Abstract
Background
In our institute in Marseille, France, we initiated early and massive screening for coronavirus disease 2019 (COVID-19). Hospitalization and early treatment with hydroxychloroquine and azithromycin (HCQ-AZ) was proposed for the positive cases.
Methods
We retrospectively report the clinical management of 3,737 screened patients, including 3,119 (83.5%) treated with HCQ-AZ (200 mg of oral HCQ, three times daily for ten days and 500 mg of oral AZ on day 1 followed by 250 mg daily for the next four days, respectively) for at least three days and 618 (16.5%) patients treated with other regimen (“others”). Outcomes were death, transfer to the intensive care unit (ICU), ≥10 days of hospitalization and viral shedding.
Results
The patients’ mean age was 45 (sd 17) years, 45% were male, and the case fatality rate was 0.9%. We performed 2,065 low-dose computed tomography (CT) scans highlighting lung lesions in 592 of the 991 (59.7%) patients with minimal clinical symptoms (NEWS score = 0). A discrepancy between spontaneous dyspnoea, hypoxemia and lung lesions was observed. Clinical factors (age, comorbidities, NEWS-2 score), biological factors (lymphocytopenia; eosinopenia; decrease in blood zinc; and increase in D-dimers, lactate dehydrogenase, creatinine phosphokinase, troponin and C-reactive protein) and moderate and severe lesions detected in low-dose CT scans were associated with poor clinical outcome. Treatment with HCQ-AZ was associated with a decreased risk of transfer to ICU or death (Hazard ratio (HR) 0.18 0.11–0.27), decreased risk of hospitalization ≥10 days (odds ratios 95% CI 0.38 0.27–0.54) and shorter duration of viral shedding (time to negative PCR: HR 1.29 1.17–1.42). QTc prolongation (>60 ms) was observed in 25 patients (0.67%) leading to the cessation of treatment in 12 cases including 3 cases with QTc> 500 ms. No cases of torsade de pointe or sudden death were observed.
Conclusion
Although this is a retrospective analysis, results suggest that early diagnosis, early isolation and early treatment of COVID-19 patients, with at least 3 days of HCQ-AZ lead to a significantly better clinical outcome and a faster viral load reduction than other treatments.
Keywords: SARS-CoV-2, COVID-19, Hydroxychloroquine, Azithromycin
1. Introduction
In December 2019, a novel coronavirus emerged in the central Chinese province of Hubei, causing an outbreak of pneumonia [1]. As of June 11th, 2020, more than 7 million persons were infected with SARS-CoV-2, and more than 400,000 have died [2]. Management of this infection was heterogeneous across countries regarding i) indications for virological testing of patients and asymptomatic contacts, ii) indications for low-dose computed tomography (LDCT), and iii) therapeutic options and follow-up. Based on preliminary data from Chinese physicians [3,4], in Marseille, France, we designed a strategy including early massive screening by PCR, LDCT of the chest for positive patients, and early treatment with hydroxychloroquine (HCQ) to which we rapidly added azithromycin (AZ) after we found that the combination had a synergistic effect against the virus in vitro [5] and in vivo [[6], [7], [8]]. This led our institute to face a dramatic increase in workload but allowed us to generate real-life data allowing us to comprehensively describe the disease and management at our institute, despite the inherent limitations of such an observational study (Table 1 ).
Table 1.
Key numbers of activities at IHU Méditerranée Infection (2020, February 27th – 2020 May 12th).
| Patients tested for SARS-CoV-2 | 31,003 individuals including 1,277 health care workers |
| Patients hospitalized in day-care hospital | 3,525 |
| Patients hospitalized in infectious diseases units | 705 |
| Serology SARS-CoV-2 | 6,000 samples tested including 643 samples from health care workers |
| Culture | 4,786 samples inoculated 1,908 SARS-CoV-2 strains isolated |
| Genome | 466 genomes sequenced and analysed |
| Low-dose CT scan | 2,218 performed |
| Electrocardiograms | 7,800 performed |
| Serum drug dosages | 1,939 hydroxychloroquine dosages |
Indeed, among the candidate treatments, only three main drugs (remdesivir, lopinavir-ritonavir and HCQ) have been tested in large comparative studies [[11], [12], [13]]. Lopinavir-ritonavir and remdesivir have not clearly demonstrated efficacy but are associated with many adverse events [11,12,14]. HCQ has demonstrated its efficacy in reducing viral shedding persistence [6] and improving clinical status in observational or randomized clinical trials [13,15,16]. In addition, we performed a recent meta-analysis of 20 available reports, including 105,040 patients demonstrating that, in clinical studies, chloroquine and its derivatives improve clinical and biological outcomes and reduce mortality by a factor 3 in coronavirus disease 2019 (COVID-19) patients [10]. In addition, we recently reported a very low mortality rate in a retrospective analysis of more than 1,000 patients early treated with a combination of HCQ-AZ, with a very low mild adverse event rate (2.3%) [8]. Conversely, in a recent observational study, patients treated with HCQ showed no difference regarding risk of death or intubation compared with patients under other treatments [17]. However, the patients included in the group receiving HCQ had more severe disease and had more comorbidities than those who did not receive the drug [17].
Here, we report on more than 3,700 cases treated in our institute, including those previously reported [7,8], to give a comprehensive analysis of our strategy. Outcomes were death, transfer to intensive care unit (ICU), hospitalization stay ≥10 days and viral shedding persistence.
2. Materials and methods
2.1. Patients and study design
The study was conducted in the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection (https://www.mediterranee-infection.com/), Assistance Publique-Hôpitaux de Marseille (AP-HM), Southern France. As previously described, we performed early massive PCR screening both for patients suspected of having COVID-19 and for contacts of confirmed cases [8]. We proposed standardized treatment and follow-up for all individuals >18 years of age with PCR-documented SARS-CoV-2 RNA from a nasopharyngeal sample. Data were collated from all patients from March 3rd to April 27th and were analysed retrospectively.
2.2. Clinical, biological and radiological data and follow-up
Demographics (age, sex), chronic conditions (cancer, diabetes mellitus, chronic heart disease, hypertension, chronic respiratory disease and obesity) and concomitant medications were documented. Symptoms, including rhinitis, anosmia, ageusia, fever, cough, dyspnoea and chest pain, were systematically recorded. Severity was assessed using the National Early Warning Score adapted to COVID-19 patients (NEWS-2) at admission and during follow-up [18]. Three categories of clinical worsening were defined: low score (NEWS-2 = 0–4), medium score (NEWS-2 = 5–6), and high score (NEWS-2≥7).
We recorded lymphocyte, eosinophil and platelet counts; fibrinogen; D-dimer and other coagulation factors; electrolytes; zinc; lactate dehydrogenase (LDH); creatine phosphokinase (CPK); C-reactive protein; and HCQ serum dosage [19]. Viral load was analysed by qPCR from nasopharyngeal swabs [8] at admission and during the follow-up, and an indirect immunofluorescence quantitative assay was used to assess the serological status against SARS-CoV-2 [20]. Viral culture was attempted for PCR-positive patients [5]. A LDCT was proposed for all patients when possible. Radiological lung lesions were classified into three categories: minimal, intermediate and severe involvement [8].
2.3. COVID-19 management and outcomes
The treatment consisted of the combination of HCQ (200 mg of oral HCQ, three times daily for ten days) and AZ (500 mg on day 1 followed by 250 mg daily for the next four days). This regimen was proposed as standard care for all patients without contraindications to these drugs [8]. Patients were informed of the off-label character of the prescription of HCQ and AZ prior to receiving treatment. Treatment was initiated among inpatients in our day-care hospital (i.e. here are patient kept just during the day) or in our infectious disease hospitalization units. All patients underwent electrolyte analysis and an electrocardiogram (EKG) with corrected QT measurement (Bazett's formula) before starting treatment [8]. EKGs with any abnormalities were systematically referred to a cardiologist for further assessment. In addition, broad-spectrum antibiotics (ceftriaxone or ertapenem) were included in the regimen for patients with pneumonia and/or NEWS scores ≥ 5. Standard care included systematic oxygen supplementation and preventive anticoagulation when necessary.
As it is common practice to assess the clinical evolution at 72 h for pneumonia [21], we selected this time-point to evaluate the clinical efficacy of HCQ-AZ [8]. Therefore, we defined two groups of patients: i) those receiving HCQ-AZ for at least three days and ii) the others comprising treatment with HCQ alone, AZ alone, HCQ-AZ for less than 3 days before defined clinical outcome, and those receiving neither HCQ either AZ.
Poor clinical outcome was defined as one of the following outcomes (transfer to ICU, death, hospitalization lasting ≥10 days), while others were considered as having a good clinical outcome.
2.4. Statistical methods
We used the Wilcoxon Mann-Whitney U test, χ2 test, or Fisher's exact test to compare differences between groups of patients where appropriate. We performed multiple correspondence analysis (MCA) to investigate the associations between clinical data, biological data, radiological data, poor clinical outcome and the treatment received (HCQ-AZ for at least three days, other treatments). Visual observations of Kaplan-Meier curves and log-rank tests were used for survival analyses. Multivariate logistic regression and the Cox proportional hazard model were used to identify independent predictors of each outcome. Considering that death was a main outcome and that only 35 patients died in our cohort (0.9%), the number of covariates to be included in multivariate analyses was a priori limited to three variables: previous health status (modified Charlson combined comorbidity index) [22], severity of the disease (NEWS-2 score) and treatment (HCQ-AZ for at least 3 days). Association between treatment (HCQ + AZ≥3days) and death was estimated by Cox regression models using three different methods. In the primary analysis, a multivariable Cox regression adjusted on the combined comorbidity index and the NEWS score was performed. We conducted a secondary analysis that used propensity-score matching. The propensity score was calculated using multivariable logistic regression on the combined comorbidity index and the NEWS score. Each patient of the “other treatment” group was matched to a patient selected of the “HCQ-AZ ≥ 3 days” group using the 1:1 nearest-neighbour propensity score matching method to create a matched sample. The third analysis used inverse probability weighting (https://cdn1.sph.harvard.edu/wp-content/uploads/sites/343/2013/03/msm-web.pdf). Association between treatment and death was estimated using stratified and weighted Cox regression. A two-sided p-value of less than 0.05 was considered statistically significant. MCA was performed using R Statistical Software and the FactoMineR package. All other analyses were carried out using SAS 9.4 statistical software (SAS Institute, Cary, NC).
2.5. Ethics statement
Data presented herein were collected retrospectively from the routine care setting using the electronic health recording system of the hospital. This non-interventional retrospective study was approved by our institutional review board committee (Mediterranée Infection N°: 2020–021). In France, at the time the study was conducted, HCQ for COVID-19 treatment was approved off-label for hospital delivery only. As previously reported [8], for all patients, the prescription of HCQ-AZ was made during either complete hospitalization or at day-care hospital by one of the physicians, after collegial decision based on the most recent scientific data available and after assessment of the benefit/harm ratio of the treatment. According to European General Data Protection Regulation No 2016/679, patients were informed of the potential use of their medical data and that they could refuse the use of their data. The analysis of collected data followed the reference methodology MR-004 registered on N° MR 5010010520 in the AP-HM register.
3. Results
3.1. Selection of the current cohort
From March 3rd to April 27th, we performed 101,522 SARS-CoV-2 PCR tests among 65,993 people (including more than 25,302 sampled in the IHU). Among them, 6,831 patients tested positive (10.4%). Of these, 3,024 patients (comparable in age and sex) were excluded: 1,399 whose samples were sent to our laboratory but who were followed up outside Marseille and 1,363 patients who were managed in Marseille, outside IHU. Among the patients diagnosed and treated in the IHU, 3,737 were analysed in this study after the exclusion of 332 younger than 18 years of age (described elsewhere).
3.2. Overall characteristics of patients
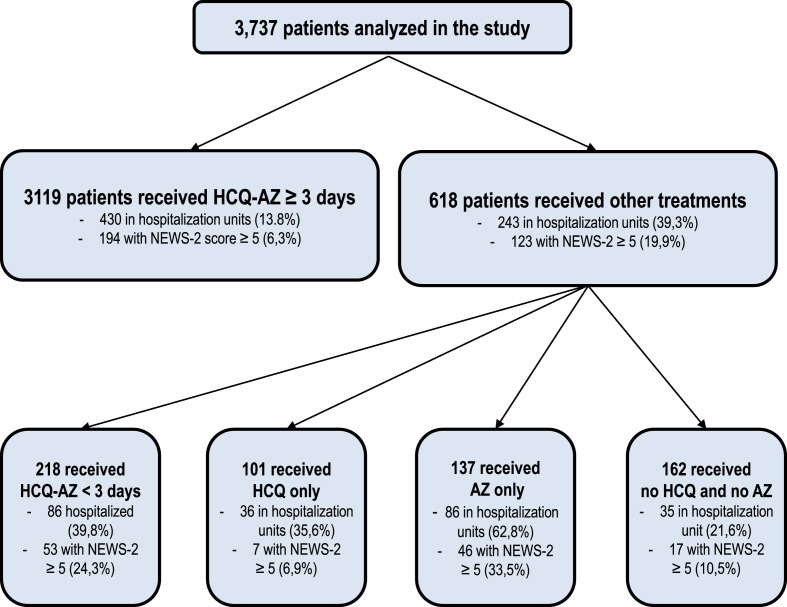
Among the 3,737 included patients, 3,284 (87.9%) were younger than 65 years, and 453 were older (12.1%), with a mean age of 45.3 years (standard deviation (sd), 16.8). A total of 1,704 patients (45.6%) were male. Regarding therapeutic management, 3,119 (83.5%) patients received at least a 3-day course of HCQ-AZ. Among the 618 others, 218 received a shorter course of HCQ-AZ (35.3%), 137 received AZ alone (22.2%), 101 received HCQ alone (16.3%) and 162 did not receive either drug (26.2%) (Fig. 1 ). The baseline characteristics of patients according to treatment groups are summarized in Table 2 . We paid a rigorous attention to avoiding HCQ-AZ in patients with cardiac diseases, abnormal EKG, dyskaliemia or current use of other interacting medications (Table 3 ).
Fig. 1.
Flowchart summarizing our study design.
Table 2.
Baseline characteristics of the patients according to treatment.
| All |
HCQ-AZ ≥ 3 days |
Other treatments |
HCQ-AZ < 3 days |
HCQ |
AZ |
No HCQ, No AZ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 3,737 |
(n = 3,119 83.5%) |
(n = 618 16.5%) |
(n = 218 5.8%) |
(n = 101 2.7%) |
(n = 137 3.7%) |
(n = 162 4.3%) |
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age | ||||||||||||||
| Age 18-44 | 1874 | 50.2 | 1649 | 52.8 | 225 | 36.4* | 76 | 34.9* | 46 | 45.5 | 24 | 17.5* | 79 | 48.8* |
| Age 45-54 | 804 | 21.5 | 671 | 21.5 | 133 | 21.5 | 50 | 22.9 | 29 | 28.7 | 22 | 16.1 | 32 | 19.7 |
| Age 55-64 | 606 | 16.2 | 503 | 16.1 | 103 | 16.7 | 38 | 17.4 | 17 | 16.8 | 25 | 18.2 | 23 | 14.2 |
| Age 65-74 | 241 | 6.5 | 183 | 5.9 | 58 | 9.4 | 21 | 9.6 | 4 | 4 | 20 | 14.6 | 13 | 8 |
| Age >74 | 212 | 5.7 | 113 | 3.6 | 99 | 16 | 33 | 15.1 | 5 | 4.9 | 46 | 33.6 | 15 | 9.3 |
| Sex | ||||||||||||||
| Men | 1704 | 45.6 | 1416 | 45.4 | 288 | 46.6 | 105 | 48.2 | 47 | 46.5 | 64 | 46.7 | 72 | 44.4 |
| Chronic condition(s) | ||||||||||||||
| Cancer disease | 129 | 3.5 | 83 | 2.7 | 46 | 7.4* | 20 | 9.2* | 3 | 3 | 16 | 11.7* | 7 | 4.3 |
| Diabetes | 312 | 8.4 | 235 | 7.5 | 77 | 12.5* | 23 | 10.5 | 4 | 4 | 37 | 27.0* | 13 | 8 |
| Chronic heart diseases | 219 | 5.9 | 125 | 4 | 94 | 15.2* | 23 | 10.5* | 7 | 6.9 | 46 | 33.6* | 18 | 11.1* |
| Hypertension | 561 | 15 | 410 | 13.1 | 151 | 24.4* | 50 | 22.9* | 13 | 12.9 | 57 | 41.6* | 31 | 19.1* |
| Chronic respiratory diseases | 338 | 9 | 267 | 8.6 | 71 | 11.5* | 21 | 9.6 | 9 | 8.9 | 25 | 18.2* | 16 | 9.9 |
| Obesity | 418 | 11.2 | 345 | 11.1 | 73 | 11.8 | 25 | 11.5 | 5 | 4.9 | 28 | 20.4* | 15 | 9.3 |
| Symptom(s) declared by patienta | 3397 | 90.9 | 2862 | 91.8 | 535 | 86.6* | 202 | 92.7 | 86 | 85.1* | 116 | 84.7* | 131 | 80.9* |
| Fever | 574 | 15.6 | 468 | 15.1 | 106 | 18.6* | 42 | 22.5* | 25 | 25.0* | 20 | 16.3 | 19 | 11.9 |
| Cough | 1846 | 50.2 | 1578 | 50.8 | 268 | 47.1* | 88 | 47.1 | 56 | 56 | 51 | 41.5* | 73 | 45.9 |
| Rhinitis | 1202 | 32.7 | 1065 | 34.3 | 137 | 24.1* | 46 | 24.6* | 28 | 28 | 21 | 17.1* | 42 | 26.4* |
| Anosmia | 1442 | 39.2 | 1277 | 41.1 | 165 | 29.0* | 59 | 31.5* | 28 | 28.0* | 25 | 20.3* | 53 | 33.3 |
| Ageusia | 1389 | 37.8 | 1213 | 39 | 176 | 30.9* | 61 | 32.6 | 33 | 33 | 27 | 21.9* | 55 | 34.6 |
| Dyspnea | 1038 | 28.2 | 901 | 29 | 137 | 24.1* | 65 | 34.8 | 16 | 16.0* | 26 | 21.1 | 30 | 18.9* |
| Thoracic pain | 811 | 22.1 | 745 | 24 | 66 | 11.6* | 27 | 14.4* | 12 | 12.0* | 7 | 5.7* | 20 | 12.6* |
| NEWS score | ||||||||||||||
| 0-4 | 3420 | 91.5 | 2925 | 93.8 | 495 | 80.1* | 165 | 75.7* | 94 | 93.1 | 91 | 66.4* | 145 | 89.5* |
| 5-6 | 172 | 4.6 | 114 | 3.7 | 58 | 9.4 | 22 | 10.1 | 5 | 4.9 | 25 | 18.2 | 6 | 3.7 |
| >6 | 145 | 3.9 | 80 | 2.6 | 65 | 10.5 | 31 | 14.2 | 2 | 2 | 21 | 15.3 | 11 | 6.8 |
| Pulmonary CT-scannerb | ||||||||||||||
| Normal | 616 | 29.8 | 540 | 31.6 | 76 | 21.4* | 28 | 19.3* | 9 | 23.1 | 19 | 18.3* | 20 | 29.9 |
| Minimal | 928 | 44.9 | 780 | 45.6 | 148 | 41.7 | 55 | 37.9 | 25 | 64.1 | 38 | 36.5 | 30 | 44.8 |
| Intermediate | 414 | 20.1 | 329 | 19.2 | 85 | 23.9 | 36 | 24.8 | 4 | 10.2 | 30 | 28.8 | 15 | 22.4 |
| Severe | 107 | 5.2 | 61 | 3.6 | 46 | 13 | 26 | 17.9 | 1 | 2.6 | 17 | 16.3 | 2 | 3 |
*: p < 0.05 (Fisher's exact test). Reference group is “HCQ-AZ ≥ 3 days”, aData available for 3676 patients. One patient may present several symptoms. bData available for 1710 patient in the “HCQ-AZ ≥ 3 days” group, 145 in the “HCQ-AZ < 3 days” group, 39 in the “HCQ only” group, 104 in the “AZ only” group and 67 in the “other treatments” group.
Table 3.
Baseline characteristics of patients with contraindication to or non-prescription of hydroxychloroquine and azithromycin combination.
| 88 patients with cardiac contraindication to the combined treatment | 24 prolonged QTc |
| 3 Brugada syndrome | |
| 1 myocarditis history | |
| 16 severe cardiopathy | |
| 12 left bundle branch block | |
| 4 right bundle branch block | |
| 5 atrio-ventricular block | |
| 23 others EKG abnormalities | |
| 139 patients for whom the combined treatment was not proposed by the physician* | |
| 55 patients who refused the combined treatment | |
| 45 patients with potential risk for drug interactions with the combined treatment | Cardiac drugs |
| 4 flecainide | |
| 9 amiodarone | |
| 1 celiprolol | |
| 1 bisoprolol | |
| 1 nicardipine | |
| 1 hydrochlorothiazide | |
| Neuropsychiatric drugs | |
| 10 escitalopram | |
| 2 paroxetine | |
| 1 citalopram | |
| 3 levetiracetam | |
| 2 aripiprazole | |
| 1 cyamemazine | |
| 1 venlafaxine | |
| 1 lamotrigine | |
| 2 valproate | |
| 2 lithium | |
| Others | |
| 1 cabergoline | |
| 1 dolutegravir/rilpivirine | |
| 1 methotrexate | |
| 10 patients with hypokalaemia/hyperkalaemia | |
| 6 patients with ophthalmologic contraindications to hydroxychloroquine treatment | 3 retinopathy |
| 2 glaucoma | |
| 1 other disorder | |
| 16 patients with known allergies to hydroxychloroquine or azithromycin or known gastrointestinal intolerance to hydroxychloroquine or azithromycin | |
| 2 breastfeeding patients | |
| 3 patients with G6PD deficiency | |
| 36 patients with unspecified reasons for non-prescription of the combined treatment | |
The reasons mentioned here are those retained by physicians who followed up with the patients and should not be considered formal contraindications.
*Most of these patients were seen at the early beginning of the epidemic in Marseille when the decision of systematically proposing combination of HCQ-AZ was still not taken by our team.
Overall, 673 patients (18%) were hospitalized in our infectious disease units, and 3,064 patients were followed in our day-care hospital (Fig. 1). Most of the patients (3,507, 93.8%) had a good clinical outcome, while 230 (6.2%) had a poor clinical outcome, including 67 who were transferred to ICU (1.8%), 35 who died (0.9%) and 197 with a hospital stay ≥10 days (5.3%) (Table 4 ).
Table 4.
Bivariate analyses of associations between combined treatment (HCQ-AZ ≥ 3 days) and clinical outcomes (death, hospitalization >10 days, and transfer to the intensive care unit) of COVID-19 patients, Marseille, France (n = 3,737).
| All |
HCQ-AZ ≥3 days |
Other treatments |
HCQ-AZ <3 days |
HCQ |
AZ |
No HCQ – No AZ |
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 3,737, 100%) |
(n = 3,119, 83.5%) |
(n = 618, 16.5%) |
(n = 218, 5.8%) |
(n = 101, 2.7%) |
(n = 137, 3.7%) |
(n = 162, 4.3%) |
|||||||||||||||||||||||||||||
| na | %a | na | %a | na | %a | pc | na | %a | pc | na | %a | pc | na | %a | pc | na | %a | pc | |||||||||||||||||
| Hospitalization | 673 | 18 | 430 | 13.8 | 243 | 39.3 | <0.001 | 86 | 39.4 | <0.001 | 36 | 35.6 | <0.001 | 86 | 62.8 | <0.001 | 35 | 21.6 | 0.0077 | ||||||||||||||||
|
Duration of hospitalization (days) - Mean (std) Q1-Median-Q3b |
8.0(7.5) 3-6-11 | 7.3(7.0) 2-5-10 | 9.2 (8.1) 3-7-13 | <0.001 | 11.8 (9.8) 4-9-17 | <0.001 | 5.7 (4.0) 3-5-7 | 0.5963 | 8.8 (7.1) 4-7-12 | 0.0135 | 7.5 (6.9)2-4-12 | 0.9885 | |||||||||||||||||||||||
| Hospitalization ≥10 days | 197 | 5.3 | 109 | 3.5 | 88 | 14.2 | <0.001 | 41 | 18.8 | <0.001 | 6 | 5.9 | 0.1741 | 30 | 21.9 | <0.001 | 11 | 6.8 | 0.0481 | ||||||||||||||||
| Intensive care unit (ICU) | 67 | 1.8 | 25 | 0.8 | 42 | 6.8 | <0.001 | 31 | 14.2 | <0.001 | 2 | 2 | 0.2069 | 8 | 5.8 | <0.001 | 1 | 0.6 | 1 | ||||||||||||||||
| Death | 35 | 0.9 | 16 | 0.5 | 19 | 3.1 | <0.001 | 8 | 3.7 | <0.001 | 2 | 2 | 0.1077 | 5 | 3.6 | 0.0014 | 4 | 2.5 | 0.0149 | ||||||||||||||||
| Death and/or ICU | 93 | 2.5 | 35 | 1.1 | 58 | 9.4 | <0.001 | 37 | 17 | <0.001 | 3 | 3 | 0.1149 | 13 | 9.5 | <0.001 | 5 | 3.1 | 0.0449 | ||||||||||||||||
|
Poor clinical outcome (Death, ICU and/or Hospitalization ≥10 days) |
230 | 6.2 | 121 | 3.9 | 109 | 17.6 | <0.001 | 51 | 23.4 | <0.001 | 8 | 7.9 | 0.0625 | 37 | 27 | <0.001 | 13 | 8 | 0.0218 | ||||||||||||||||
#An additional death occurred, unrelated to COVID-19 or treatment, but was not included in the analyses because no information can be described for forensic reasons.
Otherwise stated.
Time from treatment start (inclusion date otherwise).
Fisher's exact test, Wilcoxon Mann-Whitney test. Reference group is “HCQ-AZ ≥3 days”.
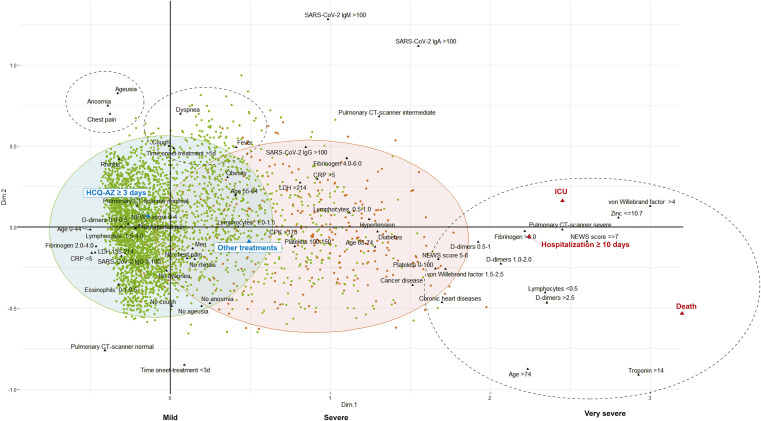
The multiple correspondence analysis (MCA) (Fig. 2 ) immediately allowed for the identification of a number of groups. Most patients with a good clinical outcome are grouped with young age and centred on HCQ-AZ treatment. The patients with a poor clinical outcome are all grouped with older age, with some biological criteria (lymphocytopenia, thrombocytopenia, eosinopenia, low zinc level and increased D-dimers and troponin) and with other treatments. Finally, the two modes of clinical presentation are highlighted: upper respiratory tract infection (URTI) symptoms with ageusia, anosmia, rhinitis, and thoracic pain, and lower respiratory tract infection symptoms (LRTIs) with dyspnoea, cough and fever (Fig. 2).
Fig. 2.
Multiple correspondence analysis (MCA) including all the clinical and biological radiological data and the outcomes. Each dot represents a patient with good clinical outcome in green or poor clinical outcome in red (HCQ-AZ: hydroxychloroquine and azithromycin; ICU = intensive care unit). Unsupervised approaches (such as multiple correspondence analysis for qualitative variables) allow graphical representation without a priori that takes together the variables and observations (biplot). Observations (individuals) can be identified and analysed according to an additional variable (such as their good or poor clinical course). Red ellipse: 90% confidence ellipse for patients with poor clinical outcome “Death/ICU/Hospitalization=>10 days”. Green ellipse: 90% confidence ellipse for patients with good clinical outcome. Dotted ellipses were added to the MCA to better figure the 2 main clinical presentations and the severe evolutionary stage of the disease. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Clinical characteristics
Underlying conditions, comedications and clinical symptoms are comprehensively described in Table S1. The prevalence of poor clinical outcome significantly increased with age, comorbidities, several comedications and male sex.
Most of the patients had a NEWS-2 score ranging from 0 to 4 (3,420, 91.5%) at admission. Cough was the most frequent symptom (50.2%), followed by anosmia (39.2%), ageusia (37.8%), rhinitis (32.7%), dyspnoea (28.2%) and thoracic pain (22.1%). A total of 15.6% of patients were febrile, and 9.1% were asymptomatic. Interestingly, anosmia, ageusia and chest pain were significantly more frequent in patients under 65 years (42.9% vs 11.4%, 40.9% vs. 14.5% and 24.3% vs. 4.9%, respectively).
Symptoms suggestive of URTI, including rhinitis, anosmia and ageusia, were significantly more common in patients with a good clinical outcome (33.8% vs. 15.6%, 40.9% vs. 11.9% and 39.3% vs. 14.2%, respectively). The symptoms suggestive of LRTI, including fever and dyspnoea, were significantly more frequent in patients with poor clinical outcome (32.1% vs. 14.6% and 40.8% vs. 27.4%, respectively).
3.4. Biological characteristics
Several biological parameters were significantly associated with poor clinical outcome, including lymphocytopenia, thrombocytopenia, low zinc level, and increased D-dimers, troponin, CRP, CPK and LDH. Indeed, eosinopenia was very marked and significantly worse in patients with poor clinical outcome (Table S2). The mean HCQ serum concentration measured at day 2 was significantly lower in patients with poor clinical outcome than in patients with good clinical outcome (Table S3). Serology was performed in 2,302 patients. Immunoglobulin G (IgG) to SARS-CoV-2 was detected in 726 patients (31.5%). Immunoglobulin A (IgA) was detected in 12.9% of patients under 65 years with poor clinical outcome, compared to 2.3% of patients with good clinical outcome (p < 0.05) (Table S2). IgG, immunoglobulin M (IgM) and IgA titres were significantly higher in the poor clinical outcome group (Table S3). Surprisingly, we observed an increase in seroprevalence and specific antibody titres in patients with poor clinical outcome during evolution (data not shown) [20].
3.5. Low-dose CT scan characteristics
We performed 2,065 LDCTs, including 1,449 (70.1%) that detected abnormalities, which were classified as minimal (928, 64%), intermediate (414, 28.6%) and severe involvement (107, 7.4%). Among 991 patients with a NEWS-2 score = 0 who underwent LDCT, 592 (59.7%) had radiological abnormalities, including 470 (47.4%) with minimal lung lesions, 115 (11.6%) with intermediate lesions and 7 (1%) with severe lesions (Fig. S1). Moreover, among 1,370 LDCT scans performed on patients without subjective perceived dyspnoea, 937 (68%) had pneumonia. Because of this intriguing result, we investigated the relationships between perceived dyspnoea, oxygen saturation and LDCT results among the patients for whom information was available. Among 1,108 patients who perceived themselves as non-dyspnoeic, 157 (14.2%) actually had oxygen saturation ≤95%, and 130/157 (82.9%) had pneumonia. A normal LDCT was significantly associated with good clinical outcome, and a CT scan with severe or intermediate lesions was significantly associated with poor clinical outcome (23.5% versus 1.5% and 37.8% versus 9.3%, respectively, p < 0.05).
3.6. Adverse events associated with treatments
Adverse events were observed in 167 (4.5%) patients (Table S4). All adverse events were mild and included mostly gastrointestinal symptoms. Discontinuation of treatment was required in 35 patients (0.93%), mostly because of gastrointestinal symptoms.
We paid specific attention to QTc prolongation, which was observed (>60 ms) in 25 patients (0.67%), including 2 treated with HCQ (2%), 3 treated with AZ (2.2%) and 20 treated with HCQ-AZ (0.6%). The cessation of treatment for QT prolongation was needed in 12 cases including 3 cases with a QTc ≥500 ms (2 treated with AZ and 1 treated with HCQ-AZ). No cases of torsade de pointe or sudden death were observed.
3.7. Clinical outcomes
The mean duration of hospitalization was significantly shorter in the HCQ-AZ group (7.3 days (sd 7) vs 9.2 (sd 8.1) than in the other treatment groups. The proportion of patients hospitalized ≥10 days was 3.5% in the HCQ-AZ group and 14.2% in the other treatment groups (Table 4). We observed that 9 of the 35 patients who died (25.7%) developed a concurrent bacterial infection, including community-acquired Streptococcus pneumoniae in 2 patients, ventilation-acquired pneumonia in 4 patients, catheter-associated septicaemia in 2 patients and cholecystitis-related septicaemia in 1 patient (Table S5).
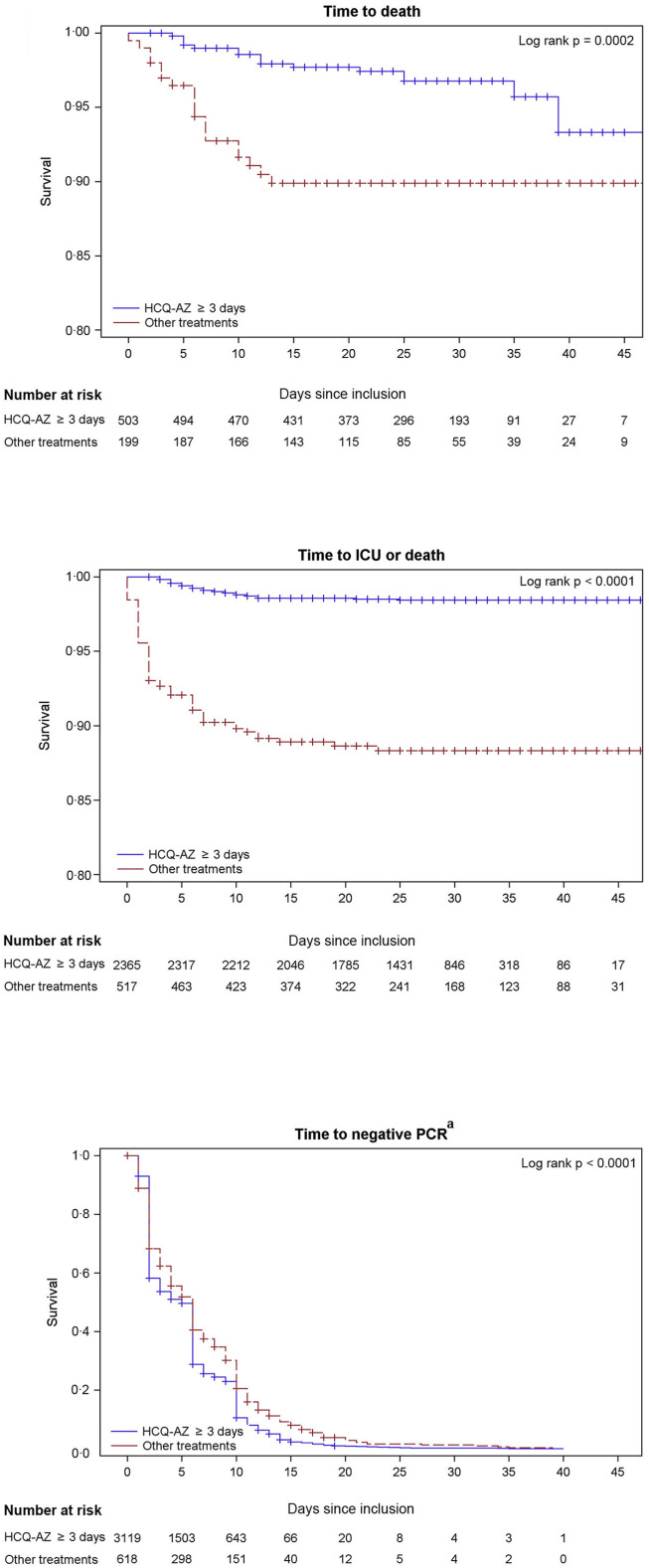
As the youngest patient who died was 60 years old, we analysed risk factors for death in the population ≥60. We recorded 35 deaths among 702 patients older than 60 (5.0%). As the youngest patient transferred to ICU was 31 years old, we analysed risk factors for this outcome in the 2,856 patients ≥31. Previous health status (combined age and comorbidity score) and disease severity (NEWS-2 score) were independent predictors of death and/or transfer to ICU (Table S6, S7). HCQ-AZ ≥3 days was an independent protective factor against death and/or transfer to ICU (death hazard ratio (HR) 0.49, 95% confidence interval (0.25–0.97)) (Table 5 , Fig. 3 ). Finally, the significant association between treatment with HCQ-AZ≥3days and reduction of risk of death was confirmed to be independent of age, comorbidities and severity of the disease, by two different propensity score methods (Table 5, Table S8).
Table 5.
Age stratified multivariable analyses adjusted on comorbidities and severity of the disease addressing associations between treatment (HCQ-AZ ≥ 3 days) and clinical outcomes/viral shedding clearance (n = 3,737).
| Cox proportional hazard modelsa | HCQ-AZ ≥ 3 days n event/n total (%) | Other treatment n event/n total (%) | Hazard ratio 95% confidence interval (ref. Other treatment) |
p-value |
|---|---|---|---|---|
| Mortalityb | 16/503 (3.2%) | 19/199 (9.6%) | ||
| Multivariable Cox regression on unmatched sample (n = 702) | 0.49 0.25–0.97 | 0.0406 | ||
| Stratified Cox regression on matched sample (n = 398)c | 0.41 0.17–0.99 | 0.0482 | ||
| Weighted Cox regression on unmatched sample (n = 702)c | 0.49 0.31–0.79 | 0.0030 | ||
| ICU transferd | ||||
| Patients ≥ 31 years (n = 2,856) | 25/2,355 (1.1%) | 42/501 (8.4%) | 0.19 0.11–0.33 | <0.0001 |
| Patients between 31 and 59 years (n = 2,180) | 10/1,862 (0.5%) | 23/318 (7.2%) | 0.13 0.05–0.31 | <0.0001 |
| Patients aged ≥ 60 years (n = 676) | 15/493 (3.0%) | 19/183 (10.4%) | 0.17 0.07–0.38 | 0.0003 |
| Death and/or ICU transfere | ||||
| Patients ≥ 31 years (n = 2,882) | 35/2,365 (1.5%) | 58/517 (11.2%) | 0.18 0.11–0.27 | <0.0001 |
| Patients aged ≥ 60 years (n = 702) | 25/503 (5.0%) | 35/199 (17.6%) | 0.30 0.18–0.51 | <0.0001 |
| Viral shedding persistence ≥ 10 daysf | ||||
| All patients (n = 3,737) | 10.6% | 20.6% | 1.29 1.17–1.42 | <0.0001 |
| Patients aged < 60 years (n = 3,035) | 10.0% | 17.4% | 1.23 1.10–1.38 | 0.0003 |
| Patients aged ≥ 60 years (n = 702) |
13.4% |
27.2% |
1.44 1.19–1.73 |
0.0002 |
|
Logistic regressiona |
HCQ-AZ ≥ 3 days n event/n total (%) |
Other treatment n event/n total (%) |
Odds ratio 95% confidence interval |
p-value |
| Hospitalization ≥ 10 days | ||||
| All patients (n = 3737) | 109/3,119 (3.5%) | 88/618 (14.2%) | 0.38 0.27–0.54 | <.0001 |
| Death and/or ICU transfer/hospitalization ≥ 10 days | ||||
| All patients (n = 3737) | 121/3,119 (3.9%) | 109/618 (17.6%) | 0.30 0.22–0.42 | <.0001 |
Models were adjusted for the combined comorbidity index and the severity of the disease (NEWS-2 score).
Mortality was evaluated among patients aged 60 years old and older (n = 702) because the youngest patient who died was 60 years old.
These two models based on propensity score methods were performed only for mortality (see methods).
ICU transfer was evaluated among patients aged 31 years and older (n = 2,856) because the youngest patient who was transferred to the ICU was 31 years old. Patients who died without ICU transfer were excluded (n = 26).
Death and/or ICU transfer was evaluated among patients aged 31 years and older (n = 2,856) because the youngest patient who was transferred to the ICU was 31 years old.
Proportion of patients with non-negative PCR within 10 days following inclusion (Kaplan-Meier estimates, see Fig. 3). Some patients did not have a PCR testing at day 10 and were still considered positive if previous sample was positive (event was defined as first negative PCR during follow-up).
Fig. 3.
Kaplan-Meier curve of clinical outcomes/viral shedding clearance according to treatment groups (n = 3,737). HCQ: hydroxychloroquine, AZ: azithromycin, ICU: Intensive care unit, PCR: polymerase chain reaction. a: For time to negative PCR, event was defined as first negative PCR during follow-up. Accordingly, patients were still considered positive at each time point if previous sample was positive.
Our global mortality rate was 0.9%, and the mortality rate was 0.5% among patients treated with HCQ-AZ ≥ 3days. Whereas no death was observed in patients <60 years old in our study, the proportion of deaths under 60 years was 3.5, 4.3, 9.8 and 19% in Italy, in grand Est region (France), in Ile de France region (France) and in China, respectively (Table 6 ) [23].
Table 6.
Numbers of deaths in hospitalized COVID-19 patients and distribution by age class in Italy, China, IHU Méditerranée Infection, Marseille France, Grand Est region and Ile de France regions of France.
| Age class | Italy as of March 17, 2020 (Onder, 2020)a | China as of February 11, 2020 (Onder, 2020)a | IHU All patients April 30, 2020 |
IHU April 30, 2020 HCQ-AZ at least 3 days |
IHU April 30, 2020 Other treatments |
Grand-Est region, France May 18, 2020b | Ile-de-France, France May 18, 2020b |
|---|---|---|---|---|---|---|---|
| All | 1,624 | 1,023 | 35 | 16 | 19 | 3,277 | 6,713 |
| 0–9 | 0 | 0 | 0 | 0 | 0 | 1 (0.03%) | 2 (0.03%) |
| 10–19 | 0 | 1 (0.1%) | 0 | 0 | 0 | 0 | 3 (0.04%) |
| 20–29 | 0 | 7 (0.7%) | 0 | 0 | 0 | 2 (0.06%) | 11 (0.2%) |
| 30–39 | 4 (0.2%) | 18 (1.8%) | 0 | 0 | 0 | 15 (0.5%) | 45 (0.7%) |
| 40–49 | 10 (0.6%) | 38 (3.7%) | 0 | 0 | 0 | 32 (1.0%) | 124 (1.8%) |
| 50–59 | 43 (2.6%) | 130 (12.7%) | 0 | 0 | 0 | 91 (2.8%) | 470 (7.0%) |
| 60–69 | 139 (8.6%) | 309 (30.1%) | 2 (5.7%) | 1 (6.25%) | 1 (5.3%) | 350 (10.7%) | 982 (14.6%) |
| 70–79 | 578 (35.6%) | 312 (30.5%) | 14 (40%) | 7 (43.75%) | 7 (36.8%) | 818 (25.0%) | 1,586 (23.6%) |
| ≥80 |
850 (52.3%) |
208 (20.3%) |
19 (54.3%) |
8 (50%) |
11 (57.9%) |
1,968 (60.1%) |
3,490 (52.0%) |
| <60 | 57 (3.5%) | 194 (19.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 141 (4.3%) | 655 (9.8%) |
| ≥60 | 1567 (96.5%) | 829 (81.0%) | 35 (100%) | 16 (100%) | 19 (100%) | 3136 (95.7%) | 6058 (90.2%) |
Mortality data provided in this study are likely to be global and not only that of hospitalized patients.
These data are collected by Sante Publique France (https://geodes.santepubliquefrance.fr/#view=map2&c=indicator); IHU (Institut Hospitalo Universitaire), Marseille, France.
3.8. Virological outcome
Kaplan-Meier estimates show that the proportion of patients with positive PCR 10 days after inclusion was significantly lower among patients treated with HCQ-AZ (10.6%; (95% CI: 8.1%–13.4%) than among those who received other treatments (20.6%; (95% CI: 14.7%–27.2%; p < 0.05) (Fig. 3, Table 5). In a multivariate Cox regression adjusted for combined comorbidity index and disease severity at admission (NEWS-2 score), HCQ-AZ treatment remained significantly associated with viral shedding clearance (HR = 1.29: 1.17–1.42, p < 0.0001) (Table 5). We inoculated samples obtained from 130 patients with positive PCR at day 10. Among them, only 16 had a positive culture at day 10 (12.3%).
4. Discussion
This work highlights that it is hazardous to make strategic decisions a priori regarding the management of a new disease when no reliable information about this disease is available. Political and public health decisions in this context should be regularly adapted to observations collected in other countries when available [24]. The decision of the government of France to recommend staying at home (lockdown) without testing while waiting for dyspnoea was not supported by our results [25]. As with other clinicians, we have seen patients with hypoxia, including some with very low blood oxygen levels, who described themselves as feeling well and comfortable (“happy hypoxemia”) [26]. Since these patients may develop severe symptoms based on our observations, the use of inexpensive pulse oximeters (around 20€) in primary-care health settings and/or by family doctors might be considered a triage tool on which to base hospitalization referral for further investigation. We propose that the initial disease severity assessment cannot rely only on clinical examination but should also take into account oxygen saturation testing and blood sampling (haemogram, CRP, LDH, troponin, D-dimers) (Fig. 2).
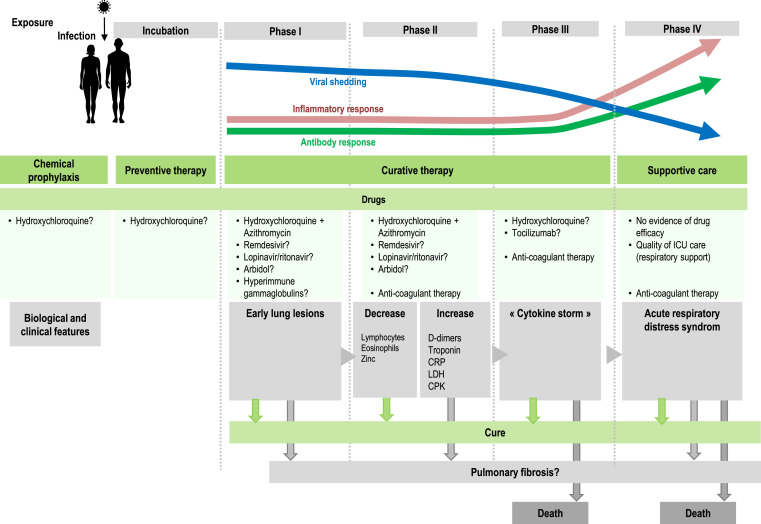
We confirm here that COVID-19 has several evolutionary stages (Fig. 4 ). After the incubation period, the first clinical stage, including LRTI and URTI symptoms, is associated with a high viral load and the occurrence of early lung lesions on LDCT, for which it is reasonable to use a compound with antiviral activity. HCQ-AZ has demonstrated its effectiveness in reducing viral shedding [6] and preventing disease progression and death particularly when prescribed at early stages [10,27,28]. Other antiviral compounds, including remdesivir and hyperimmune gamma globulins [29], may have antiviral activity at an early stage of the disease, although there is to date no convincing published report, comparable to that of oseltamivir at the early stage of influenza [30]. Taking into account the association between low blood zinc levels and poor clinical outcomes, zinc supplementation should be also considered, as recently reported [31]. However, the choice of the best treatment should be made according to its safety profile, which is much better for HCQ-AZ than for remdesivir (adverse events leading to cessation of treatment in 0.9% in our study vs. 12% for remdesivir [12]). Nevertheless, we were surprised by the large discrepancy on efficacy and toxicity of HCQ in recent studies compared to ours [32]. As a matter of fact, all patients reported here have been followed by the physicians authors named in our study. Altogether, we found only 0.67% of QTc prolongations and no death related to treatment. In our opinion, this excellent safety profile of HCQ-AZ in our real-life medical experience much better reflects the reality than registry studies such as those recently retracted from high profile medical journals [9].
Fig. 4.
Evolutionary stages of SARS-CoV-2 infection, including major clinical and biological features and possible therapies.
The second stage includes both an immune reaction and the persistence of the virus [1]. At this stage, extreme caution should be required for patients with risk factors (particularly hypertension), severe clinical presentation (NEWS CoV ≥ 5), intermediate-to-severe lesions in LDCT and biological parameters such as lymphocytopenia, eosinopenia, elevated troponin or D-dimers higher than 0.5 μg/L. Systemic coagulation activation and thrombotic complications were probably overlooked in COVID-19 patients. In our study, the youngest person who died was 60 years old, and the death was associated with generalized thrombosis. A recent study reported that among 198 hospitalized COVID-19 patients, 39 (20%) were diagnosed with venous thromboembolism (VTE), and of these patients 25 (13%) had symptomatic VTE, despite routine thrombosis prophylaxis [33]. The third stage consists of an inflammatory stage linked to pro-inflammatory cytokine release with a high risk of transfer to ICU [34]. Moreover, the strong specific antibody response observed at this stage questions the use of hyperimmune gamma globulins [29]. The fourth stage with acute respiratory distress syndrome (ARDS) is characterised by pulmonary tissue injury and requires supportive intensive care. To date, no drug has proven effective at this stage. While most surviving patients may be definitely cured, an unknown proportion may evolve towards pulmonary fibrosis constituting the late stage of the disease, as described by Chinese physicians caring for COVID-19 patients and as previously described for severe acute respiratory syndrome (SARS) in 2003 [35]. Long-term follow-up aiming to screen for fibrosis will be the next challenge in the management of COVID-19. Our experience and suggestions regarding the various stages of COVID-19 are summarized in Fig. 4.
The strength of our study is its monocentric design with a relative homogeneity of both diagnosis procedure and standard care provided to patients, allowing us to assess the impact of different therapeutic options on the evolution of the disease, in real time. Virological diagnosis, radiological investigations and clinical assessment were conducted by single teams of trained virologists, radiologists, infectious diseases specialists and cardiologists, all directly involved in patient care. Daily staff meeting ensured assessing the reliability of the data collected and adjusting medical procedures overtime, in the context of a newly emerging disease that was totally unknown three months before we started our study. In the context of pandemic, such a study allows more flexibility that a randomized controlled trial (RCT) with stringent methodological constraints and is much more economical. It is also more reliable that big-data studies conducted by external investigators dealing with incomplete information retrieved from medical files that have led to recent retraction of papers from the two major medical journals [9]. Indeed, when inconsistencies appeared in our database of the study, the authorized person in our team was able to return to patients files to reassess the data. In addition, we were able to conduct interim analysis of our data [[6], [7], [8]] and ensure early release of our preliminary results to be shared with the medical community, at an early phase of the pandemic [36].
Our study has a retrospective observational design, and such characteristics may be presented as a limitation of the study [37]. Patients were not enrolled in perfectly homogeneous groups with regards to demographics, chronic conditions and clinical status at admission. Treatments were not allocated randomly but according to the clinical status of patients and contra-indications to drugs, or preference of patients with regards to therapeutic options. As we have aimed to tests and treat all positive patients presenting to our institution, the patient population comprised a majority of patients with mild diseases and a minority of patients with severe disease, with the former managed at our day-care hospital and the latter as in-patients. We enrolled all patients including those who started their treatment with delay or stopped it early. Because of the crisis situation we had to face, clinical, virological and radiological data were not documented in 100% patients. However, missing data may also be a limitation of RCT. Furthermore, RCT are not useful in the context of an emerging pandemic when commercially available drugs known to be active in vitro are available for immediate treatment [38,39].
Our approach of early diagnosis and care of as many patients as possible results in much lower mortality rates than other strategies. The test-and-treat strategy adopted in Marseille also seems capable of shortening the duration of the outbreak when compared to data from France overall by identifying infected people and reducing their viral shedding duration. In fact, more people were tested in Marseille than in most other areas, and the outbreak lasted only 9 weeks. In addition, patients under HCQ-AZ treatment for at least 3 days had a better clinical outcome, based on mortality rates among patients >60 years, less transfer to ICU and shorter length of stay at the hospital, and these patients also had a shorter duration of viral shedding than patients who did not receive this drug combination. Finally, a global strategy for the management of the COVID-19 outbreak may help to limit both the number of cases and fatalities and guide countries where this pandemic has not yet peaked.
Author's note
Since this analysis was completed, and as of the 11t h June, 2020, 6 more patients died including 1 patient treated with HCQ-AZ for at least 3 days and 5 in the other group, resulting in an overall 1.1% case fatality rate for the 3,737 patients included in our study.
Funding
This work was funded by ANR “Investissements d'avenir”, Méditerranée infection 10-IAHU-03, and was also supported by Région Provence-Alpes-Côte d’Azur. This work had received financial support from the Mediterranean Infection Foundation.
CRediT authorship contribution statement
Jean-Christophe Lagier: Conceptualization, Investigation, Formal analysis, Writing - original draft. Matthieu Million: Conceptualization, Formal analysis, Writing - original draft. Philippe Gautret: Investigation, Formal analysis, Writing - original draft. Philippe Colson: Investigation. Sébastien Cortaredona: Formal analysis. Audrey Giraud-Gatineau: Investigation. Stéphane Honoré: Investigation. Jean-Yves Gaubert: Investigation. Pierre-Edouard Fournier: Investigation, Formal analysis. Hervé Tissot-Dupont: Investigation. Eric Chabrière: Investigation. Andreas Stein: Investigation. Jean-Claude Deharo: Investigation. Florence Fenollar: Investigation. Jean-Marc Rolain: Investigation. Yolande Obadia: Formal analysis. Alexis Jacquier: Investigation. Bernard La Scola: Investigation. Philippe Brouqui: Investigation, Formal analysis. Michel Drancourt: Investigation. Philippe Parola: Conceptualization, Formal analysis, Writing - original draft. Didier Raoult: Conceptualization, Formal analysis, Writing - original draft.
Declaration of competing interests
The authors declare no competing interests. Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Our group used widely available generic drugs distributed by many pharmaceutical companies.
Acknowledgements
We thank the 7 reviewers who helped in substantially improving and clarifying the manuscript with their many comments and suggestions.
We are thankful to:
Julien Andreani, Marion Bechet, Amin Ben Lassoued, Corinne Blasco, Céline Boschi, Laurent Boyer, Jean-Paul Casalta, Hervé Chaudet, Florian Correard, Pierre Dudouet, Grégory Dubourg, Carole Eldin, Vera Esteves-Vieira, Véronique Filosa, Léa Fuster, Marc Gainier, Lugdivine Ganci, Céline Gazin, Stephanie Gentile, Clio Grimaldier, Véronique Jacomo, Marie-Thérèse Jimeno, Elisabeth Jouve, Alexandra Kotovtchikhine, Béatrice Labric, Marc Léone, Nicolas Levy, Pierre-Yves Lévy, Léa Luciani, Cléa Melenotte, Lucille Pinault, Pierre Pinzelli, Elsa Prudent, Laurence Thomas, Joana Vitte, Nathalie Wurtz.
All medical students from Aix Marseille University, all nurses, laboratory staff, administrative, technical and security staff of Assistance Publique-Hôpitaux de Marseille and IHU Méditerranée Infection, all volunteer medical doctors, and the Bataillon des Marins Pompiers de Marseille for their help.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2020.101791.
Contributor Information
IHU COVID-19 Task force:
Sophie Amrane, Camille Aubry, Matthieu Bardou, Cyril Berenger, Laurence Camoin-Jau, Nadim Cassir, Claire Decoster, Catherine Dhiver, Barbara Doudier, Sophie Edouard, Stéphanie Gentile, Katell Guillon-Lorvellec, Marie Hocquart, Anthony Levasseur, Morgane Mailhe, Isabelle Ravaux, Magali Richez, Yanis Roussel, Piseth Seng, Christelle Tomei, and Christine Zandotti
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Coronavirus resource center. COVID-19 dashboard by the center for systems science and engineering (CSSE) at johns hopkins university (JHU) https://coronavirus.jhu.edu/map.html
- 3.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 5.Andreani J., Le Bideau M., Duflot I. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautret P., Lagier J.C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study. Trav Med Infect Dis. 2020 Apr 11:101663. doi: 10.1016/j.tmaid.2020.101663. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Million M., Lagier J.C., Gautret P. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Trav Med Infect Dis. 2020 May 5:101738. doi: 10.1016/j.tmaid.2020.101738. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledford H., Van Noorden R. High-profile coronavirus retractions raise concerns about data oversight. Nature. 2020 Jun 5 doi: 10.1038/d41586-020-01695. [DOI] [PubMed] [Google Scholar]
- 10.Million M., Gautret P., Colson P. Clinical efficacy of chloroquine derivatives in COVID-19 infection : comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(9):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu B., Li C., Chen P. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020:1–7. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 - preliminary report. N Engl J Med. 2020 May 22 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020. medRxiv 2020.03.22.20040758. [DOI]
- 16.Huang M., Tang T., Pang P., Li M., Ma R., Lu J., Shu J., You Y., Chen B., Liang J., Hong Z., Chen H., Kong L., Qin D., Pei D., Xia J., Jiang S., Shan H. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020;12(4):322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med. 2020;46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong N., Richez M., Raoult D. Simultaneous UHPLC-UV analysis of hydroxychloroquine, minocycline and doxycycline from serum samples for the therapeutic drug monitoring of Q fever and Whipple's disease. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:166–172. doi: 10.1016/j.jchromb.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Edouard S., Colson P., Melenotte C. Evaluating the serological status of COVID-19 patients using an indirect immunofluorescent assay, France. medRxiv. 2020 doi: 10.1101/2020.05.05.20092064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.uptodate.com/contents/treatment-of-community-acquired-pneumonia-in-adults-who-require-hospitalization
- 22.Charlson M., Szatrowski T.P., Peterson J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994 Nov;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 23.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;23 doi: 10.1001/jama.2020.4683. Published online March. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Qian B.Y. Making decisions to mitigate COVID-19 with limited knowledge. Lancet Infect Dis. 2020 Apr 7;(20):30280–30282. doi: 10.1016/S1473-3099(20)30280-2. pii: S1473-3099. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000041746694&categorieLien=id Décret n°2020-293 du 23 mars 2020.
- 26.Couzin-Frankel J. The mystery of the pandemic's 'happy hypoxia. Science. 2020;368(6490):455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 27.Davido B., Lansaman T., Bessis S. Hydroxychloroquine plus azithromycin: a potential interest in reducing in-hospital morbidity due to COVID-19 pneumonia (HI-ZY-COVID)? medRxiv. 2020 doi: 10.1101/2020.05.05.20088757. [DOI] [Google Scholar]
- 28.Mahévas M., Viet-Thi Tran, Roumier M. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Q.L., Yu Z.J., Gou J.J. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020;22(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzen J., Kohn R., Houk J.L. Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis. 2019 Jun 18;69(1):52–58. doi: 10.1093/cid/ciy860. [DOI] [PubMed] [Google Scholar]
- 31.Carlucci P., Ahuja T., Petrilli C.M. Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.05.02.20080036. 05.02.20080036. [DOI] [Google Scholar]
- 32.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemostasis. 2020 May 5 doi: 10.1111/jth.14888. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 Mar 12 doi: 10.1093/cid/ciaa248. pii: ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T., Sun L.X., Feng R.E. [Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019] Zhonghua Jiehe He Huxi Zazhi. 2020 Apr 3;43:E040. doi: 10.3760/cma.j.cn112147-20200311-00312. 0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Risch H.A. Early outpatient treatment of symptomatic, high-risk covid-19 patients that should be ramped-up immediately as Key to the pandemic crisis. Am J Epidemiol. 2020 May 27 doi: 10.1093/aje/kwaa093. kwaa093. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Concato J., Shah N., Horwitz R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000 Jun 22;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frieden T.R. Evidence of health decision making-beyond randomized controlled trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]
- 39.Gautret P., Raoult D. Nullane salus extra ecclesiam. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100714. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.