Sterility testing of cellular therapy products along with the associated environmental monitoring requirements for aseptic facilities, including compounding pharmacies, continues to impact clinical microbiology laboratories, as evidenced by the numerous discussions recurring on American Society for Microbiology Division C and ClinMicroNet listservs. This minireview provides an overview of this complex field of current good manufacturing practices (cGMP) based on biopharmaceutical industry standards and summarizes the compendial and alternative rapid microbial test methods available for product sterility and Mycoplasma testing.
KEYWORDS: biopharmaceuticals, cell therapy, rapid detection, sterility testing, compounding pharmacy, environmental monitoring
ABSTRACT
Sterility testing of cellular therapy products along with the associated environmental monitoring requirements for aseptic facilities, including compounding pharmacies, continues to impact clinical microbiology laboratories, as evidenced by the numerous discussions recurring on American Society for Microbiology Division C and ClinMicroNet listservs. This minireview provides an overview of this complex field of current good manufacturing practices (cGMP) based on biopharmaceutical industry standards and summarizes the compendial and alternative rapid microbial test methods available for product sterility and Mycoplasma testing. In addition, this minireview highlights major overarching regulatory requirements governing any laboratory performing product testing as regulated by the United States Food and Drug Administration (FDA). These requirements are different from the more familiar clinical requirements of the Clinical Laboratory Improvement Act of 1988 (CLIA ’88), the College of American Pathologists (CAP), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), all of which have no jurisdiction in this area. As the cellular therapy field continues to advance and an increasing number of medical centers participate in clinical trials of these novel therapies, it is critical that laboratories have a sound understanding of the major regulations and cGMP practices governing microbiological testing in the biopharmaceutical industry.
INTRODUCTION
Cell, gene, and immune therapies (collectively known as biopharmaceuticals) are rapidly emerging as favorable alternatives for the treatment of a variety of hematological malignancies due to their targeted efficacy and high potency. These types of therapies are often referred to as advanced therapeutic medicinal products (ATMPs) and are gaining traction in the public arena due to the recent United States Food and Drug Administration (FDA) approval of chimeric antigen receptor (CAR) T cell therapies for the treatment of certain types of leukemia, non-Hodgkin lymphoma, and diffuse large B-cell lymphoma (1–3). Cellular engineering and other ATMP developments over recent years have led to significant advances in precision gene editing, cellular reprogramming, and functional manipulations, thus revolutionizing the future of numerous disease treatments and patient outcome. Several large academic clinical centers are currently pursuing phase I and phase II clinical trials of these novel therapies, in the form of investigational new drug (IND) applications to the FDA. Some centers currently have facilities and capabilities on site to manufacture various IND ATMPs under current good manufacturing practices (cGMP).
Sterility testing of the ATMP is an important component in ensuring the safety of the cellular product prior to patient infusion, especially because terminal sterilization is not possible for live therapeutics. In 2002, the International Society for Cellular Therapy (ISCT) showed that 76% of facilities performed sterility testing in hospital microbiology laboratories based on a survey of 98, mostly North American, participants (4). While a more recent figure is currently unavailable, numerous discussions recurring on clinical microbiology forums, including ClinMicroNet and that of Division C of the American Society for Microbiology, suggest that product sterility testing and pharmaceutical environmental monitoring are continuing to impact the clinical microbiology field. This is most likely attributable to the increased adoption of automated blood culture systems in lieu of compendial (i.e., biopharmaceutical industry standard) culture methods for sterility testing (5–11) and the close proximity of hospital microbiology laboratories that provide environmental monitoring cultures to the regulated compounding pharmacies. As advances in the cell therapy field continue to progress through academic clinical trials and hospital pharmacies continue to rely on on-site laboratories for environmental monitoring cultures of regulated compounding areas, it is important that clinical microbiology laboratories have a sound understanding of the major regulations and practices governing microbiological testing in the biopharmaceutical industry. We write from our experience at the NIH Clinical Center and provide insight into a program where product sterility testing and environmental monitoring practices were routine in the clinical microbiology laboratory until events in 2015 (12, 13) prompted leadership to develop a robust and holistic cGMP program with a separate testing laboratory dedicated solely to cGMP activities.
This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for bacterial, fungal, and mycoplasma sterility testing for product release; (ii) the performance of noncompendial rapid microbial methods (RMM) evaluated to date; and (iii) the regulatory requirements for qualification and validation of all testing aspects (e.g., facility, equipment, raw materials, test system, and personnel) prior to routine operations for any laboratory performing biopharmaceutical environmental monitoring cultures and/or cGMP product release testing. The standards regulating the testing laboratory are referenced throughout, as they are significantly different from those governing clinical laboratories. Most importantly, the Clinical Laboratory Improvement Act of 1988 (CLIA ’88), the College of American Pathologists (CAP), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) have no jurisdiction in the area of biopharmaceutical product testing and compounding pharmacy environmental monitoring. All components relating to cellular therapies, including sourcing of raw materials, consumables, manufacturing, facility and engineering controls, quality systems, and product safety and testing, fall under the governance of the Food, Drug, and Cosmetic Act (14), which is unrelated to clinical microbiology governance.
OVERVIEW OF THE COMPENDIAL PRODUCT STERILITY TESTS
Product sterility testing is required for any manufactured biological material under the Code of Federal Regulations, Title 21, parts 210 and 211 for cGMP for finished pharmaceuticals and parts 600 to 680 for additional biological product standards. Sections 610.12 and 610.30 relate specifically to sterility and Mycoplasma testing, respectively (15, 16). In any IND application, sterility testing methods are addressed as part of the chemistry, manufacturing, and control (CMC) data to ensure stability and safety of the product (17). The frequency at which a product is tested for sterility during the manufacturing process is based on a risk assessment. At a minimum, a sample representative of the final product must be obtained for sterility testing. Additional in-process sampling during product manufacture may be performed if it is determined that there is a moderately high risk for introducing microbiological contamination during the manufacturing process, such as open processes requiring multiple product manipulation steps rather than fully enclosed systems, and/or lengthy manufacturing processes. Because the turnaround time for compendial sterility testing culture results is long (14 days for sterility testing and 28 days for Mycoplasma) and incompatible with the short shelf life of ATMPs (e.g., fresh infusion products), sterility testing results for the in-process product may be used as a proxy to determine microbiological safety and at-risk product release while waiting for the final product culture results.
The rapid growth of the biopharmaceutical field these past 2 decades has led to a lag in guidance documents that describe, in detail, test procedures for product sterility testing designed specifically for cellular therapy products. As such, the industry has adopted and accepted test methods that were originally designed for the sterility testing of large-batch sterile pharmaceutical drugs, found in United States Pharmacopeia chapters 71 and 63 (referred to here as USP<71> and USP<63>) for sterility testing and Mycoplasma testing, respectively (18, 19).
Description of compendial USP <71> product sterility testing.
USP <71> (18) is the industry standard for product sterility testing. In an effort to support the global trade of pharmaceutical agents for health care improvement and patient care, and to ensure the universal safety of these products, many components of the USP <71> have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia (20, 21). Highlights of the methods of USP <71> are summarized in Table 1.
TABLE 1.
Comparative summary of compendial USP <71> and automated blood culture systems for sterility testing of biopharmaceutical products
| Testing aspect | Compendial USP <71> method | Automated blood culture systems |
|---|---|---|
| Sample handling | Sample is often inoculated by the testing laboratory due to the need for preprocessing steps for membrane filtration. | Bottles are inoculated by the manufacturing facility much like blood culture bottles are inoculated at the patient bedside and transported to the laboratory |
| Inoculation | Direct inoculation or membrane filtration. Membrane filtration may aid in removing potential culture inhibitors. | Direct inoculation only |
| Product vol | Depends on the total product vol and the no. of product containers. Refer to tables in USP <71> (18) for guidance. | Depends on the total product vol and the no. of product containers. Refer to tables in USP <71> (18) for guidance. |
| Medium | Tryptic soy broth (TSB) and fluid thioglycolate medium (FTM) | Aerobic and anaerobic bottles. A bottle containing antimicrobial adsorbing resin is preferable to standard bottles. It is well reported in the clinical setting that blood culture bottles have poor sensitivity for detecting mold. Addition of a Sabouraud dextrose agar plate for fungal culture has been shown to significantly improve detection of mold contaminants (5). |
| Incubation conditions | TSB at 20–25°C for at least 14 days; FTM at 30–35°C for at least 14 days | Aerobic bottle at 20–25°C for at least 14 days. (Note, a 20–25°C blood culture bottle chamber is not common in clinical laboratories. England et al. showed in a comparative study that superior performance was obtained when both the aerobic and anaerobic bottles were incubated at 30–35°C, which is a design that is readily available for all blood culture systems [5].) Anaerobic bottle at 30–35°C for at least 14 days. |
| Culture observation intervals | Manual visual inspection at defined intervals during the incubation period, typically days 3, 5, 7, and 14. | Automated continuous monitoring based on colorimetric or fluorometric detection of CO2. A terminal visual inspection of the bottle is recommended to detect gross mold contamination that fails to be automatically detected by the system (5). These colonies are often in the neck of the bottle or attached to the base of the bottle. |
| Advantages | Gold standard method | Faster detection than USP <71> (5, 37); closed system; existing instrumentation in most clinical laboratories |
| Disadvantages | Visual inspection may be confounded by the already turbid nature of the cell product. Any sign of turbidity triggers subculture and increases the risk of introducing a laboratory contaminant. Requires manual product manipulation by the testing laboratory. | Considered an alternative method by the FDA. Requires comparative method and product qualification studies to ensure that the system provides performance that is equivalent to or better than USP <71> for each product matrix. Blood culture bottles alone have poor sensitivity for mold detection (5). |
The USP <71> sterility test consists of two test procedures: (i) direct inoculation of the culture medium and (ii) membrane filtration. Both procedures are used equally in the industry, with the choice of which one to use being determined by the type of product under examination, the need to remove potential culture inhibitors from the product, costs, and equipment resources.
The volume of in-process and/or final product tested for sterility is defined in USP <71> and is based on total product volume and the total number of articles (i.e., containers) in a batch. Briefly, for direct inoculation, an appropriate volume of product (refer to the tables in USP <71> for guidance) is inoculated directly into tryptic soy broth (TSB) and fluid thioglycolate medium (FTM) and incubated for 14 days at 20 to 25°C and 30 to 35°C, respectively. For membrane filtration, the product (either neat or in diluent, as appropriate) is passed through a ≤0.45-μm membrane filter. If the product has antimicrobial properties (as is common with cell culture media), the filter may be washed at least three times (but no more than five times per filter) with 100 ml of a sterile diluent. The membrane filter is then transferred to TSB at 20 to 25°C and FTM at 30 to 35°C for at least 14 days. Alternatively, liquid media may be transferred onto the membrane. For both direct inoculation and membrane filtration, the broths containing product are manually inspected for turbidity at intervals during the incubation period and at its conclusion. A common manual observation schedule is days 3, 5, 7, and 14, although this may vary between laboratories.
Before routine testing can proceed, the regulatory authorities require method suitability testing (also known as the qualification test) to ensure that the product does not interfere with the detection of low levels (<100 CFU) of viable microorganisms. For method suitability testing, six organisms defined in USP <71> must be challenged (Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Bacillus subtilis ATCC 6633, Clostridium sporogenes ATCC 19404, Candida albicans ATCC 10231, and Aspergillus brasiliensis ATCC 16404). Testing of additional isolates representative of the manufacturing and testing environment is also highly recommended and viewed favorably by regulatory reviewers. For method suitability testing, the maximum volume of product proposed for routine testing is inoculated into the system and spiked with <100 CFU of organism. Positive controls consisting of inoculated organism without product and negative controls comprising product alone are tested in parallel. The test method is considered suitable if growth is detected within 5 days of incubation in the presence of product and within 3 and 5 days for bacterial and fungal controls, respectively. If growth inhibition is detected, the product has failed method suitability testing and the laboratory is responsible for modifying the method through the addition of diluents, neutralizing agents, and/or filters, until a suitable method is identified that will detect <100 CFU of at least the six organisms defined in USP <71> in that product. Method suitability testing must be performed on all new products that have not undergone suitability testing and any time changes are made to the manufacturing process or formulation of a previously qualified product. Typically, the request for method suitability testing is overseen by the product manufacturing facility, unless the testing laboratory has knowledge of the composition of all incoming products.
Description of compendial USP <63> Mycoplasma testing.
Mycoplasma species and their close relatives, Acholeplasma and Spiroplasma, are the most common contaminants in cellular therapies, with an estimated 4% to 35% of biologics and raw materials demonstrating the presence of Mycoplasma, even after terminal filter sterilization (22–26). Sources of contamination have been associated with cell culture reagents such as fetal bovine serum and cell lines, starting donor material, the environment, and personnel (25, 27). The small size of these organisms (0.2 to 0.8 μm) allows them to pass through bacterial filters, and their ready adherence to glass and plastics contributes to their ubiquitous nature. The presence of Mycoplasma in a product can significantly impact product quality and safety. As such, the regulations governing the safety of cellular products uniformly require that cell cultures must be free of Mycoplasma (17). The addition of antibiotics to cell culture media has proven effective in controlling Mycoplasma in cell lines, although resistance has been observed in 3% to 20% of cases (28). Also, even though many cell manufacturing processes have moved toward use of disposable consumables and closed systems, the presence of Mycoplasma does not always result in cell culture turbidity or obvious changes to the cell morphology and may go unnoticed for several cell passages (19, 25). Over 120 species of Mycoplasma have been identified to date, with the most common contaminating species being identified as Mycoplasma fermentans, Mycoplasma hyorinis, Mycoplasma arginini, Mycoplasma orale, and Acholeplasma laidlawii. Despite the significant concerns regarding Mycoplasma from a manufacturing quality and patient safety standpoint, only a few species have been associated with known human clinical disease, including Mycoplasma hominis, Mycoplasma orale, Mycoplasma genitalium, and Mycoplasma pneumoniae (29).
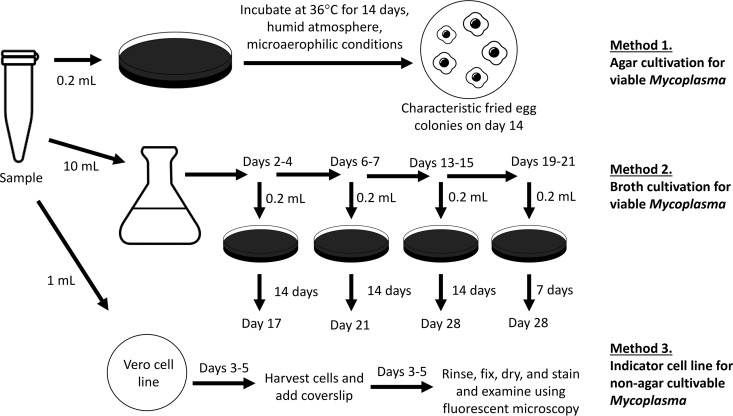
The compendial USP <63> test for Mycoplasma consists of a combination of three methods: (i) agar cultivation for viable organisms, (ii) broth cultivation for viable organisms, and (iii) inoculation of indicator Vero cell lines with fluorescence staining for non-agar-cultivable Mycoplasma, which requires living eukaryotic cells for growth. All three methods must be performed to satisfy the requirements for compendial USP <63> Mycoplasma testing (Fig. 1). The enriched media used in these studies, such as Hayflick medium, Frey medium, and/or Friis medium, are most suited for the detection of slow-growing Mycoplasma species. Briefly, for agar cultivation, 0.2 ml of the biopharmaceutical product is cultured directly onto solid medium and incubated at 36°C in a humid atmosphere under microaerophilic conditions (5% to 10% CO2). Mycoplasma colonies grow with a characteristic fried egg appearance and are observed at 14 days of incubation. Briefly, for broth cultivation, at least 10 ml of the biopharmaceutical product is inoculated into liquid medium. At periodic intervals during the incubation process (days 2 to 4, 6 to 7, 13 to 15, and 19 to 21), 0.2 ml of the primary broth culture is subcultured onto solid medium, which is then incubated for 14 days (or 7 days for broth subcultured on days 19 to 21) to observe for the presence of Mycoplasma colonies. The indicator cell culture method involves inoculating 1 ml of the product into a Vero cell culture. After 3 to 5 days of incubation, a subculture is made on coverslips and incubated for an additional 3 to 5 days to reach 50% cellular confluence. The cells are then rinsed, fixed, dried, stained with bisbenzimide, and examined using fluorescence microscopy to observe for the characteristic particulate, pinpoint, or filamentous pattern on the cell surface indicative of Mycoplasma. The limits of detection for optimized broth culture and indicator cell culture methods are in the range of 3 to 10 CFU/10 ml and 10 to 100 CFU/ml, respectively (30). Altogether, testing for Mycoplasma via the compendial USP <63> method is slow (28-day turnaround) and laborious, and it requires at least 12 ml of cell product, all factors that are not suited to the biopharmaceutical field, where the shelf life of the cellular product is short and product volume is generally conserved for patient infusion to maximize therapeutic effect. Of note, the FDA’s points-to-consider (PTC) method (31) is also considered a reference method for the detection of Mycoplasma in biological products. This PTC method is similar to the compendial USP <63> method, with only minor differences (32).
FIG 1.
Summary of methods required for USP <63> Mycoplasma testing.
As with product sterility testing, method suitability testing is also a requirement for Mycoplasma testing to ensure the recovery of low-level Mycoplasma in the presence of the product and potential inhibitors. Here, <100 CFU of Acholeplasma laidlawii ATCC 23206, Mycoplasma gallisepticum ATCC 19610, Mycoplasma fermentans ATCC 19989, Mycoplasma hyorhinis ATCC 17981, M. orale ATCC 23714, M. pneumoniae ATCC 15531, or Mycoplasma synoviae ATCC 25204 is used to spike the product in independent experiments. Selection of test organisms is based on product origin and risk as defined in USP <63>. The test method is considered suitable for that particular product if spiked Mycoplasma is recovered within the specified incubation period. If growth inhibition is detected, the product has failed method suitability testing and the laboratory is responsible for modifying the method through the addition of diluents or neutralizing agents, until a method is identified that will detect <100 CFU of Mycoplasma in that product. As with sterility testing, method suitability testing must be performed on all new products and any time changes are made to the manufacturing process or formulation of a previously qualified product.
ALTERNATIVE TESTING METHODS: PERFORMANCE OF RAPID MICROBIAL METHODS
The compendial USP <71> and USP <63> methods described above for sterility testing and Mycoplasma testing, respectively, were designed for the pharmaceutical industry, where there is the luxury of time and large batch sizes. They are not, however, very well suited to the safety and release testing of single-batch, low-volume cellular therapy products, where the turnaround time for results is of utmost importance due to critical patient status and the short shelf life of the product. As such, the industry has focused on the development and evaluation of alternative test methods, namely, rapid microbial test methods (RMMs), in an effort to shorten turnaround time and potentially increase test sensitivity. The use of alternative test methods is supported by global governing bodies such as the FDA and the European Medicines Agency (EMA) as long as the alternative test system has undergone sufficient performance qualification to demonstrate that the alternative test is equivalent to or better than the compendial reference method, with appropriate adjustments made to acceptance levels and specifications to account for increased sensitivity of the alternative test (19, 33–35).
Performance of alternative test methods for sterility testing.
The use of blood culture systems as an alternative method for product sterility testing has been overwhelmingly favorable over the last 20 years, with most studies focusing on the performance of Bactec (Becton, Dickinson) (7, 11, 36, 37) and BacT/Alert (bioMérieux) (5, 10, 38–40) systems (Table 1). These platforms are advantageous compared with the manual compendial USP <71> method, as they provide automated continuous monitoring and objective detection of microbial growth, which is often confounded by the already turbid nature of the cell therapy products, leading to difficulties in culture interpretation via the USP <71> method. In the 2002 ISCT survey mentioned above, blood culture systems were used for sterility testing in 86% of cases for minimally manipulated products and 56% of cases for cultured and expanded products (4). A similar survey conducted in France in 2007 under the direction of the National Agency for Medicines and Health Product Safety (ANSM) showed that >91% of laboratories had replaced manual compendial methods with blood culture systems for product sterility testing (41). Currently, the European Pharmacopeia (chapter 2.6.27) formally recognizes the use of aerobic and anaerobic enriched media at a minimum incubation at 35 to 37°C for 7 days as an alternative testing method (35, 42). In the United States, blood culture systems are not formally approved by the FDA for testing of ATMPs, but they are considered an acceptable alternative if the system-product combination is qualified and demonstrates performance equivalent to or better than that of the compendial method (33, 34). A comparison study of RMMs published by the FDA in 2011 demonstrated that detection of growth by ATP bioluminescence technology (Rapid Milliflex detection system) or CO2 monitoring systems (Bactec and BacT/Alert) were suitable alternatives to turbidity detection as described in the Code of Federal Regulations Title 21, section 610.12 (21CFR610.12) and USP <71> (43).
A major limitation of published studies thus far is the small spectrum and number of organisms evaluated (36–40). In the United States, equivalency qualification studies require comparison testing of the six organisms listed in USP <71> at a minimum. These organisms, however, are not truly representative of those in the manufacturing environment, which include predominantly skin flora and environmental organisms (10). Also, as expected, the flora of a clinical microbiology laboratory is even more diverse than that of a controlled cGMP setting (6, 8, 11); thus, it is viewed favorably by regulators that product qualification studies include test organisms that are reflective of environmental risk and exposure. Unfortunately, there are very few method comparison studies that examine a broad spectrum of organisms beyond the six defined in USP <71>. More recently, England et al. challenged findings that had been previously limited by small organism study sets through an extensive evaluation of the Bactec, BacT/Alert, and USP <71> methods (a total of seven different culture combinations evaluated) using 118 organisms recovered from previously contaminated culture-positive products and the cGMP facility environment (5). In that study, the authors demonstrated excellent performance of the BacT/Alert system and poor performance of Bactec FX when these systems were challenged beyond routine clinical bloodstream isolates and the six USP <71> reference organisms (5). This finding contrasts previous works from various laboratories, including their own (7, 11, 36, 37), thus demonstrating the power of more expansive organism study sets for the rigorous evaluation of test system performance. A critical component of this study, especially in the aftermath of the 2012 New England Compounding Center fungal meningitis outbreak, was the expansive evaluation of 41 fungal isolates, which showed significantly poor performance of the Bactec system and suboptimal performance of the compendial USP <71> method and BacT/Alert platform (5). Addition of a Sabouraud dextrose agar plate increased the sensitivity of mold detection to 100% in that study (5). Blood culture systems for product sterility testing have shown faster detection than the compendial USP <71> method (5, 37), although shortening the overall incubation time from recommended USP <71> and European Pharmacopoeia standards (14 days and ≥7 days, respectively) should be considered carefully for slower-growing but frequent contaminants, such as Cutibacterium acnes. Time to detection is also dependent on the product matrix (39), highlighting the continued importance and requirement for method suitability testing (as described above for compendial testing) for alternative test methods. Visual inspection of the blood culture bottles at the end of incubation may also aid in detecting gross mold contamination that failed to trigger automatic detection by the instrument (5).
Given the many reasons for using blood culture systems as alternative testing methods for product sterility testing, it is easy to see how clinical microbiology laboratories may become involved in the testing process (5–11). Golay et al. described an experience in which product sterility testing was shifted away from the clinical microbiology laboratory to a cGMP setting to accommodate a change in French regulatory requirements in 2014 (10). This resulted in more than a doubling in costs for routine testing (71 versus 200 euros) and validation testing (1,000 versus 2,100 euros), as well as a delay in product inoculation and culture turnaround time (10). A significant decrease in the percentage of contaminated apheresis products was noted between 2009-2010 and 2015-2016; however, this was attributed to improved collection practices rather than changes in laboratory settings (10).
With regard to other alternative testing methods, Surrette et al. described a proof-of-concept rapid microfluidic assay for the detection of microbial contaminants for direct in-line testing during the pharmaceutical production process (44). This is an exciting new area of study that may significantly reduce the dependence on final product sterility testing for the biopharmaceutical industry.
Performance of alternative test methods for Mycoplasma testing.
The main disadvantage of the compendial USP <63> reference culture method for Mycoplasma testing is the slow turnaround (28-day culture). Molecular testing for Mycoplasma has therefore become a favorable alternative in the industry, with several laboratory developed (45–49) and commercial assays available on the market, including MycoTOOL (Roche) (50, 51), MycoSEQ (Applied Biosystems), and VenorGeM PCR (Minerva Biolabs). The availability of commercial assays has removed laborious assay validation requirements, as documented qualification points already covered by the kit manufacturer can replace qualification by the user (52). Minimal in-house assay validation and method suitability testing for each product matrix, however, are still required.
Despite its advantages, a major limitation of molecular Mycoplasma testing is the high inter- and intralaboratory variability demonstrated across a wide range of assays studied by the FDA, the World Health Organization (WHO), and participating laboratories worldwide (30, 53). This led to the development of a WHO international standard to harmonize the detection of Mycoplasma, which consists of M. fermentans at 2.0 × 105 IU/ml for nucleic acid test assay development and validation (53). A repeat analysis to measure the effect of such a standard has not been performed since the original publication in 2015; as such, test results received across laboratories continue to show discrepancies as demonstrated in the 2019 Quality Control for Molecular Diagnostics (QCMD) proficiency testing survey for Mycoplasma (54). In this survey, which included 12 unknown samples, detection of Mycoplasma ranged from 57.1% to 100% depending on the testing laboratory, test system, organism, and organism concentration tested (54). Wide ranges in performance were also observed in the 2016, 2017, and 2018 QCMD Mycoplasma proficiency testing surveys.
The availability of faster tests for Mycoplasma clearly improves cell therapy safety through earlier detection of contamination events. However, as discussed previously, an acceptable alternative RMM must be equivalent to or better than the compendial method (33, 34). Given the potential for increased test sensitivity and detection of nonviable nucleic acid with use of molecular assays, PCR-positive-culture-negative cases have been reported (23), and a risk-based approach encompassing patient safety, risk, and benefit would need to be applied in these circumstances.
OTHER IMPORTANT CONSIDERATIONS FOR TESTING LABORATORIES
In addition to performing sterility and/or Mycoplasma testing for cellular therapy product release, there are many other components that must be considered by any laboratory providing cGMP testing services. These include cGMP regulatory expectations, facility and engineering controls, qualification and validation requirements, environmental monitoring programs (viable and nonviable), the development and maintenance of a robust quality management system (QMS), and auditing (internal and external).
Regulatory framework for good manufacturing practices.
In the United States, the regulatory authority over any laboratory performing cGMP testing services is the FDA, which is authorized to enforce Title 21 of the Code of Federal Regulations (CFR). For pharmaceutical products and biologics (cell and gene therapies); parts 210, 211, and 600 to 680 are particularly relevant. However, compliance with other parts of Title 21 (e.g., part 11 for electronic records and signatures) is required and commonly cited during regulatory inspections. The GMP regulations as we understand them today originated in 1978 with the finalization of 21CFR210 and 21CFR211 and have since undergone continued revision and refinement. These regulations set forth the minimum requirements that manufacturers (and, by extension, testing facilities) must adhere to in order to ensure the safety, integrity, strength, purity, and quality (SISPQ) of pharmaceuticals and biologics. Adherence by manufacturers and testing labs to these regulations ultimately protects consumers from purchasing and/or using products that may be ineffective or dangerous.
The GMP regulations as described in Title 21 establish the framework of compliance for pharmaceutical and biologics manufacturers but do not provide a step-by-step guide for how to implement the requirements. To maintain compliance with the GMP regulations, it is critical to remain current with the FDA’s thinking, which is why the GMP regulations are frequently referred to as current good manufacturing practices (cGMP). The best way to remain “current” is to follow industry trends, read the FDA guidance for industry documents, and review FDA inspection findings. Utilization of industry resources (e.g., Parenteral Drug Association [PDA] technical reports or International Society for Pharmaceutical Engineering [ISPE] baseline guides) or international standards (e.g., United States Pharmacopeia [USP] chapters, International Council for Harmonization [ICH] guidelines, or International Standards Organization [ISO] standards) can help organizations implement compliant systems and remain current with industry trends. It is important to remember that established GMP systems that were compliant in the past may no longer be adequate because of changes in the FDA’s thinking or because of revisions to guidelines or standards.
Facility considerations.
While the science behind sterility and Mycoplasma testing methods has been well established, testing must be performed in a compliant facility. The CFR requires that “adequate laboratory facilities for testing and approval (or rejection) of components, drug product containers, closures, packaging materials, in-process materials, and drug products shall be available to the quality control unit” (55). Regulations further require that operations occur in specifically designated areas that are adequately sized for their purpose and prevent contamination and potential mix-up during operation (56). This regulation alone makes it difficult for clinical microbiology laboratories with mixed and open operations to remain cGMP compliant. The FDA expectation is that the testing facility “…should employ facilities and controls comparable to those used for aseptic filling operations” (57). This means that a clinical laboratory performing sterility or Mycoplasma testing must do so in a facility using testing practices and quality systems comparable to those of the facility in which the product was manufactured.
In general, a cGMP-compliant testing facility will need dedicated rooms for various operations (testing versus support functions), positive-pressure HEPA-filtered air, sufficient unidirectional airflow (to move particles away from critical areas), cleanable surfaces, appropriate gowning practices, environmental monitoring programs to ensure a state of control, a rigorous cleaning program (including ceiling, walls, and floor), and adequate procedures to describe operations (57). These criteria make it difficult for clinical microbiology laboratories to easily implement product sterility testing in their routine environment. Because the regulations are general, more specific information can be found in the FDA guidance documents, industry standards, and technical documents. Clinical laboratories currently performing or considering bringing sterility and/or Mycoplasma testing in-house should determine whether a separate aseptic facility (different than the manufacturing space) with adequate space is available, along with supporting infrastructure and resources.
If space and resources are available, user requirements, including lab personnel and organizational structure, types of testing to be performed, anticipated current and future test volume, ideal workflow (e.g., personnel, sample, waste, materials, and gowning), cleaning requirements, and facility technical requirements (derived from industry guidelines and standards) need to be considered. Such items are documented in a user requirement specification (URS), which forms the basis for the facility design process, building, qualification, and continued operation.
In general, a laboratory providing cGMP testing services will need at least three separate rooms to support the staging, gowning, and testing workflows, although a design with four rooms is ideal to provide optimized unidirectional personnel, waste, and gowning workflows. The ISO classifications for clean rooms are defined in the ISO 14644-1 standard (Table 2) (58), but other standards within the ISO 14644 series are valuable references for the design, building, and operation of clean rooms. The function and classification of support rooms will vary based on particular workflow but are recommended to be at least ISO class 7 if they are adjacent to critical operations or ISO class 8 if the area is to be used for less critical operations (57). Any critical operation, such as sterility testing or open product manipulations, must be performed in an ISO class 5 area (usually a biosafety cabinet or isolator) to prevent contamination of the product during operations (57).
TABLE 2.
ISO classifications for clean rooms
| ISO classification | Description | Recommended gowning (gowning builds from ISO 8 to ISO 5) | Acceptable particle count (≥0.5-μm particles/m3) (ISO 14644-1) | Recommended microbial sampling frequency | Recommended active air action levels (GFIa aseptic processing, USP<1116>) | Recommended settling plate action levels (GFI aseptic processing, USP<1116>) |
|---|---|---|---|---|---|---|
| 5 | Critical area; used for aseptic manipulations during manufacturing/testing operations (e.g., sterility testing inoculation, filling, or stoppering) | Sterile sleeves; sterile gloves | 3,520 | During each production/testing shift | 1 CFU/m3 or <1% incident rate | 1 CFU/4 h or <1% incident rate |
| 7 | Immediate buffer to ISO class 5 environment, direct support area to aseptic processing (e.g., staging area for materials entering ISO 5 area) | Sterile overalls or frock (process dependent); sterile shoe/ankle covers; sterile head cover; face mask | 352,000 | Risk based; if immediately adjacent to ISO class 5 area, typically each operating shift; if not directly adjacent to ISO class 5 area, typically 1 or 2 times/wk depending on criticality of processes | 10 CFU/m3 or <5% incident rate | 5 CFU/4 h or <5% incident rate |
| 8 | Support area where less critical functions occur, such as equipment cleaning and initial gowning | Dedicated scrubs and shop shoes; gloves; bouffant (hair cover); beard cover (as required) | 3,520,000 | Risk based; typically 1 or 2 times/wk depending on criticality of processes | 100 CFU/m3 or <10% incident rate | 50 CFU/4 h or <10% incident rate |
GFI, guidance for industry.
A successful cGMP testing facility must be qualified for intended usage and continually monitored to ensure that a state of control is maintained. Prior to use, a cGMP facility must undergo qualification to ensure that the components and utilities are built and installed correctly (known as installation qualification [IQ]), operate as intended (known as operation qualification [OQ]), and perform to the intended function (known as performance qualification [PQ]) (59). Collectively, this process is referred to as installation, operation, and performance qualification (IOPQ) and is applied to any cGMP facility and cGMP equipment. IQ and OQ are typically performed only once, but they may need to be repeated if significant changes are made to the facility design. A well-designed PQ will demonstrate that the required cleanliness levels and airflow patterns at rest (i.e., static conditions, with no operations occurring) and under dynamic conditions (i.e., with routine operations occurring) can be maintained by the facility. Use of airflow visualization studies (commonly known as smoke studies), nonviable particulate monitoring, and viable particulate (air and surface microbial sampling, commonly referred to as environmental monitoring) are all key components of the PQ. Design and execution of these qualifications can be labor-intensive, time-consuming, and resource demanding. However, a properly designed and executed qualification is required by the regulatory agencies to demonstrate that the facility is adequate for its intended purpose. The data collected during the IOPQ process will form the backbone for establishing a prospective, continuous monitoring program and assist with trend analysis.
The cGMP facilities are highly specialized and require a robust system of controls and checks to ensure that “adequate control over air pressure, microorganisms, dust, humidity, and temperature” is maintained during operation (60). Many of these parameters can be monitored and maintained through validated building automation systems and/or environmental monitoring systems. Viable and nonviable environmental monitoring (EM) is a continuous process and provides surveillance data that is used to assess the state of control over the facility or process and can be leveraged to make critical operational decisions. Design of the EM program must take into consideration the intended use of the facility, room classifications, room sizes, airflow patterns, sampling locations and the rationale for them (based on risk assessment and understanding of the process), sampling frequencies, alert and action levels (based on regulatory requirements and statistical evaluation of previously collected data), and periodic review of the EM data to assess trends (61). cGMP facility qualification and EM program requirements (including rationale) must be documented and maintained and must be available for inspection during regulatory and client audits (59, 62–64).
Quality management system considerations.
To support GMP operations, a robust quality management system (QMS) and a team of trained quality professionals managing these systems are required. As part of the FDA’s Pharmaceutical cGMPs for the 21st Century Initiative, the FDA has sought to integrate quality systems (QS) and quality risk management (QRM) practices into the existing GMP programs (65). Organizations performing cGMP manufacturing and/or testing are required to apply holistic approaches that integrate quality into their processes instead of relying on quality control testing to ensure product quality. As such, the quality assurance (QA) group is a critical partner of cGMP operations and QC.
QA primarily involves (i) review and approval of all procedures related to production and maintenance, (ii) review of associated records, and (iii) auditing and performing/evaluating trend analysis (65). The QA group must be supported by the organization’s management and have the autonomy and authority to perform regulatory functions (55). A robust QMS must be created and managed by the QA group in order to support the GMP functions of the organization. The QMS must contain procedures and processes to investigate, remediate, and monitor deviations, discrepancies (sometimes referred to as “out of specifications”), complaints, corrective and preventive actions (CAPA), and change control (62, 65–68). Unfortunately, the regulations and guidance documents only state the requirement to have these systems and are not a road map to implementation. International standards such as ISO 9000 and ISO 9001 (69, 70) and the FDA medical device quality system regulations (21CFR820) can provide additional information and guidance on the requirements for QMS implementation. In addition to these required systems, the QA group is responsible for document management (e.g., creation and review of standard operating procedures [SOP], documentation practices and review, and document retention), equipment control, training, validation/qualification, product/material release, management review, QRM, and audits. When properly executed, all of these systems align with the FDA’s expectation that quality be built into the process and provide a holistic approach to managing cGMP operations.
The FDA uses on-site inspections as their primary tool to enforce the GMP requirements set forth in CFR Title 21, sections 210, 211, and 600 to 680. If the decision is made by the clinical laboratory to take on GMP testing activities, it must come with an understanding of all of the considerations outlined previously and with the expectation that the laboratory may be inspected by the FDA. Unlike the more familiar CLIA and CAP requirements, there is no regulatory requirement that mandates that testing laboratories self-identify to the FDA or establish an independent FDA establishment identification (FEI) number that is separate from that of the manufacturing facility. FEI number paperwork filed by the product manufacturer lists the testing laboratory. Thus, by default, the laboratory can be inspected by the FDA if it performs testing for products that fall under cGMP requirements. This has occurred twice in our laboratory in recent years in response to FDA inspections of the NIH Clinical Center Pharmacy (12, 13). Inspections in the United States tend to be unannounced and typically occur approximately every 2 years. Because the FDA uses a risk-based model to develop their audit schedule, some lower-risk facilities may be inspected less frequently while higher-risk facilities may be inspected more frequently. The data gathered from review of the warning letters available on the FDA’s website (71) can provide insight into the FDA’s current thinking and potential inspection foci. In fiscal year 2019, there were notable trends in warning letter citations particularly with regard to supply chain control, microbial contamination of nonsterile drug products, aseptic processing line design, data integrity (primarily focused on electronic system vulnerabilities), and out-of-specification investigations (e.g., inadequate investigations or failure to identity the root cause) (72). Performing internal audits can provide the opportunity to assess quality systems and processes to determine if they are functioning as intended and can also highlight areas that are deficient or in need of improvement. These internal audits form the foundation of a laboratory’s compliance and continuous improvement programs and should be treated as constructive opportunities to assess internal systems.
CONCLUSION
In conclusion, sterility and Mycoplasma testing for biopharmaceutical products is complex. A clinical microbiology laboratory performing such testing must consider the different cGMP regulatory responsibilities for the facility as well as testing components described here, which are unlike the more familiar requirements of CLIA, CAP, and JCAHO. Any laboratory providing cGMP testing services for ATMPs, or environmental monitoring cultures for regulated areas such as compounding pharmacies, are open and subject to inspection by the FDA and any other relevant jurisdictions. Examples of common acceptable practices in the clinical microbiology laboratory, including streamlined QC (e.g., for commercially prepared media) and individualized quality control plans (IQCP), do not align with cGMP testing requirements. In the end, optimized analytical testing alone without integration of all cGMP testing aspects is suboptimal for patient safety in the cGMP setting, as we know that test systems have limitations and a subset of contaminating organisms may not be detected even after 14 days of incubation (24). Out-of-specification results will occur, and it is important that the organization has established strategic risk-based approaches for products that have failed microbiologic release criteria (8, 10, 11, 36, 73). Sterility and Mycoplasma testing in-house will require dedicated expertise, space, and resources but will provide the advantages of lower costs, faster turnaround, and simpler product handling logistics. Outsourcing to a qualified reference lab is extremely expensive, is often slower, and is complicated by product transport logistics and chain of custody. Careful consideration that encompasses the program size is critical in determining the feasibility of establishing and maintaining a cGMP microbiology laboratory in the clinical setting.
ACKNOWLEDGMENTS
We thank Karen Frank and Niki Putnam for their critical review of the manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health. The content is solely our responsibility and does not represent the official views of the National Institutes of Health.
REFERENCES
- 1.Ledford H. 2017. Engineered cell therapy for cancer gets thumbs up from FDA advisers. Nature 547:270–270. doi: 10.1038/nature.2017.22304. [DOI] [PubMed] [Google Scholar]
- 2.United States Food and Drug Administration. 2017. FDA approval brings first gene therapy to the United States.
- 3.United States Food and Drug Administration. 2017. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma.
- 4.Davis-Sproul J. 2002. ISCT sterility testing survey. ISCT Telegraph Newsl 9:1–3. [Google Scholar]
- 5.England MR, Stock F, Gebo JET, Frank KM, Lau AF. 2019. Comprehensive evaluation of compendial USP<71>, BacT/Alert Dual-T, and Bactec FX for detection of product sterility testing contaminants. J Clin Microbiol 57:e01548. doi: 10.1128/JCM.01548-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khuu HM, Patel N, Carter CS, Murray PR, Read EJ. 2006. Sterility testing of cell therapy products: parallel comparison of automated methods with a CFR-compliant method. Transfusion 46:2071–2082. doi: 10.1111/j.1537-2995.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 7.Khuu HM, Stock F, McGann M, Carter CS, Atkins JW, Murray PR, Read EJ. 2004. Comparison of automated culture systems with a CFR/USP-compliant method for sterility testing of cell-therapy products. Cytotherapy 6:183–195. doi: 10.1080/14653240410005997. [DOI] [PubMed] [Google Scholar]
- 8.Panch SR, Bikkani T, Vargas V, Procter J, Atkins JW, Guptill V, Frank KM, Lau AF, Stroncek DF. 2019. Prospective evaluation of a practical guideline for managing positive sterility test results in cell therapy products. Biol Blood Marrow Transplant 25:172–178. doi: 10.1016/j.bbmt.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomasen H, Mosel F, Steuhl KP, Meller D. 2015. Evaluation of a new protocol for sterility controls of corneal culture medium. Cell Tissue Bank 16:343–350. doi: 10.1007/s10561-014-9477-2. [DOI] [PubMed] [Google Scholar]
- 10.Golay J, Pedrini O, Capelli C, Gotti E, Borleri G, Magri M, Vailati F, Passera M, Farina C, Rambaldi A, Introna M. 2018. Utility of routine evaluation of sterility of cellular therapy products with or without extensive manipulation: best practices and clinical significance. Cytotherapy 20:262–270. doi: 10.1016/j.jcyt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Padley DJ, Dietz AB, Gastineau DA. 2007. Sterility testing of hematopoietic progenitor cell products: a single-institution series of culture-positive rates and successful infusion of culture-positive products. Transfusion 47:636–643. doi: 10.1111/j.1537-2995.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi TK. 2016. Safety lessons from the NIH Clinical Center. N Engl J Med 375:1705–1707. doi: 10.1056/NEJMp1609208. [DOI] [PubMed] [Google Scholar]
- 13.Reardon S. 2015. Contamination shuts down NIH pharmacy center. Nature News. [Google Scholar]
- 14.United States Food and Drug Administration. 1938. Federal Food, Drug, and Cosmetic Act (FD&C Act), United States Code, Title 21.
- 15.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 610.12: sterility (21CFR610.12).
- 16.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 610.30: test for Mycoplasma (21CFR610.30).
- 17.United States Food and Drug Administration. 2018. Chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs) - draft guidance for industry.
- 18.United States Pharmacopeia Convention. 2016. Sterility tests (USP<71>) In United States Pharmacopeia and National Formulary (USP 32/NF 27). United States Pharmacopeia Convention, Bethesda, MD. [Google Scholar]
- 19.United States Pharmacopeia Convention. 2009. Mycoplasma tests (USP<63>) In United States Pharmacopeia and National Formulary (USP 33/NF 28). United States Pharmacopeia Convention, Bethesda, MD. [Google Scholar]
- 20.EDQM Council of Europe. 2012. Sterility In European Pharmacopoeia. EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 21.Ministry of Health, Labour, and Welfare. 2016. Sterility test In The Japanese Pharmacopoeia, 17th ed The Japanese Pharmacopoeia, Tokyo, Japan. [Google Scholar]
- 22.Drexler HG, Uphoff CC. 2002. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39:75–90. doi: 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo F, Cortes JL, Cabrera C, Nieto A, Concha A. 2007. Microbiological contamination in stem cell cultures. Cell Biol Int 31:991–995. doi: 10.1016/j.cellbi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Mirjalili A, Parmoor E, Moradi Bidhendi S, Sarkari B. 2005. Microbial contamination of cell cultures: a 2 years study. Biologicals 33:81–85. doi: 10.1016/j.biologicals.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Windsor HM, Windsor GD, Noordergraaf JH. 2010. The growth and long term survival of Acholeplasma laidlawii in media products used in biopharmaceutical manufacturing. Biologicals 38:204–210. doi: 10.1016/j.biologicals.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Barile MF. 1981. Mycoplasma infections of cell cultures. Isr J Med Sci 17:555–562. [PubMed] [Google Scholar]
- 27.Nikfarjam L, Farzaneh P. 2012. Prevention and detection of Mycoplasma contamination in cell culture. Cell J 13:203–212. [PMC free article] [PubMed] [Google Scholar]
- 28.Uphoff CC, Meyer C, Drexler HG. 2002. Elimination of mycoplasma from leukemia-lymphoma cell lines using antibiotics. Leukemia 16:284–288. doi: 10.1038/sj.leu.2402364. [DOI] [PubMed] [Google Scholar]
- 29.Dixit A, Alexandrescu S, Boyer D, Graf EH, Vargas SO, Silverman M. 2017. Mycoplasma hominis empyema in an 18-year-old stem cell and lung transplant recipient: case report and review of the literature. J Pediatric Infect Dis Soc 6:e173–e176. doi: 10.1093/jpids/pix049. [DOI] [PubMed] [Google Scholar]
- 30.Dabrazhynetskaya A, Volokhov DV, Lin TL, Beck B, Gupta RK, Chizhikov V. 2013. Collaborative study report: evaluation of the ATCC experimental mycoplasma reference strains panel prepared for comparison of NAT-based and conventional mycoplasma detection methods. Biologicals 41:377–383. doi: 10.1016/j.biologicals.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 31.United States Food and Drug Administration. 1993. Points to consider in the characterization of cell lines used to produce biologicals.
- 32.Nims RW, Meyers E. 2010. USP <63> mycoplasma tests: a new regulation for mycoplasma testing. BioPharm International 23. [Google Scholar]
- 33.Miller MJ. 2013. PDA technical report no. 33: evaluation, validation and implementation of alternative and rapid microbiological methods. Parenteral Drug Association, Bethesda, MD. [Google Scholar]
- 34.United States Pharmacopeia Convention. 2015. Validation of alternative microbiological methods (USP<1223>) In United States Pharmacopeia and National Formulary (USP 32/NF 27). United States Pharmacopeia Convention, Bethesda, MD. [Google Scholar]
- 35.EDQM Council of Europe. 2012. Alternative methods for control of microbiological quality In European Pharmacopoeia. EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 36.Hocquet D, Sauget M, Roussel S, Malugani C, Pouthier F, Morel P, Gbaguidi-Haore H, Bertrand X, Grenouillet F. 2014. Validation of an automated blood culture system for sterility testing of cell therapy products. Cytotherapy 16:692–698. doi: 10.1016/j.jcyt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Lysak D, Holubova M, Bergerova T, Vavrova M, Cangemi GC, Ciccocioppo R, Kruzliak P, Jindra P. 2016. Validation of shortened 2-day sterility testing of mesenchymal stem cell-based therapeutic preparation on an automated culture system. Cell Tissue Bank 17:1–9. doi: 10.1007/s10561-015-9522-9. [DOI] [PubMed] [Google Scholar]
- 38.Arlt N, Rothe R, Sielaff S, Juretzek T, Peltroche H, Moog R. 2018. Sterility release testing of peripheral blood stem cells for transplantation: impact of culture bottles and incubation temperature. Transfusion 58:2918–2923. doi: 10.1111/trf.14910. [DOI] [PubMed] [Google Scholar]
- 39.Klarmann D, Sireis W, Hogardt M, Kempf VA, Seifried E, Bonig H. 2015. A validation protocol and evaluation algorithms to determine compatibility of cell therapy product matrices in microbiological testing. Cell Tissue Bank 16:311–318. doi: 10.1007/s10561-014-9474-5. [DOI] [PubMed] [Google Scholar]
- 40.Mastronardi C, Yang L, Halpenny M, Toye B, Ramirez-Arcos S. 2012. Evaluation of the sterility testing process of hematopoietic stem cells at Canadian Blood Services. Transfusion 52:1778–1784. doi: 10.1111/j.1537-2995.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 41.Agence nationale de securite du et des produits de sante. 2007. Etudes collaboratives pour le controle bacteriologique des produits cellulaires.
- 42.EDQM Council of Europe. 2012. Microbiological examination of cell-based preparations In European Pharmacopoeia. EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 43.Parveen S, Kaur S, David SA, Kenney JL, McCormick WM, Gupta RK. 2011. Evaluation of growth based rapid microbiological methods for sterility testing of vaccines and other biological products. Vaccine 29:8012–8023. doi: 10.1016/j.vaccine.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 44.Surrette C, Scherer B, Corwin A, Grossmann G, Kaushik AM, Hsieh K, Zhang P, Liao JC, Wong PK, Wang TH, Puleo CM. 2018. Rapid microbiology screening in pharmaceutical workflows. SLAS Technol 23:387–394. doi: 10.1177/2472630318779758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhi Y, Mayhew A, Seng N, Takle GB. 2010. Validation of a PCR method for the detection of mycoplasmas according to European Pharmacopoeia section 2.6.7. Biologicals 38:232–237. doi: 10.1016/j.biologicals.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Tabatabaei-Qomi R, Sheykh-Hasan M, Fazaely H, Kalhor N, Ghiasi M. 2014. Development of a PCR assay to detect mycoplasma contamination in cord blood hematopoietic stem cells. Iran J Microbiol 6:281–284. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Kong F, Jelfs P, James G, Gilbert GL. 2004. Simultaneous detection and identification of common cell culture contaminant and pathogenic mollicutes strains by reverse line blot hybridization. Appl Environ Microbiol 70:1483–1486. doi: 10.1128/aem.70.3.1483-1486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong F, James G, Gordon S, Zelynski A, Gilbert GL. 2001. Species-specific PCR for identification of common contaminant mollicutes in cell culture. Appl Environ Microbiol 67:3195–3200. doi: 10.1128/AEM.67.7.3195-3200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stormer M, Vollmer T, Henrich B, Kleesiek K, Dreier J. 2009. Broad-range real-time PCR assay for the rapid identification of cell-line contaminants and clinically important mollicute species. Int J Med Microbiol 299:291–300. doi: 10.1016/j.ijmm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Chisholm J, Bhatt S, Chaboureau A, Viswanathan S. 2017. Strategy for an abbreviated in-house qualification of a commercially available Rapid Microbiology Method (RMM) for canadian regulatory approval. Cytotherapy 19:1529–1536. doi: 10.1016/j.jcyt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Deutschmann SM, Kavermann H, Knack Y. 2010. Validation of a NAT-based Mycoplasma assay according European Pharmacopoiea. Biologicals 38:238–248. doi: 10.1016/j.biologicals.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 52.EDQM Council of Europe. 2012. Mycoplasmas In European Pharmacopoeia. EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 53.Nubling CM, Baylis SA, Hanschmann KM, Montag-Lessing T, Chudy M, Kress J, Ulrych U, Czurda S, Rosengarten R, Mycoplasma Collaborative Study Group. 2015. World Health Organization International Standard to harmonize assays for detection of Mycoplasma DNA. Appl Environ Microbiol 81:5694–5702. doi: 10.1128/AEM.01150-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quality Control for Molecular Diagnostics. 2019. QCMD 2019 Mycoplasma spp. (cell contamination) EQA programme.
- 55.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.22: responsibilities of quality control unit (21CFR211.22).
- 56.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.42: design and construction features (21CFR211.42).
- 57.United States Food and Drug Administration. 2004. Guidance for industry: sterile drug products produced by aseptic processing—current Good Manufacturing Practice.
- 58.International Standards Organization. 2015. ISO 14644–1:2015: cleanrooms and associated controlled environments—part 1: classification of air cleanliness by particle concentration. International Standards Organization, Geneva, Switzerland. [Google Scholar]
- 59.United States Food and Drug Administration. 2011. Guidance for industry: process validation: general principles and practices.
- 60.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.46: ventilation, air filtration, air heating and cooling (21CFR211.46).
- 61.Moldenhauer J. 2014. PDA technical report no. 13 (revised): fundamentals of an environmental monitoring program. Parenteral Drug Association, Inc, Bethesda, MD. [PubMed] [Google Scholar]
- 62.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.100: written procedures; deviations (21CFR211.100).
- 63.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.110: sampling and testing of in-process materials and drug products (21CFR211.110).
- 64.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.180: general requirements (21CFR211.180).
- 65.United States Food and Drug Administration. 2006. Guidance for industry: quality systems approach to pharmaceutical CGMP regulations.
- 66.European Medicines Agency. 2008. ICH guideline Q10 on pharmaceutical quality system.
- 67.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.198: complaint files (21CFR211.198).
- 68.United States Food and Drug Administration. 2019. Code of Federal Regulations Title 21, section 211.192: production record review (21CFR211.192).
- 69.International Standards Organization. 2015. ISO 9000: quality management. International Standards Organization, Geneva, Switzerland. [Google Scholar]
- 70.International Standards Organization. 2015. ISO 9001:2015: quality management systems—requirements. International Standards Organization, Geneva, Switzerland. [Google Scholar]
- 71.United States Food and Drug Administration. 2019. Warning letters and notice of violation letters to pharmaceutical companies. https://www.fda.gov/drugs/enforcement-activities-fda/warning-letters-and-notice-violation-letters-pharmaceutical-companies.
- 72.Friedman R. 2019. CDER OMQ update, abstr PDA/FDA Joint Regulatory Conference, Sep 16–18, Washington, DC, Parenteral Drug Association, Inc, Bethesda, MD. [Google Scholar]
- 73.Klein MA, Kadidlo D, McCullough J, McKenna DH, Burns LJ. 2006. Microbial contamination of hematopoietic stem cell products: incidence and clinical sequelae. Biol Blood Marrow Transplant 12:1142–1149. doi: 10.1016/j.bbmt.2006.06.011. [DOI] [PubMed] [Google Scholar]