Sepsis is a complex process defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It is associated with significant morbidity and mortality rates in both adults and children, and emphasis has been placed on its early recognition and prompt provision of antimicrobials. Owing to limitations of current diagnostic tests (i.e., poor sensitivity and delayed results), significant research has been conducted to identify sepsis biomarkers. Ideally, a biomarker could reliably and rapidly distinguish bacterial infection from other, noninfectious causes of systemic inflammatory illness.
KEYWORDS: adults, biomarkers, pediatrics, procalcitonin, sepsis
ABSTRACT
Sepsis is a complex process defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It is associated with significant morbidity and mortality rates in both adults and children, and emphasis has been placed on its early recognition and prompt provision of antimicrobials. Owing to limitations of current diagnostic tests (i.e., poor sensitivity and delayed results), significant research has been conducted to identify sepsis biomarkers. Ideally, a biomarker could reliably and rapidly distinguish bacterial infection from other, noninfectious causes of systemic inflammatory illness. In doing so, a sepsis biomarker could be used for earlier identification of sepsis, risk stratification/prognostication, and/or guidance of antibiotic decision-making. In this minireview, we review one of the most common clinically used sepsis biomarkers, procalcitonin, and its roles in sepsis management in these three areas. We highlight key findings in the adult literature but focus the bulk of this review on pediatric sepsis. The challenges and limitations of procalcitonin measurement in sepsis are also discussed.
INTRODUCTION
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection (1), and it remains a major cause of morbidity and death worldwide. Recent estimates indicate close to 50 million incident cases of sepsis worldwide each year, with nearly one-half of these cases in infants, children, and adolescents (2). In the United States, more than 1.7 million adults develop sepsis each year, with an associated mortality rate of approximately 20% (3), while pediatric sepsis is associated with a mortality rate between 8% and 10% (4). Organ dysfunction can progress rapidly to shock and death if the underlying cause is untreated or undertreated. Thus, educational efforts have focused on early sepsis recognition, with goals to initiate supportive therapies and antibiotic treatment as quickly as possible. However, because sepsis is a complex, heterogeneous process rather than a singular entity, accurate early diagnosis and risk stratification are challenging.
Despite the high morbidity and mortality rates associated with sepsis, it is unnecessary and unsafe to treat every patient who could have sepsis with antibiotics. Instead, clinicians need to use clinical judgement to determine the appropriateness of antibiotics for each individual patient. Due to the complexity of sepsis, however, clinicians need both a high index of suspicion and diagnostic tools that can help differentiate sepsis from other inflammatory processes. While sepsis is most often associated with bacterial infections, it can result from any type of infectious process. In most studies of patients with suspected sepsis, bacterial infections are identified in only about 50% of cases (5). Unfortunately, microbial cultures, the current gold standard for diagnosing bacterial infections, are not 100% sensitive and cannot always be readily obtained. Additionally, collecting adequate culture samples from focal sites of infection (e.g., lung or intra-abdominal cavity) is often not feasible, particularly during initial evaluations and for deep or tissue-based infections. Furthermore, the results of microbial cultures are not immediately available, even when positive, limiting their utility in informing early decisions about initial sepsis management. Finally, cessation of antibiotic therapy once an infection is appropriately treated is important in sepsis management, leading to potential reductions in the need for prolonged intravenous access and decreasing the development of antimicrobial resistance.
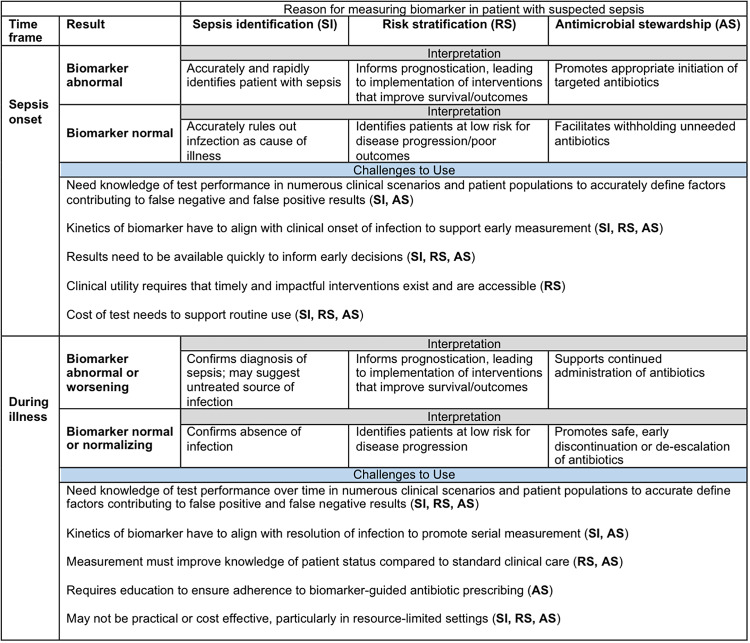
As a result of the limitations of currently available diagnostic tests, extensive research has been performed to identify surrogate tests (e.g., biomarkers) that can accurately and rapidly distinguish individuals with bacterial infections from those with critical illnesses from other causes and can stratify patients according to the risk of adverse outcomes. In doing so, an ideal sepsis biomarker could improve the care of patients with sepsis in three primary areas, (i) early diagnosis, (ii) risk stratification, and (iii) antibiotic stewardship. The goals of using a sepsis biomarker for these three indications are summarized in Fig. 1. While not necessarily exclusive of one another, these areas represent the primary settings for which biomarkers are studied and used clinically in sepsis.
FIG 1.
General goals and challenges for use of sepsis biomarkers at onset and during illness.
This aim of this review is to summarize the current literature relating to one of the most commonly utilized sepsis biomarkers, procalcitonin (PCT). We provide a brief background on why PCT is a sepsis biomarker and highlight its utility in sepsis in the context of these three areas. Because PCT has been the focus of several reviews in the adult literature (6–9), this minireview will specifically highlight PCT data for children, when possible. In doing so, we seek to provide a concise yet thorough review of the utility of PCT as a biomarker for pediatric sepsis.
WHAT IS PROCALCITONIN?
PCT is a precursor of the hormone calcitonin that is produced by C cells in the thyroid gland and, to a lesser extent, other neuroendocrine cells throughout the body (10). PCT is expressed in the central nervous system very early in fetal development (11), but the function of PCT itself is unclear; it is converted in the thyroid to calcitonin, a hormone involved in calcium homeostasis. Because PCT is nearly exclusively produced in the thyroid under normal physiological conditions, it is typically undetectable in the serum in healthy patients. In the setting of bacterial infection, however, proinflammatory cytokines (tumor necrosis factor alpha [TNF-α], interleukin-1β [IL-1β], and IL-6) trigger expression of CALC-1, the gene responsible for PCT production, in numerous cells throughout the body (10). In primates injected with lipopolysaccharide, mRNA expression was detected in several tissues, including liver, lung, kidney, brain, intestine, skin, spleen, adrenals, and pancreas (12). However, since most cells are unable to convert PCT to calcitonin, PCT enters the circulation and blood levels rise while calcitonin levels are unchanged. Additionally, because PCT is produced by tissues in the setting of bacterial infection, in addition to immune cells, it should be reliably produced in both immunocompetent and immunocompromised patients. Importantly, cytokines more selectively produced in response to viral infection, such as interferon gamma, attenuate CALC-1 upregulation such that viral infections tend not to induce the same degree of blood PCT level elevation. As a result, PCT is more specific to bacterial infections than viral infections and offers a potentially attractive biomarker to differentiate bacterial causes of infection from other etiologies.
Although viral infections are less likely to cause elevations in PCT levels, several other inflammatory conditions aside from bacterial infections can lead to increased blood PCT concentrations (13). Nonbacterial infections, such as severe fungal infections and malaria, surgery, burns, and bowel wall ischemia can all cause increases in PCT levels, as can some paraneoplastic syndromes and cancers (10). Meanwhile, clearance of PCT from the blood may be prolonged among patients with severe kidney disease, even though PCT does not require renal elimination to be cleared from the blood (14). In that study (14), the authors suggested that the delayed PCT clearance may actually be due to prolonged production of PCT in patients with severe renal dysfunction, as a result of ongoing inflammation, rather than decreased elimination.
Because there is a normal physiological increase in PCT levels immediately after birth, neonates have detectable PCT levels that peak around 24 h of life and decline over the subsequent 2 days (15). Thus, different cutoff values are necessary for the diagnosis of infection in the immediate postpartum period. Meanwhile, gestational age influences PCT levels; the normal physiological rise that takes place following birth is blunted in preterm infants, leading to delayed normalization (16). This has led to the development of different age-specific reference ranges for PCT levels among preterm infants (17). Beyond the neonatal period, however, PCT should not be detectable in the blood under normal conditions, and thus the adult reference range should apply to children. However, the performance of PCT likely differs between adults and children because of age-related differences in the epidemiology, microbiology, and immune responses in sepsis, as well as maturational variability in comorbid conditions that affect the production and clearance of PCT directly and indirectly through cytokines. Consequently, it is likely that a given rise in PCT levels should be interpreted differently for a child, young adult, or geriatric patient (18).
PROCALCITONIN IN THE DIAGNOSIS OF SEPSIS
Since PCT levels were originally found to be elevated in patients with staphylococcal toxic shock syndrome (19), numerous studies have investigated the utility of PCT as a biomarker to differentiate bacterial infection and sepsis from nonbacterial causes of critical illness that cause systemic inflammation. One of the earliest studies to evaluate PCT as a biomarker for bacterial infection was performed with hospitalized children with suspected infections (20). In that study, 19 infants and children with severe bacterial infections had high PCT levels, compared to those with no infection and those with localized bacterial infections or viral infections. Proof of principle for the concept of PCT as a biomarker of bacterial infection was then provided by demonstrating that endotoxin injection into healthy volunteers produced increases in PCT levels (21). The kinetics revealed that PCT levels peaked 6 h after endotoxin injection and remained elevated through 24 h, making PCT a promising early biomarker to differentiate bacterial infections from nonbacterial infections prior to the results of microbiological cultures (21). Further, while several other processes that cause inflammation may cause transient rises in PCT levels, these elevations are generally somewhat smaller than those seen in bacterial infections and typically are sustained over the course of days only when concomitant infection is present (13).
Multiple studies in adults have confirmed that PCT is a sensitive and specific biomarker for severe bacterial infections, providing insights into clinical situations in which PCT measurements are most useful. A prospective study of 101 medical intensive care unit (ICU) patients with systemic inflammatory response syndrome (SIRS) and anticipated lengths of stay of ≥24 h showed that PCT levels were higher in patients with sepsis and positive cultures, with 0.89 sensitivity and 0.94 specificity for sepsis using a PCT concentration cutoff value of 1 ng/ml (22). PCT outperformed C-reactive protein (CRP), IL-6, and lactate as a diagnostic biomarker for sepsis in that study (22). Meta-analyses subsequently confirmed those findings. One meta-analysis of 30 studies involving 3,244 emergency department or ICU patients with SIRS calculated a pooled sensitivity of 0.77 and a pooled specificity of 0.79, with an area under the receiver operating characteristic curve (AUROC, or AUC) of 0.85 (95% confidence interval [CI], 0.81 to 0.88), for PCT to differentiate sepsis from noninfectious SIRS (23). In that meta-analysis, PCT performed better among surgical patients, compared to medical patients. A more recent meta-analysis of 58 studies involving 16,514 patients with suspected infection or sepsis calculated a pooled sensitivity and specificity to detect bacteremia of 0.76 (95% CI, 0.72 to 0.80) and 0.69 (95% CI, 0.64 to 0.72), respectively, with a cutoff value of 0.5 ng/ml (AUC of 0.79) (24). In that meta-analysis, PCT performed best among ICU patients (AUC, 0.89) and worst among immunocompromised/neutropenic patients (AUC, 0.71) (24). Meanwhile, Wu et al. performed a meta-analysis of PCT to detect bacterial infections in patients with autoimmune diseases and calculated a summary AUC of 0.91 (95% CI, 0.88 to 0.93), better than that for CRP (AUC, 0.81 [95% CI, 0.78 to 0.93]), providing evidence that PCT may even be useful for patients with known systemic inflammatory conditions that are frequently treated with immunosuppressive therapies (25).
Similar to adults, many studies have investigated the use of PCT among children in various clinical settings. PCT can help differentiate serious bacterial infections in children with fever and central venous catheters or neutropenia (26, 27), distinguish bacterial from viral/aseptic meningitis (28, 29), and identify the presence of meningococcal disease in febrile children with rashes (30). PCT is particularly well studied, however, in critically ill children in the pediatric ICU (PICU). In a prospective study of 80 children with suspected sepsis in a PICU, PCT levels had better diagnostic performance for severe infections than did CRP levels or leukocyte counts (31). Among 78 PICU patients with sepsis, PCT levels were elevated in those with bacterial sepsis but not in those with fungal, viral, or culture-negative sepsis, and PCT levels were persistently elevated over time in children with multiple organ dysfunction syndrome and nonsurvivors (32).
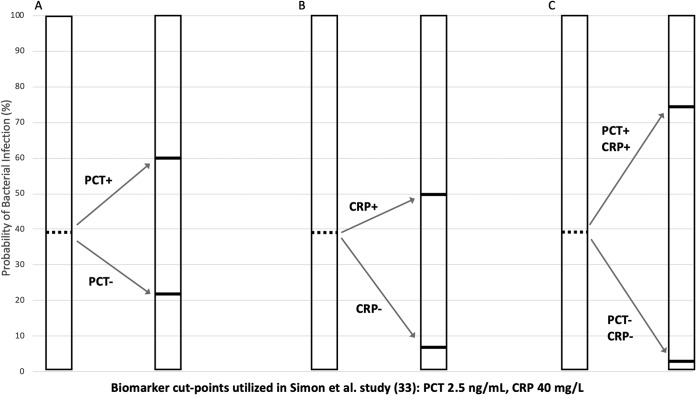
A prospective cohort study of 64 PICU patients with SIRS also showed that PCT can identify those with bacterial infections better than CRP as a single biomarker, although the performance of PCT alone was only moderate (AUC, 0.71) (33). However, measuring both PCT and CRP levels improved diagnostic accuracy; the posttest probability of bacterial infection was 74% when PCT and CRP measurements were both indicative of infection and was only 3% when both were negative (33). Using data from that study (33), Fig. 2 visually demonstrates how the combination of PCT and CRP measurements improved the posttest probabilities of bacterial infection in children with SIRS, compared to the use of either test alone. Similarly, in a prospective study of 85 PICU patients with SIRS and suspected infection, measurement of multiple biomarkers accurately identified critically ill children with low risk of bacterial infection; a combination of CRP levels of <4 mg/dl and PCT levels of <1.75 ng/ml had a negative predictive value of 0.9 (95% CI, 0.79 to 1.0) (34).
FIG 2.
Example of how measurement of PCT and CRP levels in combination strengthens the performance of biomarkers in pediatric SIRS. Data in figure are derived from the report by Simon et al. (33). In that study of 69 children with SIRS, 24 had bacterial infections (pretest probability, 39%). (A) Use of PCT alone had a positive LR (LR+) of 2.65, increasing the posttest probability to 60%, and a LR− of 0.43, decreasing the posttest probability to 22%. (B) Use of CRP alone had a LR+ of 1.63 (posttest probability, 50%) and a LR− of 0.10 (posttest probability, 6%). (C) Use of the combination of PCT and CRP had a LR+ of 4.32 (posttest probability, 74%) and a LR− of 0.043 (posttest probability, 3%). Discordant CRP and PCT results (not shown) had a LR of 0.27 to 0.70 (posttest probability, 15 to 30%).
Other studies with the PICU population have also demonstrated the utility of PCT measurements to exclude bacterial infections, which is useful to guide treatment decisions and to limit unnecessary antibiotic use. A retrospective study of 646 PICU patients with PCT levels measured within 48 h after admission showed that PCT levels peaked within 24 h after PICU admission, outperformed leukocyte counts, and had better performance to rule out rather than rule in infection, with a negative likelihood ratio (LR−) of 0.3 (35). In a detailed analysis of false-negative results, the authors found that PCT measurements were less helpful to distinguish bacterial infections in cases of localized central nervous system, soft tissue, bone, and severe lower respiratory tract infections (35).
Because PCT has a good LR−, PCT measurements can identify lower-risk patients who are very unlikely to have bacterial infections. Sensitivity and specificity are metrics that describe a test’s utility as a screening test in a population but are less useful for interpreting an individual patient’s test results. LRs, which are interpreted in the context of pretest probability, are more useful clinically. In adult patients with suspected sepsis, using a PCT concentration cutoff value of 0.5 ng/ml, the LR− reported in a meta-analysis of 19 studies (3,012 patients) was 0.27 (5). Thus, in the population of all patients with suspected sepsis, if 50% have bacterial infections (pretest probability of infection, 0.50), then a LR− of 0.27 means that an individual with a PCT level below 0.5 ng/ml would still have an approximately 20% chance of having an infection (posttest probability). As a result, a low PCT value determined at the onset of suspected sepsis would be insufficient to withhold antibiotics if no additional information was considered. In clinical practice, however, the actual pretest probability of bacterial infection varies from patient to patient and clinicians always have additional data available aside from the PCT levels. Using other information (such as CRP levels and clinical judgement) can alter the pretest probability and allow interpretation of a low PCT level as highly reflective of an absence of infection.
Several gaps remain in the understanding of the utility of PCT as a rapid biomarker to detect serious bacterial infections in critically ill children. While there are some data supporting its use for patients with neutropenia (36), it is not well studied in other types of immunocompromising conditions and specifically in immunocompromised critically ill children. PCT measurements may miss patients with localized, but still clinically relevant, infections (30, 35, 37). Finally, PCT has variable utility in patients with fungal infections and, when elevated, PCT levels are typically lower than in bacterial infections (32, 38, 39). Therefore, the precise utility of PCT measurements for children at high risk for nonbacterial causes of sepsis remains unclear.
RISK STRATIFICATION
Multiple studies have demonstrated that PCT exhibits a “dose-response” gradient that tracks with the overall severity of bacterial infection in both adults (40) and children (41, 42). In general, localized bacterial infections (e.g., lower urinary tract infections) tend to induce a minimal rise in circulating PCT levels, while there is a progressive increase in PCT levels in more invasive bacterial infections that cause SIRS (e.g., pyelonephritis with bacteremia) and with clinical progression to sepsis and septic shock. A similar rise in PCT levels may even be apparent among critically ill children with increasing severity of viral infections (35). Furthermore, among hospitalized patients, nonsurvivors tend to have higher PCT levels than survivors (43, 44). These observations led, in part, to the initial approval of PCT by the U.S. Food and Drug Administration in 2008 (45), “For use in conjunction with other laboratory findings and clinical assessments to aid in the risk assessment of critically ill patients on their first day of ICU admission for progression to severe sepsis and septic shock.”
An important challenge to the application of PCT as a biomarker of illness severity at the bedside, however, is the lack of clear discrimination across individual patients. For example, among 646 children treated in a PICU, patients with bacterial septic shock exhibited higher median PCT levels than patients with bacterial infections without shock (7.16 versus 1.51 ng/ml) (35). However, the interquartile ranges overlapped considerably in the range of 2 to 4 ng/ml (2.21 to 42.28 ng/ml with shock and 0.41 to 4.04 ng/ml without shock) (35). Similarly, Van der Kaay et al. found that median PCT levels at PICU admission were significantly higher in children with meningococcal disease who had septic shock than in children with sepsis without shock (270 versus 64 ng/ml; P < 0.01) but with notable overlap in the ranges for the groups (6 to 672 ng/ml for septic shock versus 21 to 284 ng/ml for sepsis) (46). These studies highlight the large “gray zone” for PCT measurements, in which results are indeterminate when used to distinguish septic shock from sepsis.
The discriminatory prognostic value of PCT may be even less pronounced among patients with similar categories of illness severity. For example, in a meta-analysis of all hospitalized adults with infection and SIRS, the weighted mean difference of initial PCT values between survivors and nonsurvivors was −6.02 ng/ml (95% CI, −10.01 to −2.03 ng/ml; P = 0.003) (47). However, this difference was no longer significant when the analysis was limited to patients with the highest clinical illness severity of severe sepsis or septic shock (pooled mean difference, −1.71 ng/ml [95% CI, −10.16 to 6.73 ng/ml]; P = 0.69). In a study of 75 children with septic shock, Hatherill et al. reported a higher median PCT value for nonsurvivors, compared to that for survivors (273 versus 82 ng/ml; P = 0.03), but the ranges were nearly identical (5.1 to 736 ng/ml for nonsurvivors versus 3.3 to 760 ng/ml for survivors) (48). Thus, the prognostic capacity of a single elevated PCT measurement may be limited. However, a very low or normal PCT value (<0.5 to 1 ng/ml) may more reliably indicate a reassuring prognosis, with negative predictive values for adverse outcomes (e.g., death) that approached 100% in several pediatric studies (44, 46, 48).
Serial testing of PCT levels likely provides more accurate prognostic information than a single test. For example, Hatherill et al. demonstrated that the absence of a decline in PCT levels after 24 h of treatment for pediatric septic shock was associated with an increased mortality rate (44% versus 9%; P = 0.02) (48). Biomarker data from the REsearching severe Sepsis and Organ dysfunction in children: A gLobal perspective (RESOLVE) study, a phase III trial of drotrecogin alfa (activated) in pediatric severe sepsis, demonstrated a greater decline in PCT values over the first 6 days of septic illness among 324 survivors, compared to 63 nonsurvivors (49). Similarly, in a small study of pediatric severe sepsis/septic shock performed in India, Poddar et al. reported a 76% reduction in PCT levels among 14 survivors, compared to an increase of 200% for 6 nonsurvivors (P = 0.006) (50). Serial testing is especially necessary for pediatric conditions in which an initial PCT level may be elevated due to the underlying insult, such as after trauma, burn injuries, or cardiac surgery. For example, in a single-center study of 33 children following open heart surgery, persistent elevation of PCT levels through postoperative day 4 was associated with severity of organ failure and death (51).
While additional pediatric data are needed, a threshold decrease of at least 80% from initial PCT measurements has been tested in adults with severe sepsis and septic shock in the Multicenter Procalcitonin Monitoring Sepsis (MOSES) study (52). Among 646 patients who were alive and in the hospital on day 4, there was a significantly higher risk of death if PCT levels did not decline by at least 80% from baseline levels to day 4, after adjustment for initial illness severity and other clinical variables associated with adverse outcomes (adjusted hazard ratio, 1.97 [95% CI, 1.18 to 3.30]; P < 0.01). Ultimately, while PCT measurements are useful to distinguish illness severity across groups of patients, the additional informative data provided by a single elevated PCT measurement at the bedside, beyond other clinical distinguishing factors (e.g., SIRS versus shock), is not yet clear. In particular, for predicting risk of death among patients, clinicians must incorporate the precipitating cause of illness, the clinical milieu, and other clinical information (including other test results and underlying conditions) when estimating illness severity or prognosis for an individual patient. In this setting, serial measurements of PCT likely yield more accurate predictive validity than initial values.
ANTIMICROBIAL STEWARDSHIP
Among adult patients meeting the current definition of sepsis, bacterial infections were identified in approximately 50% of cases (5). Among children, according to a nationally representative administrative data set (53), bacterial or fungal pathogens were identified in only 43% of sepsis cases. Despite important limitations in current methodologies for identifying pathogens, it follows that not all patients with suspected sepsis will benefit from antibiotic treatment. Knowing that inappropriate antibiotic use contributes to the development of antimicrobial resistance and leads to increased adverse events and hospital costs, an important clinical use of any sepsis biomarker is its ability to guide antibiotic decisions. Antimicrobial stewardship focuses on the judicious use of antibiotics, and biomarkers such as PCT can help aid clinicians regarding who needs antibiotics and for how long. As a result, there are three avenues related to antibiotic stewardship for which PCT has been predominantly studied, (i) antibiotic initiation, (ii) escalation of antibiotics (i.e., broadening the antibiotic spectrum), and (iii) antibiotic cessation.
Antibiotic initiation.
To facilitate early identification of sepsis and timely treatment, clinicians seek a biomarker that optimizes sensitivity. Several meta-analyses summarize the test characteristics of PCT and other biomarkers for the diagnosis of sepsis in critically ill adult patients (5, 54, 55). In a recent meta-analysis of 19 observational studies involving 3,012 adult patients (5), the pooled sensitivity for the diagnosis of sepsis, according to the Sepsis-3 definition (1), was 0.80 (95% CI, 0.75 to 0.84). Based on this pooled test performance, if clinicians were to rely solely on PCT values to initiate antibiotics for patients with suspected sepsis, then 1 in 5 patients with sepsis would go untreated. As described earlier, these data suggest that PCT is a helpful biomarker for identifying patients with sepsis but is inadequate to serve as a stand-alone test to guide decisions about antibiotic initiation for acutely ill patients with clinical features of septic shock for whom a low threshold for empirical antibiotic treatment is appropriate. In the current quest to ensure early initiation of antibiotics for patients with sepsis, PCT does not yet play an effective role.
Similar data exist for neonates with early- and late-onset sepsis. In a 2018 meta-analysis of 28 studies that included 2,661 neonates with suspected sepsis, PCT had a pooled sensitivity of 0.85 (95% CI, 0.79 to 0.89) for distinguishing sepsis from nonsepsis (56). The diagnostic accuracy of PCT for neonatal sepsis improved when results were combined with CRP values, yielding a pooled sensitivity of 0.91 (95% CI, 0.84 to 0.95) (56). As it does in adults, PCT has insufficient accuracy by itself for the diagnosis of neonatal sepsis. When combined with other biomarkers, however, such as CRP, PCT is more helpful in identifying patients who would most benefit from antibiotics.
Even fewer data exist for older children. To our knowledge, no meta-analyses have examined the test performance of PCT for sepsis exclusively in older children. Thus, data are limited to single-center observational studies. While in theory PCT should perform similarly among children and adults, since PCT values are not age dependent, it is unlikely that PCT performs sufficiently well to serve as an independent sepsis screening test for antibiotic initiation in ill-appearing children.
Escalation of antibiotics.
Because patients whose PCT levels fail to decline in serial measurements have increased mortality rates (48, 49), it has been postulated that persistently elevated concentrations over time may signal the presence of an inadequately treated infection. Under this theory, serial measurements could help identify patients who would benefit from escalation of antibiotics (i.e., broader-spectrum therapy) to ensure adequate antimicrobial coverage of potential pathogens. The goal of utilizing PCT in this respect is to facilitate the early identification of an inadequate response to initial antibiotic therapy in patients with infection, rather than early identification of the infection itself.
There has only been one randomized clinical trial (RCT) studying the utility of PCT in this context and none involving children (57). In the PASS trial, which was conducted across 9 ICUs in Denmark, investigators asked whether the availability of PCT results and an obligatory guideline for antimicrobial escalation would result in earlier provision of appropriate antibiotic therapy for infected critically ill patients and would improve survival (57). The investigators randomly assigned 1,200 patients, upon ICU admission, to a standard-of-care arm or a PCT-guided drug escalation arm and collected PCT measurements daily. In the standard-of-care arm, clinicians were not informed of PCT results; in the PCT arm, clinicians were instructed to escalate antibiotics if the PCT level was >1 ng/ml and not decreasing by at least 10% from the previous day. Additional diagnostic testing was also directed in the PCT arm to identify uncontrolled sources of infection. De-escalation was permitted when PCT levels were less than 1 ng/ml for 3 consecutive days. By design, subjects in the PCT arm received significantly more (2,925 versus 1,893 days; P < 0.001) and broader antibiotics than patients in the standard-of-care arm. However, among patients with microbiologically confirmed infections, the times to appropriate therapy were similar in the two groups, i.e., 0.2 days for the PCT group versus 0.4 days for the standard-of-care group (P = 0.61). There was no difference in 28-day survival (hazard ratio, 0.98 [95% CI, 0.83 to 1.16]), even across a number of prespecified subgroup analyses such as gender, age, APACHE II score, severity of infection, and prior surgery. Meanwhile, the PCT arm had worse secondary outcomes, including more ICU days on mechanical ventilation, days with renal failure, and days in the ICU. The authors concluded that escalation of antibiotics based on PCT results did not improve survival rates while leading to administration of more and broader antibiotics, longer hospital stays, and negative effects on organ function.
To our knowledge, there are no pediatric studies that have utilized PCT in this manner. Because the PASS study had a very specific algorithm for how antibiotics were to be escalated and interventions followed, the outcomes were heavily dependent on the specifics of the algorithm. It is possible that a different algorithm, one that escalates breadth of coverage based on different criteria or that broadens to different antimicrobial agents, would have less deleterious outcomes. However, mandatory escalation of therapy in the setting of rising or persistently elevated PCT values does not appear to be an effective therapeutic strategy.
De-escalation/antibiotic cessation.
Data are supportive of PCT use in ICU settings to inform decisions regarding safe cessation of antibiotics. In a patient-level meta-analysis of RCTs evaluating the effects of PCT-guided treatment on mortality rates among adults with acute respiratory tract infections, the use of a PCT algorithm was associated with decreased overall mortality rates among adults in an ICU (odds ratio, 0.88 [95% CI, 0.77 to 1.00]) (58). Similarly, a Cochrane review of PCT measurements to initiate or to discontinue antibiotics for acute respiratory tract infections found decreased 30-day mortality rates (odds ratio, 0.88 [95% CI, 0.77 to 1.00]) among ICU trials (59). As reported above, PCT has a good negative predictive value, particularly when combined with other clinical data such as CRP levels. Therefore, low PCT values determined at suspected sepsis onset can support early antibiotic cessation when other data similarly suggest that infection is absent. Because PCT levels decrease by 50% every 1 to 2 days in the absence of active infection (i.e., when infection is adequately treated) (21), serial PCT measurement can also be useful to guide antibiotic duration in patients with documented or proven infections. Meta-analyses of RCTs of PCT-based antibiotic cessation found that implementation of an algorithm that specifies stopping antibiotic treatment when PCT levels are low (usually <0.5 ng/ml), or when concentrations have decreased by ≥80% from peak values, leads to a significant reduction in antibiotic duration, compared to the standard of care, without an increase in mortality rates (6, 7). Two recent meta-analyses both concluded that the use of a PCT-based cessation algorithm led to 1.3 fewer days of antibiotics in critically ill adults (6, 7). PCT-guided cessation was also associated with decreased short-term mortality rates, compared with standard-of-care groups (relative risk, 0.82 [95% CI, 0.70 to 0.96]; P = 0.01) (6). While there was significant heterogeneity across included studies and variable levels of adherence to PCT-defined algorithms in the included trials, implementation of a PCT-based cessation guideline appears to be an effective strategy to decrease antibiotic usage in critically ill adults. This has led to publication of an international consensus document supporting measurement of PCT levels every 24 to 48 h, with recommendations to discontinue antibiotics when PCT levels are <0.5 ng/ml or have dropped by 80% from peak values (60).
Fewer RCTs have been conducted in children. The NeoPIns study was a large RCT across 18 hospitals among neonates with suspected early-onset sepsis (61). The trial stratified more than 1,700 infants based on their risk for early-onset sepsis and randomly assigned subjects to standard-of-care treatment or PCT-guided discontinuation. Based on the specific algorithm implemented, only subjects with unlikely or possible infections were eligible for PCT-based discontinuation, because those with probable or proven infections were treated with a standard regimen of 7 to 21 days of antibiotics. Similar to adult studies, the duration of antibiotics was reduced in the PCT group (intention to treat, 55.1 versus 65.0 h; P < 0.0001) without an increase in adverse outcomes. Although the difference in antibiotic duration was only 10 h, this is a substantial and important reduction in overall use (15% shorter) in neonates.
To our knowledge, there are no published RCTs comparing PCT-based discontinuation to standard-of-care treatment in older pediatric patients. However, observational studies are available (62). One study evaluated the impact of an algorithm on antibiotic use in critically ill children with SIRS (62). The algorithm indicated cessation of antibiotics at 24 to 48 h if PCT levels determined at SIRS onset were <1 ng/ml and CRP levels were <4 mg/dl, microbiological cultures were negative, and there were no focal signs of infection present on evaluation. Although antibiotic use was not decreased among all patients with SIRS (P = 0.76), implementation of this algorithm was associated with a significant reduction in days of therapy in the subset of patients who had both CRP and PCT levels below the defined cutoff points, i.e., 129.9/1,000 days versus 301.2/1,000 days (incidence rate ratio, 0.43 [95% CI, 0.26 to 0.71]) (62). While observational, this study demonstrates the potential utility of PCT measurements to allow for safe antibiotic de-escalation in pediatric patients at low risk for bacterial infection.
CONCLUSIONS
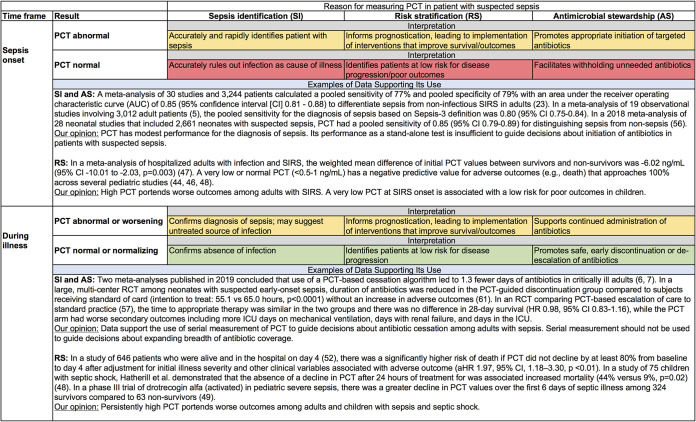
PCT has become a biomarker used frequently in sepsis due to its relative specificity for bacterial over viral infections, compared to other biomarkers available for clinical use. Despite its superior test performance, compared to more traditional biomarkers such as CRP levels and white blood cell counts, PCT measurement is not sufficiently sensitive or specific to serve as a stand-alone test for the diagnosis of sepsis. Given the diverse nature of sepsis and the moderate performance of PCT as a sepsis biomarker, clinicians should be careful not to be overly reliant on the results of any single PCT measurement. Similarly, while an elevated PCT concentration at the time of initial sepsis evaluation portends a worse outcome for patients with severe sepsis or septic shock, serial measurements provide more reliable prognostic information, as values decline more readily in survivors/responders to treatment. Figure 3 summarizes the performance of PCT with respect to each of the clinical scenarios highlighted in this review.
FIG 3.
Performance of PCT measurement and data supporting its use at sepsis onset and during illness. Green shading denotes the presence of good evidence to support the use of PCT in that scenario. Yellow shading denotes the presence of weak evidence to support the use of PCT in that scenario. Red shading denotes the presence of evidence against the use of PCT in that scenario.
With an understanding of its limitations and prognostic ability, PCT can help guide safe antibiotic cessation decisions for children who are initially suspected to have a bacterial infection but show clinical improvement without confirmation of a true infection. Among low-risk patients for whom additional information or clinical assessment makes bacterial infection unlikely, a very low PCT level can provide further evidence that stopping antibiotics early is safe for critically ill adult and pediatric patients. For higher-risk patients, including those with documented infections, serial measurements can provide data to guide the duration of therapy, leading to reductions in overall antibiotic use, compared to standard-of-care practices (e.g., arbitrary 7- to 14-day courses). In adults, a decline in PCT levels of 80% from peak concentrations or to values of <0.5 ng/ml has been supported as a means to reduce antibiotic duration (60). Confirmation that these targets are safe and effective for pediatric patients will need to be verified.
Finally, while biomarker-based algorithms employ a cutoff point to assist with interpretation of PCT results, clinicians must remember that results should not be accepted in absolute terms (i.e., a value above the cutoff point is diagnostic of sepsis while a value below the cutoff point rules out sepsis). Cutoff points have been identified as inflection points that best influence the likelihood that bacterial infection is present or absent. Because PCT measurement is not a diagnostic test, however, it should be used only to supplement and not to replace clinical judgement.
ACKNOWLEDGMENTS
This study received no specific funding and was conducted as part of the authors’ routine work. K.J.D. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award K23 HD091365. J.C.F. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award K23 DK119463.
K.J.D. has received research support from Merck, Inc., and Pfizer, Inc., unrelated to the current work.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. 2020. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M. 2017. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. 2013. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 14:686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. 2019. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: a systematic review and meta-analysis. J Intensive Care 7:22. doi: 10.1186/s40560-019-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng F, Chang W, Xie JF, Sun Q, Qiu HB, Yang Y. 2019. Ineffectiveness of procalcitonin-guided antibiotic therapy in severely critically ill patients: a meta-analysis. Int J Infect Dis 85:158–166. doi: 10.1016/j.ijid.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Pepper DJ, Sun J, Rhee C, Welsh J, Powers JH III, Danner RL, Kadri SS. 2019. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: a systematic review and meta-analysis. Chest 155:1109–1118. doi: 10.1016/j.chest.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covington EW, Roberts MZ, Dong J. 2018. Procalcitonin monitoring as a guide for antimicrobial therapy: a review of current literature. Pharmacotherapy 38:569–581. doi: 10.1002/phar.2112. [DOI] [PubMed] [Google Scholar]
- 9.Iankova I, Thompson-Leduc P, Kirson NY, Rice B, Hey J, Krause A, Schonfeld SA, DeBrase CR, Bozzette S, Schuetz P. 2018. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: a systematic review and meta-analysis. Crit Care Med 46:691–698. doi: 10.1097/CCM.0000000000002928. [DOI] [PubMed] [Google Scholar]
- 10.Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. 2004. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 89:1512–1525. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- 11.Canudas J, Sorribas V, Lacosta AM, Sarasa M. 2013. The expression of procalcitonin in the central nervous system of the rat embryo. Cell Dev Biol 2:3. doi: 10.4172/2168-9296.1000126. [DOI] [Google Scholar]
- 12.Morgenthaler NG, Struck J, Chancerelle Y, Weglöhner W, Agay D, Bohuon C, Suarez-Domenech V, Bergmann A, Müller B. 2003. Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. Horm Metab Res 35:290–295. doi: 10.1055/s-2003-41304. [DOI] [PubMed] [Google Scholar]
- 13.Becker KL, Snider R, Nylen ES. 2008. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 36:941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 14.Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K. 2001. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol 18:79–87. doi: 10.1046/j.0265-0215.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Chiesa C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, Pacifico L. 1998. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis 26:664–672. doi: 10.1086/514576. [DOI] [PubMed] [Google Scholar]
- 16.Turner D, Hammerman C, Rudensky B, Schlesinger Y, Goia C, Schimmel MS. 2006. Procalcitonin in preterm infants during the first few days of life: introducing an age related nomogram. Arch Dis Child Fetal Neonatal Ed 91:F283–F286. doi: 10.1136/adc.2005.085449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuzumi N, Osawa K, Sato I, Iwatani S, Ishino R, Hayashi N, Iijima K, Saegusa J, Morioka I. 2016. Age-specific percentile-based reference curve of serum procalcitonin concentrations in Japanese preterm infants. Sci Rep 6:23871. doi: 10.1038/srep23871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stucker F, Herrmann F, Graf JD, Michel JP, Krause KH, Gavazzi G. 2005. Procalcitonin and infection in elderly patients. J Am Geriatr Soc 53:1392–1395. doi: 10.1111/j.1532-5415.2005.53421.x. [DOI] [PubMed] [Google Scholar]
- 19.Chesney RW, McCarron DM, Haddad JG, Hawker CD, DiBella FP, Chesney PJ, Davis JP. 1983. Pathogenic mechanisms of the hypocalcemia of the staphylococcal toxic-shock syndrome. J Lab Clin Med 101:576–585. [PubMed] [Google Scholar]
- 20.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. 1993. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C. 1994. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 22.Müller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R. 2000. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 28:977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. 2013. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 13:426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 24.Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. 2015. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 21:474–481. doi: 10.1016/j.cmi.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Wu JY, Lee SH, Shen CJ, Hsieh YC, Yo PH, Cheng HY, Chan RC, Lee CC, Chang SS. 2012. Use of serum procalcitonin to detect bacterial infection in patients with autoimmune diseases: a systematic review and meta-analysis. Arthritis Rheum 64:3034–3042. doi: 10.1002/art.34512. [DOI] [PubMed] [Google Scholar]
- 26.Kasem AJ, Bulloch B, Henry M, Shah K, Dalton H. 2012. Procalcitonin as a marker of bacteremia in children with fever and a central venous catheter presenting to the emergency department. Pediatr Emerg Care 28:1017–1021. doi: 10.1097/PEC.0b013e31826caac2. [DOI] [PubMed] [Google Scholar]
- 27.Demirkaya M, Tugcu D, Akcay A, Aydogan G, Akici F, Salcioglu Z, Ekmekci H, Sevinir B, Balci Ekmekci O. 2015. Adrenomedullin: a new marker in febrile neutropenia: comparison with CRP and procalcitonin. Pediatr Hematol Oncol 32:482–489. doi: 10.3109/08880018.2015.1057310. [DOI] [PubMed] [Google Scholar]
- 28.Dubos F, Moulin F, Gajdos V, De Suremain N, Biscardi S, Lebon P, Raymond J, Breart G, Gendrel D, Chalumeau M. 2006. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr 149:72–76. doi: 10.1016/j.jpeds.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C. 1997. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis 24:1240–1242. doi: 10.1086/513633. [DOI] [PubMed] [Google Scholar]
- 30.Carrol ED, Newland P, Riordan FA, Thomson AP, Curtis N, Hart CA. 2002. Procalcitonin as a diagnostic marker of meningococcal disease in children presenting with fever and a rash. Arch Dis Child 86:282–285. doi: 10.1136/adc.86.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casado-Flores J, Blanco-Quiros A, Asensio J, Arranz E, Garrote JA, Nieto M. 2003. Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med 4:190–195. doi: 10.1097/01.pcc.0000059420.15811.2d. [DOI] [PubMed] [Google Scholar]
- 32.Han YY, Doughty LA, Kofos D, Sasser H, Carcillo JA. 2003. Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. Pediatr Crit Care Med 4:21–25. doi: 10.1097/00130478-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. 2008. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med 9:407–413. doi: 10.1097/PCC.0b013e31817285a6. [DOI] [PubMed] [Google Scholar]
- 34.Downes KJ, Weiss SL, Gerber JS, Klieger SB, Fitzgerald JC, Balamuth F, Kubis SE, Tolomeo P, Bilker WB, Han X, Nachamkin I, Garrigan C, Han JH, Lautenbach E, Coffin SE. 2017. A pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in Sepsis (OASIS) Study. J Pediatr Infect Dis Soc 6:134–141. doi: 10.1093/jpids/piw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lautz AJ, Dziorny AC, Denson AR, O'Connor KA, Chilutti MR, Ross RK, Gerber JS, Weiss SL. 2016. Value of procalcitonin measurement for early evidence of severe bacterial infections in the pediatric intensive care unit. J Pediatr 179:74–81.e2. doi: 10.1016/j.jpeds.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SG, Hou TY, Huang DH, He SY, Lin YD, Zhang LY, Hsieh PS. 2012. Role of procalcitonin in the diagnosis of severe infection in pediatric patients with fever and neutropenia: a systemic review and meta-analysis. Pediatr Infect Dis J 31:e182–e188. doi: 10.1097/INF.0b013e31825da45d. [DOI] [PubMed] [Google Scholar]
- 37.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, Taneja S, Ray S. 2015. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care 30:218.e7–218.e12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Cortegiani A, Misseri G, Ippolito M, Bassetti M, Giarratano A, Martin-Loeches I, Einav S. 2019. Procalcitonin levels in candidemia versus bacteremia: a systematic review. Crit Care 23:190. doi: 10.1186/s13054-019-2481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas-Ruddel DO, Poidinger B, Kott M, Weiss M, Reinhart K, Bloos F. 2018. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care 22:128. doi: 10.1186/s13054-018-2050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, Vadas L, Pugin J. 2001. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 41.Milcent K, Faesch S, Gras-Le Guen C, Dubos F, Poulalhon C, Badier I, Marc E, Laguille C, de Pontual L, Mosca A, Nissack G, Biscardi S, Le Hors H, Louillet F, Dumitrescu AM, Babe P, Vauloup-Fellous C, Bouyer J, Gajdos V. 2016. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr 170:62–69. doi: 10.1001/jamapediatrics.2015.3210. [DOI] [PubMed] [Google Scholar]
- 42.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. 2008. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics 122:701–710. doi: 10.1542/peds.2007-3503. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Su L, Han G, Yan P, Xie L. 2015. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 10:e0129450. doi: 10.1371/journal.pone.0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casado-Flores J, Blanco-Quirós A, Nieto M, Asensio J, Fernández C. 2006. Prognostic utility of the semi-quantitative procalcitonin test, neutrophil count and C-reactive protein in meningococcal infection in children. Eur J Pediatr 165:26–29. doi: 10.1007/s00431-005-1761-5. [DOI] [PubMed] [Google Scholar]
- 45.Food and Drug Administration. 2008. 510(k) substantial equivalence determination: decision summary, assay and instrument combination template. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/cdrh_docs/reviews/K071146.pdf. [Google Scholar]
- 46.Van der Kaay D, De Kleijn E, De Rijke Y, Hop W, De Groot R, Hazelzet J. 2002. Procalcitonin as a prognostic marker in meningococcal disease. Intensive Care Med 28:1606–1612. doi: 10.1007/s00134-002-1505-1. [DOI] [PubMed] [Google Scholar]
- 47.Arora S, Singh P, Singh PM, Trikha A. 2015. Procalcitonin levels in survivors and nonsurvivors of sepsis: systematic review and meta-analysis. Shock 43:212–221. doi: 10.1097/SHK.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 48.Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. 2000. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 28:2591–2594. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 49.Dalton HJ, Carcillo JA, Woodward DB, Short MA, Williams MD. 2012. Biomarker response to drotrecogin alfa (activated) in children with severe sepsis: results from the RESOLVE clinical trial. Pediatr Crit Care Med 13:639–645. doi: 10.1097/PCC.0b013e318250ad48. [DOI] [PubMed] [Google Scholar]
- 50.Poddar B, Gurjar M, Singh S, Aggarwal A, Baronia A. 2016. Reduction in procalcitonin level and outcome in critically ill children with severe sepsis/septic shock: a pilot study. J Crit Care 36:230–233. doi: 10.1016/j.jcrc.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Celebi S, Koner O, Menda F, Balci H, Hatemi A, Korkut K, Esen F. 2006. Procalcitonin kinetics in pediatric patients with systemic inflammatory response after open heart surgery. Intensive Care Med 32:881–887. doi: 10.1007/s00134-006-0180-z. [DOI] [PubMed] [Google Scholar]
- 52.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, Runyon MS, Self WH, Courtney DM, Nowak RM, Gaieski DF, Ebmeyer S, Johannes S, Wiemer JC, Schwabe A, Shapiro NI. 2017. Serial procalcitonin predicts mortality in severe sepsis patients: results from the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med 45:781–789. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prout AJ, Talisa VB, Carcillo JA, Decker BK, Yende S. 2020. Bacterial and fungal etiology of sepsis in children in the United States: reconsidering empiric therapy. Crit Care Med 48:e192–e199. doi: 10.1097/CCM.0000000000004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan M, Lu Y, Jiang H, Zhang L. 2019. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: a systematic review and meta-analysis. J Cell Biochem 120:5852–5859. doi: 10.1002/jcb.27870. [DOI] [PubMed] [Google Scholar]
- 55.Yeh CF, Wu CC, Liu SH, Chen KF. 2019. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann Intensive Care 9:5. doi: 10.1186/s13613-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruan L, Chen GY, Liu Z, Zhao Y, Xu GY, Li SF, Li CN, Chen LS, Tao Z. 2018. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care 22:316. doi: 10.1186/s13054-018-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, Thornberg KJ, Loken J, Steensen M, Fox Z, Tousi H, Soe-Jensen P, Lauritsen AO, Strange D, Petersen PL, Reiter N, Hestad S, Thormar K, Fjeldborg P, Larsen KM, Drenck NE, Ostergaard C, Kjaer J, Grarup J, Lundgren JD. 2011. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 39:2048–2058. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]
- 58.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Kristoffersen KB, Burkhardt O, Welte T, Schroeder S, Nobre V, Wei L, Bucher HC, Annane D, Reinhart K, Falsey AR, Branche A, Damas P, Nijsten M, de Lange DW, Deliberato RO, Oliveira CF, Maravic-Stojkovic V, Verduri A, Beghe B, Cao B, Shehabi Y, Jensen JS, Corti C, van Oers JAH, Beishuizen A, Girbes ARJ, de Jong E, Briel M, Mueller B. 2018. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 59.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Kristoffersen KB, Burkhardt O, Welte T, Schroeder S, Nobre V, Wei L, Bucher HC, Bhatnagar N, Annane D, Reinhart K, Branche A, Damas P, Nijsten M, de Lange DW, Deliberato RO, Lima SS, Maravic-Stojkovic V, Verduri A, Cao B, Shehabi Y, Beishuizen A, Jensen JS, Corti C, Van Oers JA, Falsey AR, de Jong E, Oliveira CF, Beghe B, Briel M, Mueller B. 2017. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 10:CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, Gonzalez Del Castillo J, Jensen JU, Kanizsai PL, Kwa ALH, Krueger S, Luyt CE, Oppert M, Plebani M, Shlyapnikov SA, Toccafondi G, Townsend J, Welte T, Saeed K. 2019. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med 57:1308–1318. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]
- 61.Stocker M, van Herk W, el Helou S, Dutta S, Fontana MS, Schuerman FABA, van den Tooren-de Groot RK, Wieringa JW, Janota J, van der Meer-Kappelle LH, Moonen R, Sie SD, de Vries E, Donker AE, Zimmerman U, Schlapbach LJ, de Mol AC, Hoffman-Haringsma A, Roy M, Tomaske M, Kornelisse RF, van Gijsel J, Visser EG, Willemsen SP, van Rossum AMC, Bakry A, Dutta S, el Helou S, Kalaniti K, Pogorzelski D, Alliston S, Roy M, Grey V, Hauff K, Hill S, Kittanakom S, Janota J, Visnovska M, Fontana M, Lanz N, Stocker M, Glauser D, Zimmerman U, Tomaske M, Nelle M, Schlapbach LJ, Schuerman F, Sie SD, van Weissenbruch MM, van den Dungen FAM, Strik M, van den Tooren-de HK, van Rossum GA, Batstra M, van der Meer-Kappelle LH, de Vries E, de Mol AC, Bolt-Wieringa J, Stok D, Moonen R, Donker S, van Gijsel J, Gondriet IPE, van Herk W, Hoekstein S, Hofhuis M, Hop W, de Ligt L, Manai B, Kornelisse R, de Rijke Y, van Rossum A, Siiskonen S, van der Velden J, Visser EG, van Wijk JA, Willemsen S, van der Geijn GJ, Haringsma A, Andriessen PA, Broeren MAC, Donker A. 2017. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns.). Lancet 390:871–881. doi: 10.1016/S0140-6736(17)31444-7. [DOI] [PubMed] [Google Scholar]
- 62.Downes KJ, Fitzgerald JC, Schriver E, Boge CLK, Russo ME, Weiss SL, Balamuth F, Kubis SE, Tolomeo P, Bilker WB, Han JH, Lautenbach E, Coffin SE, Gerber JS. 2020. Implementation of a pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in Sepsis (OASIS) II Study. J Pediatr Infect Dis Soc 9:36–43. doi: 10.1093/jpids/piy113. [DOI] [PMC free article] [PubMed] [Google Scholar]