Abstract
Coronavirus disease-19 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is now a pandemic threat. This virus is supposed to be spread by human to human transmission. Cellular angiotensin-converting enzyme 2 (ACE2) is the receptor of SARS-CoV-2 which is identical or similar in different species of animals such as pigs, ferrets, cats, orangutans, monkeys, and humans. Moreover, a recent study predicted that dogs might be secondary hosts during the evolution of SARS-CoV-2 from bat to human. Therefore, there is a possibility of spreading SARS-CoV-2 through domestic pets. There are now many reports of SARS-CoV-2 positive cases in dogs, cats, tigers, lion, and minks. Experimental data showed ferrets and cats are highly susceptible to SARS-CoV-2 as infected by virus inoculation and can transmit the virus directly or indirectly by droplets or airborne routes. Based on these natural infection reports and experimental data, whether the pets are responsible for SARS-CoV-2 spread to humans; needs to be deeply investigated. Humans showing clinical symptoms of respiratory infections have been undergoing for the COVID-19 diagnostic test but many infected people and few pets confirmed with SARS-CoV-2 remained asymptomatic. In this review, we summarize the natural cases of SARS-CoV-2 in animals with the latest researches conducted in this field. This review will be helpful to think insights of SARS-CoV-2 transmissions, spread, and demand for seroprevalence studies, especially in companion animals.
Keywords: SARS-CoV-2, COVID-19, Animals, Pets, Transmission
Introduction
Coronaviruses are group viruses belong to Coronaviridae family can produce diseases in human and animal. There are four genera under this family; Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. However, most of the human coronaviruses produce mild illness in the upper respiratory tract while some strains are lethal and can cause severe acute respiratory syndrome (SARS), middle East respiratory syndrome (MERS) and coronavirus disease 2019 (COVID-19).1, 2, 3 The most recent and rapidly evolved coronavirus associated disease in human is COVID-19 caused by SARS-Corona virus-2 (SARS-CoV-2).4 The nucleotide variations of the COVID-19 causing coronavirus are closely related with SARS coronavirus (SARS-CoV), therefore it has been named SARS-CoV-2.5 The SARS-CoV-2 is extremely infectious and contagious compared to SARS-CoV which was affected in 2003.3 Though the clinical symptoms and severity of COVID-19 depend on the age, health condition of the infected patients but mild to high fever, cough, dyspnea, headache, diarrhea, etc might be revealed.6 SARS-CoV-2 supposed to be transmitted to healthy individual by direct contact from infected patient's coughs and sneezes through respiratory droplets.7 Indirect transmission may also occur by surface or feces contaminations.8, 9, 10 It is already reported that many of the infected patients do not show any clinical symptoms but may shed the virus through their respiratory droplets.11 On the other hand, the infected person may shed virus before the onset of the symptoms.12 Therefore, SARS-CoV-2 can be transmitted to healthy individuals in three possible ways; symptomatic, pre-symptomatic, and asymptomatic COVID-19 patients.12 Moreover, it is hypothesizing, SARS-CoV-2 might be transmitted as airborne and closed environments contribute to the secondary transmission of the virus thereby promote the super-spreading phenomenon.13 , 14
SARS-CoV-2 is a spherical shaped and enveloped virus under betacoronavirus genus which is 50–200 nm in diameter. The viral genome is a ∼30 kb sized single-stranded positive-sense RNA materials from which four structural proteins and 16 nonstructural proteins are produced.15 The structural proteins are spike (S), envelope (E), membrane (M), and nucleocapsid (N).15 The viral envelope consists of E and M proteins on which S is anchored.16 The SARS-CoV-2 enter into the host cells by using the receptor-binding domain (RBD) of S protein which interacts with the cellular receptor angiotensin-converting enzyme 2 (ACE2).17
SARS-CoV-2 outbreaks occurred at the end of December, 2019; has been thought to be originated from the bats as shown by evolutionarily similar genome sequence.18 It is now a serious pandemic threat and international public health concern according to World Health Organization (WHO). Till May 20, 2020, around 213 countries and territories have been affected by SARS-CoV-2 and ∼5 million people infected with more than 334 thousand deaths. The SARS-CoV and MERS-CoV supposed to be originated from bat through an intermediate animal host.19, 20, 21 MERS-CoV-2 has been reported having zoonotic transmission.22 Many reports showed that SARS-CoV might infect animals such as cats, dogs, and ferrets.23, 24, 25 However, there is very limited information regarding the investigations on SARS-CoV-2 in animals. A recent study found that SARS-CoV-2 can recognize the host cells receptor ACE2 of pigs, ferrets, cats, orangutans, monkeys, and humans with similar efficiencies.26 Several cases of SARS-CoV-2, that causes COVID-19 in human; have been confirmed in animals. Laboratory studies also showed that cats and ferrets are highly susceptible to SARS-CoV-2 that were isolated from humans.27 , 28 To date, there is no published report so far on the investigation of SARS-CoV-2 in domestic pets which were in contact with COVID-19 patients. In this review, we summarized the SARS-CoV-2 infected animal cases and recent researches conducted in this field. This review will be helpful to think insights of SARS-CoV-2 transmissions, spread, and demand for seroprevalence studies in, especially companion animals.
Methods
Data and information collection
The recent information on SARS-CoV-2 infections in animals was collected by google searches. The published articles regarding the SARS-CoV-2 and animals, or cells originated from animals were systematically screened and checked using google scholar, PubMed, etc. The SARS-CoV-2 genome sequences were obtained from GISAID (https://www.gisaid.org/).
Phylogenetic tree construction
The retrieved sequences were aligned using ClustalW and the phylogenetic tree was constructed using MEGA X version 10.1.8. The neighbor-joining algorithm was used with 1,000 replicates of bootstrap analysis to build the tree.
History of human coronaviruses
In 1960, the coronavirus was characterized for the first time isolated from a child with upper respiratory tract infections.29 Then, the HCoV-OC43 and HCoV-229E strains of coronaviruses were identified by Hamre and Procknow, 1996 and McIntosh et al., 1967. From the persons suffering from colds.30 , 29 , 31 However, these two viruses and infectious bronchitis virus, mouse hepatitis virus, and swine transmissible gastroenteritis virus were morphologically similar under electron microscopy; therefore, this new group of viruses was named coronavirus in the late 1960s.29 The term “corona” used to mean crown-like structure surrounded by a surface projection of the viruses under electron microscopy.29 The HCoV-OC43 and HCoV-229E strains of coronavirus cause common cold in human prevalent worldwide.29 , 32
Coronavirus (SARS-CoV) causing pandemic severe acute respiratory syndrome (SARS) was first identified in Foshan, Guangdong, China in November 2002. Around 29 different countries have been affected by SARS-CoV and at least 831 people were died worldwide among over 8000 infected cases.3 The human coronavirus NL63 (HCoV-NL63) was first identified in 2004 in Netherland from a seven-month-old child with bronchiolitis.33 A novel strain of coronavirus HKU1 (HCoV-HKU1) has been discovered in 2005 which was isolated and characterized from an adult human suffering from chronic pulmonary disease in Hong Kong.34 The Middle East respiratory syndrome-related coronavirus (MERS-CoV) is another species of coronavirus; first reported in 2012 in Saudi Arabian patients suffering from pneumonia.1 As of April, 2019, globally 2374 MERS-CoV confirmed cases have been reported with a total of 823 deaths from 27 countries worldwide.35
The latest strain of a pandemic coronavirus is SARS-CoV-2 causes coronavirus disease-19 (COVID-19); reported on December 31, 2019 at Wuhan of China.36 Due to extremely high contagiousness of SARS-CoV-2; as of May 20, 2020, more than 5000,000 cases of COVID-19 have been confirmed from 213 affected countries and territories with more than 334,000 deaths (https://coronavirus.jhu.edu/map.html).
SARS-CoV host diversity
SARS outbreak in 2003 was supposed to be originated from the masked palm civets. Genetic sequence analysis also revealed the viruses isolated from this civet as highly homologous to the SARS-CoV genome.20 There was no record of new SARS cases in the community after the destruction and/or quarantine of all civets reared for human consumption in China.20 Guan et al. characterized SARS-CoV like particles from Himalayan palm civets in a live-animal market in Guangdong, China.24 This is also evident to infect with this strain in raccoon dog and human which suggests an interspecies transmission of SARS-CoV-like viruses.23 , 24 Domestic cats and ferrets could be experimentally infected with severe acute respiratory syndrome coronavirus (SARS-CoV).25 The researchers used the SARS virus which caused the death of a human.25 They demonstrated that the infected animals could transmit the virus to other healthy animals housed together through the virus shedding from the pharynx.25 However, the infected cat showed no clinical signs but the ferrets become lethargic which suggests that the pet might act as reservoirs of SARS-CoV. According to WHO, domestic cats living in the Amoy Gardens in Hong Kong were infected with SARS-CoV during the spring of 2003 where a hundred people contracted SARS.37 This report suggests that cats may naturally get infected with SARS-CoV from the infected human.
SARS-CoV-2 host diversity
SARS-CoV-2 natural infections in animals
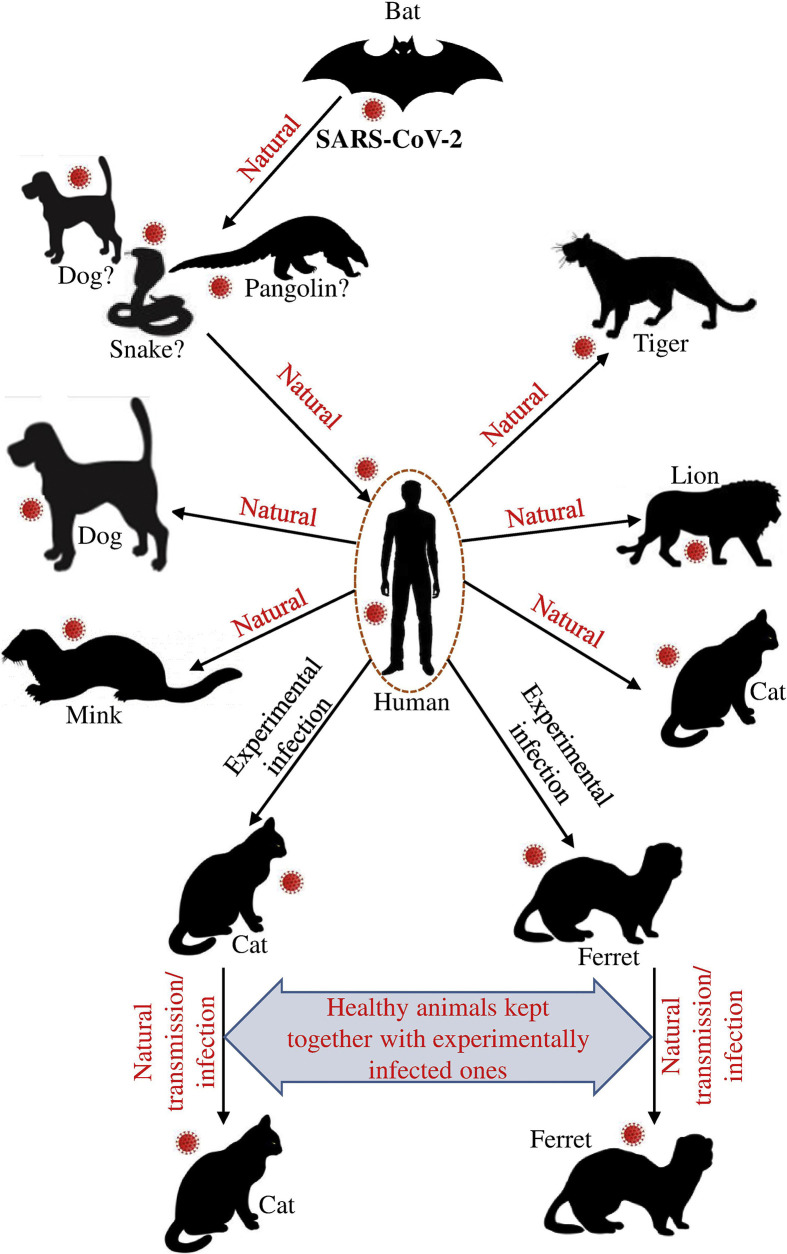
There is no confirmed report on the animal to human transmission of SARS-CoV-2 but few reports already showed that some carnivores might be infected with SARS-CoV-2. A pet dog had been confirmed as weak positive to the COVID-19 virus on February 28, 2020, in Hong Kong reported by Agriculture, Fisheries, and Conservation Department (AFCD).38 , 39 A German shepherd breed of dog has found to be SARS-CoV-2 positive in Hong Kong which is the second case though the dog showed no clinical symptoms.40 , 41 Importantly, the dog owner had been previously diagnosed as COVID-19 positive.41 However, no other dogs in the same group found to be positive for SARS-CoV-2. Recently, one dog in the Netherlands found to be positive for SARS-CoV-2 whose owner was a COVID-19 patient.42
A cat in Belgium that was infected with SARS-CoV-2 might be from its owner who traveled to northern Italy with a history of COVID-19 illness. Laboratory test from feces and vomit samples showed high levels of SARS-CoV-2 RNA as the cat showed respiratory illness, nausea, and diarrhea.43 The AFCD announced on March 31, 2020 that, SARS-CoV-2 has been detected from the oral cavity, nasal, and rectal samples of a pet cat lived with a person COVID-19 patient in Hong Kong and no clinical signs was observed.44 , 45 CDC and US National Veterinary Services Laboratories (NVSL) announced that two cats have found as confirmed for SARS-CoV-2 on April 21, 2020 in New York. One of them showed mild respiratory signs with no history of COVID-19 patients of the cat's owners or other households. The owner of the second cat was COVID-19 positive and the cat showed respiratory signs.46 The first cat with SARS-CoV-2 has been tested in French on May 2 whose owner was suffering from COVID-19. Interestingly, the nasal swab of this cat showed negative whereas rectal swab was tested positive for viral RNA though it is suffering from the clinical signs of respiratory and digestive problems. Three cats have been tested positive for SARS-CoV-2 in Netherland in May, 2020. Surprisingly these cats were lived on a mink farm; where minks were previously infected with SARS-CoV-2.42 , 47
The first case of a tiger infected with SARS-CoV-2 is on April 5, 2020 in a zoo in New York announced by USDA National Veterinary Services Laboratories.45 The tiger was shown signs of respiratory illness such as dry cough and decreased appetite.45 It was thought that the tiger probably got infected by a caretaker of the zoo who did not show any symptoms of the disease but actively shedding virus. According to USDA, a lion has been tested positive for SARS-CoV-2 on April 15, 2020 in New York.46 However, on April 22, the Wildlife Conservation Society (WCS) announced that all tigers and lions of the above-mentioned zoo have been tested positive for SARS-CoV-2 as confirmed by the detection of viral RNA from fecal samples.45
Several minks of two different farms in the Netherlands have been reported to be positive for SARS-CoV-2 as announced by Dutch Minister of Agriculture, Nature, and Food Quality on 26 April.45 , 47 The mink of these farms have been suffering from gastrointestinal and respiratory diseases. The caretakers of the farms were positive for COVID-19 and it is believed that they are the sources on infection for mink and mink-to-mink transmission helped the spread of the virus.45 , 47 The SARS-CoV-2 has been detected in the two more mink farms in the Netherlands (total of four farms) announced on May 7, 2020.45
A cohort study conducted by Zhang et al. from January to March 2020 in Wuhan, China after the outbreak of COVID-19 showed that 15 among 102 cats were seropositive for SARS-CoV-2.48 The sera samples were collected from animal shelters or pet hospitals of Wuhan. Virus neutralization test (VNT) confirmed that 11 of them contained the SARS-CoV-2 specific antibody. The sera of 39 cats retrieved from the serum bank collected from Wuhan from march to May 2019 before the COVID-19 outbreak were negative for the SARS-CoV-2 antibody. However, no viral RNA was detected from the cats with SARS-CoV-2 specific antibody.48 These results suggested that cats might be naturally infected with the SARS-CoV-2 that caused COVID-19 in humans.
These reports suggest that SARS-CoV-2 could be transmitted from humans to animals (Fig. 1 ). A microbiologist of Pennsylvania School of Veterinary Medicine also explained a concern whether pets can act as reservoir of SARS-CoV-2 need to be investigated when she talked to the science magazine.39 Based on an experimental infection study the authors also suggested to survey for SARS-CoV-2 in cats for minimizing the COVID-19 in humans.28
Figure 1.
Host range of SARS-CoV-2. Different pets/animals are susceptible to SARS-CoV-2 might be occurred naturally and/or experimentally.
Experimental infections in animals by SARS-CoV-2 and molecular evidences
Shi et al. reported that ferrets and cats might be experimentally infected with SARS-CoV-2 strains of both environments (SARS-CoV-2/F13/environment/2020/Wuhan; 13-E) and human (SARS-CoV-2/CTan/human/2020/Wuhan; CTan-H) isolates as confirmed by virus detection and specific respiratory clinical symptoms appeared on the infection.28 Moreover, they found infected cats may transmit SARS-CoV-2 into the healthy cats lived together through respiratory droplets or airborne routes.28 Results of similar experiments showed that dogs are less susceptible to SARS-CoV-2. However, pigs, chickens, and ducks are not susceptible to SARS-CoV-2 as no viral RNA was detected and these animals were seronegative.28 Moreover, the ACE2 molecules responsible for binding with SARS-CoV-2 spike are almost identical or similar in pigs, ferrets, cats, orangutans, monkeys, and humans.26 Accordingly, SARS-CoV-2 spike RBD domain binds with ACE2 of pigs, ferrets, cats, orangutans, monkeys, and humans in similar efficiencies.26 Phylogenetic clustering and sequence alignment study conducted by Qui et al., 2020 found that ACE2 of several domestic animals such as cat, cow, buffalo, goat, sheep, and pigeon could be used by SARS-CoV-2.49 A Ferret can be experimentally infected with causal agents of COVID-19.27 The infected ferrets showed respiratory signs and shed the virus through nasal washes, saliva, urine, and feces though none of them died.27 Infected ferrets rapidly transmitted the virus to the non-infected ones by direct or indirect contact which suggests that ferrets are highly susceptible to SARS-CoV-2.27 , 50 Moreover, recent research showed that animal cell lines such as Vero and MDCKII are susceptible to VSV pseudotypes surrounded SARS-CoV-2 spike protein.51 A recent study reported that among all known Betacoronaviruses, SARS-CoV-2 has an extreme deficiency of CpG that might be associated with the novel host intermediate host.52 Xia, 2020 hypothesized that SARS-CoV-2 originated from bat through the intermediate host dog which ingested bat meat. The virus evolved by reducing CpG content in the canid intestine thereby contaminated the respiratory system and finally become a severe human pathogen by evading the ZAP-mediated immune response.52 The author also suggested monitoring the coronavirus in stray dogs for proper fighting against SARS-CoV-2.52 In addition, Damas et al., 2020 analyzed the ACE2 cross-species conservation for the functional properties as a receptor for SARS-CoV-2.53 Among 252 mammals, humans and different types of monkeys are at high risk of SARS-CoV-2 whereas cat, dog, cattle and goat, etc. at moderate risk.
Genetic relationship of SARS-CoV-2 of human and animals
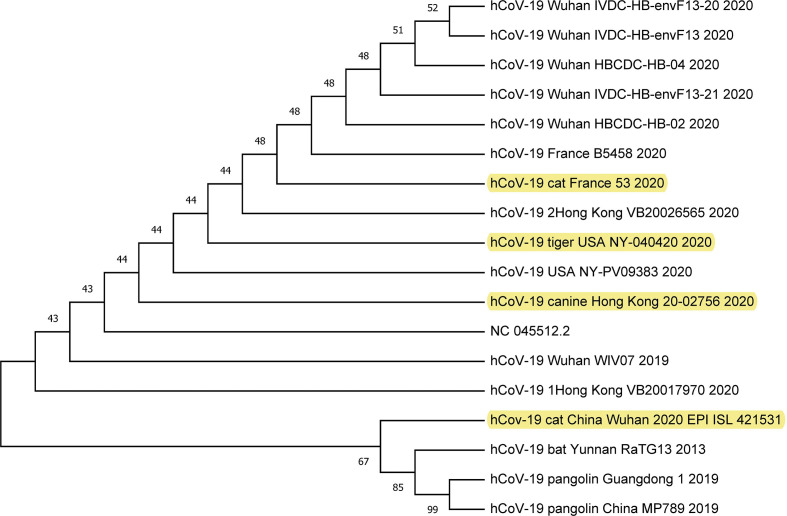
SARS-CoV-2 isolated from humans until now are very much related to coronaviruses isolated from bat populations.18 SARS-CoV outbreak in 2003 was also closely related to coronavirus from bats.54 Genetic analysis of all of these viruses suggests that they all have their ecological origin in bat population, and transmission of the virus to humans has likely occurred through an intermediate animal host. This intermediate animal host might be a domestic animal, a wild animal, or a domesticated wild animal that has not been identified clearly yet. Although the zoonotic sources of the SARS-CoV-2 are currently unknown pangolins and snakes are the main suspects. Till now, several animals (Table 1 ) have been found to be positive for SARS-COV-2, during reviewing the data and/or articles, we tried to compare the evolutionary relationship of these animal isolates with humans, bat, pangolin and environment, etc. Unfortunately, the genome sequences of all animal isolates of SARS-CoV-2 have not been found in GISAID (https://www.gisaid.org/), GenBank (https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/) and China National Center of Bioinformation (https://bigd.big.ac.cn/ncov/) except few; hCoV-19/canine/Hong Kong/20–02756/2020 and hCov-19/cat/China/Wuhan/2020, hCoV-19/tiger/USA/NY-040420/2020, and >hCoV-19_France_B5458_2020. Results of the phylogenetic tree showed that SARS-CoV-2 isolate of a cat in Wuhan is closely related to bat and pangolin isolates of the same region (Fig. 2 ). Similarly, the dog, cat, and tiger isolates were found to be clustered with the human isolates in the respective countries or regions (Fig. 2). These results further support the local and human to animal transmission occurrence of SARS-CoV-2. However, according to the Agriculture, Fisheries and Conservation Department (AFCD), the cat was found positive for SARS-CoV-2 in Hong Kong but in the GISAID (https://www.gisaid.org/) samples location of cat isolate was Wuhan.44
Table 1.
Summary of natural infections by SARS-CoV-2 in animals.
| Cases | Host | Date of reports | Region | References |
|---|---|---|---|---|
| 1 | Dog | February 28, 2020 | Hong Kong | 38,39 |
| 2 | Dog | March 18, 2020 | Hong Kong | 40,41 |
| 3 | Cat | March 27, 2020 | Belgium | 43 |
| 4 | Cat | March 31, 2020 | Hong Kong | 44,45 |
| 5 | Tiger | April 5, 2020 | New York | 45,46 |
| 6 | Lion | April 15, 2020 | New York | 46 |
| 7 | Cat | April 21, 2020 | New York | 46 |
| 8 | Cat | April 21, 2020 | New York | 46 |
| 9 | Mink | April 26, 2020 | Netherlands | 45 |
Figure 2.
Evolutionary relationship of SARS-CoV-2 isolates of dog, cat, tiger, human, bat, and pangolin. The genome sequences were retrieved from the GISAID. The sequences were aligned and the phylogenetic tree was constructed using MEGA X version 10.1.8. The neighbor-joining algorithm was used with 1,000 replicates of bootstrap analysis to build the tree.
Conclusions and recommendations
Several cats and one dog were infected during the SARS outbreak 2002–2003. SARS was caused by a coronavirus that transferred from a wild animal, the civet cat to humans. Data are accumulating and scientists believe that the SARS-CoV-2 may follow a similar path like SARS-CoV. Cats, dogs, and ferrets are susceptible to SARS-CoV-2 according to natural infections history and experimental results. All of the studies suggest a diversified host range might be involved in the SARS-CoV-2 pandemic outbreak. Whether the domestic pets might be the reservoir of SARS-CoV-2 should be carefully investigated. Seroprevalence study should be conducted in the COVID-19 affected regions to monitor the SARS-CoV-2 silent infections in the domestic pets especially cats, ferrets, and dogs. Moreover, it would also be suggested to test the companion animals which were with COVID-19 patients. SARS-CoV-2 infected owners should avoid close contact with their pets. Humans should take special protection during handling animals/pets showing respiratory symptoms.
Author contributions
MGH conceptualized, searched and reviewed the literature, and wrote the original draft manuscript. AJ, SA and SS revised and edited the manuscript. MGH, AJ, SA, and SS revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
None.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Nothing to disclose.
References
- 1.Abdel-Moneim A.S. Middle East respiratory syndrome coronavirus (MERS-CoV): evidence and speculations. Arch Virol. 2014;159:1575–1584. doi: 10.1007/s00705-014-1995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Ho W., Huang Y., Jin D.Y., Li S., Liu S.L. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarner J. Three Emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am J Clin Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindson J. COVID-19: faecal–oral transmission? Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X., Yang R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Coronavirus disease 2019 (COVID-19) situation report-73. World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-20200473-covid-20200419.pdf?sfvrsn=20200405ae20200425bc20200407_20200402 [Google Scholar]
- 13.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiura H., Oshitani H., Kobayashi T., Saito T., Sunagawa T., Matsui T. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19) medRxiv. 2020 [Google Scholar]
- 15.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133:74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R.-H., He J.-F., Evans M.R., Peng G.W., Field H.E., Yu D.W. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudas G., Carvalho L.M., Rambaut A., Bedford T. MERS-CoV spillover at the camel-human interface. eLife. 2018;7 doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enserink M. Clues to the animal origins of SARS. Science. 2003;300:1351. doi: 10.1126/science.300.5624.1351a. [DOI] [PubMed] [Google Scholar]
- 24.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 25.Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020 doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24:S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 30.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci Unit States Am. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S.F., Tuo J.L., Huang X.B., Zhu X., Zhang D.M., Zhou K. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. PloS One. 2018;13:e0191789. doi: 10.1371/journal.pone.0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadzadeh J., Mobaraki K. Epidemiological status of the Middle East respiratory syndrome coronavirus in 2019: an update from January 1 to March 31, 2019. Int J Gen Med. 2019;12:305–311. doi: 10.2147/IJGM.S215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lun Z.-R., Qu L.-H. Animal-to-human SARS-associated coronavirus transmission? Emerg Infect Dis. 2004;10:959. doi: 10.3201/eid1005.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AFCD . 2020. Detection of low level of COVID-19 virus in pet dog. Agriculture, Fisheries and Conservation Department.https://www.info.gov.hk/gia/general/202002/202028/P2020022800013.htm [Google Scholar]
- 39.David G. Quarantine the cat? Disinfect the dog? The latest advice about the coronavirus and your pets. Sci Magna. 2020 https://www.sciencemag.org/news/2020/2003/quarantine-cat-disinfect-dog-latest-advice-about-coronavirus-and-your-pets [Google Scholar]
- 40.AFCD . Agriculture, Fisheries and Conservation Department; 2020. Pet dog tests positive for COVID-19 virus.https://www.info.gov.hk/gia/general/202003/19/P2020031900606.htm [Google Scholar]
- 41.Goumenou M., Spandidos D., Tsatsakis A. Possibility of transmission through dogs being a contributing factor to the extreme Covid-19 outbreak in North Italy. Mol Med Rep. 2020 doi: 10.3892/mmr.2020.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delong J. Dutch Minister confirms dog, three cats have caught novel coronavirus. American Reporter. 2020 https://www.reporter.am/dutch-minister-confirms-dog-three-cats-have-caught-novel-coronavirus/ [Google Scholar]
- 43.Thompson H. A cat appears to have caught the coronavirus, but it's complicated. Sci News. 2020 https://www.sciencenews.org/article/cats-animals-pets-coronavirus-covid19 [Google Scholar]
- 44.AFCD . Agriculture, Fisheries and Conservation Department; 2020. Pet cat tests positive for COVID-19 virus.https://www.info.gov.hk/gia/general/202003/31/P2020033100717.htm [Google Scholar]
- 45.AVMA . American Veterinary Medical Association; 2020. SARS-CoV-2 in animals, including pets.https://wwwavmaorg/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets 2020 American Veterinary Medical Association. [Google Scholar]
- 46.USDA . United States Department of Agriculture; 2020. Confirmed cases of SARS-CoV-2 in animals in the United States.https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/SA_One_Health/sars-cov-2-animals-us [Google Scholar]
- 47.Oreshkova N., Molenaar R.J., Vreman S., Harders F., Munnink B.B.O., Hakze R. April 2020. SARS-CoV2 infection in farmed mink, Netherlands. bioRxiv 20202020.2005.2018.101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q., Zhang H., Huang K., Yang Y., Hui X., Gao J. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 2020 doi: 10.1101/2020.04.01.021196. [DOI] [Google Scholar]
- 49.Qiu Y., Zhao Y.B., Wang Q., Li J.Y., Zhou Z.J., Liao C.H. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microb Infect. 2020 doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richard M., Kok A., de Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A. SARS-CoV-2 is transmitted via contact and via the air between ferrets. bioRxiv. 2020 doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell Entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia X. Extreme genomic CpG deficiency in SARS-CoV-2 and evasion of host antiviral defense. Mol Biol Evol. 2020 doi: 10.1093/molbev/msaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. bioRxiv. 2020 doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L.F., Shi Z., Zhang S., Field H., Daszak P., Eaton B.T. Review of bats and SARS. Emerg Infect Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]