Abstract
Magnetic resonance imaging (MRI) is a widely used method for the diagnosis of multiple sclerosis (MS) that is essential for the detection and follow-up of the disease. The Polish Medical Society of Radiology (PLTR) and the Polish Society of Neurology (PTN) present the second version of the recommendations for examinations routinely conducted in magnetic resonance imaging departments in patients with MS, which include new data and practical comments for electroradiology technicians and radiologists. The recommended protocol aims to improve the MRI procedure and, most importantly, to standardise the method of conducting scans in all MRI departments. This is crucial for the initial diagnostics that are necessary to establish a diagnosis as well as monitor patients with MS, which directly translates into significant clinical decisions.
MS is a chronic idiopathic inflammatory demyelinating disease of the central nervous system (CNS), the aetiology of which is still unknown. The nature of the disease lies in the CNS destruction process disseminated in time and space. MRI detects focal lesions in the white and grey matter with high sensitivity (with significantly less specificity in the latter). It is also the best tool to assess brain atrophy in patients with MS in terms of grey matter volume and white matter volume as well as local atrophy (by measuring the volume of thalamus, corpus callosum, subcortical nuclei, hippocampus) as parameters that correlate with disability progression and cognitive dysfunctions. Progress in magnetic resonance techniques, as well as the abilities of postprocessing the obtained data, has become the basis for the dynamic development of computer programs that allow for a more repeatable assessment of brain atrophy in both cross-sectional and longitudinal studies.
MRI is unquestionably the best diagnostic tool used to follow up the course of the disease and to treat patients with MS. However, to diagnose and follow up the patients with MS on the basis of MRI in accordance with the latest standards, an MRI study must meet certain quality criteria, which are the subject of this paper.
Keywords: multiple sclerosis, magnetic resonance imaging, imaging protocol, guideline
General comments
It is recommended that patients with multiple sclerosis (MS) undergo magnetic resonance imaging (MRI) with intravenous administration of a paramagnetic contrast medium (gadolinium) solely as a part of the initial diagnostics. A follow-up gadolinium-enhanced MRI with the use of macrocyclic contrast agents is recommended only in cases of clinical progression of the disease, if the need for another differential diagnosis of MS arises, or in other clinically justified cases. The use of linear contrast agents based on gadolinium (GBCA) is not recommended in the follow-up of the treatment of MS in clinically and radiologically stable patients, due to the possible occurrence of long-term side effects associated with the accumulation of a contrast agent within the central nervous system (CNS).
Patients with clinically isolated syndrome (CIS) or suspected MS should undergo head MRI before and after an intravenous administration of a contrast agent (Table 1), and it is recommended that an additional scanning of the cervical and thoracic sections of the spinal cord is performed (in accordance with a referral/indication of the neurologist) after an intravenous administration of a contrast agent, especially when head MRI does not meet the diagnostic criteria or clinical symptoms suggest the lesions are located in the spinal cord (Table 2). MRI studies of the brain and the cervical section of the spinal cord should be performed during one stay at the MRI department. The thoracic section should be examined immediately in the next procedure, and, if justified, the MRI examination should cover the entire spinal cord [1-6].
Table 1.
Head magnetic resonance imaging protocol
| Parameters | Description |
|---|---|
| Electromagnetic field | Images should be of good quality with an appropriate SNR value and resolution (≤ 1 × 1 mm) |
| Reference setting | When setting the scanning plane, use a line parallel to the lower edges of the rostrum and splenium of the corpus callosum, also to have an identical angulation of the planned slices to the angulation of the slices in the previous study (Figure 1) |
| Scanning range | Whole brain scanned |
| Slice thickness and gaps | ≤ 3 mm, with no gaps (for 2D and 3D acquisitions) |
| Basic sequences | 1. 3DT1 axial isotropically 2. T2 axial 3. Axial DWI (with ADC map) Administration of a contrast medium1 (T1 sequence 5-10 minutes after administration!) 4. FLAIR + C sagittal 5. FLAIR + C axial 6. 3DT1 + C axial isotropically2 1The recommended dose of a contrast medium is 0.1 mmol/kg body mass, see General comments. 2 It is recommended that sagittal reconstructions are performed and recorded, and archived on a CD and in the PACS system, if available, as an integral part of the examination. |
| Optional sequences | 1. PD 2. SWI (for the identification of central veins in lesions and microbleedings) 3. DIR – for evaluation of cortical and subcortical foci |
1.The direction of scanning in axial scans must be upwards, whereas in sagittal scans it must be from the right to the left (also when scanning the spine).
2. Gaps between slices should be as small as possible (proposed 0.3 mm, i.e. 10% slice thickness).
3. 3DT1 – it is recommended that this sequence be performed first, to avoid motion artefacts in the course of the examination. This is a sequence necessary for precise volumetric evaluation of the brain.
4. Both FLAIR sequences should be performed after the administration of a contrast medium, to delay the onset of T1 + C acquisition (within the range of 5-10 minutes), in order to achieve better contrast enhancement. A contrast medium does not affect the quality of FLAIR images, and at the same time the patient’s time spent in the scanner is used optimally.
5. If scanning with 3DT2 and 3D FLAIR sequences is possible, they should be used with subsequent axial reconstruction with 3 mm slices in the plane set up to the lower edge of the corpus callosum.
6. If a software which automatically determines the angulation/range of the layers can be used, as in the previous examination, such a function is recommended.
Table 2.
Spinal cord section magnetic resonance imaging protocol
| Parameters | Description |
|---|---|
| Electromagnetic field | Images should be of good quality with an appropriate SNR value and resolution (≤ 1 × 1 mm) |
| Scanning range | Whole cervical spinal cord scanned |
| Slice thickness and gaps | Sagittal: ≤ 3 mm, no gaps (for 2D and 3D) Axial: 3 mm, no gaps |
| Basic sequences | 1. T2 sagittal 2. T1 sagittal Administration of a contrast medium1 (T1 sequence 5-10 minutes after administration!) 3. Sagittal T2 FAT-SAT, PD or PST1-IR 4. T2 axially at the level of the lesions visible in SAG sequences 5. T1 sagittal 6. T1 axial 1The recommended dose of a contrast medium is 0.1 mmol/kg body mass, see General comments. |
| Optional sequences | 1. T2 coronal at the level of the lesions visible in the following sequences: SAG AX PD or SAG T2 FAT-SAT, PD or PST1-IR 2. 3DT1 sagittal (to assess brain atrophy) |
MRI is particularly important in the diagnosis of primary progressive MS according to current disease diagnosis criteria (see Appendix).
In patients with multifocal damage to the nervous system involving the symptomatology associated with both brain and spinal cord impairment, in order to shorten the diagnostic time, according to a referring neurologist’s recommendation, it is possible to perform simultaneously an MRI of the head and of a selected section of the spinal cord using a combined protocol (Table 3).
Table 3.
Head and spinal cord section magnetic resonance imaging protocol (combined)
| Parameters | Description |
|---|---|
| Electromagnetic field | Images should be of good quality with an appropriate SNR value and resolution (≤ 1 × 1 mm) |
| Scanning range | Brain and cervical spinal cord scanned |
| Slice thickness and gaps | Head and spinal cord (sagittal) ≤ 3 mm, no gaps (for 2D and 3D) Cord (axial): 3 mm, no gaps |
| Basic sequences | 1. Use protocol prior to the administration of a contrast medium for the head and spinal cord section. Administration of a contrast medium1 (T1 sequence 5-10 minutes after administration!) 2. FLAIR + C sagittal (head) 3. FAT-SAT, PD or PST1-IR + C sagittal (spinal cord section) 4. T2 + C axially at the level of the lesions visible in SAG sequences (spinal cord section) 5. FLAIR + C axial (head) 6. 3DT1 + C axial isotropically (head)2 7. T1 + C sagittal (spinal cord section) 8. T1 + C axial (spinal cord section) 1The recommended dose of a contrast medium is 0.1 mmol/kg body mass, see General comments. 2 It is recommended that sagittal reconstructions are performed and recorded, and then archived on a CD and in the PACS system, if available, as an integral part of the examination. |
| Optional sequences | As in the specified protocols of the head and spinal cord. |
Recommendations for disease progression follow-up based on MRI:
Head MRI to show new/enlarging demyelinating lesions (Table 1) at least every 12 months during the first years of treatment and possibly less frequently later in patients with complete clinical stability.
Cervical and/or thoracic spinal cord scan is recommended, according to a neurologist’s referral/indication.
For patients under 18 years of age, the MRI protocol for brain and spinal cord examination remains unchanged, the same as in the adult population [7-12].
Brain magnetic resonance imaging protocol for patients with multiple sclerosis
In order to use the same scanning planes during the follow- up examinations, it is recommended to achieve slices in the true midline plane.
For this purpose, once three localisation slices have been performed, five slices with a thickness of 3 mm should be planned as accurately as possible in the sagittal plane on T2-weighted images.
The planned slices should be set exactly parallelly to the longitudinal cerebral fissure using the localisation slices, in the transverse and frontal planes. The third of the five slices should pass through the median fissure as accurately as possible.
Axial slices should be set on the thus obtained midline slice in parallel to the lower limits of the rostrum (anterior commissure – AC) and splenium of the corpus callosum (posterior commissure – PC), according to the AC-PC reference line (Figure 1).
Figure 1.
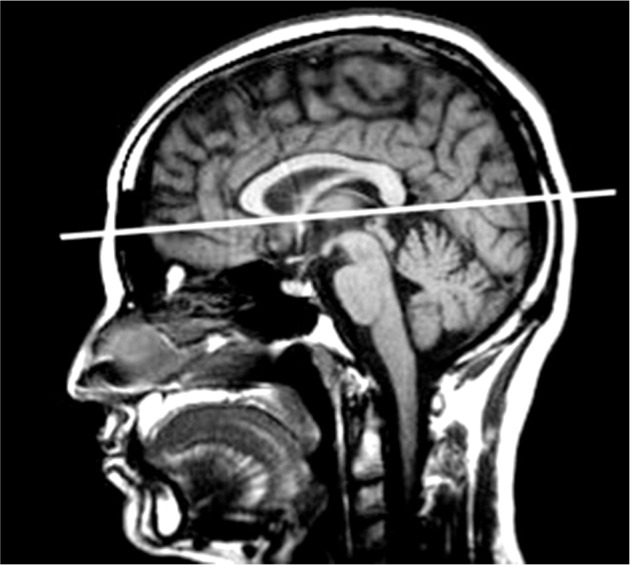
A pre-specified reference line parallel to the corpus callosum
NOTE! During the follow-up examinations, when the scanning plan is set in the reference to the corpus callosum, it is necessary to compare the angulation of the planned slices with the angulation of the slices in the previous study.
Some scanners do not offer the possibility to remember a single slice, which is the best for such a comparison; in which case the slice should be carefully defined as shown in the Figure 1.
A radiological report should include the standard terminology used in brain assessment.
Assessment of focal lesions:
Location (supratentorial region: cortical, paracortical, central white matter, periventricular, subtentorial, corpus callosum, brainstem, spinal cord).
Size – when multiple lesions are detected, the range of the longest dimension from–to. In the case of multiple lesions, the size of the largest lesions only should be reported. According to the current definition, demyelinating lesions are defined as lesions ≥ 3 mm in diameter.
The number of demyelinating lesions – specify according to the following scheme: 1, 2, 3–8, ≥ 9.
The nature of the lesion, i.e. specify whether the appearance is typical for MS demyelination, or whether differential diagnosis is required, e.g. ischaemic lesion.
Whether the lesions are disseminated in space (DIS) and meet the 2017 McDonald criteria (see Appendix and Table 4).
Comparison to the previous head and spinal cord MRI (if such an examination was conducted). In patients with suspected MS, comparison to the previous MRI to assess disease activity and eligibility for treatment. In on-treatment patients, comparison to the previous examination and baseline examination performed prior to treatment initiation.
Activity assessment, i.e. the number of contrast-enhancing foci in the current examination and the number of new/enlarging lesions compared to the baseline and the previous examination – please specify according to the scheme: 1, 2, 3-8, ≥ 9.
Assessment of brain atrophy. It is recommended that expressions such as “brain atrophy” or “cerebral atrophy” be avoided in the text. If possible, provide current whole brain volume, grey matter volume, white matter volume, corpus callosum volume, and the volume of the right and left thalami.
Table 4.
Disseminated in space (DIS) and disseminated in time (DIT) damage to the nervous system according to the 2017 McDonald criteria
| Damage to the central nervous system DIS | Damage to the central nervous system DIT |
|---|---|
| Minimum one T2 lesion present in at least two typical locations: 1. Paracortical/cortical 2. Periventricular 3. Infratentorial 4. In the spinal cord |
Occurrence of new lesions on T2-weighted images and/or contrast-enhanced lesions on a subsequent MRI scan compared to a reference examination, regardless of the time since the baseline examination, or concomitant occurrence of contrast-enhancing and non-enhancing lesions, regardless of the time of this examination in relation to the time of the onset of neurological signs and symptoms (may also be a basic examination), or immunological equivalent of radiological dissemination over time: confirmation of the presence of specific oligoclonal bands (absent in serum) in the cerebrospinal fluid. |
Volumetric analysis of the brain should be performed using certified software; in the future, it can alternatively be carried out by a central centre in order to standardise the results.
Appendix
Criteria for multiple sclerosis diagnosis according to McDonald 2017
| Clinical presentation | Additional criteria required for diagnosis |
|---|---|
| Minimum two relapses, clinical signs from two foci | Not required* |
| Minimum two relapses, clinical signs from one focus | DIS damage to the nervous system on MRI (Appendix 2) or another relapse of another clinical location |
| One relapse, clinical signs from two or more foci | DIT damage to the nervous system on MRI (Appendix 2), presence of specific oligoclonal bands in cerebrospinal fluid (absent in serum) or subsequent relapse |
| One relapse, clinical signs from one focus (isolated central nervous system damage) | DIS and DIT damage to the nervous system on MRI, or presence of specific oligoclonal bands (absent in serum) in cerebrospinal fluid |
| Primary progressive multiple sclerosis | The year of neurological disability progression diagnosed prospectively or retrospectively and 2 out of 3 of the following conditions fulfilled: 1. MRI dissemination in space (but 1 and not 2 typical locations required) 2. MRI dissemination in space in the spinal cord (minimum 2 lesions) 3. Positive CSF test (presence of oligoclonal bands, absent in serum, and/or elevated immunoglobulin index) |
Once other possible causes of symptoms have been excluded; in practice, every patient with suspected MS should undergo at least an MRI of the head and cervical spinal cord MRI, as well as lumbar puncture.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Rovira A, Wattjes MP, Tintore M, et al. MAGNIMS consensus guideliness on the use of MRI in multiple sclerosis–clinical implementation in the diagnostic process. Nat Rev Neurol 2015; 11: 471-482. [DOI] [PubMed] [Google Scholar]
- 2.Miller DH, Filippi M, Fazekas F, et al. Role of magnetic resonance imaging within diagnostic criteria for multiple sclerosis. Ann Neurol 2004; 56: 273-278. doi: 10.1002/ana.20156. [DOI] [PubMed] [Google Scholar]
- 3.Simon JH, Traboulsee A, Coyle PK, et al. Standardized MR imaging protocol for multiple sclerosis: consortium of MS centers consensus guidelines. AJNR Am J Neuroradiol 2006; 27: 455-461. [PMC free article] [PubMed] [Google Scholar]
- 4.Polaman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to McDonald criteria. Ann Neurol 2011; 69: 2292-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alroughani R, Al Hashel J, Lamdhade S, et al. Predictors of conversion to multiple sclerosis in patients with clinical isolated syndrome using the 2010 revised McDonald criteria. ISRN Neurol 2012; 2012: 792192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly S, Kinsella K, Duggan M, et al. A proposed modification to the McDonald 2010 criteria for the diagnosis of primary progressive multiple sclerosis. Mult Scler 2013; 19: 1095-1100. [DOI] [PubMed] [Google Scholar]
- 7.Traboulsee A, Simon JH, L Stone, et al. Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016; 37: 394-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson A, Bandwel B, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162-173. [DOI] [PubMed] [Google Scholar]
- 9.De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147-156. [DOI] [PubMed] [Google Scholar]
- 10.Zivadinov R, Jakimovski D, Gandhi S, et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 2016; 16: 777-793. [DOI] [PubMed] [Google Scholar]
- 11.Zivadinov R, Dwyer MG, Bergsland N. Brain atrophy measurements should be used to guide therapy monitoring in MS–YES. Mult Scler 2016; 22: 1522-1524. [DOI] [PubMed] [Google Scholar]
- 12.Sąsiadek M, Katulska K, Majos A, et al. Guidelines of the Polish Medical Society of Radiology for the routinely used MRI protocol in patients with multiple sclerosis. Neurol Neurochir Pol 2018; 52: 638-642. [DOI] [PubMed] [Google Scholar]


