Members of the Mycobacterium abscessus complex (MABC) are multidrug-resistant nontuberculous mycobacteria and cause opportunistic pulmonary infections in individuals with cystic fibrosis (CF). In this study, genomic analysis of MABC isolates was performed to gain greater insights into the epidemiology of circulating strains in Ireland. Whole-genome sequencing (WGS) was performed on 70 MABC isolates that had been referred to the Irish Mycobacteria Reference Laboratory between 2006 and 2017 across nine Irish health care centers.
KEYWORDS: Mycobacterium abscessus complex, clusters, cystic fibrosis, epidemiological analysis, transmission, whole-genome sequencing
ABSTRACT
Members of the Mycobacterium abscessus complex (MABC) are multidrug-resistant nontuberculous mycobacteria and cause opportunistic pulmonary infections in individuals with cystic fibrosis (CF). In this study, genomic analysis of MABC isolates was performed to gain greater insights into the epidemiology of circulating strains in Ireland. Whole-genome sequencing (WGS) was performed on 70 MABC isolates that had been referred to the Irish Mycobacteria Reference Laboratory between 2006 and 2017 across nine Irish health care centers. The MABC isolates studied comprised 52 isolates from 27 CF patients and 18 isolates from 10 non-CF patients. WGS identified 57 (81.4%) as M. abscessus subsp. abscessus, 10 (14.3%) as M. abscessus subsp. massiliense, and 3 (4.3%) as M. abscessus subsp. bolletii. Forty-nine (94%) isolates from 25 CF patients were identified as M. abscessus subsp. abscessus, whereas 3 (6%) isolates from 2 CF patients were identified as M. abscessus subsp. massiliense. Among the isolates from non-CF patients, 44% (8/18) were identified as M. abscessus subsp. abscessus, 39% (7/18) were identified as M. abscessus subsp. massiliense, and 17% (3/18) were identified as M. abscessus subsp. bolletii. WGS detected two clusters of closely related M. abscessus subsp. abscessus isolates that included isolates from different CF centers. There was a greater genomic diversity of MABC isolates among the isolates from non-CF patients than among the isolates from CF patients. Although WGS failed to show direct evidence of patient-to-patient transmission among CF patients, there was a predominance of two different strains of M. abscessus subsp. abscessus. Furthermore, some MABC isolates were closely related to global strains, suggesting their international spread. Future prospective real-time epidemiological and clinical data along with contemporary MABC sequence analysis may elucidate the sources and routes of transmission among patients infected with MABC.
INTRODUCTION
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms that commonly cause chronic pulmonary infections, particularly in patients with preexisting inflammatory lung diseases, such as cystic fibrosis (CF), but they can also cause infections outside of the respiratory tract in immunologically susceptible individuals (1). An increasing number of CF patients infected with NTM is being reported (2). The majority of NTM infecting CF individuals globally are members of the Mycobacterium avium complex and M. abscessus complex (MABC), with the latter appearing more commonly in Europe within the CF population (3). Three subspecies of MABC have been described: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii.
MABC is a group of rapidly growing mycobacteria with intrinsic multiple-antibiotic resistance, necessitating prolonged antimicrobial treatment with potential toxic side effects (4). MABC infections are associated with an accelerated decline in lung function and increased morbidity and mortality and remain a contraindication to lung transplantation in many centers (2, 5). Until recently, NTM infections were thought to be acquired by individuals through exposure to environmental sources, such as soil or water (6). However, recent studies have shown that MABC can be transmitted from patient to patient or can be acquired from a single environmental source and spread to patients within health care facilities (5, 7).
Ireland has the highest incidence rate of CF in Europe, and therefore, a significant number of people may be at risk of MABC infection (8). A previous study on the molecular epidemiology of MABC in Ireland was performed using rpoB fragment analysis typing and multilocus sequence typing (9). That study showed a predominance of M. abscessus subsp. abscessus isolates among the isolates investigated, with M. abscessus subsp. massiliense isolates forming a minority of isolates (9). The aim of this study was to perform whole-genome sequencing (WGS) on MABC isolates recovered from both CF and non-CF patients to characterize at a genomic level the circulating strains of MABC in Ireland.
MATERIALS AND METHODS
MABC isolates.
A total of 72 MABC isolates, recovered from Irish patients, were referred to the Irish Mycobacteria Reference Laboratory (IMRL) and included in this study. The MABC isolates had been recovered from both pulmonary (n = 68) and extrapulmonary (n = 2) specimens, while two isolates were from unknown sites. The MABC isolates comprised 54 isolates from 27 CF patients and 18 isolates from 10 non-CF patients (Table 1).
TABLE 1.
MABC isolates recovered from both CF and non-CF patients investigated in this studya
| Patient group | No. of MABC isolates |
No. of isolates from the following specimen types: |
||||
|---|---|---|---|---|---|---|
| Paired | Single | Total | Pulmonary | Extrapulmonary | Unknown | |
| CF patients (n = 27) | 54 | 0 | 54 | 52 | 0 | 2 |
| Non-CF patients (n = 10) | 16 | 2 | 18 | 16 | 2 | 0 |
| Total | 70 | 2 | 72 | 68 | 2 | 2 |
Data are for 72 MABC isolates.
The American Thoracic Society guidelines recommend screening CF patients at least yearly for NTM infection (10). Therefore, in the interest of this study, the first and most recent MABC isolates from each patient were selected. There were 70 paired MABC isolates from 35 of 37 patients and single isolates from two non-CF patients (70 + 2 = 72). Each pair of isolates had been collected a minimum of 6 months apart, while a single isolate from each of two patients was also included (see Table S1 in the supplemental material). The MABC isolates had been referred to the IMRL for identification from nine different health care centers (centers A to I) between 2006 and 2017. All MABC isolates had been previously identified using the GenoType Mycobacterium CM assay (Bruker-Hain Diagnostics, Germany).
Whole-genome sequencing.
The MABC isolates were cultured in BD Bactec MGIT 960 liquid medium (Becton, Dickinson, NJ, USA) and heat inactivated at 95°C for 30 min. Genomic DNA was extracted using a QuickGene-Mini80 device (Kurabo Industries Ltd., Osaka, Japan) according to the manufacturer’s instructions. DNA libraries were prepared using a Nextera XT DNA library preparation kit (Illumina, Cambridge, UK) according to the manufacturer’s instructions. Paired-end sequencing was performed on an Illumina MiniSeq platform using a High Output 300 cycle kit (Illumina, Cambridge, UK).
WGS was performed on 70 MABC isolates (paired isolates from 33 patients and single isolates from each of 4 patients). Two isolates were lost due to technical issues; therefore, only single isolates from two CF patients (patients 25 and 26) were investigated.
Whole-genome SNP analysis.
MABC FASTQ files and the sequences of 11 published MABC strains (shown in Table S2) were mapped to the sequence of M. abscessus type strain ATCC 19977 using the Burrows-Wheeler alignment tool (v0.7.17-r1188) (11). Variant calling was performed using the Freebayes program (v1.1), a minimum coverage of 10× was used, and a maximum likelihood tree was constructed using the PhyML program in Seaview software (v4.7). Additionally, MABC FASTQ files were analyzed using BioNumerics software (v7.6; Applied Maths, Sint-Martens-Latem, Belgium). The SPAdes assembly of the earliest recovered isolate (2006) was used as the reference genome to construct the minimum spanning tree (MST). Single nucleotide polymorphisms (SNPs) were called exclusively in positions shared by all samples. Only SNPs with at least 5× coverage (including 1× coverage in each direction) were considered. Potential indel-related SNPs occurring within 12 bp of each other were removed. Positions with ambiguous base calls were excluded. A distance matrix was generated, and an MST was constructed using permutation resampling (1,000 replicates).
Ethics.
All isolates used in this study had been previously referred for identification to the IMRL and stored as an archival collection. WGS analysis was performed on pseudonymized data, and the study was completed in March 2018. No personal patient data are reported in this study, and patient consent was considered to not be required.
Data availability.
Raw sequence reads of all 70 sequenced MABC genomes in this study were submitted to the European Nucleotide Archive database under project accession number PRJEB37730.
RESULTS
Relatedness of MABC subspecies to published strains.
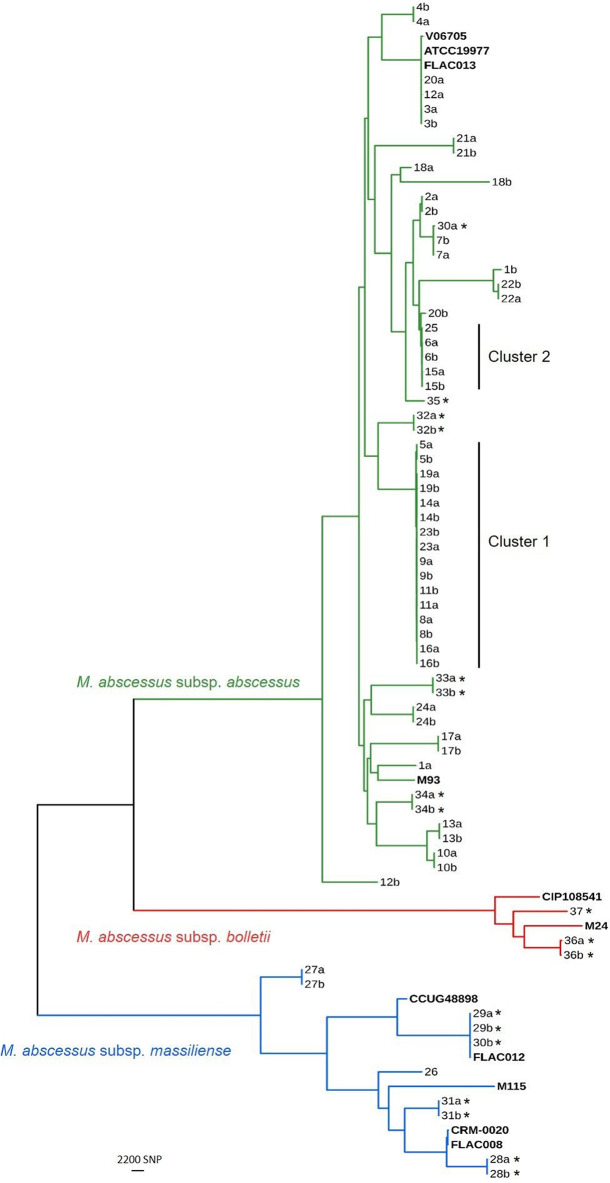
The paired and single MABC isolates recovered from CF and non-CF patients from different health care centers and investigated by WGS are listed in Table S1 in the supplemental material. WGS analysis distinguished the three subspecies of MABC, and the sequences of the isolates aligned with those of previously published MABC strains, as shown in Fig. 1. The analysis identified 57/70 (81.4%) isolates as M. abscessus subsp. abscessus, 10/70 (14.3%) as M. abscessus subsp. massiliense, and 3/70 (4.3%) as M. abscessus subsp. bolletii. Four M. abscessus subsp. abscessus isolates from three patients had genomic sequences that were related (with an average of 79 SNP differences) to those of published strains that originated in France (M. abscessus subsp. abscessus strain ATCC 19977 and M. abscessus subsp. abscessus strain V06705) and the United States (M. abscessus subsp. abscessus strain FLAC013) (11–15). In contrast, M. abscessus subsp. abscessus strain M93 was distinct from these M. abscessus subsp. abscessus isolates, with approximately 11,317 SNP variations detected (16). Among the M. abscessus subsp. massiliense strains, three isolates from two non-CF patients (isolates 29a, 29b, and 30b) had genomic sequences that differed from the sequence of M. abscessus subsp. massiliense strain FLAC012, originating in the United States, by 59 SNP variations (14). These were distinct from strains originating from France (M. abscessus subsp. massiliense strain CCUG48898), Malaysia (M. abscessus subsp. massiliense strain M115), Brazil (M. abscessus subsp. massiliense strain CRM-0020), and the United States (M. abscessus subsp. massiliense strain FLAC008) (14, 17–19). Our three M. abscessus subsp. bolletii isolates were distinct from the M. abscessus subsp. bolletii strains that originated from France (M. abscessus subsp. bolletii strain CIP108541) and Malaysia (M. abscessus subsp. bolletii strain M24) (20, 21).
FIG 1.
Maximum likelihood tree of Mycobacterium abscessus complex. A maximum likelihood tree was built from 222,210 core SNP sites of 70 M. abscessus complex (MABC) isolates from 37 patients and 11 published strains (see Tables S1 and S2 in the supplemental material). All sequenced genomes were mapped to the M. abscessus ATCC 19977 genome as a reference genome, and the mean coverage was 82×. MABC isolates classified as M. abscessus subsp. abscessus are represented in green. Likewise, M. abscessus subsp. massiliense isolates are represented in blue and M. abscessus subsp. bolletii isolates are represented in red on the tree. The first and last isolate from each patient is indicated by a and b, respectively. Single isolates were available only from patients 25, 26, 35, and 37. *, isolates recovered from non-CF patients in this study. Published MABC strains are highlighted in bold.
Distribution of MABC isolates among CF and non-CF patients. (i) CF patients.
Forty-nine isolates (94%) collected from 25/27 CF patients were identified as M. abscessus subsp. abscessus, whereas only 3 (6%) isolates from 2/27 CF patients were identified as M. abscessus subsp. massiliense (Fig. 1 and Table S1). No M. abscessus subsp. bolletii isolate was identified among the isolates collected from CF patients, and no mixed MABC infections were detected in this cohort.
(ii) Non-CF patients.
There was a greater diversity among the isolates (n = 18) collected from the 10 non-CF patients than among the isolates collected from the CF patients; eight M. abscessus subsp. abscessus isolates (44%) were recovered from 5 patients; seven M. abscessus subsp. massiliense isolates (39%) were recovered from 4 patients, and three M. abscessus subsp. bolletii (17%) were recovered from 2 patients. One non-CF patient (patient 30) was infected with both subspecies M. abscessus subsp. abscessus and M. abscessus subsp. massiliense, which were recovered within the same year from two different pulmonary specimens (Table S1).
Relatedness among MABC isolates from CF and non-CF patients.
Overall, 85% (28/33) of the patients’ paired isolates were highly related, indicating homogeneous populations (Table S1). Identical genomic sequences were found in 39% (13/33) of the paired isolates collected at different time points. Among the CF patients, 84% (21/25) of the isolates from the same patient differed by ≤11 SNPs, while among the non-CF patients, 87.5% (7/8) of the isolates from the same patient differed by less than 10 SNPs. Large SNP differences (ranging from 6,770 to 29,350) between paired isolates were found in five patients (patients 1, 12, 18, 20, and 30), suggesting infection with a different MABC strain in each case. These included four CF patients from center A and one non-CF patient (patient 30) from center I. M. abscessus subsp. massiliense was identified from a pulmonary specimen taken 6 months after M. abscessus subsp. abscessus had been recovered from a pulmonary specimen from the same patient.
While M. abscessus subsp. abscessus was the predominant (81.4%) subspecies of MABC identified, isolates collected from different patients were clearly segregated from one another when investigated by WGS (Fig. 2). Two distinct clusters of M. abscessus subsp. abscessus strains, named clusters 1 and 2, were identified. In total, 21 (37%) M. abscessus subsp. abscessus isolates were grouped in these two clusters, and all these isolates were from CF patients. Cluster 1 comprised eight pairs of M. abscessus subsp. abscessus isolates each from eight CF patients and had been collected between 2006 and 2017. The M. abscessus subsp. abscessus isolates within cluster 1 were from centers A (n = 7), E (n = 6), C (n = 2), and D (n = 1) (Fig. 2B). There was evidence from genomic analysis of both probable and possible recent transmission events (whether direct or indirect) between patients in centers A and E when a previously defined threshold for indicating probable (<20 SNPs) and possible (20 to 38 SNPs) transmission events between patients was applied (3).
FIG 2.
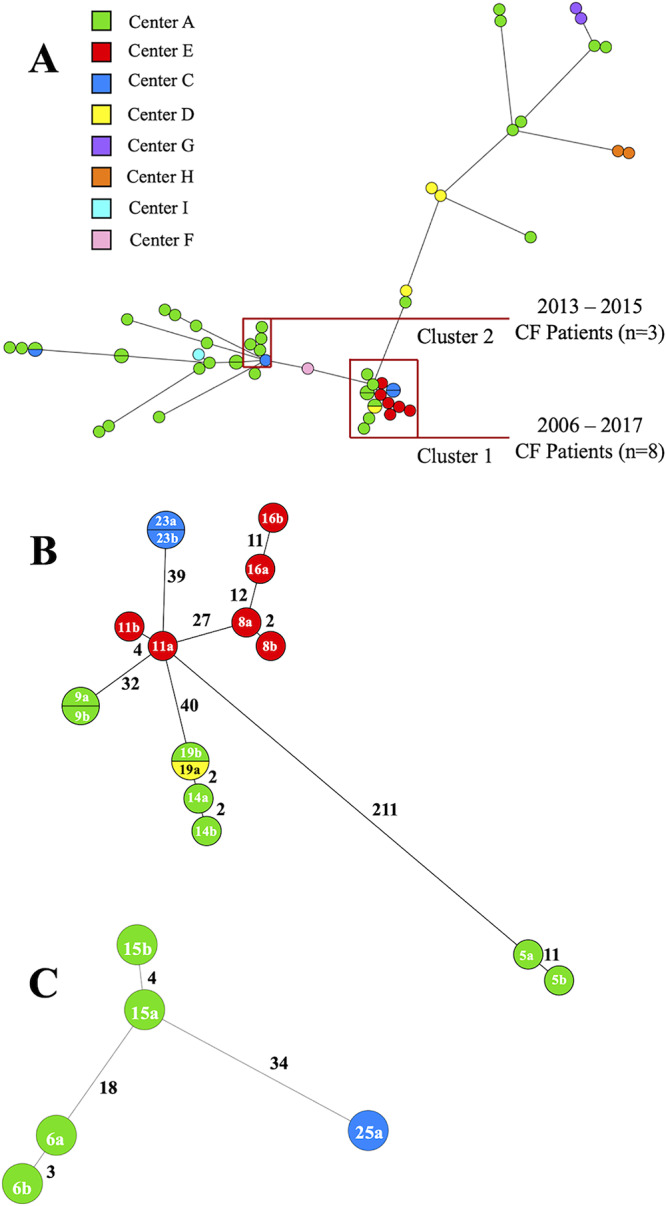
Minimum spanning tree (MST) of M. abscessus subsp. abscessus. (A) MST representing 57 M. abscessus subsp. abscessus whole-genome SNP differences among CF and non-CF patient isolates. SPAdes assembly of the earliest isolate resulted in 68 contigs with a mean coverage of 104× across the assembly. The MST was constructed from 87,491 core SNP sites. Isolates were recovered from multiple centers, as indicated by the colored nodes. While disperse, two distinct groups of isolates were noted as clustering together and are represented as cluster 1 and cluster 2. Cluster 1 comprised isolates recovered from multiple centers, while cluster 2 comprised isolates recovered from 2 centers. (B) MST of cluster 1, representing the SNP difference among the isolates. While these isolates clustered closely together, the average SNP difference was 88, with a minimum and maximum SNP difference of 2 and 211, respectively. (C) MST of cluster 2, representing the SNP difference among the isolates. The average SNP difference was 2, with a minimum and maximum SNP difference of 3 and 34, respectively.
In cluster 2, there were five M. abscessus subsp. abscessus isolates from three CF patients, dating between 2013 and 2015 and consisting of two pairs of M. abscessus subsp. abscessus isolates recovered from each of two CF patients and one M. abscessus subsp. abscessus isolate from one CF patient (Fig. 2C). Similar to cluster 1, the majority of isolates in cluster 2 were recovered from specimens collected in center A (n = 4); one isolate was collected in center C. Within this cluster, there was also evidence of both probable and possible recent transmission events between patients (3).
Three M. abscessus subsp. abscessus isolates recovered from three CF patients (patients 3, 12, and 20) collected between 2008 and 2015 differed by 39 to 62 SNPs (Fig. 1). M. abscessus subsp. abscessus isolates from one patient (patient 3) had been collected at different centers (centers C and A) 144 days apart, and there were no SNP variations between the isolates, as measured by pairwise SNP comparison. Among the M. abscessus subsp. massiliense strains, three isolates from two non-CF patients (patients 29 and 30) collected between 2009 and 2017 in centers A and I, respectively, were closely related and distinguished by only 111 SNPs (Fig. 1).
There was no clustering between the three M. abscessus subsp. bolletii isolates collected from 2 non-CF patients (patients 36 and 37) in 2013, 2014, and 2016 from center A (n = 2) and center G (n = 1).
DISCUSSION
This is the first comprehensive investigation of the molecular epidemiology of MABC in Ireland based on WGS analysis. There was a predominance of M. abscessus subsp. abscessus (81.4%), followed by M. abscessus subsp. massiliense (14.3%) and M. abscessus subsp. bolletii (4.3%), which is consistent with reports from other countries (1, 12, 13).
In some cases, our MABC isolates aligned with previously published strains from the United States and France, consistent with the global spread and human-to-human transmission of MABC organisms (5). The low genetic variation and the presence of clusters among M. abscessus subsp. abscessus isolates collected from more than one center over prolonged time periods suggest that there are dominant circulating strains in Ireland. The genomic similarities observed within an individual patient’s isolates and between the patients’ isolates suggest the possibility of M. abscessus subsp. abscessus transmission between patients rather than point source or independent acquisition from environmental sources. This finding is consistent with earlier reports (5, 7). In a previous global study, it was observed that the majority of patients are infected with clustered rather than unclustered MABC isolates and that less than 20 SNPs between patient isolates could indicate a probable recent transmission event (5). MABC isolates recovered from 14 Irish patients in that study were represented in dense clusters (5). Prospectively collected epidemiological and clinical data for MABC-infected patients along with contemporaneous WGS of environmental MABC isolates may help to elucidate reservoirs and routes of acquisition and transmission among patients infected with MABC.
Our study has shown that CF patients were mainly infected with M. abscessus subsp. abscessus (93%) rather than M. abscessus subsp. massiliense (7%) or M. abscessus subsp. bolletii (0%). Infections caused by M. abscessus subsp. abscessus are associated with worse clinical outcomes following lung transplantation, partly due to the high frequency of macrolide resistance, and other factors, such as immunological status, can also impact clinical outcomes (22). Further work is needed to elucidate the presence of virulence factors or pathogenic mechanisms linked to MABC infection in CF patients.
There was greater diversity among MABC isolates recovered from non-CF than among those recovered from CF patients. However, the clinical significance of this finding is uncertain, in view of the relatively small number of isolates from non-CF patients.
We have shown the high discriminatory power of WGS for analyzing MABC strains circulating in Ireland, where MABC infection is a nonnotifiable disease. This results in knowledge gaps regarding antimicrobial resistance, clinical characteristics, and epidemiology. However, the high prevalence of CF in Ireland and the genomic data presented here may influence a change in the MABC public health policy to monitor MABC infections and detect the transmission of dominant clones of MABC occurring across different geographical settings. The implementation of both a national and an international MABC surveillance system based on conventional and WGS surveillance would allow greater insights into the prevalence of MABC, would guide infection prevention and control policies within health care facilities, and may help avoid the onward transmission of MABC between patients.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the many Irish hospitals, clinicians, and laboratory staff, in addition to our own, for referring isolates and/or specimens which generated the collection of MABC isolates used in this study. We acknowledge the Microbiology Research Unit at The Dublin Dental University Hospital, particularly Megan Earls and the Public Health Laboratory (Dublin-HSE), for the use of BioNumerics software. We thank Aura Andreasen for critically reviewing the manuscript.
We declare that we have no conflict of interest.
Natalia Redondo was funded by European Centre for Disease Prevention and Control. Consumable costs were supported by the Department of Clinical Microbiology, School of Medicine, Trinity College, Dublin, Ireland.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, Conlan S, McNulty S, Brown-Elliott BA, Wallace RJ, Olivier KN, Holland SM, Sampaio EP. 2009. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol 47:1985–1995. doi: 10.1128/JCM.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scoffone VC, Trespidi G, Chiarelli LR, Barbieri G, Buroni S. 2019. Quorum sensing as antivirulence target in cystic fibrosis pathogens. Int J Mol Sci 20:1838. doi: 10.3390/ijms20081838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skolnik K, Kirkpatrick G, Quon BS. 2016. Nontuberculous mycobacteria in cystic fibrosis. Curr Treat Options Infect Dis 8:259–274. doi: 10.1007/s40506-016-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luthra S, Rominski A, Sander P. 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol 9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkinham JO. 2015. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell PM. 2008. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros 7:450–453. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 9.O'Driscoll C, Konjek J, Heym B, Fitzgibbon MM, Plant BJ, Ní Chróinín M, Mullane D, Lynch-Healy M, Corcoran GD, Schaffer K, Rogers TR, Prentice MB. 2016. Molecular epidemiology of Mycobacterium abscessus complex isolates in Ireland. J Cyst Fibros 15:179–185. doi: 10.1016/j.jcf.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 11.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann JL, Daffé M, Brosch R, Risler JL, Gaillard JL. 2009. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, Kim BJ, Kook YH. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol 46:3384–3390. doi: 10.1128/JCM.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, Gutierrez C, Vincent V, Fauroux B, Rottman M, Guillemot D, Gaillard JL, Herrmann JL, OMA Group. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 47:4124–4128. doi: 10.1128/JCM.01257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caverly LJ, Spilker T, LiPuma JJ. 2016. Complete genome sequences of 17 rapidly growing nontuberculous mycobacterial strains. Genome Announc 4:e01009-16. doi: 10.1128/genomeA.01009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang S, Renvoisé A, Perret C, Guinier M, Chelghoum N, Brossier F, Capton E, Jarlier V, Sougakoff W. 2013. Whole-genome sequence of Mycobacterium abscessus clinical strain V06705. Genome Announc 1:e00690-13. doi: 10.1128/genomeA.00690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo SW, Wong YL, Yusoff AM, Leong ML, Wong GJ, Ong CS, Ng KP, Ngeow YF. 2012. Genome sequence of the Mycobacterium abscessus strain M93. J Bacteriol 194:3278. doi: 10.1128/JB.00492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngeow YF, Wong YL, Lokanathan N, Wong GJ, Ong CS, Ng KP, Choo SW. 2012. Genomic analysis of Mycobacterium massiliense strain M115, an isolate from human sputum. J Bacteriol 194:4786. doi: 10.1128/JB.01104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson RM, Hasan NA, de Moura VC, Duarte RS, Jackson M, Strong M. 2013. Phylogenomics of Brazilian epidemic isolates of Mycobacterium abscessus subsp. bolletii reveals relationships of global outbreak strains. Infect Genet Evol 20:292–297. doi: 10.1016/j.meegid.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tettelin H, Sampaio EP, Daugherty SC, Hine E, Riley DR, Sadzewicz L, Sengamalay N, Shefchek K, Su Q, Tallon LJ, Conville P, Olivier KN, Holland SM, Fraser CM, Zelazny AM. 2012. Genomic insights into the emerging human pathogen Mycobacterium massiliense. J Bacteriol 194:5450. doi: 10.1128/JB.01200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong YL, Choo SW, Tan JL, Ong CS, Ng KP, Ngeow YF. 2012. Draft genome sequence of Mycobacterium bolletii strain M24, a rapidly growing mycobacterium of contentious taxonomic status. J Bacteriol 194:4475. doi: 10.1128/JB.00916-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi GE, Cho YJ, Koh WJ, Chun J, Cho SN, Shin SJ. 2012. Draft genome sequence of Mycobacterium abscessus subsp. bolletii BD(T). J Bacteriol 194:2756–2757. doi: 10.1128/JB.00354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris KA, Kenna DT. 2014. Mycobacterium abscessus infection in cystic fibrosis: molecular typing and clinical outcomes. J Med Microbiol 63:1241–1246. doi: 10.1099/jmm.0.077164-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads of all 70 sequenced MABC genomes in this study were submitted to the European Nucleotide Archive database under project accession number PRJEB37730.