Integration of genetic networks allows bacteria to rapidly adapt to changing environments. This is particularly important in bacteria that interact with multiple hosts. Erwinia carotovora is a plant pathogen that uses Drosophila melanogaster as a vector. To interact with these two hosts, Ecc15 uses different sets of virulence factors: plant cell wall-degrading enzymes to infect plants and the Erwinia virulence factor (evf) to infect Drosophila. Our work shows that, despite the virulence factors being specific for each host, both sets are coactivated by homoserine lactone quorum sensing and by the two-component GacS/A system in infected plants. This regulation is essential for Ecc15 loads in the gut of Drosophila and minimizes the developmental delay caused by the bacteria with respect to the insect vector. Our findings provide evidence that coactivation of the host-specific factors in the plant may function as a predictive mechanism to maximize the probability of transit of the bacteria between hosts.
KEYWORDS: Drosophila, Ecc15, homoserine lactones, bacterial infections, host-pathogen interactions, insect development, invertebrate-microbe interactions, quorum sensing
ABSTRACT
Multihost bacteria have to rapidly adapt to drastic environmental changes, relying on a fine integration of multiple stimuli for an optimal genetic response. Erwinia carotovora spp. are phytopathogens that cause soft-rot disease. Strain Ecc15 in particular is a model for bacterial oral-route infection in Drosophila melanogaster as it harbors a unique gene, evf, that encodes the Erwinia virulence factor (Evf), which is a major determinant for infection of the D. melanogaster gut. However, the factors involved in the regulation of evf expression are poorly understood. We investigated whether evf could be controlled by quorum sensing as, in the Erwinia genus, quorum sensing regulates pectolytic enzymes, the major virulence factors needed to infect plants. Here, we show that transcription of evf is positively regulated by quorum sensing in Ecc15 via acyl-homoserine lactone (AHL) signal synthase ExpI and AHL receptors ExpR1 and ExpR2. We also show that the load of Ecc15 in the gut depends upon the quorum sensing-mediated regulation of evf. Furthermore, we demonstrate that larvae infected with Ecc15 suffer a developmental delay as a direct consequence of the regulation of evf via quorum sensing. Finally, we demonstrate that evf is coexpressed with plant cell wall-degrading enzymes (PCWDE) during plant infection in a quorum sensing-dependent manner. Overall, our results show that Ecc15 relies on quorum sensing to control production of both pectolytic enzymes and Evf. This regulation influences the interaction of Ecc15 with its two known hosts, indicating that quorum sensing signaling may impact bacterial dissemination via insect vectors that feed on rotting plants.
INTRODUCTION
Insects play an important role in the dissemination of microorganisms that cause both human and plant diseases. This dissemination may be an active process whereby microbes develop strategies to interact with insects and use them as vectors (1, 2). To do so, bacteria must have the ability to persist within the host (either lifelong or transiently), evading or resisting its immune system in order to abrogate their elimination (3, 4). The host vector responds with a battery of innate defenses, such as production of antimicrobial peptides and reactive oxygen species as well as behavioral strategies (e.g., avoidance) and physiological responses (e.g., increased peristalsis) (5–9). The successful establishment of these interactions, from the bacterial perspective, ultimately depends on maximizing the fitness of the microorganism and minimizing the impact on the fitness of the vector host (1). Phytopathogenic bacteria such as Phytoplasma spp., Xylella fastidiosa, Pantoea stewartii (formerly Erwinia stewartii), and Erwinia carotovora (also known as Pectobacterium carotovorum) are among those known to establish close associations with insects and to rely on these hosts as vectors, presumably to facilitate rapid dissemination among plants (10–13). Thus, understanding the molecular mechanisms governing the establishment of these interactions is crucial to prevent insect-borne diseases.
Bacteria from the Erwinia genus produce pectolytic enzymes that degrade plant tissue, causing soft root disease (14). These bacteria survive poorly in soil, overwinter in decaying plant material (14), and use insects, including Drosophila species (12, 15) as vectors. Specifically, the nonlethal interaction between the phytopathogen Erwinia carotovora (strain Ecc15) and Drosophila melanogaster has been used as a model to study bacterium-host interactions. Oral infections with Ecc15 lead to a transient systemic induction of the immune system in D. melanogaster and consequent production of antimicrobial peptides (7, 16). During infection, Ecc15 causes damage and loss of epithelial cells, leading to an overall shrinkage of the gut (7). To reestablish the normal functions of the gut, there is activation of tissue repair programs with proliferation and differentiation of stem cells, which have been correlated with a developmental delay in the larval stage of Drosophila (7, 17). These responses are strain specific and highly dependent on the expression levels of the Erwinia virulence factor gene (evf) (18), which promotes bacterial infection of the Drosophila gut by an unknown mechanism (19). Additionally, expression of evf requires the transcriptional regulator Hor (18), but the signals required for the activation of this regulator remain unknown.
Quorum sensing has recently been shown to be important in the regulation of bacterial traits that affect the persistence and/or virulence of bacteria in insects (20–23). Many bacteria use quorum sensing to regulate gene expression as a function of population density (24, 25). This cell-cell signaling mechanism relies on the production, secretion, and response to extracellular signaling molecules called autoinducers (25–27). Bacteria from the Erwinia genus produce a mixture of plant cell wall-degrading enzymes (PCWDE), which are the major virulence factors used to degrade plant tissues and potentiate bacterial invasion of the plant host (28–31). In these bacteria, expression of these PCWDE is tightly regulated by two main signaling pathways: the acyl-homoserine lactone (AHL) quorum sensing system and the GacS/A two-component (GAC) system (Fig. 1) (32–36). Typically, the AHL quorum sensing system present in Erwinia spp. includes the AHL synthase ExpI (37) and two AHL receptors, ExpR1 and ExpR2 (38), which are homologues of the canonical LuxI/R quorum sensing system first identified in Vibrio fischeri (39–41). The GacS/A two-component system is also activated at high cell density, and, like the AHL quorum sensing system, regulates virulence in many Gram-negative pathogenic bacteria (42–47). Given the importance of these two signal transduction pathways for the expression of the major plant virulence factors in Erwinia spp., we investigated whether quorum sensing and the GacS/A system also regulate evf expression in Ecc15. Additionally, we tested whether these signaling pathways are important for Ecc15 infection and determined the consequences of this interaction for the insect host. Our results show that PCWDE expression and evf expression in Ecc15, which are required for the interactions with plants and insects, respectively, are regulated by the same quorum sensing signaling pathway. Moreover, we demonstrate that the quorum sensing-dependent evf expression has a negative effect on the insect host as it leads to a developmental delay in larvae infected with Ecc15. Finally, we show that evf and the PCWDE are coexpressed during the plant infection, which may function as a predictive mechanism to maximize the probability of transmission of Erwinia to a new host.
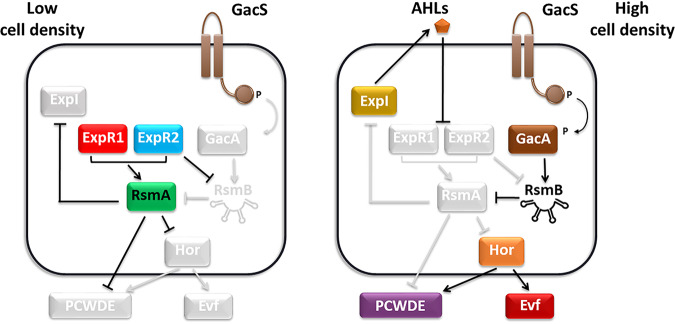
FIG 1.
Signaling pathways regulating PCWDE and evf production in Erwinia spp. At low cell density, when the concentration of AHL signaling molecules is low, ExpR1 and ExpR2 induce transcription of rsmA, repressing expression of both PCWDE and evf. As cell density increases, AHLs accumulate and when the concentration threshold is reached these signal molecules bind to ExpR1 and ExpR2 receptors, inhibiting their DNA binding ability. As a result, rsmA transcription is no longer induced. The GacS/A two-component system is also active at high cell density and promotes transcription of rsmB, a noncoding RNA that has high binding affinity to RsmA and inhibits the remaining available RsmA. Inhibition of RsmA results in increased production of both PCWDE and hor and, consequently, evf, leading to full induction of virulence. We show here that evf is regulated by quorum sensing and the GacS/A system via hor. While evf is not necessary to infect the plant host, we also show that there is coexpression of evf and PCWDE during plant infection. Gray and black lines indicate inactive and active pathways, respectively. Arrows indicate activation, while intersecting lines indicate repression.
RESULTS
Expression of evf is regulated by both AHL-dependent quorum sensing and the GAC system.
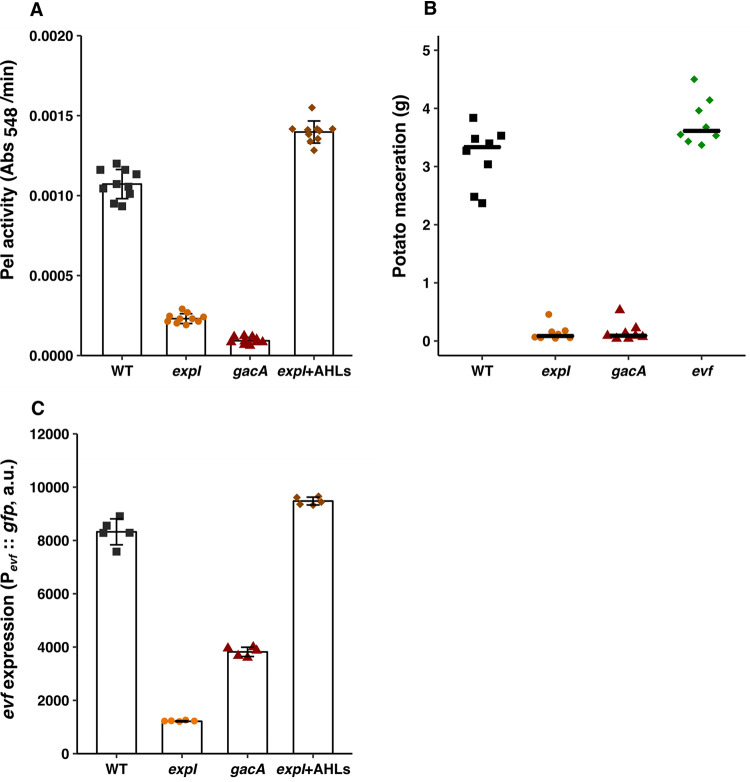
We first investigated whether activation of the production of PCWDE in Ecc15 requires both the AHL quorum sensing system and the GacS/A two-component (GAC) system, as occurs in other members of the Erwinia (or Pectobacterium) genus (33, 35, 37, 48). We constructed deletion mutants of expI and gacA, the genes encoding homologues of the AHL synthase and the response regulator of the GAC system, respectively. We determined whether any of these mutations cause a growth defect in Ecc15, and observed no difference in growth compared to the wild-type (WT) strain (see Fig. S1 in the supplemental material). We then measured pectate lyase (Pel) activity in supernatants of cultures from the Ecc15 WT strain or the expI or gacA mutants, as this is one of the PCWDE typically secreted by Erwinia spp. As shown in Fig. 2A (and for replicate experiments in Fig. S2), both the expI and the gacA mutants exhibited pronounced reductions in pectate lyase activity compared to the WT strain (Tukey honestly significant difference [HSD] test, P < 0.001; see also Fig. S2C). Addition of a mixture of exogenous 3-oxo-C6-HSL and 3-oxo-C8-HSL, the major AHLs produced by Erwinia carotovora (48), to an expI mutant culture was sufficient to restore production of this PCWDE to levels higher than those seen with the WT (Fig. 2A, Tukey HSD test, P < 0.001; see also Fig. S2C). In addition, both the expI and gacA mutants were impaired in virulence for the plant host, which we tested by measuring the mass of macerated tissue in potato tubers inoculated with these genotypes (Fig. 2B, Tukey HSD test, P < 0.001; see also Fig. S2F). In contrast, the evf mutant showed no statistically significant difference in maceration in comparison to the WT (Fig. 2B; see also Fig. S2D to F). Altogether, these results show that production of pectate lyase and plant host virulence are regulated by both the AHL and GAC systems in Ecc15, as occurs in other Erwinia spp., where expI and gacA mutants have been shown to be avirulent (35, 36, 49, 50). Moreover, we show that evf is not necessary for plant infection (Fig. 2B; see also Fig. S2D to F).
FIG 2.
Production of pectate lyase and expression of evf are dependent on both quorum sensing and the GAC system. (A) Pectate lyase activity in cell-free supernatants of WT Ecc15 and expI and gacA mutants at 6 h of growth in LB plus 0.4% PGA, n = 10. (B) Potato maceration quantification (grams) in potatoes infected with WT Ecc15 and expI, gacA, and evf mutants, 48 h postinfection, n = 8. (C) Pevf::gfp expression in WT Ecc15, expI and gacA mutants, and expI+AHLs at 6 h of growth in LB + Spec, n = 5. Complementation with AHLs (expI+AHLs) was performed with a mixture of 1 μM 3-oxo-C6-HSL and 3-oxo-C8-HSL. Growth curves of the strains used are shown in Fig. S1. Error bars represent standard deviations of the means. For each panel, results from a representative experiment from three independent experiments are shown (the results from the other two experiments are shown in Fig. S2). Results of statistical analysis taking the data of all three experiments are shown in Fig. S2. a.u., arbitrary units.
Growth curves of WT Ecc15, expI, gacA, and expI + AHL mutants carrying a Pevf::gfp reporter fusion. Download FIG S1, TIF file, 0.1 MB (108.6KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicates of the experiments described in the Fig. 2 legend (production of pectate lyase and expression of evf are dependent on both quorum sensing and the GAC system). (A and B) Replicates of experiments described in the Fig. 2A legend. (C) Statistical groups of results of all three experiments described in the Fig. 2A legend. (D and E) Replicates of experiments described in the Fig. 2B legend. (F) Statistical groups representing all three experiments described in the Fig. 2B legend. (G and H) Replicates of experiments described in the Fig. 2C legend. (I) Statistical groups representing all three experiments described in the Fig. 2C legend. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S2, TIF file, 0.9 MB (899.2KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
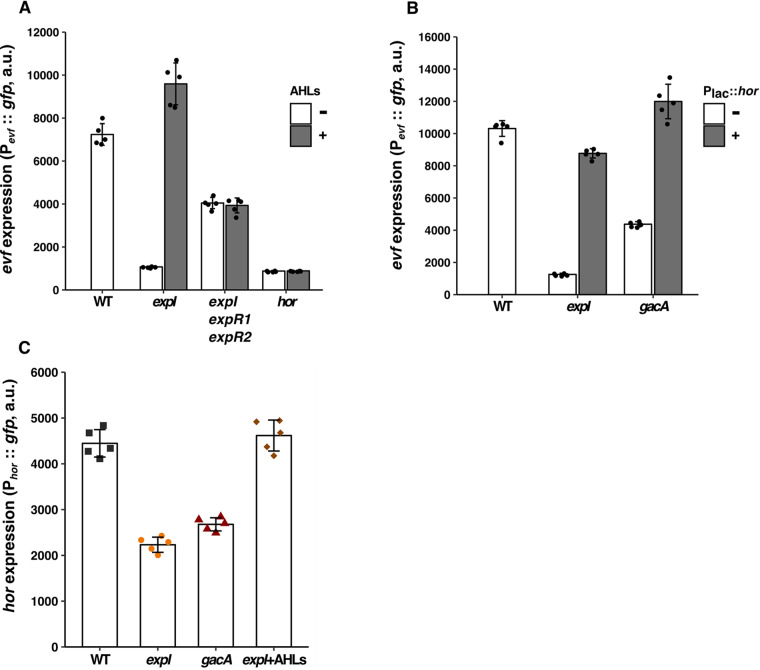
To investigate whether evf expression is also regulated by these two systems, we analyzed the expression of a transcriptional reporter consisting of a green fluorescent protein (GFP) fused to the promoter of evf (Pevf::gfp) in mutants of either AHL quorum sensing or GAC signaling systems. We observed that the expression of Pevf::gfp was reduced in the expI mutant compared to the WT (Tukey HSD test, P < 0.001) and that this expression was restored when exogenous AHLs were supplied to the culture (Fig. 2C; see also Fig. S2G to I). In the gacA mutant, expression of the evf promoter was also reduced compared to the WT, but not as much as in the expI mutant (Fig. 2C, Tukey HSD test, P < 0.001; see also Fig. S2G to I). Since it was previously shown that mutants in the GAC system produce lower levels of AHLs (35, 51), we asked if the difference observed between the WT and the gacA mutant could be explained solely by the lower levels of AHLs produced by the latter. However, addition of exogenous AHLs to the cultures of a gacA mutant did not restore the levels of Pevf::gfp expression to WT levels (Fig. S3). Therefore, we conclude that the gacA phenotype regarding evf expression is mostly independent of AHLs. Moreover, complementation of the gacA mutant with a gacA gene in trans restored the levels of evf expression (Fig. S4). Overall, these results show that full activation of both evf expression and PCWDE activity is dependent on quorum sensing regulation via AHLs and, to a lesser extent, on activation of the GAC system.
AHLs cannot complement intermediate levels of evf expression in a gacA mutant. Data represent Pevf::gfp expression in WT Ecc15 and the gacA mutant at 6 h of growth in LB + Spec, n = 3. Complementation with AHLs was performed with a mixture of 1 μM 3-oxo-C6-HSL and 3-oxo-C8-HSL. Error bars represent standard deviations of the means. Download FIG S3, TIF file, 0.1 MB (131.5KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complementation of gacA, expR1, and expR2 genes. Data represent evf expression at 6 h of growth in WT Ecc15 and in expI, expI expR1 expR2, and gacA mutants (white bars) or in the gacA mutant complemented with the gacA gene in trans (light gray) and the expI expR1 expR2 mutant complemented with expR1 and expR2 genes in trans (dark gray), n = 5. “Vector” stands for pOM1 Pevf::gfp, while “p(gacA+)” and “p(expR1+ expR2+)” refer to the same plasmid expressing gacA and expR1 expR2, respectively. Error bars represent standard deviations of the means. Download FIG S4, TIF file, 0.2 MB (213KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In the absence of AHLs, the AHL receptors ExpR1 and ExpR2 were found to be associated with repression of virulence traits such as PCWDE (Fig. 1) (37, 52). These receptors are DNA binding proteins that act as transcriptional activators of rsmA, which encodes a global repressor of quorum sensing-regulated genes in Erwinia spp. (38, 52, 53). Upon AHL binding, these receptors lose their ability to bind DNA, resulting in decreased expression of rsmA and, consequently, increased expression of virulence traits (Fig. 1) (54, 55). To determine whether ExpR1 and ExpR2 also mediate AHL-dependent regulation of evf expression, we constructed deletions of these two genes in the expI background. We measured expression of the Pevf::gfp reporter in this expI expR1 expR2 triple mutant, with or without exogenous AHLs. Because AHLs block activation of RsmA via ExpR1 and ExpR2 (54, 55), deletion of expR1 and expR2 in the expI background was expected to result in the derepression of evf. Consistent with this prediction, Pevf::gfp expression was higher in the expI expR1 expR2 triple mutant than in the expI single mutant (Fig. 3A, Tukey HSD test, P < 0.001; see also Fig. S5A to C) and the levels of evf expression in an expI expR1 expR2 mutant complemented with the expR1 and expR2 genes in trans were similar to those seen with the expI mutant (Fig. S4). However, the expression levels of Pevf::gfp were lower in the expI expR1 expR2 mutant than in the WT (Fig. 3A, Tukey HSD test, P < 0.001; see also Fig. S5A to C). The fact that deletion of these two receptors in the expI background was not sufficient to fully restore expression of evf to WT levels indicates that additional regulators control the expression of evf. Nonetheless, while addition of exogenous AHLs to a culture of an expI mutant increased Pevf::gfp expression, it remained unaltered in the triple expI expR1 expR2 mutant (Fig. 3A, Tukey HSD test, P = 1; see also Fig. S5A to C). Therefore, AHL-dependent regulation of evf expression is mediated by expR1 and expR2, as is also the case for the regulation of PCWDE in other Erwinia spp. (34, 36, 49).
FIG 3.
evf regulation by quorum sensing is dependent on ExpR receptors and hor. (A) Pevf::gfp expression without (white bars) or with (gray bars) addition of exogenous AHLs in the Ecc15 WT and in expI, expI expR1 expR2, and hor mutants at 6 h of growth in LB + Spec, n = 5. (B) Pevf::gfp expression in the Ecc15 WT strain and expI and gacA mutants containing a plasmid with the Pevf::gfp fusion (white bars) or with both Plac::hor and Pevf::gfp fusions (gray bars) at 6 h of growth in LB + Spec, n = 5. (C) Phor::gfp expression in WT Ecc15 and expI and gacA mutants at 6 h of growth in LB + Spec, n = 5. Complementation with AHLs was performed with a mixture of 1 μM 3-oxo-C6-HSL and 3-oxo-C8-HSL. Error bars represent standard deviations of the means. For each panel, results from a representative experiment from three independent experiments are shown (results from the other two experiments are shown in Fig. S5). Results of statistical analysis taking the data of all three experiments are shown in Fig. S5. a.u., arbitrary units.
Independent replicates of the experiment shown in Fig. 3a (evf regulation by quorum sensing is dependent on ExpR receptors and hor). (A and B) Replicates of experiments described in the Fig. 3A legend. (C) Statistical groups representing all three experiments described in the Fig. 3A legend. (D and E) Replicates of experiments described in the Fig. 3B legend. (F) Statistical groups representing all three experiments described in the Fig. 3B legend. (G and H) Replicates of experiments described in the Fig. 3C legend. (I) Statistical groups representing all three experiments described in the Fig. 3C. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S5, TIF file, 0.9 MB (926.4KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Regulation of evf by AHL quorum sensing is mediated by hor.
It was previously shown that Hor, a global regulator of diverse physiological processes in many animal-pathogenic and plant-pathogenic bacterial species (56), is a positive regulator of evf (18) and that, as in other Erwinia spp., hor is regulated by quorum sensing (57). Therefore, we asked if AHL-dependent regulation of evf is mediated via hor. We analyzed the expression levels of the Pevf::gfp reporter in a hor mutant and found that the levels were lower than in the WT and as low as those in the expI mutant (Fig. 3A). Moreover, we observed that addition of exogenous AHLs to a hor mutant did not restore the expression of evf (Fig. 3A, Tukey HSD test, P = 1; see also Fig. S5A to C). We next cloned the hor gene under the control of a lac promoter in the plasmid containing the Pevf::gfp fusion and measured evf expression levels in the expI and gacA mutants expressing or not the hor gene. We observed that expression of hor in either the expI or the gacA mutant restored evf expression to levels similar to those seen with the WT (Fig. 3B, Tukey HSD test, P < 0.001; see also Fig. S5D to F). Therefore, regulation of evf is mediated by both the AHL and the GAC systems and occurs via hor. Next, we asked whether these systems regulate hor itself by analyzing the expression of a hor promoter fusion (Phor::gfp) in expI and gacA mutants. As with the evf reporter, we observed that Phor::gfp expression was lower in an expI mutant than in the WT strain (Fig. 3C, Tukey HSD test, P < 0.001; see also Fig. S5G to I). Moreover, expression of Phor::gfp was complemented to WT levels by the addition of exogenous AHLs to the growth medium of the expI mutant (Fig. 3C, Tukey HSD test, P = 0.08; see also Fig. S5G to I). These data demonstrate that hor expression is regulated by AHLs and is necessary for the increase of evf expression mediated by AHLs.
Infection by Ecc15 causes a developmental delay in D. melanogaster larvae in a manner dependent on quorum sensing and GAC regulation of evf expression.
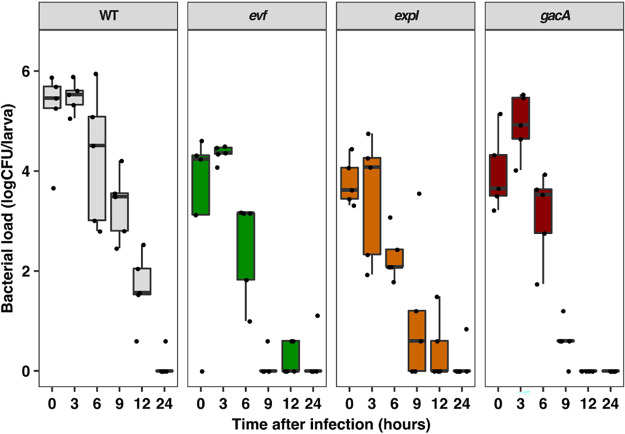
It is known that Evf promotes infection in the D. melanogaster gut (18, 19). To examine the effects of downregulation of evf on quorum sensing and GAC mutants, we measured Ecc15 loads upon oral infection. We inoculated Ecc15 WT or evf, expI, or gacA mutant cells into D. melanogaster L3 stage larvae and assessed the dynamics of bacterial loads by counting the number of CFU of Ecc15 over time. As previously reported (19), Ecc15 infection was transient and larvae were able to clear it after 24 h (Fig. 4). Additionally, we observed that the rates of elimination of the bacteria from the larval gut were not statistically significantly different between the WT and the evf, gacA, and expI mutants (Fig. 4, linear mixed model [lmm], chi-square test P = 0.27). However, we also observed that Ecc15 WT loads were approximately 10 times higher than the evf mutant loads considering the entire infection period (Fig. 4, Tukey HSD test, P < 0.001; see also Fig. S6), confirming that evf is required for optimal infection of the larval gut by Ecc15. Importantly, a similar trend was observed in comparisons of the WT to the gacA and expI mutants, the two mutants impaired in evf expression (Fig. 4, Tukey HSD test, P < 0.001; see also Fig. S6), revealing the importance of quorum sensing regulation and the GAC system in the infection process. Taken together, our data show that evf provides Ecc15 with the ability to reach high loads in the insect gut but does not increase its capacity to survive inside it.
FIG 4.
Ecc15 loads are higher in D. melanogaster larvae orally infected with the WT than in those infected with mutants impaired in evf expression. D. melanogaster L3 stage larvae were infected with WT Ecc15 and evf, expI, and gacA mutants for 30 min and then transferred to fresh media. Following the infection period, CFU levels of Ecc15 were measured at the specified time points. Each dot represents CFU of one single larvae (5 larvae per time point). The time point indicated as 0 h after infection corresponds to 30 min of confined exposure to 200 μl of an OD600 of 200. Results from a representative experiment from three independent experiments are shown (results from the other two experiments are shown in Fig. S5). Results of statistical analysis of the comparisons of data from the entire infection period for each condition tested in all three experiments are shown in Fig. S6.
Independent replicates of the experiment shown in Fig. 4 (Ecc15 loads are higher in D. melanogaster larvae orally infected with WT than in mutants impaired in evf expression). (A and B) Replicates of experiments described in the Fig. 4 legend. (C) Statistical groups representing all three experiments described in the Fig. 4 legend. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S6, TIF file, 0.3 MB (265.1KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
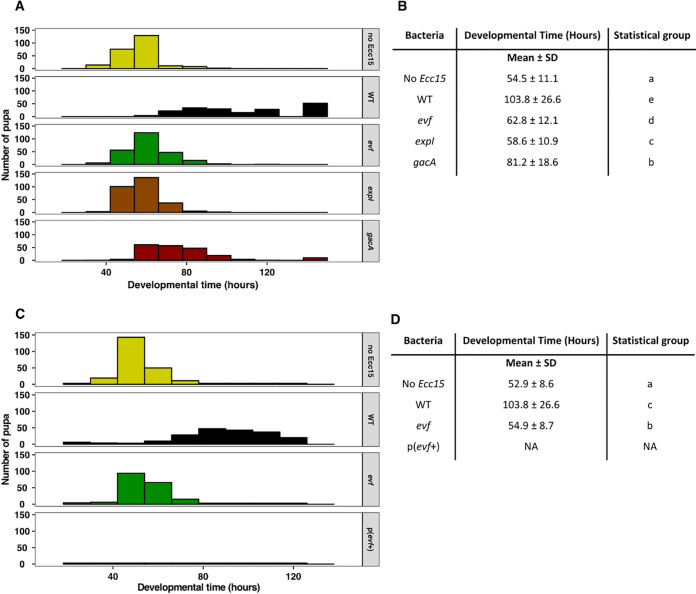
Next, we asked if infection of D. melanogaster larvae by Ecc15 has an effect on larval development given that larvae infected with Ecc15 are shorter than noninfected larvae (58). To investigate this possibility, we infected D. melanogaster L3 stage larvae orally with Ecc15 WT or an evf mutant and followed their development over time. We found that infection by WT Ecc15 delayed passage of D. melanogaster larvae to the pupal stage an average of 49 h, compared to noninfected larvae (Fig. 5A and B; see also Fig. S7; Tukey HSD test, P < 0.001). Moreover, we show that this strong delay was evf dependent, since larvae exposed to an evf mutant showed a delay of only 8 h compared to noninfected larvae (Tukey HSD test, P < 0.001, Fig. 5A and B). We then asked if the mutants in the quorum sensing pathway and GAC system, which have low expression of evf, would show a similar phenotype. We observed that larvae exposed to the expI mutant, which has very low expression of evf, also show a delay of only 4 h with respect to noninfected larvae, similarly to the evf mutant (Tukey HSD test, P < 0.001, Fig. 5A and B). Interestingly, larvae infected with the gacA mutant, which has intermediate levels of evf expression, showed an intermediate developmental delay, taking an average of 27 h longer than noninfected larvae to reach the pupal stage (Tukey HSD test, P < 0.001, Fig. 5A and B). Since the developmental delay correlated with the levels of evf expression in the strains tested, we next examined whether constitutive overexpression of evf would exacerbate the phenotype. We observed that larvae infected with a WT Ecc15 overexpressing evf died before reaching the pupal stage (Fig. 5C and D). These results show that Ecc15 has a negative impact on larval development and that this effect requires both evf and the quorum sensing and GAC regulatory systems.
FIG 5.
Ecc15 causes a developmental delay in D. melanogaster larvae that is dependent on evf, quorum sensing, and the GAC system. (A and C) L3 stage Drosophila larvae pupariation time after exposure to (A) WT Ecc15 and evf, expI, and gacA mutants or (C) WT Ecc15 overexpressing Evf [p(evf+)], compared with noninfected larvae. (B and D) Average developmental time (in hours) with standard deviation (data correspond to the results shown in panels A and C, respectively). Overexpression of Evf was lethal, as larva exposed to WT Ecc15 overexpressing Evf (C) died without reaching the pupa stage. NA, not applicable. Results from a representative experiment of three independent experiments are shown (results from the other two experiments are shown in Fig. S7). The statistical groups represented in panels B and D were determined using a linear mixed-effect model taking into consideration the data from the three experiments. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model.
Independent replicates of the experiment shown in Fig. 5 (Ecc15 causes a developmental delay in D. melanogaster larvae that is dependent on evf, quorum sensing, and the GAC system). (A and B) Replicates of experiments described in the Fig. 5A legend. (C and D) Replicates of experiments described in the Fig. 5C. Larvae exposed to WT Ecc15 overexpressing Evf died without reaching the pupa stage. Download FIG S7, TIF file, 0.3 MB (274.4KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
evf and pelA are coexpressed during plant infection.
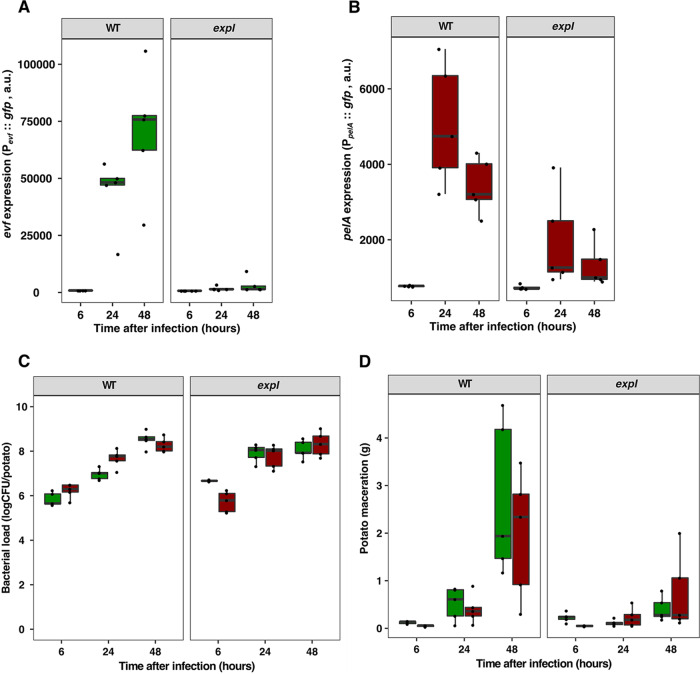
Considering that the interaction of Ecc15 with both insects and plants is thought to be crucial for the life cycle of this bacterial species and that quorum sensing is essential for the expression of both evf and PCWDE, we asked if evf expression is coactivated with pelA (one of the PCWDE) in a quorum sensing-dependent manner during plant infection. We observed that in WT Ecc15, both evf and pelA promoter fusions were expressed 24 h postinfection of potato tubers (Fig. 6A and B). Moreover, we observed that the levels of both evf expression and pelA expression were higher in the WT Ecc15 strain than in an expI mutant (t test, P < 0.01, Fig. 6A and B; see also Fig. S8), even though the bacterial loads at the site of infection were similar for the WT and expI strains (t test, P = 0.37, Fig. 6C; see also Fig. S8). As expected, the levels of plant tissue maceration were higher in potatoes infected with Ecc15 WT than in potatoes infected with the expI mutant (t test, P < 0.01, Fig. 6D; see also Fig. S8). Overall, our results show that there is quorum sensing-dependent coactivation of evf and pelA during plant infection as well as plant tissue maceration.
FIG 6.
Coactivation of evf and pelA expression in plant infections. Potatoes were infected for 6, 24, or 48 h with a WT Ecc15 strain or an expI mutant carrying an evf or pelA gfp reporter plasmid with a constitutive mCherry promoter. At each specified time point, pelA or evf expression, CFU levels, and weight of macerated plant tissue were determined. (A and B) Pevf::gfp expression (A) and PpelA::gfp expression (B) in potatoes infected with WT Ecc15 or expI mutant. (C) CFU of WT Ecc15 or expI mutant expressing mCherry and carrying the Pevf::gfp (green boxes) or the PpelA::gfp (red boxes) reporter plasmids. (D) Potato maceration (in grams) in potatoes infected with WT Ecc15 or expI mutant. Each dot represents an independent potato, n = 5. Results are from a representative experiment of two independent experiments (results from the second experiment are shown in Fig. S8). Results of statistical analysis of comparisons of data from the entire infection period for each condition tested using the data from both experiments are shown in Fig. S8.
Independent replicate of the experiment described in the Fig. 6 legend. (evf expression and pelA expression are coactivated during plant infection). (A) Replicate of experiment shown in Fig. 6A legend. (B) Replicate of experiment shown in Fig. 6B legend. (C) Replicate of experiment shown in Fig. 6C legend. (D) Replicate of experiment shown in Fig. 6D legend. (E) Statistical groups representing the two experiments described in the Fig. 6 legend. Statistical analysis was performed using a linear mixed-effect model. A t test was applied using the estimates from the model. Download FIG S8, TIF file, 0.7 MB (735.7KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Erwinia spp. are phytopathogenic bacteria thought to depend on insects to spread among plant hosts (1, 12, 13). To interact with both plants and insects, Ecc15 relies on different traits that seem to be specific for the interaction with each host. In this bacterium, PCWDE are the major virulence factors required for plant infection (42) and Evf is required to infect D. melanogaster but is not necessary to infect potato tubers (Fig. 2B) (16, 18). It was not known whether WT Ecc15, which relies on multiple hosts for survival, regulates host-specific traits using the same or different signal transduction networks. Here, we showed that the AHL-dependent ExpI/ExpR system, which regulates plant virulence factors (Fig. 1), is also essential for the expression of the insect virulence factor evf, suggesting that the signal transduction networks regulating traits required across hosts are the same. An expI mutant had lower levels of evf expression than the WT, and WT levels could be restored by addition of exogenous AHLs to the growth medium. We also demonstrated that the GAC system, which is thought to respond to the physiological state of the cell (44) and is involved in regulation of plant virulence factors (43, 59), is also necessary for full expression of evf. Additionally, we showed that regulation by these two networks occurs through hor, a conserved transcriptional regulator of the SlyA family (60), previously found to be regulated by quorum sensing in another E. carotovora strain (57). ExpR1 and ExpR2 AHL receptors function as activators of rsmA, the global repressor of the AHL regulon; therefore, we expected the expI expR1 expR2 mutant to have the same levels of evf expression as the expI mutant supplemented with AHLs. However, we found that the expI expR1 expR2 mutant has lower levels of evf expression than both the expI mutant supplemented with AHLs and the WT. Moreover, we showed that complementation of the expI expR1 expR2 mutant with AHLs does not change the level of evf expression. These results show that expR1 and expR2 are required for the response of Ecc15 to AHLs but also indicate that an additional AHL-independent regulator is playing a role in the regulation of evf in this bacterium. One possibility is that Ecc15 has additional orphan luxR genes encoding a DNA binding protein homologous to LuxR that lack a cognate AHL synthase. These orphan genes are divided into two categories: those that have both a LuxR DNA and an AHL binding domain, such as ExpR2, and those that have only the typical LuxR DNA binding domain (61), such as vqsR in Pseudomonas aeruginosa. In this bacterium, in response to an unknown signal, vqsR has been found to downregulate expression of virulence through binding to the promoter region of the quorum sensing receptor qscR, inhibiting its expression without responding to AHLs (62). Because addition of exogenous AHLs to the expI expR1 expR2 mutant does not change the level of evf expression, this unknown regulator is more likely to lie within the second category of orphan LuxR receptors. Our data also suggest that this unknown regulator could be repressed by rsmA, since the expI mutant shows lower levels of evf expression than the expI expR1 expR2 mutant. Another layer of regulation required for PCWDE expression in Erwinia spp. is represented by the detection of external environmental signals such as pectin, a component of the plant cell wall (63, 64). In the absence of plant signals, transcription of PCWDE is repressed. Unlike in the regulation of PCWDE in Erwinia spp., we have no evidence for the need of a host signal in our experimental setting for evf, since we were able to detect evf expression in cells grown in LB without the need for other signals. However, this does not exclude the possibility that environmental signals, perhaps related to insect-derived compounds, have a role in the overall levels of evf expression.
It has been hypothesized that evf was horizontally acquired by Ecc15 and a few other Erwinia spp. As these phytopathogens often use insects as vectors, one hypothesis for the selective benefit of acquiring evf is that this gene might be important for favoring bacterial transmission by strengthening the interaction of Ecc15 with Drosophila. This hypothesis is supported by our results showing that the presence of evf allows the Ecc15 strain to have higher loads at the initial stage of Drosophila larval infection. However, the rate of Ecc15 elimination postinfection was the same in the WT and an evf mutant. This suggests that evf is promoting transmission of Ecc15 by increasing the overall number of bacteria that reach the gut. Moreover, we show that larvae infected with Ecc15 are developmentally delayed compared to noninfected larvae. This delay in larval development is most probably deleterious in an ecological scenario with strong competition between larvae and has been observed also in infection with Pseudomonas fluorescens (65). Although this delay is evf dependent, we do not know its molecular mechanism. Our results are, however, in agreement with previous reports showing that larvae infected with WT Ecc15 had epithelial cell damage (7, 16, 17) and were smaller due to inhibition of gut proteolytic activity in turn promoted by Drosophila-associated Lactobacillus species (58). These studies, together with our results, show that evf expression in Ecc15 has an overall deleterious effect on the host and thus that acquisition of evf, which enables higher host loads and is presumably beneficial for bacterial transmission, seems to have resulted in a trade-off for host fitness. Interestingly, evf homologues with low (below 40%) amino acid sequence identity, but with a predicted secondary structure highly similar to that of the Evf (66), can be found in other multihost bacteria such as the insect pathogen Photorhabdus luminescens (19) (locus PLU2433). P. luminescens colonizes the gut of Heterorhabditis bacteriophora, a nematode preying on insects (67, 68). The nematode enters through the insect’s respiratory and/or digestive tract and regurgitates the bacteria into its hemolymph. Once in the hemolymph, P. luminescens produces a battery of toxins that kill the insect, allowing the nematode to feed on the corpse and favoring P. luminescens recolonization (69–71). However, the role of this evf homologue in the establishment of interactions with either of P. luminescens hosts was never addressed.
Quorum sensing regulation is associated with tight control of density-dependent activation of genes encoding functions that are often essential for the establishment of host-microbe interactions (27). For instance, in the interaction between the squid Euprymna scolopes and V. fischeri, mutants with mutations in the quorum sensing system are less efficient in persisting in the light organ, being outcompeted by other strains (72, 73). Here, we show that Ecc15 relies on quorum sensing to regulate both PCWDE and the evf-mediated developmental delay in infected Drosophila larvae. Moreover, overexpression of evf leads to a complete developmental arrest of larvae, eventually killing them. Therefore, one possible benefit of having evf expression under the control of these networks might be minimization of the detrimental effect that the evf-dependent infection has on the insect host while still enabling a transient infection. On the other hand, insects are attracted to rotten plant tissue, and if evf is important for promoting the interaction of Ecc15 with its insect vector (Drosophila), synchronization of the expression of evf with that of the PCWDE might have been selected for as advantageous for bacterial dissemination. Indeed, we observed that evf and pelA (one of the PCWDE genes) are coexpressed during plant infection, even though evf is not required for plant maceration. Interestingly, although the quorum sensing system was necessary for expression of these genes and for maceration, lack of quorum sensing and of these traits did not affect bacterial loads during plant infection. One possibility is that maceration of the plant tissue is not necessary to sustain bacterial growth in the potato but might be needed to attract the insect and ensure transmission of bacteria to the next plant host. Therefore, it is possible that control of PCWDE expression and control of evf expression are intertwined such that, following colonization of the plant, evf expression is triggered, anticipating the appearance of the insect vector which is attracted to rotten plant tissue and thus maximizing the probability of establishing the interaction with this host vector. This phenomenon, called predictive behavior, is particularly common in symbiotic relationships where the microbe often experiences predictable cyclic environments (74). In mammalian hosts, a very predictable change that occurs during the transition from the outside environment to the oral cavity is an immediate increase in temperature followed by a decrease in the oxygen level. This phenomenon has been described for Escherichia coli gut colonization, where, coupled to an increase in temperature, downregulation of genes related to aerobic respiration is observed (75). Yersinia pestis, the etiological agent of plague, also experiences rapid environmental changes when transitioning between its two hosts. This bacterium colonizes the gut of fleas, particularly the proventriculus, forming a biofilm and blocking the digestive tract. This blockage induces a feeding behavior characterized by repetitive biting that increases the chances of successful transmission of Yersinia to the mammal host (76, 77). In the flea gut, Yersinia upregulates the expression of genes which do not provide an advantage in the colonization of the flea gut but which promote an increased resistance to phagocytosis by mammalian macrophages (78). This preconditioning mechanism, akin to a predictive behavior, provides Y. pestis with the ability to resist the first encounter with the host defenses, allowing subsequent expression of the remaining traits necessary to overcome the host immune system (79–81). Quorum sensing may play a role in activating the anticipatory genes in this bacterium because it has been shown to regulate genes important for colonization of flea and mammals (82, 83). Additionally, quorum sensing regulation in this bacterium is altered by changes in the metabolic environment related to different stages of the infection (84). Interestingly, P. luminescens also relies on different sets of genes to interact with each of its hosts (85, 86). This bacterium possesses two phenotypic variants: a primary one that is virulent to the insect and able to colonize the nematode and a secondary one that, while still virulent to the insect, is unable to support the development of the nematode (87, 88). However, it is not known if the traits involved in these processes are coregulated or not, but it is known that quorum sensing is required for the regulation of genes important in the interactions with the two hosts and thus that these genes might also be coregulated (89–92). Therefore, P. luminescens and Y. pestis are examples of bacteria which rely on different sets of virulence factors for the interactions with the different hosts and where quorum sensing plays a major role in regulating these traits. It is possible that anticipatory expression of virulence factors for the next stage may be regulated by quorum sensing in these bacteria, and in others that rely on multihost infections, as we have shown here for evf in Ecc15.
Our results show that, in Ecc15, the regulatory networks responding to self-produced quorum sensing signals and physiological cues sensed by the GAC system are used to coregulate expression of traits required to infect different hosts. Thus, the signal transduction mechanisms are the same even though the functions involved in the interactions with each plant or insect host are largely different. Therefore, our findings reinforce the idea of a central role of quorum sensing in the regulatory circuitry controlling the array of traits used by bacteria to interact with diverse hosts.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table S1 of the supplemental material. All bacterial strains used were derived from the wild-type (WT) Ecc15 strain (7). Ecc15 and mutants were grown at 30°C with aeration in Luria-Bertani medium (LB). When specified, medium was supplemented with 0.4% polygalacturonic acid (PGA; Sigma catalog no. P3850) to induce expression of PCWDE. Escherichia coli DH5α was used for cloning procedures and was grown at 37°C with aeration in LB. When required, antibiotics were used at the following concentrations (mg liter−1): ampicillin (Amp), 100; kanamycin (Kan), 50; spectinomycin (Spec), 50; chloramphenicol (Cm), 25. To assess bacterial growth, optical density at 600 nm (OD600) was determined in a Thermo Spectronic Helios delta spectrophotometer.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (16.5KB, docx) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genetic and molecular techniques.
All primer sequences used in this study are listed in Table S2 in supplemental material. The Ecc15 deletion mutants listed in Table S1 were constructed by chromosomal gene replacement with an antibiotic marker using the λ-Red recombinase system (93). Plasmid pLIPS, able to replicate in Ecc15 and carrying the arabinose-inducible λ-Red recombinase system, was used (35). Briefly, the DNA region of the target gene, including approximately 500 bp upstream and downstream from the gene, was amplified by PCR and cloned into pUC18 (94) using restriction enzymes. These constructs, containing the target gene and its flanking regions, were divergently amplified by PCR, to introduce an XhoI restriction site in the 5′ and 3′ regions and to remove the native coding sequence of the target gene. The kanamycin cassette from pkD4 was amplified with primers also containing the XhoI restriction site. The fragment containing the kanamycin cassette was then digested with XhoI and was introduced into the XhoI-digested PCR fragment carrying the flanking regions of the target gene. The final construct, containing the kanamycin cassette flanked by the upstream and downstream regions of the target gene, was then amplified by PCR, and approximately 2 μg of DNA was electroporated into the parental strain (FDV31) expressing the λ-Red recombinase system from pLIPS, to favor recombination. To construct the plasmid carrying the evf promoter fused to GFP (pFDV54), a fragment of 503 bp containing the evf promoter was amplified from WT Ecc15 DNA with the primers P1194 and P1195. This fragment was then digested with HindIII and SphI and ligated to pUC18. GFP was amplified from the pCMW1 (95) vector using primers P0576 and P0665. Both the GFP and pUC18-Pevf were digested with SphI and BamHI followed by ligation, and a 2-μl volume of the ligation reaction mixture was used to transform Dh5α (pFDV54). The same procedure was used for the Phor::gfp fusion using primers P1351 and P1352 for promoter amplification (493 bp) and primers P1353 and P1354 for GFP amplification. Digestions were made with enzymes HindIII/PstI and PstI/XbaI (pFDV84). For PpelA, primers P1941 and P1942 were used for promoter amplification (300 bp) and GFP was amplified using P1333 and P1334. Digestions were made using HindIII/XbaI and XbaI/SacI. For hor overexpression and for expR1, expR2, and gacA complementation, a NcoI site was introduced in pOM1-Pevf::gfp with primers P1309 and P1310. hor was amplified using primers P1311 and P1312 from WT template DNA. Then, both the plasmid and the fragment carrying hor were digested with NcoI and SacI and subsequently ligated (pFDV104). expR1 and gacA were amplified using primers P1943 and P1944 and primers P1947 and P1948 from WT template DNA. Then, both the plasmid and the fragment carrying the desired gene were digested with EcoRI and SacI and subsequently ligated (pFDV104). For expR2 complementation, a fragment was amplified from WT template DNA with primers P1958 and P1959, digested with XmnI, and ligated into the plasmid containing the expR1 and Pevf::gfp expression reporter. pOM1-mCherry was constructed by digesting pOM1 with XmnI and ligating a fragment of 825 bp amplified with primers P1789 and P1790 from genomic DNA of strain RB290 containing the constitutive mCherry fusion.
Primers used in this study. Download Table S2, DOCX file, 0.01 MB (14.2KB, docx) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PCR for cloning purposes was performed using the proofreading enzyme Bio-X-ACT (Bioline). Other PCRs were performed using Dream Taq polymerase (Fermentas). Digestions were performed with Fast Digest enzymes (Fermentas), and ligations were performed with T4 DNA ligase (New England Biolabs). All cloning steps were performed in either E. coli DH5α or WT Ecc15. All mutants and constructs were confirmed by PCR amplification and subsequent Sanger sequencing performed at the Instituto Gulbenkian de Ciência sequencing facility.
Pectate lyase activity assay.
Ecc15 and mutants were grown overnight in LB with 0.4% PGA, inoculated into fresh media to a starting OD600 of 0.05, and incubated at 30°C with aeration. After 6 h of incubation, aliquots were collected to evaluate growth and to analyze pectate lyase (Pel) activity in cell-free supernatants, using the previously described procedure (59) based on the thiobarbituric acid colorimetric method (96). Each experiment included at least 5 independent cultures per genotype and was repeated on 3 independent days.
Plant virulence assay.
Plant virulence was analyzed by assessing the maceration of potato tubers with a protocol adapted from previous studies (35, 97). Potatoes were washed and surface sterilized by soaking for 10 min in 10% bleach, followed by 10 min in 70% ethanol. Overnight cultures in LB broth were washed twice and diluted to an OD600 of 0.05 in phosphate-buffered saline (PBS). Thirty-microliter aliquots were then used to inoculate the previously punctured potatoes. Potato tubers were incubated at 28°C at a relative humidity above 90% for 6, 24, or 48 h. After incubation, potatoes were sliced, and macerated tissue was collected and weighed.
Promoter expression assays.
Ecc15 bacterial cells carrying the different plasmid-borne promoter reporter fusions were grown overnight in LB supplemented with spectinomycin (LB + Spec), inoculated into fresh medium at a starting OD600 of 0.05, and incubated at 30°C with aeration. At the indicated time points, aliquots were collected to assess growth and the expression of the reporter fusion. For the analyses of reporter expression, aliquots of the cultures were diluted 1:100 in PBS and expression was measured by flow cytometry (LSRFortessa; BD) and analyzed with Flowing Software v 2.5.1, as previously described (59). A minimum of 10,000 green fluorescent protein (GFP)-positive single cells were acquired per sample. Expression of the promoter-gfp fusions is reported as the median GFP expression of GFP-positive single cells in arbitrary units. Each experiment included at least 5 independent cultures per genotype and was repeated on 3 independent days.
Drosophila stocks.
DrosDel w1118 isogenic stock (w1118 iso) was used in all experiments (98, 99). Stocks were maintained at 25°C in standard corn meal fly medium composed of water (1.1 liter), 45 g molasses, 75 g of sugar, 10 g agar, 70 g cornmeal, and 20 g yeast. Food was autoclaved and cooled to 45°C before addition of 30 ml of a solution containing 0.2 g of carbendazim (Sigma), 100 g of methylparaben (Sigma), and 1 liter of absolute ethanol. Experiments were performed at 28°C
Developmental delay and bacterial CFU assays.
Egg-laying took place in cages containing adult flies at a ratio of 3 females to 1 male. To synchronize the embryo stages, flies were initially incubated for 1 h at 25°C to lay prior fertilized eggs. After this initial incubation, flies were transferred to new cages where eggs were laid for 4 to 6 h in the presence of standard corn meal fly medium. After this period, eggs were removed and incubated at 25°C for 72 h to obtain L3-stage larvae. For bacterial infections, third-instar larvae were placed in a 2-ml Eppendorf tube containing 200 μl of concentrated bacterium pellet (OD600 = 200) from an overnight culture and 400 μl of standard corn meal fly medium. Larvae, bacteria, and food were then thoroughly mixed using a spoon, and the Eppendorf tube was closed with a foam plug and incubated at room temperature (RT) for 30 min. The mix was then transferred to a 25-ml plastic tube containing 7.5 ml of standard corn meal fly medium and incubated at 28°C. To assess development of the larvae postinfection, pupa were counted every 12 h for 5 days. For CFU counts, larvae were inoculated as described above. At each time point, 5 larvae were randomly collected, surface sterilized for 10 s in ethanol 70%, and washed with Milli-Q water. Individual larvae were then transferred to Eppendorf tubes containing 300 μl of 1× PBS and homogenized with a blender. The homogenate was diluted 100-fold, and serial dilutions were plated in LB. Plates were incubated overnight at 30°C.
Promoter expression assays in plant infections.
Ecc15 bacteria carrying either of the different plasmid-borne promoter GFP reporter fusions (Pevf::gfp or PpelA::gfp) and a constitutive mCherry fusion were grown overnight in LB supplemented with spectinomycin (LB + Spec). Bacterial cells (2 ml) were collected and washed twice in 1× PBS. Potatoes were infected as described in the plant virulence assay section. At the indicated time points, potatoes were sliced, and macerated tissue was weighted. To isolate bacterial cells from potato tissue, approximately 0.5 g (or all the soft tissue if the weight was lower than 0.5 g) was introduced into an Eppendorf tube containing 250 μl of 1× PBS. Bacterial cells were isolated from the plant tissue by adapting the previously described protocol (100). Briefly, tissue was mechanically disrupted using a pipette tip before addition of 750 μl of 1× PBS to the collection tube. The disrupted pellets were then subjected to 4 iterations of mixing using a vortex mixer for 15 s at medium speed followed by centrifugation at 2,000 rpm at RT for 1 min, recovery of the 750 μl of 1× PBS into a new tube, and replacement of that volume of PBS before the next iteration. The resulting 3 ml of isolated cells was pelleted for 5 min at 4,000 × g for 5 min at RT, the supernatant was discarded, and the cells were resuspended in 1 ml of 1× PBS. For CFU counts at the infection site, 100 μl of the recovered cells was diluted, plated in LB + Spec, and incubated overnight at 30°C and red colonies were counted. For the analyses of reporter expression, aliquots of the recovered cells were diluted 1:100 in PBS and expression was measured by flow cytometry (LSRFortessa; BD) and analyzed with Flowing Software v 2.5.1, as previously described (59). Potatoes were also infected with an Ecc15 strain carrying a plasmid with a constitutive mCherry promoter fusion and no GFP fusion; bacteria collected from these infected potatoes were used to define the gating that allows distinguishing between potato debris and bacterial cells. A minimum of 10,000 GFP and mCherry doubly positive single cells were acquired per sample. Expression of the promoter-gfp fusions is reported as the median level GFP expression of doubly fluorescence-positive single cells in arbitrary units. Each experiment included at least 5 independent cultures per genotype, and all experiments were repeated on 2 independent days.
Statistical analysis.
Statistical analyses were performed in R (101), and graphs were generated using the package ggplot2 (102). All experiments were analyzed using linear mixed-effect models (package lme4, updated version 1.1-20 [103]). Significance of interactions between factors was tested by comparing models fitting the data with and without the interactions using analysis of variance (ANOVA). Models were simplified when interaction data were not statistically significant. Multiple comparisons of the estimates from fitted models were performed with a Tukey HSD (honestly significant difference) test (packages lmerTest [104] and multicomp [105, 106]). A letter is assigned to each statistical group; differing letters stand for statistically significant differences.
ACKNOWLEDGMENTS
We thank Joana Amaro for technical assistance and Rita Valente, Vitor Cabral, Tanja Dapa, Inês Torcato, and André Carvalho for suggestions and helpful comments on the manuscript. We thank Roberto Balbontín for helpful comments on the manuscript and for sharing of the E. coli mCherry strain. We are very grateful to Bruno Lemaitre (EPFL) for sharing protocols and Ecc15 strain.
K.B.X. and F.J.D.V. acknowledge support from Portuguese national funding agency Fundação para a Ciência e Tecnologia (FCT) for individual grants IF/00831/2015 and SRFH/BD/113986/2015 within the scope of the Ph.D. program Molecular Biosciences PD/00133/2012, respectively. This work was supported by the research infrastructure ONEIDA projects (LISBOA-01-0145-FEDER-016417 and LISBOA-01-0145-FEDER-022170) cofunded by Fundos Europeus Estruturais e de Investimento from Programa Operacional Regional Lisboa 2020 to K.B.X. and L.T. and Marie Curie (PIEF-GA-2011-301365) to P.N.-J.
Footnotes
Citation Vieira FJD, Nadal-Jimenez P, Teixeira L, Xavier KB. 2020. Erwinia carotovora quorum sensing system regulates host-specific virulence factors and development delay in Drosophila melanogaster. mBio 11:e01292-20. https://doi.org/10.1128/mBio.01292-20.
Contributor Information
Christopher M. Waters, Michigan State University.
Edward G. Ruby, University of Hawaii at Manoa.
REFERENCES
- 1.Eigenbrode SD, Bosque-Pérez NA, Davis TS. 2018. Insect-borne plant pathogens and their vectors: ecology, evolution, and complex interactions. Annu Rev Entomol 63:169–191. doi: 10.1146/annurev-ento-020117-043119. [DOI] [PubMed] [Google Scholar]
- 2.Nadarasah G, Stavrinides J. 2011. Insects as alternative hosts for phytopathogenic bacteria. FEMS Microbiol Rev 35:555–575. doi: 10.1111/j.1574-6976.2011.00264.x. [DOI] [PubMed] [Google Scholar]
- 3.Vallet-Gely I, Lemaitre B, Boccard F. 2008. Bacterial strategies to overcome insect defences. Nat Rev Microbiol 6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 4.Matthews KR. 2011. Controlling and coordinating development in vector-transmitted parasites. Science 331:1149–1153. doi: 10.1126/science.1198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 6.Buchon N, Broderick NA, Lemaitre B. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 7.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Leulier F, Parquet C, Pili-Floury S, Ryu J-H, Caroff M, Lee W-J, Mengin-Lecreulx D, Lemaitre B. 2003. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol 4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- 9.Bae YS, Choi MK, Lee W-J. 2010. Dual oxidase in mucosal immunity and host–microbe homeostasis. Trends Immunol 31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Redak RA, Purcell AH, Lopes JRS, Blua MJ, Mizell RF, Andersen PC. 2004. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu Rev Entomol 49:243–270. doi: 10.1146/annurev.ento.49.061802.123403. [DOI] [PubMed] [Google Scholar]
- 11.Menelas B, Block CC, Esker PD, Nutter FW. 2006. Quantifying the feeding periods required by corn flea beetles to acquire and transmit Pantoea stewartii. Plant Dis 90:319–324. doi: 10.1094/PD-90-0319. [DOI] [PubMed] [Google Scholar]
- 12.Kloepper JW, Brewer JW, Harrison MD. 1981. Insect transmission of Erwinia carotovora var.carotovora and Erwinia carotovora var.atroseptica to potato plants in the field. Am Potato J 58:165–175. doi: 10.1007/BF02854416. [DOI] [Google Scholar]
- 13.Molina JJ, Harrison MD, Brewer JW. 1974. Transmission of Erwinia carotovora var.atroseptica by Drosophila melanogaster Meig. I. Acquisition and transmission of the bacterium. Am Potato J 51:245–250. doi: 10.1007/BF02851435. [DOI] [Google Scholar]
- 14.Perombelon MCM, Kelman A. 1980. Ecology of the soft rot erwinias. Annu Rev Phytopathol 18:361–387. doi: 10.1146/annurev.py.18.090180.002045. [DOI] [Google Scholar]
- 15.Shapiro L, De Moraes CM, Stephenson AG, Mescher MC, van der Putten W. 2012. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol Lett 15:1430–1438. doi: 10.1111/ele.12001. [DOI] [PubMed] [Google Scholar]
- 16.Basset A, Khush RS, Braun A, Gardan L, Boccard F, Hoffmann JA, Lemaitre B. 2000. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc Natl Acad Sci U S A 97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houtz P, Bonfini A, Bing X, Buchon N. 2019. Recruitment of adult precursor cells underlies limited repair of the infected larval midgut in Drosophila. Cell Host Microbe 26:412–425.e5. doi: 10.1016/j.chom.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Basset A, Tzou P, Lemaitre B, Boccard F. 2003. A single gene that promotes interaction of a phytopathogenic bacterium with its insect vector, Drosophila melanogaster. EMBO Rep 4:205–209. doi: 10.1038/sj.embor.embor730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muniz CA, Jaillard D, Lemaitre B, Boccard F. 2007. Erwinia carotovora Evf antagonizes the elimination of bacteria in the gut of Drosophila larvae. Cell Microbiol 9:106–119. doi: 10.1111/j.1462-5822.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 20.Kamareddine L, Wong ACN, Vanhove AS, Hang S, Purdy AE, Kierek-Pearson K, Asara JM, Ali A, Morris JG Jr, Watnick PI. 2018. Activation of Vibrio cholerae quorum sensing promotes survival of an arthropod host. Nat Microbiol 3:243–252. doi: 10.1038/s41564-017-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enomoto S, Chari A, Clayton AL, Dale C. 2017. Quorum sensing attenuates virulence in Sodalis praecaptivus. Cell Host Microbe 21:629–636.e5. doi: 10.1016/j.chom.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perchat S, Talagas A, Poncet S, Lazar N, Li de la Sierra-Gallay I, Gohar M, Lereclus D, Nessler S. 2016. How quorum sensing connects sporulation to necrotrophism in Bacillus thuringiensis. PLoS Pathog 12:e1005779. doi: 10.1371/journal.ppat.1005779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S-J, Kim S-K, So Y-I, Park H-Y, Li X-H, Yeom DH, Lee M-N, Lee B-L, Lee J-H. 2014. Protease IV, a quorum sensing-dependent protease of Pseudomonas aeruginosa modulates insect innate immunity. Mol Microbiol 94:1298–1314. doi: 10.1111/mmi.12830. [DOI] [PubMed] [Google Scholar]
- 24.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassler BL. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2:582–587. doi: 10.1016/s1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 26.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, Bassler BL. 2019. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol 17:371–382. doi: 10.1038/s41579-019-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattinen L, Tshuikina M, Mäe A, Pirhonen M. 2004. Identification and characterization of Nip, necrosis-inducing virulence protein of Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact 17:1366–1375. doi: 10.1094/MPMI.2004.17.12.1366. [DOI] [PubMed] [Google Scholar]
- 29.Saarilahti HT, Henrissat B, Palva ET. 1990. CelS: a novel endoglucanase identified from Erwinia carotovora subsp. carotovora. Gene 90:9–14. doi: 10.1016/0378-1119(90)90433-R. [DOI] [PubMed] [Google Scholar]
- 30.Marits R, Koiv V, Laasik E, Mae A. 1999. Isolation of an extracellular protease gene of Erwinia carotovora subsp. carotovora strain SCC3193 by transposon mutagenesis and the role of protease in phytopathogenicity. Microbiology 145:1959–1966. doi: 10.1099/13500872-145-8-1959. [DOI] [PubMed] [Google Scholar]
- 31.Mäe A, Heikinheimo R, Palva ET. 1995. Structure and regulation of the Erwinia carotovora subspecies carotovora SCC3193 cellulase gene celV1 and the role of cellulase in phytopathogenicity. Mol Gen Genet 247:17–26. doi: 10.1007/BF00425817. [DOI] [PubMed] [Google Scholar]
- 32.Pirhonen M. 1991. Identification of pathogenicity determinants of Erwinia carotovora subsp. carotovora by transposon mutagenesis. Mol Plant Microbe Interact 4:276. doi: 10.1094/MPMI-4-276. [DOI] [Google Scholar]
- 33.Eriksson ARB, Andersson RA, Pirhonen M, Palva ET. 1998. Two-component regulators involved in the global control of virulence in Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact 11:743–752. doi: 10.1094/MPMI.1998.11.8.743. [DOI] [PubMed] [Google Scholar]
- 34.Andersson RA, Eriksson AR, Heikinheimo R, Mäe A, Pirhonen M, Kõiv V, Hyytiäinen H, Tuikkala A, Palva ET. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR(Ecc). Mol Plant Microbe Interact 13:384–393. doi: 10.1094/MPMI.2000.13.4.384. [DOI] [PubMed] [Google Scholar]
- 35.Valente RS, Nadal-Jimenez P, Carvalho AFP, Vieira FJD, Xavier KB. 2017. Signal integration in quorum sensing enables cross-species induction of virulence in Pectobacterium wasabiae. mBio 8:e00398-17. doi: 10.1128/mBio.00398-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moleleki LN, Pretorius RG, Tanui CK, Mosina G, Theron J. 2017. A quorum sensing-defective mutant of Pectobacterium carotovorum ssp. brasiliense 1692 is attenuated in virulence and unable to occlude xylem tissue of susceptible potato plant stems. Mol Plant Pathol 18:32–44. doi: 10.1111/mpp.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirhonen M, Flego D, Heikinheimo R, Palva ET. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J 12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Bodman SB, Ball JK, Faini MA, Herrera CM, Minogue TD, Urbanowski ML, Stevens AM. 2003. The quorum sensing negative regulators EsaR and ExpR(Ecc), homologues within the LuxR family, retain the ability to function as activators of transcription. J Bacteriol 185:7001–7007. doi: 10.1128/jb.185.23.7001-7007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nealson KH. 1977. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol 112:73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- 40.Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol 104:313–322. doi: 10.1128/JB.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai C-S, Winans SC. 2010. LuxR-type quorum-sensing regulators that are detached from common scents. Mol Microbiol 77:1072–1082. doi: 10.1111/j.1365-2958.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeb S, Haas D. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact 14:1351–1363. doi: 10.1094/MPMI.2001.14.12.1351. [DOI] [PubMed] [Google Scholar]
- 44.Lapouge K, Schubert M, Allain F-T, Haas D. 2008. Gac/Rsm signal transduction pathway of γ-proteobacteria: from RNA recognition to regulation of social behaviour: regulation of RsmA/CsrA binding to RNA. Mol Microbiol 67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 45.Hrabak EM, Willis DK. 1992. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol 174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babitzke P, Romeo T. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol 10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Bejerano-Sagie M, Xavier KB. 2007. The role of small RNAs in quorum sensing. Curr Opin Microbiol 10:189–198. doi: 10.1016/j.mib.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee A, Cui Y, Hasegawa H, Leigh N, Dixit V, Chatterjee AK. 2005. Comparative analysis of two classes of quorum-sensing signaling systems that control production of extracellular proteins and secondary metabolites in Erwinia carotovora subspecies. J Bacteriol 187:8026–8038. doi: 10.1128/JB.187.23.8026-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Praillet T, Nasser W, Robert-Baudouy J, Reverchon S. 1996. Purification and functional characterization of PecS, a regulator of virulence-factor synthesis in Erwinia chrysanthemi. Mol Microbiol 20:391–402. doi: 10.1111/j.1365-2958.1996.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Cui Y, Mukherjee A, Chatterjee AK. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol Microbiol 29:219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- 51.Monson RE, Apagyi K, Bowden SD, Simpson N, Williamson NR, Cubitt MF, Harris S, Toth IK, Salmond G. 2019. The rsmS (ybaM) mutation causes bypass suppression of the RsmAB post-transcriptional virulence regulation system in enterobacterial phytopathogens. Sci Rep 9 doi: 10.1038/s41598-019-40970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui Y, Chatterjee A, Hasegawa H, Dixit V, Leigh N, Chatterjee AK. 2005. ExpR, a LuxR homolog of Erwinia carotovora subsp. carotovora, activates transcription of rsmA, which specifies a global regulatory RNA-binding protein. J Bacteriol 187:4792–4803. doi: 10.1128/JB.187.14.4792-4803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjöblom S, Brader G, Koch G, Palva ET. 2006. Cooperation of two distinct ExpR regulators controls quorum sensing specificity and virulence in the plant pathogen Erwinia carotovora. Mol Microbiol 60:1474–1489. doi: 10.1111/j.1365-2958.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 54.Cui Y, Chatterjee A, Liu Y, Dumenyo CK, Chatterjee AK. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-L-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol 177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee A, Cui Y, Liu Y, Dumenyo CK, Chatterjee AK. 1995. Inactivation of RsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-L-homoserine lactone. Appl Environ Microbiol 61:1959–1967. doi: 10.1128/AEM.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson NR, Cox A, Bycroft BW, Stewart GS, Williams P, Salmond GP. 1997. The rap and hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol Microbiol 26:531–544. doi: 10.1046/j.1365-2958.1997.5981976.x. [DOI] [PubMed] [Google Scholar]
- 57.Sjöblom S, Harjunpää H, Brader G, Palva ET. 2008. A novel plant ferredoxin-like protein and the regulator Hor are quorum-sensing targets in the plant pathogen Erwinia carotovora. Mol Plant Microbe Interact 21:967–978. doi: 10.1094/MPMI-21-7-0967. [DOI] [PubMed] [Google Scholar]
- 58.Erkosar B, Storelli G, Mitchell M, Bozonnet L, Bozonnet N, Leulier F. 2015. Pathogen virulence impedes mutualist-mediated enhancement of host juvenile growth via inhibition of protein digestion. Cell Host Microbe 18:445–455. doi: 10.1016/j.chom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valente RS, Xavier KB. 2016. The Trk potassium transporter is required for RsmB-mediated activation of virulence in the phytopathogen Pectobacterium wasabiae. J Bacteriol 198:248–255. doi: 10.1128/JB.00569-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf HJ, Goebel W. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol Gen Genet 249:474–486. doi: 10.1007/BF00290573. [DOI] [PubMed] [Google Scholar]
- 61.Patankar AV, González JE. 2009. Orphan LuxR regulators of quorum sensing. FEMS Microbiol Rev 33:739–756. doi: 10.1111/j.1574-6976.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 62.Liang H, Deng X, Ji Q, Sun F, Shen T, He C. 2012. The Pseudomonas aeruginosa global regulator VqsR directly inhibits QscR to control quorum-sensing and virulence gene expression. J Bacteriol 194:3098–3108. doi: 10.1128/JB.06679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reverchon S, Nasser W, Robert-Baudouy J. 1991. Characterization of kdgR, a gene of Erwinia chrysanthemi that regulates pectin degradation. Mol Microbiol 5:2203–2216. doi: 10.1111/j.1365-2958.1991.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 64.Reverchon S, Expert D, Robert-Baudouy J, Nasser W. 1997. The cyclic AMP receptor protein is the main activator of pectinolysis genes in Erwinia chrysanthemi. J Bacteriol 179:3500–3508. doi: 10.1128/jb.179.11.3500-3508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olcott MH, Henkels MD, Rosen KL, LWalker F, Sneh B, Loper JE, Taylor BJ. 2010. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS One 5:e12504. doi: 10.1371/journal.pone.0012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg M. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciche TA, Kim K.-s, Kaufmann-Daszczuk B, Nguyen KCQ, Hall DH. 2008. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl Environ Microbiol 74:2275–2287. doi: 10.1128/AEM.02646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterfield NR, Ciche T, Clarke D. 2009. Photorhabdus and a host of hosts. Annu Rev Microbiol 63:557–574. doi: 10.1146/annurev.micro.091208.073507. [DOI] [PubMed] [Google Scholar]
- 69.Blackburn M, Golubeva E, Bowen D, Ffrench-Constant RH. 1998. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl Environ Microbiol 64:3036–3041. doi: 10.1128/AEM.64.8.3036-3041.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daborn PJ, Waterfield N, Silva CP, Au CPY, Sharma S, Ffrench-Constant RH. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci U S A 99:10742–10747. doi: 10.1073/pnas.102068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dowling AJ, Daborn PJ, Waterfield NR, Wang P, Streuli CH, Ffrench-Constant RH. 2004. The insecticidal toxin Makes caterpillars floppy (Mcf) promotes apoptosis in mammalian cells. Cell Microbiol 6:345–353. doi: 10.1046/j.1462-5822.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 72.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol 182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, LaSota ED, Cecere AG, LaPenna KB, Larios-Valencia J, Wollenberg MS, Miyashiro T. 2016. Intraspecific competition impacts Vibrio fischeri strain diversity during initial colonization of the squid light organ. Appl Environ Microbiol 82:3082–3091. doi: 10.1128/AEM.04143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao M, Goodrich-Blair H. 2017. Ready or not: microbial adaptive responses In dynamic symbiosis environments. J Bacteriol 199 doi: 10.1128/JB.00883-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tagkopoulos I, Liu Y-C, Tavazoie S. 2008. Predictive behavior within microbial genetic networks. Science 320:1313–1317. doi: 10.1126/science.1154456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry RD, Fetherston JD. 1997. Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev 10:35–66. doi: 10.1128/CMR.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinnebusch BJ, Rudolph AE, Cherepanov P, Dixon JE, Schwan TG, Forsberg A. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296:733–735. doi: 10.1126/science.1069972. [DOI] [PubMed] [Google Scholar]
- 78.Vadyvaloo V, Jarrett C, Sturdevant DE, Sebbane F, Hinnebusch BJ. 2010. Transit through the flea vector induces a pretransmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog 6:e1000783. doi: 10.1371/journal.ppat.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cornelis GR. 2002. The Yersinia Ysc-Yop “type III” weaponry. Nat Rev Mol Cell Biol 3:742–752. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- 80.Burrows TW, Bacon GA. 1956. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br J Exp Pathol 37:286–299. [PMC free article] [PubMed] [Google Scholar]
- 81.Cavanaugh DC, Randall R. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol 83:348–363. [PubMed] [Google Scholar]
- 82.LaRock CN, Yu J, Horswill AR, Parsek MR, Minion FC. 2013. Transcriptome analysis of acetyl-homoserine lactone-based quorum sensing regulation in Yersinia pestis. PLoS One 8:e62337. doi: 10.1371/journal.pone.0062337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J, Madsen ML, Carruthers MD, Phillips GJ, Kavanaugh JS, Boyd JM, Horswill AR, Minion FC. 2013. Analysis of autoinducer-2 quorum sensing in Yersinia pestis. Infect Immun 81:4053–4062. doi: 10.1128/IAI.00880-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ritzert JT, Minasov G, Embry R, Schipma MJ, Satchell K. 2019. The cyclic AMP receptor protein regulates quorum sensing and global gene expression in Yersinia pestis during planktonic growth and growth in biofilms. mBio 10:e02613-19. doi: 10.1128/mBio.02613-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Somvanshi VS, Sloup RE, Crawford JM, Martin AR, Heidt AJ, Kim K, Clardy J, Ciche TA. 2012. A single promoter inversion switches Photorhabdus between pathogenic and mutualistic states. Science 337:88–93. doi: 10.1126/science.1216641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Easom CA, Joyce SA, Clarke DJ. 2010. Identification of genes involved in the mutualistic colonization of the nematode Heterorhabditis bacteriophora by the bacterium Photorhabdus luminescens. BMC Microbiol 10:45. doi: 10.1186/1471-2180-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boemare NE, Akhurst RJ. 1988. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae). Microbiology 134:751–761. doi: 10.1099/00221287-134-3-751. [DOI] [Google Scholar]
- 88.Clarke DJ. 2014. The genetic basis of the symbiosis between Photorhabdus and its invertebrate hosts, p 1–29. In Advances in applied microbiology. Elsevier, Amsterdam, the Netherlands. [DOI] [PubMed] [Google Scholar]
- 89.Brachmann AO, Brameyer S, Kresovic D, Hitkova I, Kopp Y, Manske C, Schubert K, Bode HB, Heermann R. 2013. Pyrones as bacterial signaling molecules. Nat Chem Biol 9:573–578. doi: 10.1038/nchembio.1295. [DOI] [PubMed] [Google Scholar]
- 90.Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles J-F, Dassa E, Derose R, Derzelle S, Freyssinet G, Gaudriault S, Médigue C, Lanois A, Powell K, Siguier P, Vincent R, Wingate V, Zouine M, Glaser P, Boemare N, Danchin A, Kunst F. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol 21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 91.Brameyer S, Kresovic D, Bode HB, Heermann R. 2015. Dialkylresorcinols as bacterial signaling molecules. Proc Natl Acad Sci U S A 112:572–577. doi: 10.1073/pnas.1417685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eckstein S, Dominelli N, Brachmann A, Heermann R. 2019. Phenotypic heterogeneity of the insect pathogen Photorhabdus luminescens: insights into the fate of secondary cells. Appl Environ Microbiol 85:e01910-19. doi: 10.1128/AEM.01910-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 95.Waters CM, Bassler BL. 2006. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev 20:2754–2767. doi: 10.1101/gad.1466506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sherwood R. 1966. Pectin lyase and polygalacturonase production by Rhizoctonia solani and other fungi. Phytopathology 56:279. [Google Scholar]
- 97.McMillan GP, Hedley D, Fyffe L, Pérombelon M. 1993. Potato resistance to soft-rot erwinias is related to cell wall pectin esterification. Physiol Mol Plant Pathol 42:279–289. doi: 10.1006/pmpp.1993.1026. [DOI] [Google Scholar]
- 98.Ryder E, Blows F, Ashburner M, Bautista-Llacer R, Coulson D, Drummond J, Webster J, Gubb D, Gunton N, Johnson G, O'Kane CJ, Huen D, Sharma P, Asztalos Z, Baisch H, Schulze J, Kube M, Kittlaus K, Reuter G, Maroy P, Szidonya J, Rasmuson-Lestander A, Ekström K, Dickson B, Hugentobler C, Stocker H, Hafen E, Lepesant JA, Pflugfelder G, Heisenberg M, Mechler B, Serras F, Corominas M, Schneuwly S, Preat T, Roote J, Russell S. 2004. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167:797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ronda C, Chen SP, Cabral V, Yaung SJ, Wang HH. 2019. Metagenomic engineering of the mammalian gut microbiome in situ. Nat Methods 16:167–170. doi: 10.1038/s41592-018-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Team RC. 2012. R: a language and environment for statistical computing . R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. Accessed 23 April 2018. [Google Scholar]
- 102.Wickham H. 2009. ggplot2—elegant graphics for data analysis, p 1–222. Springer Verlag New York, New York, NY. [Google Scholar]
- 103.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 104.Kuznetsova A, Brockhoff PB, Christensen R. 2017. lmerTest Package: tests in linear mixed effects models. J Stat Softw 82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 105.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J 50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 106.Lagendijk EL, Validov S, Lamers GEM, De Weert S, Bloemberg GV. 2010. Genetic tools for tagging Gram-negative bacteria with mCherry for visualization in vitro and in natural habitats, biofilm and pathogenicity studies. FEMS Microbiol Lett 305:81–90. doi: 10.1111/j.1574-6968.2010.01916.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth curves of WT Ecc15, expI, gacA, and expI + AHL mutants carrying a Pevf::gfp reporter fusion. Download FIG S1, TIF file, 0.1 MB (108.6KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicates of the experiments described in the Fig. 2 legend (production of pectate lyase and expression of evf are dependent on both quorum sensing and the GAC system). (A and B) Replicates of experiments described in the Fig. 2A legend. (C) Statistical groups of results of all three experiments described in the Fig. 2A legend. (D and E) Replicates of experiments described in the Fig. 2B legend. (F) Statistical groups representing all three experiments described in the Fig. 2B legend. (G and H) Replicates of experiments described in the Fig. 2C legend. (I) Statistical groups representing all three experiments described in the Fig. 2C legend. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S2, TIF file, 0.9 MB (899.2KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
AHLs cannot complement intermediate levels of evf expression in a gacA mutant. Data represent Pevf::gfp expression in WT Ecc15 and the gacA mutant at 6 h of growth in LB + Spec, n = 3. Complementation with AHLs was performed with a mixture of 1 μM 3-oxo-C6-HSL and 3-oxo-C8-HSL. Error bars represent standard deviations of the means. Download FIG S3, TIF file, 0.1 MB (131.5KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complementation of gacA, expR1, and expR2 genes. Data represent evf expression at 6 h of growth in WT Ecc15 and in expI, expI expR1 expR2, and gacA mutants (white bars) or in the gacA mutant complemented with the gacA gene in trans (light gray) and the expI expR1 expR2 mutant complemented with expR1 and expR2 genes in trans (dark gray), n = 5. “Vector” stands for pOM1 Pevf::gfp, while “p(gacA+)” and “p(expR1+ expR2+)” refer to the same plasmid expressing gacA and expR1 expR2, respectively. Error bars represent standard deviations of the means. Download FIG S4, TIF file, 0.2 MB (213KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicates of the experiment shown in Fig. 3a (evf regulation by quorum sensing is dependent on ExpR receptors and hor). (A and B) Replicates of experiments described in the Fig. 3A legend. (C) Statistical groups representing all three experiments described in the Fig. 3A legend. (D and E) Replicates of experiments described in the Fig. 3B legend. (F) Statistical groups representing all three experiments described in the Fig. 3B legend. (G and H) Replicates of experiments described in the Fig. 3C legend. (I) Statistical groups representing all three experiments described in the Fig. 3C. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S5, TIF file, 0.9 MB (926.4KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicates of the experiment shown in Fig. 4 (Ecc15 loads are higher in D. melanogaster larvae orally infected with WT than in mutants impaired in evf expression). (A and B) Replicates of experiments described in the Fig. 4 legend. (C) Statistical groups representing all three experiments described in the Fig. 4 legend. Statistical analysis was performed using a linear mixed-effect model. A Tukey HSD test was applied for multiple comparisons using the estimates obtained from the model. Download FIG S6, TIF file, 0.3 MB (265.1KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicates of the experiment shown in Fig. 5 (Ecc15 causes a developmental delay in D. melanogaster larvae that is dependent on evf, quorum sensing, and the GAC system). (A and B) Replicates of experiments described in the Fig. 5A legend. (C and D) Replicates of experiments described in the Fig. 5C. Larvae exposed to WT Ecc15 overexpressing Evf died without reaching the pupa stage. Download FIG S7, TIF file, 0.3 MB (274.4KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Independent replicate of the experiment described in the Fig. 6 legend. (evf expression and pelA expression are coactivated during plant infection). (A) Replicate of experiment shown in Fig. 6A legend. (B) Replicate of experiment shown in Fig. 6B legend. (C) Replicate of experiment shown in Fig. 6C legend. (D) Replicate of experiment shown in Fig. 6D legend. (E) Statistical groups representing the two experiments described in the Fig. 6 legend. Statistical analysis was performed using a linear mixed-effect model. A t test was applied using the estimates from the model. Download FIG S8, TIF file, 0.7 MB (735.7KB, tif) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (16.5KB, docx) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download Table S2, DOCX file, 0.01 MB (14.2KB, docx) .
Copyright © 2020 Vieira et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.