Immune responses to infectious agents are initiated when a pathogen or its components bind to pattern recognition receptors (PRRs). PRR binding sets off a cascade of events that activates immune responses. We now show that, in addition to activating immune responses, PRR signaling also initiates an immunosuppressive response, probably to limit inflammation. The importance of the current findings is that blockade of immunomodulatory signaling, which is mediated by the upregulation of the CD47 molecule, can lead to enhanced immune responses to any pathogen that triggers PRR signaling. Since most or all pathogens trigger PRRs, CD47 blockade could be used to speed up and strengthen both innate and adaptive immune responses when medically indicated. Such immunotherapy could be done without a requirement for knowing the HLA type of the individual, the specific antigens of the pathogen, or, in the case of bacterial infections, the antimicrobial resistance profile.
KEYWORDS: CD47, host response, immune checkpoint, innate immunity, pathogen recognition receptors
ABSTRACT
It is well understood that the adaptive immune response to infectious agents includes a modulating suppressive component as well as an activating component. We now show that the very early innate response also has an immunosuppressive component. Infected cells upregulate the CD47 “don’t eat me” signal, which slows the phagocytic uptake of dying and viable cells as well as downstream antigen-presenting cell (APC) functions. A CD47 mimic that acts as an essential virulence factor is encoded by all poxviruses, but CD47 expression on infected cells was found to be upregulated even by pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that encode no mimic. CD47 upregulation was revealed to be a host response induced by the stimulation of both endosomal and cytosolic pathogen recognition receptors (PRRs). Furthermore, proinflammatory cytokines, including those found in the plasma of hepatitis C patients, upregulated CD47 on uninfected dendritic cells, thereby linking innate modulation with downstream adaptive immune responses. Indeed, results from antibody-mediated CD47 blockade experiments as well as CD47 knockout mice revealed an immunosuppressive role for CD47 during infections with lymphocytic choriomeningitis virus and Mycobacterium tuberculosis. Since CD47 blockade operates at the level of pattern recognition receptors rather than at a pathogen or antigen-specific level, these findings identify CD47 as a novel potential immunotherapeutic target for the enhancement of immune responses to a broad range of infectious agents.
INTRODUCTION
The earliest immune responses to invasion by pathogenic microorganisms begin with the sensing of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs). Ligation of PRRs initiates signal transduction pathways that ultimately lead to the activation of broad innate and highly specific adaptive immune responses. Discoveries in recent years have demonstrated that the induction of adaptive immune responses involves not only activation mechanisms but also inhibitory mechanisms or “checkpoints,” which regulate immune function at a cellular level to prevent immunopathological damage by overactivated effector responses (1). Antibody (Ab)-mediated blockade of checkpoint molecules such as CTLA4 and PD-1 is being used therapeutically to enhance anticancer immune responses (2, 3), and blockade of CD47 is now in clinical trials to activate macrophage-mediated phagocytosis of cancer cells (4–7), which upregulate CD47 expression as an immune evasion mechanism (8–11).
CD47 is an abundantly expressed transmembrane cell surface glycoprotein that can act as a receptor for thrombospondins, form complexes with integrins, and bind to the inhibitory receptor signal-regulatory protein alpha (SIRPα) (12–14). CD47 binding to SIRPα has emerged as an important innate immune checkpoint by regulating immune cell clearance and inflammatory signaling (6). CD47 engagement of SIRPα results in the phosphorylation of cytoplasmic ITIM motifs by inhibitory protein tyrosine phosphatases, SHP-1 and SHP-2 (15). Given the well-established role of CD47 in cancer cell immune evasion, we investigated whether CD47 expression is modified in other disease contexts, specifically infectious diseases. The CD47-SIRPα axis has immunomodulatory functions that impact phagocytosis, chemokine and cytokine responses, innate and adaptive immune cell homeostasis and activation, T cell killing, and B cell antibody production (15–18).
Viruses have evolved mechanisms to evade host defenses (19) and take advantage of inhibitory signaling pathways for selective advantage. Of interest, poxviruses, which devote many genes toward immune suppression and evasion, encode a CD47 mimic (20). The CD47 mimic of myxomavirus, M128L, can be deleted with no effect on in vitro replication, but the deletion mutant loses pathogenicity in vivo. This loss of pathogenicity was associated with increased monocyte/macrophage activation (20).
The present study examines CD47 expression in the context of infectious agents that encode no CD47 mimic. Both mouse and human cells showed a significant upregulation of CD47 upon infection with various pathogens. The results indicated that stimulation of either cytosolic or endosomal PPRs resulted in CD47 upregulation. In addition, inflammatory cytokines present in the serum of hepatitis C virus (HCV)-infected patients could also induce CD47 upregulation, even in the context of no virus. In addition to viruses, clinically relevant bacteria such as Mycobacterium tuberculosis induce the upregulation of CD47 that limits host resistance. Our results indicate that CD47 upregulation is a very early innate checkpoint response and that immunological inhibitory mechanisms are activated not only at the effector phase of immune responses but also already at the induction phase of PRR sensing. Thus, CD47 is a promising target for checkpoint therapies against a wide range of infectious diseases.
RESULTS
CD47 expression is upregulated on mouse hematopoietic cells in response to in vivo infection.
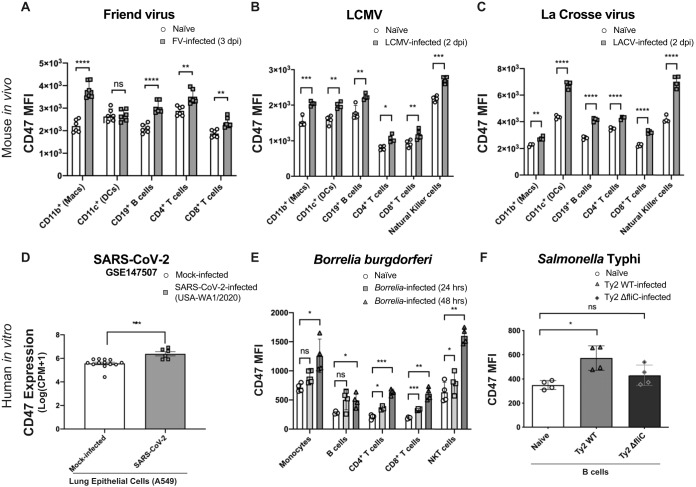
To examine the role of CD47 expression during the innate response to infection, we investigated whether hematopoietic cells upregulated CD47 expression in several unrelated infection models during the first days after infection. We began by analyzing CD47 expression on cells from mice inoculated with Friend virus (FV), a naturally occurring retroviral infection in mice (21). FV primarily infects erythroid progenitor cells in the spleen but can also infect immune cells (22). CD47 was significantly upregulated on several hematopoietic cell lineages from mouse spleens at 3 days postinfection (dpi) compared to cells from naive mice (Fig. 1A). CD47 expression was also analyzed at 2 dpi in mice infected with lymphocytic choriomeningitis virus (LCMV). Compared to naive controls, all of the spleen cell types analyzed showed significantly increased cell surface expression of CD47 (Fig. 1B). A significant upregulation of CD47 expression was also observed in response to LCMV at 3 dpi in a previous report (23). Infections with La Crosse arbovirus were also analyzed at 2 dpi, and we also observed significantly upregulated CD47 expression in hematopoietic spleen cells compared to naive controls (Fig. 1C).
FIG 1.
CD47 is broadly upregulated in immune cell types in response to several types of infection. (A and B) Comparison of CD47 median fluorescence intensities (MFI) on splenic hematopoietic cell subsets from naive mice and female (A.BY × C57BL/6)F1 mice infected intravenously with 2 × 104 SFFU Friend virus at 3 days postinfection (A) or female C57BL/6 mice infected intravenously with 2 × 106 PFU LCMV-WE at 2 days postinfection (B). (C) Female C57BL/6 mice inoculated intraperitoneally with 105 PFU La Crosse virus at 2 days postinfection. (D) CD47 expression levels analyzed from the publicly available gene expression data set from SARS-CoV-2 infection of A549 human lung tumor cells (GEO accession number GSE147507) (n = 10) comparing mock-infected (n = 13) with SARS-CoV-2 (USA-WA1/2020)-infected cells (n = 6). (E) Comparison of CD47 MFI on hematopoietic cells from Borrelia burgdorferi-GFP-infected human PBMCs 24 and 48 h after in vitro infection, compared to naive controls. GFP was used under infection conditions to identify cells with intracellular Borrelia infection (shaded). (F) Comparison of CD47 MFI on human CD19+ B cells 24 h after in vitro infection with Salmonella enterica serovar Typhi strain Ty2 (Ty2 WT) or Salmonella enterica serovar Typhi strain ΔfliC (Ty2 ΔfliC), which lacks flagella, compared to naive controls. Statistical analyses were done by Student’s t tests for panels A to D and by one-way analysis of variance (ANOVA) with a multiple-comparison posttest for panels E and F (ns [not significant], P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Error bars represent standard errors of the means (SEM).
CD47 expression is upregulated on human cells in response to in vitro infection.
Examination of a publicly available gene expression data set (Gene Expression Omnibus [GEO] accession number GSE147507) from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of A549 human lung cells also showed a significant upregulation of CD47 compared to mock-infected controls (Fig. 1D). To determine whether bacterial infection would also upregulate CD47 expression, human peripheral blood mononuclear cells (PBMCs) were examined 24 and 48 h after infection with Borrelia burgdorferi in vitro. Multiple PBMC subsets showed significantly upregulated CD47 expression in response to Borrelia burgdorferi infection compared to naive cells (Fig. 1E). We also investigated CD47 expression on human PBMCs infected in vitro with mCherry-expressing strains of Salmonella enterica serovar Typhi. Wild-type Salmonella Typhi (Ty2 WT) and Salmonella Typhi ΔfliC (Ty2 ΔfliC), a mutant strain that lacks flagella, were examined. Infections were done by centrifugation to compensate for the lack of motility of the flagellum mutant. Compared to naive cells, mCherry-positive B cells significantly upregulated CD47 expression when infected with wild-type Salmonella Typhi for 24 h (Fig. 1F). In contrast, CD47 expression was not significantly upregulated in B cells infected with the mutant strain lacking flagella, Salmonella Typhi ΔfliC (Fig. 1F). The reduced upregulation of CD47 expression by Salmonella Typhi ΔfliC compared to wild-type Salmonella Typhi suggested that the sensing of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) might play a role in CD47 upregulation, as flagellin is a potent PAMP recognized by extracellular TLR5 (24) and the NLR family of apoptosis-inhibitory proteins (NAIPs) (25). Overall, the combined in vivo and in vitro results from multiple pathogen infections in both human and mouse cells indicated that the upregulation of CD47 was a conserved host response possibly related to host sensing mechanisms.
CD47 is upregulated in response to host recognition of pathogens.
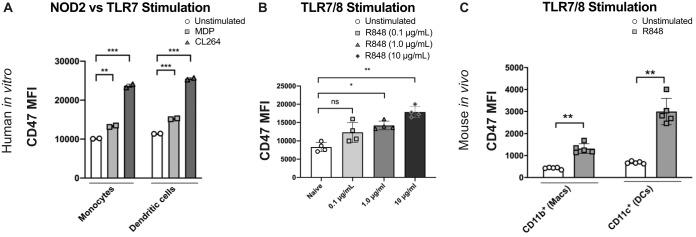
To determine whether CD47 upregulation was initiated by PRR stimulation, we specifically stimulated PRRs using small-molecule agonists rather than infectious agents. CD47 upregulation on human dendritic cells (DCs) and monocytes was tested in vitro using PBMCs stimulated with either muramyl dipeptide (MDP) to activate the bacterial peptidoglycan PRR, nucleotide-binding oligomerization domain-containing protein 2 (NOD2), or CL264 to activate the single-stranded RNA (ssRNA) endosomal PRR, TLR7. Flow cytometry was used to identify cell subsets and measure CD47 expression at 24 h poststimulation. Both human DCs and monocytes responded to either type of PRR stimulation with a significant upregulation of CD47 surface expression (Fig. 2A). We also tested TLR stimulation using a dual TLR7/8 agonist, R848. Since this dual agonist, which also has in vivo activity (26), produced dose-dependent upregulation of CD47 on human PBMCs (Fig. 2B), we proceeded to test PRR stimulation in an in vivo mouse model. Daily intraperitoneal injections of R848 into mice led to strongly upregulated CD47 expression on both macrophages and DCs isolated from spleens on day 3 (Fig. 2C). Together, these data demonstrated that CD47 was upregulated on both human and mouse immune cells via pathogen-sensing mechanisms. Furthermore, TLR7 stimulation via endosomal uptake indicated that CD47 could be upregulated not only by infected cells, as would be reflected by cytosolic NOD2 stimulation, but also by surveilling immune cells.
FIG 2.
Stimulation of pathogen-associated molecular patterns upregulates CD47 surface expression. (A) MFI of CD47 surface expression on human PBMC monocytes and dendritic cells after 48 h of stimulation with either 1 μg/ml MDP or 1 μg/ml CL264 or with no stimulation. The results are from one of three experiments with two different donors. All 3 experiments showed consistent effects. Statistics were done by a paired two-way t test with Bonferroni correction. (B) CD47 MFI on human total PBMCs from 4 separate donors stimulated with titrated concentrations of R848 from 0.1 μg/ml to 10 μg/ml, as indicated, for 48 h. Statistics were done by a paired two-way t test with Bonferroni correction. (C) Mice (n = 5/group) were injected intraperitoneally with 1 mg/kg R848 for 3 days, and on day 3, splenocytes were isolated and macrophages and DCs were analyzed for CD47 MFI. Statistics were done by an unpaired two-way t test. (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Error bars represent SEM.
CD47 expression is upregulated during HCV infection in vivo.
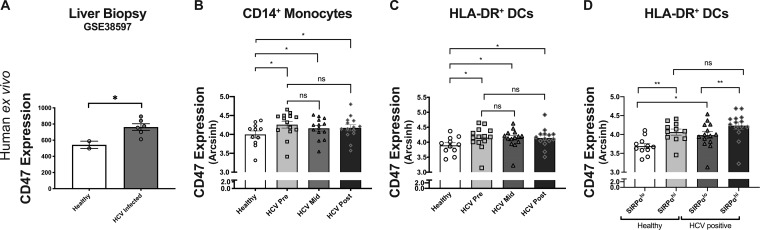
To examine CD47 expression in human viral infection, we first compared transcriptional levels of CD47 from publicly available microarray data (GEO accession number GSE38597) from healthy and hepatitis C virus (HCV) patient liver biopsy specimens. The analysis revealed significantly higher expression levels of CD47 in the liver biopsy specimens from acutely infected HCV patients than for healthy controls (Fig. 3A). We then used cytometry by time of flight (CyTOF) to examine CD47 expression on PBMCs during HCV infection in the context of sofosbuvir (SOF) therapy (27), comparing healthy controls to HCV-infected patients prior to treatment, midway through treatment, and at 6 months posttreatment. Compared to healthy controls, monocytes and DCs from HCV patients demonstrated a sustained upregulation of CD47 at all treatment time points, including the 6-month posttreatment time point (Fig. 3B and C). There were no significant differences between pre-, mid-, and posttreatment CD47 levels in either monocytes or DCs, and all of these time points were significantly different from those for healthy controls (Fig. 3B and C). Conventional dendritic cells (cDCs) are classified into cDC1s and cDC2s, which can be distinguished in part through the specific expression of the CD47 receptor, SIRPα, on cDC2s (28). When we compared CD47 expression levels within SIRPαlo and SIRPαhi DCs, we observed that CD47 expression was highest on SIRPαhi DCs (Fig. 3D). There was no correlation between viral titers and CD47 expression on either monocytes or DC subsets in this patient cohort (see Fig. S1A in the supplemental material). Between healthy controls and pretreatment HCV patients, there was significant CD47 upregulation in SIRPαlo DCs but not in SIRPαhi DCs (Fig. 3D). However, compared to healthy controls, the proportion of SIRPαhi DCs was significantly increased at both pretreatment and midtreatment, which could also contribute to higher CD47 expression levels in DCs (Fig. S1B). Thus, HCV infections were associated with the increased expression of both CD47 and its receptor, SIRPα, on antigen-presenting cells (APCs).
FIG 3.
CD47 is involved in innate licensing of adaptive immune responses in HCV patient clinical samples. (A) Comparison of CD47 expression from Affymetrix array profiles of liver biopsy specimens from two healthy controls and six patients with acute HCV infection (P = 0.03 by an unpaired two-way t test) (NCBI GEO accession number GSE38597). (B and C) Comparison of CD47 expression by CyTOF MFI on CD14+ monocytes (B) and HLA-DR+ DC subpopulations (C) isolated from HCV-infected sofosbuvir-treated patients before the initiation of treatment (Pre), midway through treatment (Mid), and after treatment (Post) compared to healthy controls. (D) Comparison of CD47 expression on SIRPαlo versus SIRPαhi DCs from healthy control and HCV patients. Statistics were done by one-way ANOVA with multiple-comparison posttests (ns, P > 0.05; *, P < 0.05; **, P < 0.01). Error bars represent SEM.
HCV viral titer does not correlate with CD47 expression levels; however, SIRPhi DCs are increased in response to HCV infection and PAMP stimulation. (A) HCV viral loads of HCV patients at the pretreatment time point compared to the CD47 MFI of their monocytes and dendritic cells from pretreatment clinical sample peripheral blood. (B) Percentages of SIRPhi dendritic cells by CyTOF analysis of HCV patient clinical peripheral blood samples pre-, mid-, and posttreatment compared to healthy controls. Download FIG S1, PDF file, 0.5 MB (568KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
HCV patient plasma-induced CD47 expression ex vivo.
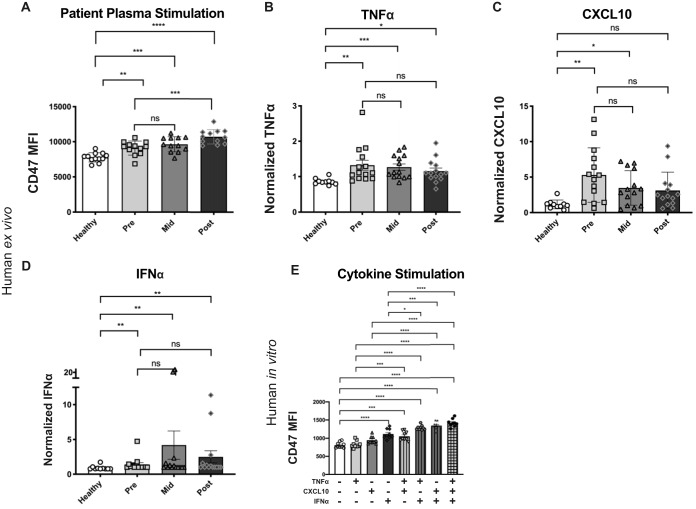
To determine whether inflammatory factors present in HCV patient plasma could affect CD47 upregulation, we derived DCs from healthy donor monocytes with plasma added from either patients in the HCV patient cohort or healthy controls. We found that monocyte-derived DCs (mDCs) cultured in the presence of HCV patient plasma significantly upregulated CD47 compared to plasma from healthy donors (Fig. 4A). Luminex analysis of patient plasma was used to characterize the inflammatory milieu over the course of HCV infection and treatment. Plasma isolated from patients at the pretreatment time point contained both virus and inflammatory cytokines, indicating that CD47 upregulation could have been due to infection of the DCs. However, plasma isolated from patients at the midtreatment and posttreatment time points contained no detectable virus (data not shown) but still had increased levels of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), CXCL10, and interferon alpha (IFN-α), compared to healthy controls (Fig. 4B to D). CD47 expression was increased under all HCV patient plasma conditions compared to healthy controls despite undetectable virus at the midtreatment and posttreatment time points. The differences between the pre-, mid-, and posttreatment time points within HCV patients were not statistically significant. These results suggested that cytokines in the inflammatory milieu of HCV patient plasma could upregulate CD47 expression.
FIG 4.
Plasma from HCV-infected patients is sufficient to increase CD47 expression on monocyte-derived DCs. (A) Quantitative comparison of CD47 CyTOF MFI of monocyte-derived DCs matured in culture medium containing plasma samples from the sofosbuvir HCV patient cohort before the initiation of treatment (Pre), midway through treatment (Mid), and after treatment (Post) compared to healthy controls. (B to D) Plasma collected from the sofosbuvir HCV patient cohort was analyzed by Luminex assays and normalized as described in Materials and Methods for TNF-α (B), CXCL10 (C), and IFN-α (D). (E) Comparison of CD47 MFI on HLA-DR+ DCs 72 h after stimulation in vitro with 10 μg/ml TNF-α, 100 μg/ml CXCL10, and 100 μg/ml IFN-α as single treatments or in combination. Statistics were done by one-way ANOVA with multiple-comparison posttests (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Error bars represent SEM. Comparisons not labeled are not significantly different.
To confirm the ability of cytokines to upregulate CD47 surface expression, we performed in vitro stimulations of human PBMCs isolated from healthy donors using TNF-α, CXCL10, and IFN-α, as single treatments and in combinations. At 72 h poststimulation, the only single cytokine that induced significant CD47 upregulation was IFN-α (Fig. 4E). Combinations of these inflammatory cytokines enhanced the upregulation of CD47 surface expression, with the triple combination of TNF-α, CXCL10, and IFN-α having the strongest effect (Fig. 4E). These experiments demonstrated that in addition to pathogen recognition, host immune cells could also upregulate CD47 surface expression in response to the inflammatory milieu induced by the pathogen.
Blockade of CD47 signaling enhances antiviral immune responses in vivo.
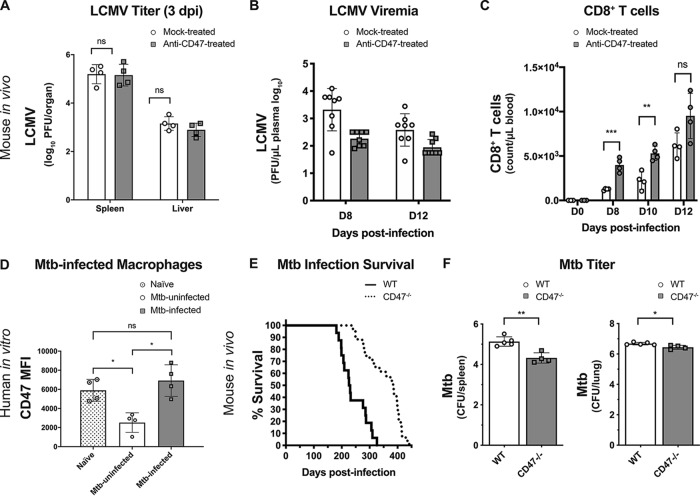
To determine the effects of CD47 blockade during a live viral infection, C57BL/6 mice were injected with anti-CD47 (blocking) antibody daily beginning 2 days prior to infection with LCMV. To ascertain whether both anti-CD47-treated and mock-treated animals were equivalently infected, LCMV titers in the spleens and liver were determined at 3 dpi. The analyses showed no significant differences in viral titers at this time point regardless of treatment (Fig. 5A). Anti-CD47 injections were continued through 5 dpi, and plasma virus levels were then measured at 8 and 12 dpi. Significantly reduced viremia levels were observed in anti-CD47-treated mice compared to mock-treated mice at both time points (Fig. 5B). Since it is known that host clearance of LCMV is highly dependent on CD8+ T cell responses (29, 30), it was of interest to determine if CD47 blockade affected those responses. Total CD8+ T cell levels were determined from blood samples of LCMV-infected mice at 8, 10, and 12 dpi in the presence or absence of CD47 blockade. At both 8 and 10 dpi, there were significantly increased CD8+ T cell numbers responding to LCMV infection in the treated mice compared to the isotype-matched antibody control-treated mice (Fig. 5C).
FIG 5.
In vivo CD47 blockade in LCMV infection and CD47 genetic inactivation in M. tuberculosis infection. (A to C) Female C57BL/6 mice 8 to 12 weeks old were treated by intraperitoneal injection with either 100 μg anti-CD47 or an isotype control antibody at days −2, −1, 0, +1, and +2 relative to the day of intravenous infection (day 0 [D0]) with 2 × 106 PFU LCMV-WE. (A) Mice were euthanized at 3 dpi, and LCMV PFU from spleen and liver were determined as described in Materials and Methods. (B) LCMV viremia levels were determined from blood samples by plaque-forming assays for control and anti-CD47-treated mice at 8 and 12 dpi. (C) CD8+ T cell counts in blood samples from control and anti-CD47-treated mice were analyzed by flow cytometry at 0, 8, 10, and 12 dpi. Statistics were done by unpaired two-way t tests (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Error bars represent SEM. (D) Human peripheral blood monocyte-derived macrophages from four different donors were infected in vitro with M. tuberculosis (Mtb) (pantothenate/lysine mutant strain) fluorescently labeled with pHrodo (to distinguish infected from uninfected cells) in triplicate and stained with anti-CD47 at 24 h postinfection. Flow cytometry was used to measure CD47 MFI in cells from uninfected cultures (naive) compared to both infected and uninfected cells from M. tuberculosis-infected cultures. Statistics were done by one-way ANOVA with multiple-comparison posttests (ns, P > 0.05; *, P < 0.05). (E) Both male and female C57BL/6 WT (n = 16) and C57BL/6.CD47−/− (n = 23) mice were analyzed for survival (humane endpoints) following M. tuberculosis infection by inhalation. The difference between C57BL/6 WT and C57BL/6.CD47−/− mice was statistically significant (****, P < 0.001 by a log rank Mantel-Cox test from pooled data from three independent experiments). (F) Analysis of M. tuberculosis CFU from lungs and spleens of endpoint animals. Statistical analyses were done by Student’s t tests, and two-sided P values are shown (*, P < 0.05; **, P < 0.01) with standard deviations.
Genetic inactivation of CD47 expression prolongs survival from Mycobacterium tuberculosis infection in vivo.
We next investigated the role of CD47 during Mycobacterium tuberculosis infection in vitro and in vivo. First, human macrophages derived from healthy PBMC samples were used for in vitro infection with M. tuberculosis to examine CD47 expression levels. CD47 expression levels are shown for four donors, and compare naive macrophages from cultures that were not infected to macrophages from cultures that were infected with pHrodo-labeled M. tuberculosis. In comparison to naive macrophages, CD47 was not upregulated on M. tuberculosis-infected (pHrodo-positive [pHrodo+]) macrophages (Fig. 5D). Interestingly, M. tuberculosis-uninfected (pHrodo-negative [pHrodo−]) macrophages from the M. tuberculosis-infected cultures downregulated CD47 expression in comparison to naive macrophages, an observation for which we do not yet have an explanation (Fig. 5D). The lack of CD47 upregulation by M. tuberculosis-infected macrophages was supported by metasignature analysis comparing microarrays for gene expression signatures specific to HCV and tuberculosis (TB) (Fig. S2). The disease-specific gene expression signature for HCV consistently showed an upregulation of CD47, whereas the disease-specific gene expression signature for TB showed downregulation.
CD47 gene expression levels are lower in tuberculosis gene arrays and higher in hepatitis C virus gene arrays. Metasignature gene expression analysis of CD47 for tuberculosis (A) and hepatitis C virus (B) data sets was performed based on standardized mean difference (log2 scale) gene expression correlation plots for TB and HCV from http://metasignature.stanford.edu/. Download FIG S2, PDF file, 0.4 MB (404.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
For an in vivo assessment of a possible functional role for CD47 in host resistance to M. tuberculosis infection, CD47 knockout (KO) mice were infected via the aerosol route with a low dose (100 to 200 CFU) of M. tuberculosis strain H37Rv. CD47-deficient mice displayed significantly increased host resistance to M. tuberculosis infection, with significantly longer survival (humane endpoints), compared to wild-type C57BL/6 mice (Fig. 5E). Furthermore, spleens and lungs from the CD47-deficient mice at humane endpoints yielded significantly lower mycobacterial CFU (Fig. 5F). These results indicated that CD47 expression can exert significant suppressive effects on immune responses to infectious pathogens in vivo.
DISCUSSION
Previous results demonstrating that malignant cells upregulate CD47 expression to evade cellular clearance (9, 31) led us to hypothesize that pathogens might also induce CD47 surface expression as an immune evasion mechanism. In fact, poxviruses encode a CD47 mimic that acts as a potent virulence factor (20). Curiously, we repeatedly observed an upregulation of CD47 surface expression across diverse infections with both viruses and bacteria, none of which are known to encode a CD47 mimic or have any CD47 sequence homology. Flagella are potent inducers of TLRs, so the finding that Salmonella Typhi ΔfliC mutants lacking flagella were poor at inducing CD47 suggested a possible connection to host sensing via PRR signaling (Fig. 1F). Indeed, direct stimulation of PRRs with synthetic ligands such as MDP, CL264, and R848 induced CD47 upregulation in vitro and in vivo (Fig. 2). Since SIRPα-mediated recognition of CD47 on infected cells by macrophages and DCs transduces an inhibitory signal that attenuates phagocytosis and downstream antigen presentation functions, it was counterintuitive that CD47 upregulation would be due to a host response. Why would the host dampen its immune response to infection? We have shown that in vivo, CD47 blockade improved the control of LCMV infection (Fig. 5A), increased CD8+ T cell responses (Fig. 5C), and, in a previous report, increased the expression of costimulatory molecules on APCs as well (23), thus confirming that CD47 induces immunosuppressive signals. Similarly, mice with genetic inactivation of CD47 had reduced bacterial loads and longer survival times when infected with M. tuberculosis (Fig. 5E and F). We conclude that, like coinhibitory molecules such as PD-1 that dampen adaptive immune responses, CD47 upregulation acts as an intrinsic governor of the innate immune response to prevent overactivation that can lead to immunopathology. Thus, the initial immune response to infections is attenuated until proinflammatory signals overcome anti-inflammatory signals.
CD47 was previously identified by microarray analysis as an interferon-stimulated gene (ISG), upregulated as part of a coordinated program of host defense mechanisms upon IFN-α stimulation (32). In addition, TNF-α was demonstrated to induce the upregulation of CD47 on vascular smooth muscle cells in vitro (33). In breast cancer, TNF-α-mediated CD47 upregulation is transcriptionally controlled by NF-κB through an NF-κB motif within an enhancer of the CD47 gene (34). These reports are consistent with our findings showing that the inflammatory milieu in patient blood during HCV infection is sufficient to upregulate CD47 surface expression (Fig. 4E). While our study focused on TNF-α, CXCL10, and IFN-α, redundant mechanisms of CD47 upregulation by additional inflammatory mediators are possible and should be examined in depth in future experiments. In the experimental models utilized, it is difficult to distinguish the relative contributions of cytokine-induced CD47 upregulation from those of direct pathogen-induced CD47 upregulation where both are present and either is sufficient. The results clearly indicate that CD47 surface expression can be upregulated either in a cell-intrinsic manner via stimulation of PRRs or by surveilling immune cells in response to extrinsic signaling by inflammatory cytokines. It may also be possible that signaling via cis interactions within a cell could occur in cells such as macrophages, which express both CD47 and SIRPα.
Of the infectious agents that we analyzed, M. tuberculosis infection was unique in failing to induce CD47 expression upon infection. Of interest, uninfected cells from M. tuberculosis-infected cultures downregulated CD47 expression. This may not be too surprising because the life cycle of M. tuberculosis is dependent on phagocytosis by alveolar macrophages, and M. tuberculosis is known to induce strong inflammatory responses via the induction of cytokines and chemokines. Further research will be required to identify the mechanisms involved in these unique responses to M. tuberculosis, but despite its failure to upregulate CD47, improved survival from M. tuberculosis infection was observed in mice genetically deficient in CD47 compared to wild-type mice (Fig. 5E). Better recoveries from malaria parasites (35, 36) and Escherichia coli infections (37) have also been shown in CD47 KO mice. In addition, CD47 KO mice have improved influenza virus vaccine responses (16). However, from an evolutionary standpoint, the upregulation of CD47 expression in response to pathogens must result in a competitive advantage for the host. As an example, CD47 KO mice show poorly controlled inflammatory responses to and increased morbidity and mortality from Candida albicans infection (38). The contrasting results from various infections in CD47 KO mice illustrate how tightly balanced the immune system has evolved to be and the care that must be taken when immune interventions are undertaken. That said, the results from CD47 KO mice, in which the entire immune system has developed in the absence of a critical immunomodulatory molecule, might produce different results than the same infection in an immune-replete mouse treated with anti-CD47 antibodies during the infectious process. Indeed, while we found that treating wild-type mice with anti-CD47 before LCMV strain WE (LCMV-WE) infection improved recovery, it has been reported that CD47 KO mice have decreased resistance to LCMV Clone-13 infections (39). Additional experiments will be required to determine which experimental factors may account for the differences in these outcomes.
The accelerated CD8+ T cell responses and clearance of LCMV infections following prophylactic blockade of CD47 were most likely due to enhanced APC function evidenced by the increased expression of costimulatory CD86 on dendritic cells observed at 3 dpi (23). However, it is also possible that there were direct effects on CD8+ T cells since it was previously shown that activated CD8+ T cells express SIRPα, the receptor for CD47 (17). It was shown that only activated effector cells and not naive CD8+ T cells express SIRPα, and any direct effects on CD8+ T cells would occur only after expansion and development of effector functions. Furthermore, in contrast to the negative signal delivered to cells of the monocytic lineage via SIRPα ligation to CD47, evidence suggested that CD47-SIRPα signaling in CD8+ T cells delivered a positive signal associated with improved cytolytic killing of infected target cells in vivo. The CD8+ T cell responses measured in this prophylactic anti-CD47 study were total CD8+ T cell responses rather than tetramer-stained cells known to be virus specific. Thus, the expansion of bystander CD8+ T cells by anti-CD47 was not excluded. However, when anti-CD47 was used in a therapeutic setting against LCMV infections, the predominant CD8+ T cell expansions were virus specific, and the mechanism of protection was dependent on DCs and CD8+ T cells (23).
The current results demonstrate that CD47 plays a prominent role in modulating inflammatory responses to infections. While these findings open new possibilities for therapeutic intervention against pathogenic agents, it is important to note that the context of the host response to specific types of infections will determine whether CD47 blockade would be protective or detrimental. There may be circumstances where host responses need boosting, and CD47 represents a novel target for host-directed therapies in such cases. Possibilities include viruses such as SARS-CoV-2, human immunodeficiency virus, human papillomavirus, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus, and Ebola virus, etc. There is also a potential application for treating infections with bacteria, including M. tuberculosis and multidrug-resistant bacterial strains that might otherwise be untreatable. Although not addressed in this study, other infectious agents, such as fungi or parasites, that elicit PRR responses might also be tractable to anti-CD47 therapy. A key factor is that infected cells also express damage-associated molecular patterns (DAMPs), which act as “eat me” signals that are being masked by the CD47 “don’t eat me” signal (11, 40). Therefore, releasing the inhibition of phagocytosis of these cells would need to be weighed cautiously with the extent of infection and the replaceability of the infected cell types.
MATERIALS AND METHODS
All animal studies were performed at NIAID Laboratories and Stanford University and were done so under animal study proposals approved by the Institutional Animal Care and Use Committees following all regulations and guidelines of the Public Health Service’s Office of Laboratory Animal Welfare.
Murine in vivo viral infections and flow cytometry analysis.
Friend virus (FV)-infected mice were female (C57BL/10 × A.BY)F1 (abbreviated Y10) (H-2b/b, Fv1b, Rfv3r/s) mice bred at the Rocky Mountain Laboratories (RML) (Hamilton, MT) and were used at between 8 and 16 weeks of age at the beginning of the experiments. The FV stock used in these experiments has been passaged in mice for more than 3 decades and contains three separate viruses: (i) replication-competent B-tropic Friend murine leukemia helper virus (F-MuLV), (ii) replication-defective polycythemia-inducing spleen focus-forming retrovirus that is packaged by F-MuLV-encoded virus particles, and (iii) lactate dehydrogenase-elevating virus (LDV), an endemic murine positive-sense ssRNA [(+)ssRNA] virus (22, 41). Mice were infected by intravenous (i.v.) injection of 0.2 ml of a phosphate-buffered balanced salt solution (PBS) containing 1,500 spleen focus-forming units (SFFU) of the FV complex. La Crosse virus (LACV) infections were performed with a 1978 human isolate provided as a gift from Stephen Whitehead (NIAID, NIH). Virus stocks were passaged no more than 3 times in Vero cells. For analysis of CD47 expression in mice during LACV infection, 21-day-old C57BL/6 (Jackson Laboratories) male or female mice were inoculated intraperitoneally with a 105-PFU dose of virus diluted into a 200-μl volume of sterile PBS. Mice of the same strain, age, and sex inoculated with an equivalent volume of a Vero cell culture supernatant in PBS were used as controls. At 2 dpi, whole blood and spleen were isolated from mice and processed for flow cytometry as described above. LCMV viral titers were detected by plaque-forming assays on MC57 fibroblasts (obtained from by the Ontario Cancer Institute, Canada). Organs were dissociated, and plasma was diluted in Dulbecco’s modified Eagle medium (DMEM) containing 2% fetal calf serum (FCS), titrated 1:3 over 12 steps, and incubated on MC57 cells. After 4 h of incubation at 37°C, a methylcellulose overlay was added, and the cells were incubated for 48 h, followed by staining of LCMV plaques using an anti-LCMV-NP antibody (clone VL4). C57BL/6 mice were infected with 2 × 106 PFU of LCMV strain WE and treated with anti-CD47 via daily intraperitoneal injections of 100 μg of anti-CD47 (clone 410, catalog number BE0283; BioXCell) or an isotype control (rat IgG2a isotype) (BioXCell) from day −2 to day 6 postinfection.
To analyze infected spleen cells, splenocytes were isolated by tissue homogenization through a 100-μm filter, and red blood cells were removed using ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 M EDTA). The gating strategy for spleen cell subset analyses was the same as the one described previously (23). All antibodies were from BD Biosciences, BioLegend, or eBioscience/Thermo Fisher Scientific, including Brilliant Violet 605-anti-CD11b (clone M1/70), phycoerythrin (PE)-CF594-anti-CD19 (clone ID3), PE-Cy7-anti-CD11c (clone HL3), PE-Cy7-anti-Ter119 (clone TER-119), Alexa Fluor 647-anti-CD47 (clone MIAP301), and fluorescein isothiocyanate (FITC)-anti-major histocompatibility complex class II (MHCII) (I-A/I-E) (catalog number FAB6118F); lymphocyte populations were initially gated on single live cells on the basis of forward scatter (FSC) versus side scatter (SSC). Mouse DCs were defined as CD11c+ CD11b−, and macrophages were defined as CD11c− CD11b+. Human DCs were also gated on the basis of high MHCII expression levels. The CD11c+ subset contained a minor population of CD11b-intermediate cells that could have been inflammatory macrophages. The multiparameter data were collected with an LSRII instrument (BD Biosciences) and analyzed using FlowJo software.
Bacterial strains.
Bacterial strains included a B31 Borrelia burgdorferi clone (GCB726) with the cp9 plasmid replaced by a cp9-based pTM61 construct containing green fluorescent protein (GFP) (42). Salmonella enterica serovar Typhi Ty2 mCherry mutant strains were generated via λ red recombination and included the Salmonella enterica serovar Typhi Ty2 mCherry ΔFla (fliC::Kan) mutant (25). M. tuberculosis H37Rv ΔlysA and ΔpanCD, used for the in vitro studies, were provided by William R. Jacobs, Jr. (43). M. tuberculosis strain H37Rv was used for the in vivo studies.
Affymetrix array profiles of liver biopsy specimens.
Affymetrix arrays were obtained as CEL files, MAS5 normalized using the “affy” package in Bioconductor, mapped to NCBI Entrez gene identifiers using a custom chip definition file (Brainarray version 19 [http://brainarray.mbni.med.umich.edu/Brainarray/]), and converted to HUGO gene symbols (44).
SARS-CoV-2.
A publicly available gene expression data set from SARS-CoV-2 infection of A549 cells (accession number GSE147507) (n = 10) was downloaded from the Gene Expression Omnibus (GEO). Independent biological replicates of transformed lung alveolar (A549) cells were mock infected (uninfected [Un]) (n = 13) or infected (Inf) (n = 6) with SARS-CoV-2 (USA-WA1/2020). cDNA libraries were sequenced from each sample using the Illumina NextSeq 500 platform. Raw sequencing reads were aligned to the human genome (hg19) using the RNA-Seq Alignment App (v2.0.1) on Basespace (Illumina, CA). Gene expression values were summarized using counts per million (CPM) and converted to a log2 scale using the formulas log2(CPM) if the CPM were >1 and CPM − 1 if the CPM were <1. A standard t test was performed using the Python scipy.stats.ttest_ind package (version 0.19.0) with Welch’s two-sample t test (unpaired, unequal variance [equal_var=False], and unequal sample size) parameters. The results were independently validated with R statistical software (R version 3.6.1, 5 July 2019). CD47 was significantly upregulated (P = 0.00332) in SARS-CoV-2-infected samples. (45).
HCV sofosbuvir cohort.
PBMCs, plasma, and serum were studied in 14 HCV-infected patients previous to direct-acting antiviral therapy (sofosbuvir [SOF] and simeprevir [SIM]; SOF and ribavirin [RBV]; and SOF, RBV, and pegylated interferon [PEG]) before treatment, during treatment, and after treatment. Ten patients underwent at least one previous treatment with interferon, and the other four were treatment naive. Thirteen patients experienced a sustained virologic response (SVR) after 12 weeks of therapy. PBMCs, plasma, and serum were collected from noninfected patients as a control (Table 1). One patient relapsed. Patients provided written informed consent for research testing under protocols by the Stanford University Institutional Review Board.
TABLE 1.
HCV sofosbuvir cohort
| Patient | Previous IFN |
Genotype | Treatments | Liver transplant waitlist |
Outcome | Sex | Viral titer (copies/ml) |
|---|---|---|---|---|---|---|---|
| 2 | Yes | 1 | SOF, SIM | No | SVR | Male | 1,790,000 |
| 4 | Yes | 1 | SOF, SIM | No | SVR | Female | 921,000 |
| 7 | No | 2 | SOF, RBV | No | SVR | Female | 2,180,000 |
| 8 | Yes | 2 | SOF, RBV | No | SVR | Female | 5,380,000 |
| 9 | Yes | 1 | SOF, SIM | No | SVR | Male | 2,230,000 |
| 12 | No | 2 | SOF, RBV | No | SVR | Female | 3,160,000 |
| 13 | No | 1 | SOF, SIM | Yes | Relapse | Female | 696,000 |
| 14 | Yes | 1 | SOF, SIM | No | SVR | Male | 9,290,000 |
| 20 | Yes | 1 | SOF, SIM | No | SVR | Male | 50,000,000 |
| 22 | Yes | 1 | SOF, PEG, RBV | No | SVR | Female | 5,030,000 |
| 27 | Yes | 1 | SOF, SIM | No | SVR | Male | 148,505 |
| 29 | Yes | 1 | SOF, SIM | Yes | SVR | Male | 2,630,000 |
| 30 | No | 4 | SOF, PEG, RBV | No | SVR | Female | 1,200,000 |
| 35 | No | 1 | SOF, SIM | No | SVR | Male | 79,900 |
| 38 | Yes | 1 | SOF, SIM | No | SVR | Male | 6,556,280 |
Phospho-CyTOF sample processing and staining.
Cryopreserved PBMCs stored at −180°C were thawed in warm RPMI medium supplemented with 10% FBS, Benzonase, and a penicillin-streptomycin mixture (complete RPMI medium). Cells were transferred into serum-free RPMI medium containing 2 mM EDTA and Benzonase, incubated with cisplatin for 1 min, and immediately quenched with 4 volumes of complete RPMI medium. Next, 1 million cells per sample were transferred into complete RPMI medium and rested for 30 min at 37°C. Following this rest period, cells were fixed in PBS with 2% paraformaldehyde (PFA) at room temperature for 10 min. Cells were then washed twice with CyFACS buffer and barcoded as previously described (46). Following barcoding, samples were combined for surface marker staining, performed at room temperature for 1 h. Subsequently, cells were washed and permeabilized in methanol (MeOH) at −80°C overnight. The next day, cells were washed and incubated with the intracellular cytokine cocktail at room temperature for 1 h. DNA staining was performed for 20 min with iridium (191/193) in PBS with 2% PFA at room temperature. Finally, cells were washed twice with CyFACS buffer and then twice with MilliQ water before data acquisition on the CyTOF2 instrument. Data were debarcoded and manually analyzed on Cytobank (www.cytobank.org/).
Monocyte-derived DC and macrophage cultures.
Healthy donor leukocyte reduction system cones were provided by the Stanford blood center. PBMCs were isolated by a 1.077-g/ml Ficoll gradient using Sep-mate tubes. Monocytes were selected for by plastic adherence after 20 min of incubation at 37°C with 5% CO2 in RPMI medium plus 10% human serum (Gemini). Selected monocytes were then cultured for 72 h in RPMI medium supplemented with 1% serum, 10 ng/ml interleukin-4 (IL-4), and 800 IU/ml granulocyte-macrophage colony-stimulating factor (GM-CSF); the concentration of GM-CSF was increased to 1,600 IU/ml for the final 24 h. Immature DCs were matured by replacing culture medium with RPMI medium supplemented with 1% healthy donor or HCV patient plasma, 10 ng/ml IL-4, 800 IU/ml GM-CSF, 10 mg/ml lipopolysaccharide (LPS), and 100 IU/ml IFN-γ. For macrophage derivation, monocytes were cultured in RPMI medium supplemented with 10% human serum for 7 days.
Human and murine Luminex assays.
The human samples were analyzed at the Human Immune Monitoring Center at Stanford University. Human 63-plex or mouse 38-plex kits were purchased from eBioscience/Affymetrix and used according to the manufacturer’s recommendations, with modifications as described below. Briefly, beads were added to a 96-well plate and washed in a BioTek ELx405 washer. Samples were added to the plate containing mixed antibody-linked beads and incubated at room temperature for 1 h, followed by incubation overnight at 4°C with shaking. Cold and room-temperature incubation steps were performed on an orbital shaker at 500 to 600 rpm. Following incubation overnight, plates were again washed in a BioTek ELx405 washer, and a biotinylated detection antibody was then added for 75 min at room temperature, with shaking. The plate was washed as described above, and streptavidin-PE was added. After incubation for 30 min at room temperature, another wash was performed as described above, and reading buffer was added to the wells. Each sample was measured in duplicate. Plates were read using a Luminex 200 instrument with a lower bound of 50 beads per sample per cytokine. Custom assay control beads by Radix Biosolutions were added to all wells.
In vitro stimulations and infections of human PBMCs and macrophages.
Healthy donor leukocyte reduction system cones were provided by the Stanford blood center. Human PBMCs were isolated by a 1.077-g/ml Ficoll gradient using Sep-mate tubes. Isolated PBMCs were cultured in RPMI medium supplemented with 10% FBS and 100 U/ml penicillin-streptomycin at a concentration of 1 × 106 cells/ml. To activate PRRs, cells were stimulated with either 1 μg/ml CL264-rhodamine (InvivoGen), 1 μg/ml muramyl dipeptide (InvivoGen), or R848 at concentrations of 0.1 μg/ml, 1 μg/ml, and 10 μg/ml or left unstimulated. Cells were collected at 48 h poststimulation prior to flow cytometry. PBMCs were stimulated with single treatments or combination treatments of 10 ng/ml TNF-α, 100 ng/ml CXCL10, and 100 ng/ml IFN-α. Cells were then analyzed for CD47 expression 72 h after cytokine stimulation. In vitro bacterial infections of PBMCs were performed at a multiplicity of infection (MOI) of 10 for Salmonella enterica serovar Typhi strains and at an MOI of 40 for Borrelia burgdorferi, for 24 and 48 h, respectively. Salmonella enterica serovar Typhi strains were spun onto the cells to compensate for the motility differences. Indeed, the strain of Salmonella enterica serovar Typhi infected a lower percentage of cells, but infected versus uninfected cells were differentiated for the analyses. For M. tuberculosis in vitro infection of macrophages, M. tuberculosis was stained for 1 h in PBS with a 1:20,000 dilution of pHrodo (Essen Biosciences) at 37°C to fluorescently label infected macrophages. Macrophages were plated into 96-well U-bottom plates. Fluorescently labeled M. tuberculosis bacteria were used to infect macrophages at an MOI of 1:10 for 24 h. For antibodies for flow cytometry, anti-CD11c (clone 3.9), anti-HLA-DR (clone L243), anti-CD11b (clone M1/70), anti-CD14 (clone M5E2), anti-CD16 (clone 3G8), and anti-SIRP (clone SE5A5) were purchased from BioLegend, except for allophycocyanin (APC)-anti-CD47 (clone B6H12; eBioscience), which was purchased from Invitrogen. Cells were analyzed with 4′,6-diamidino-2-phenylindole (DAPI) for dead-cell exclusion and then gated on single cells using FSC-a (forward scatter area) by FSC-h (forward scatter height) and SSC-a (side scatter area) by SSC-h (side scatter height). Dendritic cells were defined as MHCII/HLA-DRhi and CD11chi.
All human in vitro experiments were repeated in at least two independent experiments with a minimum of 4 biological replicates.
In vivo and in vitro stimulation of mouse cells.
Female C57BL/6 RRID:IMSR_JAX:000664 (WT) mice were bred at the Stanford University Stem Cell Institute Barrier Facility (Stanford, CA) and used at between 8 and 12 weeks of age at the beginning of the experiments. For R848 in vivo analysis, 10 naive mice were injected intraperitoneally with either 1 mg/kg of body weight of R848 or PBS, all at a volume of 0.1 ml, for three consecutive days. On day 3 after the first treatment, splenocytes were isolated. Spleens were dissociated by collagenase treatment in the presence of DNase I and mechanical dissociation to obtain a single-cell suspension of splenocytes. Red blood cells were removed using ACK lysis buffer (Gibco), and the remaining splenocytes were seeded at a density of 1 × 106 splenocytes/well in a 96-well U-bottom low-adherence plate. Cells were then stained for the macrophage and DC markers CD11b, MHCII, and CD11c, as well as SIRPα and CD47, with DAPI for live/dead exclusion. For in vitro stimulation, splenocytes were isolated from naive mice as described above and then stimulated with either 1 μg/ml CL264-rhodamine (InvivoGen) overnight or 1 μg/ml poly(I·C)-rhodamine (InvivoGen) complexed with Lipofectamine 2000 for 1 h or left unstimulated. Cells were collected at 24 h poststimulation for analysis by flow cytometry. Antibodies for flow cytometry were purchased from BioLegend or BD Biosciences.
M. tuberculosis infections.
M. tuberculosis infections were done in C57BL/6 mice or CD47 KO RRID:IMSR_JAX:003173 (CD47 KO) mice that were bred at NIAID facilities. For infections with M. tuberculosis H37Rv (100 to 200 CFU), 8- to 12-week-old male and female mice were placed in a whole-body inhalation system (Glas-Col, Terre Haute, IN) and exposed to aerosolized M. tuberculosis. Delivery doses were set by measuring lung CFU at 2 to 24 h postexposure from three to five control mice through mechanical homogenization using Precellys Evolution (Precellys, Atkinson, NH). Lung homogenates were then serially diluted in PBS-Tween 20 and cultured on Middlebrook 7H11 agar plates supplemented with oleic acid-albumin-dextrose-catalase (Difco, Detroit, MI), and CFU were counted 21 days later.
Cell isolation from M. tuberculosis-infected lung tissue and flow cytometry.
Lungs were digested and dissociated using gentle magnetically activated cell sorting (MACS) and lung cell isolation buffer (Miltenyi Biotec). The digested lung was passed through a 100-mm cell strainer, and an aliquot was removed for the determination of CFU. Cells were washed and purified with 37% Percoll. Cells for sorting were washed, counted, and subsequently surface stained in a biosafety level 3 (BSL3) containment area under sterile conditions. The following cell populations were sorted to 90 to 97% purity and plated for CFU counts: CD45.1+ (WT) or CD45.1− (Il1r12/2) CD11b+, CD11b+ Gr1high (neutrophils), and CD11b+ Gr1low (myeloid). Abs against I-Ab (clone M5/114.15.2), Ly6G (clone 1A8), CD11c (clones HL3 and N418), CD45.1 (clone A20), CD45.2 (clone 104), T cell receptor β (TCRβ) (clone H57-597), NK1.1 (clone PK136), CD11b (clone M1/70), CD45 (clone 30-F11), and Gr-1 (clones RB6 and 8C5) and live/dead fixable cell stains were obtained from eBioscience/Thermo Fisher Scientific, BioLegend, or BD Pharmingen. Samples were acquired on a 350 Symphony flow cytometer or sorted on a FACSAria instrument (BD Biosciences, San Jose, CA) and analyzed using FlowJo software.
ACKNOWLEDGMENTS
We thank members of the Weissman, Hasenkrug, Davis, and Glenn laboratories, especially Da Yoon No and Nathaniel Fernhoff, for helpful advice, discussions, and reagents. We specifically acknowledge Aaron Newman for assistance and guidance in the analysis of microarray data. We thank Susan Matlock Brewer for assistance and guidance with salmonella experiments. We thank Yael Rosenberg-Hasson and the staff of the Stanford Human Immune Monitoring Center for their technical expertise and for running the mouse and human Luminex assays. We also acknowledge the laboratory of Benjamin R. tenOever and Daniel Blanco-Melo for the rapid sharing of critical SARS-CoV-2 data at the onset of this pandemic (GEO accession number GSE147507).
Research reported in this publication was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; the Virginia and D. K. Ludwig Fund for Cancer Research; the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation; AML grant R01CA086017; the PCBC from NIHLB U01HL099999; as well as grant U19AI109662. M.C.T. and Y.Y.Y. were supported by Stanford Immunology training grant 5T32AI007290, and M.C.T. was also supported by the NIH NRSA 1 F32 AI124558-01 award and the Bay Area Lyme Foundation. L.B.T.D. was supported by a Stanford Diversifying Academia Recruiting Excellence fellowship. E.P. was supported by grant F30DK099017 and the Stanford Medical Scientist Training Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
K.J.H. and I.L.W. are listed as inventors on U.S. patent 2019/0092873 A1 CD47, Targeted Therapies for the Treatment of Infectious Disease. I.L.W. is a cofounder, director, and stockholder in Forty Seven Inc., a public company that was involved in CD47-based immunotherapy of cancer during this study but was acquired by Gilead. At the time of this submission, I.L.W. has no formal relationship with Gilead.
Footnotes
This article is a direct contribution from Kim J. Hasenkrug, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Cornelia Bergmann, Cleveland Clinic, and Anthony Rongvaux, Fred Hutchinson Cancer Institute.
Citation Tal MC, Torrez Dulgeroff LB, Myers L, Cham LB, Mayer-Barber KD, Bohrer AC, Castro E, Yiu YY, Lopez Angel C, Pham E, Carmody AB, Messer RJ, Gars E, Kortmann J, Markovic M, Hasenkrug M, Peterson KE, Winkler CW, Woods TA, Hansen P, Galloway S, Wagh D, Fram BJ, Nguyen T, Corey D, Kalluru RS, Banaei N, Rajadas J, Monack DM, Ahmed A, Sahoo D, Davis MM, Glenn JS, Adomati T, Lang KS, Weissman IL, Hasenkrug KJ. 2020. Upregulation of CD47 is a host checkpoint response to pathogen recognition. mBio 11:e01293-20. https://doi.org/10.1128/mBio.01293-20.
REFERENCES
- 1.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 2.Dyck L, Mills KHG. 2017. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 3.Wei SC, Anang N-AAS, Sharma R, Andrews MC, Reuben A, Levine JH, Cogdill AP, Mancuso JJ, Wargo JA, Pe’er D, Allison JP. 2019. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 116:22699–22709. doi: 10.1073/pnas.1821218116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer J-P, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM. 2018. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O’Rourke T, Narayanan S, Papadopoulos K, Fisher GA, Villalobos V, Prohaska SS, Howard M, Beeram M, Chao MP, Agoram B, Chen JY, Huang J, Axt M, Liu J, Volkmer J-P, Majeti R, Weissman IL, Takimoto CH, Supan D, Wakelee HA, Aoki R, Pegram MD, Padda SK. 2019. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takimoto CH, Chao MP, Gibbs C, McCamish MA, Liu J, Chen JY, Majeti R, Weissman IL. 2019. The macrophage ‘do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol 30:486–489. doi: 10.1093/annonc/mdz006. [DOI] [PubMed] [Google Scholar]
- 7.Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, Volkmer J-P, Weissman IL, Majeti R. 2020. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol 9:1380. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willingham SB, Volkmer J-P, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua M-S, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NNH, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. 2012. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majeti R, Becker MW, Tian Q, Lee T-LM, Yan X, Liu R, Chiang J-H, Hood L, Clarke MF, Weissman IL. 2009. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci U S A 106:3396–3401. doi: 10.1073/pnas.0900089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. 2010. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao A-G, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. 1996. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Jiang P, Lagenaur CF, Narayanan V. 1999. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 14.Brown EJ, Frazier WA. 2001. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 15.Barclay AN, van den Berg TK. 2014. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y-T, Ko E-J, Lee Y, Lee Y-N, Bian Z, Liu Y, Kang S-M. 2016. CD47 plays a role as a negative regulator in inducing protective immune responses to vaccination against influenza virus. J Virol 90:6746–6758. doi: 10.1128/JVI.00605-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers LM, Tal MC, Torrez Dulgeroff LB, Carmody AB, Messer RJ, Gulati G, Yiu YY, Staron MM, Angel CL, Sinha R, Markovic M, Pham EA, Fram B, Ahmed A, Newman AM, Glenn JS, Davis MM, Kaech SM, Weissman IL, Hasenkrug KJ. 2019. A functional subset of CD8+ T cells during chronic exhaustion is defined by SIRPα expression. Nat Commun 10:794. doi: 10.1038/s41467-019-08637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick-Marchand H, de Geus SJ, Pouw SM, Böhne M, Voordouw A, Weijer K, Di Santo JP, Spits H. 2011. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A 108:13224–13229. doi: 10.1073/pnas.1101398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor KE, Mossman KL. 2013. Recent advances in understanding viral evasion of type I interferon. Immunology 138:190–197. doi: 10.1111/imm.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron CM, Barrett JW, Mann M, Lucas A, McFadden G. 2005. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology 337:55–67. doi: 10.1016/j.virol.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 21.Dittmer U, Sutter K, Kassiotis G, Zelinskyy G, Bánki Z, Stoiber H, Santiago ML, Hasenkrug KJ. 2019. Friend retrovirus studies reveal complex interactions between intrinsic, innate and adaptive immunity. FEMS Microbiol Rev 43:435–456. doi: 10.1093/femsre/fuz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. 2008. Suppression of acute anti-Friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol 82:408–418. doi: 10.1128/JVI.01413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cham LB, Torrez Dulgeroff LB, Tal MC, Adomati T, Li F, Bhat H, Huang A, Lang PA, Moreno ME, Rivera JM, Galkina SA, Kosikova G, Stoddart CA, McCune JM, Myers LM, Weissman IL, Lang KS, Hasenkrug KJ. 2020. Immunotherapeutic blockade of CD47 inhibitory signaling enhances innate and adaptive immune responses to viral infection. Cell Rep 31:107494. doi: 10.1016/j.celrep.2020.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi F, Means TK, Luster AD. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 25.Kortmann J, Brubaker SW, Monack DM. 2015. Cutting edge: inflammasome activation in primary human macrophages is dependent on flagellin. J Immunol 195:815–819. doi: 10.4049/jimmunol.1403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol 3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia HK, Singh H, Grewal N, Natt NK. 2014. Sofosbuvir: a novel treatment option for chronic hepatitis C infection. J Pharmacol Pharmacother 5:278–284. doi: 10.4103/0976-500X.142464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. 2014. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung-Leung WP, Kündig TM, Zinkernagel RM, Mak TW. 1991. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med 174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann-Grube F. 1971. Lymphocytic choriomeningitis virus, 1st ed Springer-Verlag, Vienna, Austria. [Google Scholar]
- 31.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL. 2009. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BRG. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 69:912–920. [PubMed] [Google Scholar]
- 33.Kojima Y, Volkmer J-P, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. 2016. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y, Leeper NJ, Ho P, Gip P, Swigut T, Sherwood RI, Clarke MF, Somlo G, Young RA, Weissman IL. 2017. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun 8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee R, Khandelwal S, Kozakai Y, Sahu B, Kumar S. 2015. CD47 regulates the phagocytic clearance and replication of the Plasmodium yoelii malaria parasite. Proc Natl Acad Sci U S A 112:3062–3067. doi: 10.1073/pnas.1418144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayi K, Lu Z, Serghides L, Ho JM, Wang JCY, Liles WC, Kain KC. 2016. CD47-SIRPα interactions regulate macrophage uptake of Plasmodium falciparum-infected erythrocytes and clearance of malaria in vivo. Infect Immun 84:2002–2011. doi: 10.1128/IAI.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. 2008. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol 180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarathna DHMLP, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. 2015. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One 10:e0128220. doi: 10.1371/journal.pone.0128220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath PR, Gangaplara A, Pal-Nath D, Mandal A, Maric D, Sipes JM, Cam M, Shevach EM, Roberts DD. 2018. CD47 expression in natural killer cells regulates homeostasis and modulates immune response to lymphocytic choriomeningitis virus. Front Immunol 9:2985. doi: 10.3389/fimmu.2018.02985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng M, Chen JY, Weissman-Tsukamoto R, Volkmer J-P, Ho PY, McKenna KM, Cheshier S, Zhang M, Guo N, Gip P, Mitra SS, Weissman IL. 2015. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A 112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steeves RA, Mirand EA, Thomson S, Avila L. 1969. Enhancement of spleen focus formation and virus replication in Friend virus-infected mice. Cancer Res 29:1111–1116. [PubMed] [Google Scholar]
- 42.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, Chaconas G. 2008. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog 4:e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR Jr.. 2005. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun 73:1196–1203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. 2015. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Møller R, Panis M, Sachs D, Albrecht RA, tenOever BR. 2020. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv 2020.03.24.004655. doi: 10.1101/2020.03.24.004655. [DOI]
- 46.Mei HE, Leipold MD, Maecker HT. 2016. Platinum-conjugated antibodies for application in mass cytometry. Cytometry A 89:292–300. doi: 10.1002/cyto.a.22778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HCV viral titer does not correlate with CD47 expression levels; however, SIRPhi DCs are increased in response to HCV infection and PAMP stimulation. (A) HCV viral loads of HCV patients at the pretreatment time point compared to the CD47 MFI of their monocytes and dendritic cells from pretreatment clinical sample peripheral blood. (B) Percentages of SIRPhi dendritic cells by CyTOF analysis of HCV patient clinical peripheral blood samples pre-, mid-, and posttreatment compared to healthy controls. Download FIG S1, PDF file, 0.5 MB (568KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
CD47 gene expression levels are lower in tuberculosis gene arrays and higher in hepatitis C virus gene arrays. Metasignature gene expression analysis of CD47 for tuberculosis (A) and hepatitis C virus (B) data sets was performed based on standardized mean difference (log2 scale) gene expression correlation plots for TB and HCV from http://metasignature.stanford.edu/. Download FIG S2, PDF file, 0.4 MB (404.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.