length reference on electrophorese agarose gel. Clones with a potential correct length were submitted to Sanger DNA sequencing to confirm correct promoter assembly.
Promoter Activity Analysis and Data Processing
Promoter activity was measured for all 42 promoters at different genetic backgrounds and conditions. For each experiment, the plasmid containing the promoter of interest was used to transform E. coli wild type, E. coli ΔihfA mutant, or E. coli Δfis mutant, as indicated. Freshly plated single colonies were selected with sterile loops and then inoculated in 1 mL of M9 media. After 16 h 10 μL of this culture was assayed in 96 wells microplates in biological triplicate with 190 μL of M9 media. Cell growth and GFP fluorescence were quantified using a Victor X3 plate reader (PerkinElmer) that was measured for 8 h at intervals of 30 min. All graphics were constructed based on 4 h of cell growth since under our experimental setup and previous work (Monteiro et al., 2018), most promoters reach maximal activity at 4 h of growth. Therefore, this is the best time point to compare maximal promoter activity. Promoter activities were calculated as arbitrary units dividing the GFP fluorescence levels by the optical density at 600 nm (reported as GFP/OD600) after background correction. Technical triplicates and biological triplicates were performed in all experiments. Raw data were processed using ad hoc R script (https://www.r-project.org/), and plots were constructed using R (version R-3.6.3). For all analyses, we calculated fold-change expression using pMR1-NNNN as the promoter reference.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
RS-R and LM designed the experimental strategy. LM, AS-M, and CW performed the experiments. LM, AS-M, CW, and RS-R analyzed and interpreted the data. LM and RS-R wrote the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to their lab colleagues for insightful comments on this work. This manuscript has been released as a pre-print at bioRxiv (Monteiro et al., 2019a).
Footnotes
Funding. This work was supported by the São Paulo Research Foundation (FAPESP, award # 2012/22921-8 and 2017/50116-6). LM, AS-M, and CW were supported by FAPESP PhD and Master Fellowships (award # 2016/19179-9, 2018/04810-0, and 2016/05472-6).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00510/full#supplementary-material
References
- Aeling K. A., Opel M. L., Steffen N. R., Tretyachenko-Ladokhina V., Hatfield G. W., Lathrop R. H., et al. (2006). Indirect recognition in sequence-specific DNA binding by Escherichia coli integration host factor. J. Biol. Chem. 281, 39236–39248. 10.1074/jbc.M606363200 [DOI] [PubMed] [Google Scholar]
- Amores G. R., Guazzaroni M. E., Silva-Rocha R. (2015). Engineering synthetic cis-regulatory elements for simultaneous recognition of three transcriptional factors in bacteria. ACS Synth. Biol. 4, 1287–1294. 10.1021/acssynbio.5b00098 [DOI] [PubMed] [Google Scholar]
- Azam T. A., Hiraga S., Ishihama A. (2000). Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells 5, 613–626. 10.1046/j.1365-2443.2000.00350.x [DOI] [PubMed] [Google Scholar]
- Azam T. A., Ishihama A. (1999). Twelve species of the nucleoid-associated protein from Escherichia coli. J. Biol. Chem. 274, 33105–33113. 10.1074/jbc.274.46.33105 [DOI] [PubMed] [Google Scholar]
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., et al. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 1–11. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintu L., Buchler N. E., Garcia H. G., Gerland U., Hwa T., Kondev J., et al. (2005). Transcriptional regulation by the numbers: models. Curr. Opin. Genet. Dev. 15, 116–124. 10.1016/j.gde.2005.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. M., Silver P. A. (2012). Parts plus pipes: synthetic biology approaches to metabolic engineering. Metab. Eng. 14, 223–232. 10.1016/j.ymben.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning D. F., Busby S. J. (2004). The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2, 57–65. 10.1038/nrmicro787 [DOI] [PubMed] [Google Scholar]
- Browning D. F., Busby S. J. W. (2016). Local and global regulation of transcription initiation in bacteria. Nat. Rev. Microbiol. 14, 638–650. 10.1038/nrmicro.2016.103 [DOI] [PubMed] [Google Scholar]
- Browning D. F., Grainger D. C., Busby S. J. (2010). Effects of nucleoid-associated proteins on bacterial chromosome structure and gene expression. Curr. Opin. Microbiol. 13, 773–780. 10.1016/j.mib.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Cepeda-Humerez S. A., Rieckh G., Tkačik G. (2015). Stochastic proofreading mechanism alleviates crosstalk in transcriptional regulation. Phys. Rev. Lett. 115:248101. 10.1103/PhysRevLett.115.248101 [DOI] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. (1991). Control site location and transcriptional regulation in Escherichia coli. Microbiol. Rev. 55, 371–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. S., Surette M. G., Elowitz M. B. (2007). Programming gene expression with combinatorial promoters. Mol. Syst. Biol. 3:145. 10.1038/msb4100187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin A. J., Stewart V. (1995). Nitrate and nitrite regulation of the fnr-dependentaeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site. J. Mol. Biol. 251, 15–29. 10.1006/jmbi.1995.0412 [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déthiollaz S., Eichenberger P., Geiselmann J. (1996). Influence of DNA geometry on transcriptional activation in Escherichia coli. EMBO J. 15, 5449–5458. 10.1002/j.1460-2075.1996.tb00928.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Deighan P. (2003). Regulation of gene expression by histone-like proteins in bacteria. Curr. Opin. Genet. Dev. 13, 179–184. 10.1016/S0959-437X(03)00025-X [DOI] [PubMed] [Google Scholar]
- Egbert R. G., Klavins E. (2012). Fine-tuning gene networks using simple sequence repeats. Proc. Natl. Acad. Sci. U.S.A. 109, 16817–16822. 10.1073/pnas.1205693109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander T., Prizak R., Guet C. C., Barton N. H., Tkačik G. (2016). Intrinsic limits to gene regulation by global crosstalk. Nat. Commun. 7:12307. 10.1038/ncomms12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama-Castro S., Salgado H., Santos-Zavaleta A., Ledezma-Tejeida D., Muñiz-Rascado L., García-Sotelo J. S., et al. (2016). RegulonDB version 9.0: high-level integration of gene regulation, coexpression, motif clustering and beyond. Nucl. Acids Res. 44, D133–D143. 10.1093/nar/gkv1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzaroni M.-E., Silva-Rocha R. (2014). Expanding the logic of bacterial promoters using engineered overlapping operators for global regulators. ACS Synth. Biol. 3, 666–675. 10.1021/sb500084f [DOI] [PubMed] [Google Scholar]
- Hermsen R., Tans S., ten Wolde P. R. (2006). Transcriptional regulation by competing transcription factor modules. PLoS Comput. Biol. 2:e164. 10.1371/journal.pcbi.0020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen C. A., Ross W., Wozniak C. E., Marasco E., Anthony J. R., Aiyar S. E., et al. (2001). Contributions of UP elements and the transcription factor FIS to expression from the seven rrn P1 promoters in Escherichia coli. J. Bacteriol. 183, 6305–6314. 10.1128/JB.183.21.6305-6314.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. J., Lee S. Y., Lee P. C. (2018). Engineering and application of synthetic nar promoter for fine-tuning the expression of metabolic pathway genes in Escherichia coli. Biotechnol. Biofuels 11:103. 10.1186/s13068-018-1104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isalan M., Lemerle C., Michalodimitrakis K., Horn C., Beltrao P., Raineri E., et al. (2008). Evolvability and hierarchy in rewired bacterial gene networks. Nature 452, 840–845. 10.1038/nature06847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A. (2010). Prokaryotic genome regulation: multifactor promoters, multitarget regulators and hierarchic networks. FEMS Microbiol. Rev. 34, 628–645. 10.1111/j.1574-6976.2010.00227.x [DOI] [PubMed] [Google Scholar]
- Izu H., Ito S., Eilas M. D., Yamada M. (2002). Differential control by IHF and cAMP of two oppositely oriented genes, hpt and gcd, in Escherichia coli : significance of their partially overlapping regulatory elements. Mol. Genet. Genomics 266, 865–872. 10.1007/s00438-001-0608-7 [DOI] [PubMed] [Google Scholar]
- Keseler I. M., Mackie A., Santos-Zavaleta A., Billington R., Bonavides-Martínez C., Caspi R., et al. (2017). The EcoCyc database: reflecting new knowledge about Escherichia coli K-12. Nucl. Acids Res. 45, D543–D550. 10.1093/nar/gkw1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkhabwala A., Guet C. C. (2008). Uncovering cis regulatory codes using synthetic promoter shuffling. PLoS ONE 3:e2030. 10.1371/journal.pone.0002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreamer N. N., Phillips R., Newman D. K., Boedicker J. Q. (2016). Predicting the impact of promoter variability on regulatory outputs. Sci. Rep. 5:18238. 10.1038/srep18238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada-Chavez I. (2006). Bacterial regulatory networks are extremely flexible in evolution. Nucl. Acids Res. 34, 3434–3445. 10.1093/nar/gkl423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Antonio A., Collado-Vides J., Balleza E., López-Bojorquez L. N., Martínez-Antonio A., Resendis-Antonio O., et al. (2003). Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 33, 482–489. 10.1016/j.mib.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Monteiro L. M. O., Arruda L. M., Sanches-Medeiros A., Martins-Santana L., Alves L. D. F., Defelipe L., et al. (2019b). Reverse engineering of an aspirin-responsive transcriptional regulator in Escherichia coli. ACS Synth. Biol. 8, 1890–1900. 10.1021/acssynbio.9b00191 [DOI] [PubMed] [Google Scholar]
- Monteiro L. M. O., Arruda L. M., Silva-Rocha R. (2018). Emergent properties in complex synthetic bacterial promoters. ACS Synth. Biol. 7, 602–612. 10.1021/acssynbio.7b00344 [DOI] [PubMed] [Google Scholar]
- Monteiro L. M. O., Sanches-Medeiros A., Westmann C. A., Silva-Rocha R. (2019a). Modulating Fis and IHF binding specificity, crosstalk and regulatory logic through the engineering of complex promoters. bioRxiv. 1–11. 10.1101/614396 [DOI] [Google Scholar]
- Rossiter A. E., Godfrey R. E., Connolly J. A., Busby S. J. W., Henderson I. R., Browning D. F. (2015). Expression of different bacterial cytotoxins is controlled by two global transcription factors, CRP and Fis, that co-operate in a shared-recruitment mechanism. Biochem. J. 466, 323–335. 10.1042/BJ20141315 [DOI] [PubMed] [Google Scholar]
- Rydenfelt M., Garcia H. G., Cox R. S., Phillips R. (2014). The influence of promoter architectures and regulatory motifs on gene expression in Escherichia coli. PLoS ONE 9:e114347. 10.1371/journal.pone.0114347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E., Maniatis T. (1989). Molecular Cloning: A Laboratory Manual. Available online at: https://www.cabdirect.org/cabdirect/abstract/19901616061 (accessed April 2, 2020).
- Sawers G. (1993). Specific transcriptional requirements for positive regulation of the anaerobically inducible pfl operon by ArcA and FNR. Mol. Microbiol. 10, 737–747. 10.1111/j.1365-2958.1993.tb00944.x [DOI] [PubMed] [Google Scholar]
- Scalcinati G., Knuf C., Partow S., Chen Y., Maury J., Schalk M., et al. (2012). Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab. Eng. 14, 91–103. 10.1016/j.ymben.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Setty Y., Mayo A. E., Surette M. G., Alon U. (2003). Detailed map of a cis-regulatory input function. Proc. Natl. Acad. Sci. U.S.A. 100, 7702–7707. 10.1073/pnas.1230759100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shis D. L., Hussain F., Meinhardt S., Swint-Kruse L., Bennett M. R. (2014). Modular, multi-input transcriptional logic gating with orthogonal LacI/GalR family chimeras. ACS Synth. Biol. 3, 645–651. 10.1021/sb500262f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl T., Tokovenko B., Myronovskyi M., Luzhetskyy A. (2013). Design, construction and characterisation of a synthetic promoter library for fine-tuned gene expression in actinomycetes. Metab. Eng. 19, 98–106. 10.1016/j.ymben.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Steinacher A., Bates D. G., Akman O. E., Soyer O. S. (2016). Nonlinear dynamics in gene regulation promote robustness and evolvability of gene expression levels. PLoS ONE 11:e0153295. 10.1371/journal.pone.0153295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussery D., Schou Larsen T., Trevor Wilkes K., Friis C., Worning P., Krogh A., et al. (2001). Genome organisation and chromatin structure in Escherichia coli. Biochimie 83, 201–212. 10.1016/S0300-9084(00)01225-6 [DOI] [PubMed] [Google Scholar]
- Yuh C. (1998). Genomic cis-regulatory logic: experimental and computational analysis of a sea urchin gene. Science 279, 1896–1902. 10.1126/science.279.5358.1896 [DOI] [PubMed] [Google Scholar]
- Zong D. M., Cinar S., Shis D. L., Josić K., Ott W., Bennett M. R. (2018). Predicting transcriptional output of synthetic multi-input promoters. ACS Synth. Biol. 7, 1834–1843. 10.1021/acssynbio.8b00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.