Abstract
Background
The microbiological control of cellular products sometimes causes significant procedural issues for quality control laboratories. According to the European Pharmacopoeia (EP), the microbiological control of cellular products requires a 7- to 14-day incubation period at two different incubation temperatures using aerobic and anaerobic growth media. However, the suitability of these test conditions for efficient quality control can be influenced by many conditions, such as the expected microbial spectrum of contamination or the texture and composition of the cellular product. Because of interference, direct inoculation and membrane filtration as reference methods of pharmacopoeia are largely unsuitable for the microbiological control of cellular products; therefore, alternative and, above all, automated methods are the focus of interest.
Objective
The aim of our study was to evaluate the method suitability and possible effects of cell matrix, incubation temperature, and oxygen pressure on the detection performance of automated culture systems.
Methods
The BacT/ALERT® 3D<sup>TM</sup> Dual T system (bioMérieux, Nürtingen, Germany) was used to evaluate the factors influencing automated microbiological control of cellular products. The tests were performed using microbial strains recommended by the EP for microbiological method suitability testing and additional relevant possible contaminants of human-derived stem-cell products under varying culture and cell matrix conditions.
Results
All contaminants were detected by the system in the required period of 2–5 days. Low incubation temperatures (22°C) had overall negative effects on the detection kinetics of each type of microbial contamination. The adverse effects of the accompanying cell matrix on the detection properties of the system could be compensated in our study by incubation at 32°C in both the aerobic and the anaerobic culture conditions.
Conclusion
Automated culture techniques represent a sufficient approach for the microbiological control of cellular products. The negative effects of the cell matrix and microbial contamination on the detection performance can be compensated by the application of variable culture conditions in the automated culture system.
Keywords: Microbiological control of cellular products, BacT/ALERT® 3DTM Dual T, Rapid microbiological method, Quality control, Automated sterility testing
Introduction
Sterility is defined as freedom from the presence of viable microorganisms. According to the International Pharmacopoeia Ph. Int. [1], the sterility of each pharmaceutical product should be confirmed by analyzing a representative sample of the product by defined sterility testing methods. Products are classified as nonsterile when microbial growth is detected during the test period, while products with no detectable microbial growth are classified as sterile by definition. Sterility testing is one of the most well-established microbiological tests in pharmaceutical quality control. Methods for sterility testing were first introduced by the British Pharmacopeia in 1932 as direct inoculation of the test product to liquid culture media [2]. As an alternative method, membrane filtration was introduced in the late 1960s in other pharmacopoeias [3, 4].
The more recent regulatory requirements for microbiological quality control of biopharmaceuticals and cellular products as well as for alternative test methods are also based on the principles and definitions of sterility testing. However, the pharmacopoeia reference methods of direct inoculation and membrane filtration are mostly unsuitable for sterility testing or microbiological control of cellular products. The reasons for this include the inherent clouding of the nutrient medium and the lack of filterability of the products due to the included cellular components. Therefore, alternative test methods are needed that are not affected by these aspects.
Attractive test systems are therefore automated culture techniques that detect microbial growth from metabolic products of microorganisms. Examples are the automated BacT/ALERT 3D (bioMérieux) and BACTEC (Becton Dickinson, Heidelberg, Germany) culture systems, which are automated technologies with real-time reading of carbon dioxide production by microorganisms that have already been validated for the control of blood components, hematopoietic stem cells and cultured chondrocytes [5, 6, 7, 8, 9, 10, 11]. In all of these areas of application, the automated culture methods showed a good test performance at minimum. However, the growth characteristics of microorganisms and detection by automated culture systems might be affected by changes in incubation conditions and the presence of cellular products.
Several rapid microbiological methods for the detection of microorganisms used for sterility testing purposes have been evaluated in recent years [2, 11, 12, 13, 14, 15]. Experts agree widely that the use of automated sterility testing systems could enable more rapid and more reliable detection of microbial contamination by using specific indicators and consequent automated analysis for the detection of microbial growth [16]. The BacT/ALERT 3D Dual T system (bioMérieux, Nürtingen, Germany) is a possible candidate for automated growth-based sterility testing. In 2004, the BacT/ALERT system received approval by the U.S. Food and Drug Administration (FDA) (Silver Spring, MD, USA) for use in evaluating the sterility of cellular products such as cartilage or pancreatic islet cells [11, 16, 17]. This system uses the colorimetric detection of CO2 production to continuously monitor the culture media for microbial growth. The BacT/ALERT 3D Dual T system is a fully automated system capable of incubating, shaking, and colorimetrically monitoring cultures in which readings are taken every 10 min throughout the incubation period of the inoculated liquid culture media [16].
The aim of our study was to evaluate the applicability of the BacT/ALERT 3D Dual T system for the rapid automated detection of microbial contaminants in cellular products depending on the presence of cellular matrices, microorganisms, temperature, and aerobic or anaerobic conditions.
For this purpose, we evaluated the systems for microbiological control according to the European Pharmacopoeia (EP) with regard to microbial growth in the culture medium used and matrix validation as proof that microorganisms can grow or be detected even in the presence of a test sample at different incubation conditions. In addition, we examined the influence of these factors on the detection kinetics and detection limits. These components are essential to know all possible factors influencing the detection performance of the system to be able to completely rule out possible negative influences on the test and, in particular, the generation of false negative results.
Materials and Methods
Instruments
The BacT/ALERT 3D Dual T system (bioMérieux) was used for the validation of automated microbiological control of cellular products. The system consisted of a BacT/ALERT 3D 240 Low-Temperature Module for automated incubation at 21–24°C and a BacT/ALERT 3D 120 Combo Module for incubation at 31–34°C. The equipment used for the validation of sterility testing methods was qualified by the manufacturer according to United States Pharmacopoeia (USP) <1058>, Analytical Instrument Qualification [18], which consisted of the following parts: installation, operational, and performance qualifications (IQ, OQ, and PQ).
Growth Media
Growth media were supplied by bioMérieux and consisted of aerobic iAST and anaerobic iNST media supplied in disposable culture bottles containing 40 mL of media and an internal sensor that detects CO2 as an indicator of microbial growth. The aerobic media component consists of casein (pancreatic digest) (1.7% w/v), papain-digested soybean meal (0.3% w/v), sodium polyanethol sulfonate (SPS) (0.035% w/v), pyridoxine HCl (0.001% w/v), and other complex amino acid and carbohydrate substrates in purified water. The bottles contain an atmosphere of CO2 in oxygen under a vacuum. The anaerobic media component consists of casein (pancreatic digest) (1.36% w/v), papain-digested soybean meal (0.24% w/v), SPS (0.035% w/v), menadione (0.00005% w/v), hemin (0.0005% w/v), yeast extract (0.38% w/v), pyridoxine hydrochloride (0.0008% w/v), pyruvic acid sodium salt (0.08% w/v), reducing agents, and other complex amino acids and carbohydrate substrates purified in water. The bottles contain an atmosphere of CO2 in nitrogen under a vacuum. Growth promotion testing was performed for each media batch by separately inoculating a culture with 100 µL of medium containing fewer than 100 colony-forming units (cfu) into iAST and iNST media (data not shown).
Bacterial Strains and Growth Conditions
The tests were performed using microbial strains recommended by the EP for microbiological method suitability testing [1] and additional relevant possible contaminants of human-derived stem-cell products. Different representative aerobic Gram-positive and Gram-negative bacteria, as well as yeasts and molds, were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). Strains were cultivated on Columbia blood agar plates (bioMérieux). The following strains of microorganisms were used as indicated in the EP chapter 2.6.1 on sterility testing [1]: aerobic and anaerobic: Staphylococcus aureus(ATCC 6538), Staphylococcus epidermidis(ATCC 12228), Candida albicans (ATCC 10231), Pseudomonas aeruginosa(ATCC 9027), Escherichia coli (ATCC 8739); strictly aerobic: Bacillus subtilis (ATCC 6633), Aspergillus brasiliensis(ATCC 16404); strictly anaerobic: Clostridium sporogenes(ATCC 19404), Bacteroides fragilis (ATCC 25285) and Cutibacterium acnes (ATCC 11827). The cultures were incubated for 24 h at 36°C for aerobic or anaerobic bacteria, 48 h at 22–25°C for C. albicans, and 5 days at 20–25°C for A. brasiliensis.
Preparation of Leukocyte Suspensions
For the preparation of leukocyte suspensions comparable to human whole blood, buffy coats (German Red Cross Blood Donor Service, Mannheim, Germany) from healthy human donors were separated using Polymorphprep (Axis-Shield, Oslo, Norway) according to the manufacturer's instructions. After fractionation, the cell preparations were washed twice in sterile PBS (Sigma Aldrich, Taufkirchen, Germany) and were finally resuspended in RPMI 1640 medium. Peripheral blood cell (PBC) preparations were adjusted to a final concentration of 2 × 106 cells/mL containing approximately 35% peripheral blood mononuclear cells (PBMC) and 65% polymorphonuclear neutrophils according to their fractions in human whole blood.
Inoculation and Incubation of Media
Inocula with defined cfu numbers of approximately 100, 10, and 1 cfu in 100 µL were prepared by serial dilution of microorganisms in tryptic soy broth (Merck Millipore, Darmstadt, Germany) containing 20% w/v glycerol (Sigma Aldrich) and were frozen immediately at −80°C after preparation. Microbial cfu counts were verified by plating an equal volume of 100 µL of each suspension on agar plates and counting colonies after 24 h of incubation as described above. The inocula, in aliquots of 100 µL, were used immediately after rapid thawing. For BacT/ALERT testing, the flasks were inoculated using different matrices and inocula and were loaded into the BacT/ALERT 3D DualT system immediately. The iNST medium was incubated at 32.5 ± 2°C, and the iAST medium was incubated at 22.5 ± 2°C under continuous shaking with automatic readings taken every 10 min over 7 days or until microbial growth was detected in the BacT/ALERT 3D DualT system. Each positive culture detected by the system was subcultured on Columbia blood agar plates (bioMérieux) and incubated at 36°C under aerobic or anaerobic conditions for 24 h to identify microorganisms. The obtained cultures were visually screened for contamination, and the accordance of the cultured microorganisms to the inoculum was verified by species verification using the microflex MALDI-TOF system (Bruker Daltonics, Bremen, Germany). Media without detection of microbial growth during the incubation period were subcultured on Columbia blood agar plates to exclude microbial growth not detected by the BacT/ALERT 3D DualT system.
Analysis of Cellular Matrix Effects on Detection Performance
To analyze the cellular matrix effects on the detection performance, growth behavior, and detection of selected microorganisms by the BacT/ALERT 3D DualT system, cultures were analyzed using matrix preparations containing 106, 105, and 104 PBCs and different microbial inocula in iAST and iNST media.
Analysis of Contamination Level Effects
To analyze the effects of different contamination levels for each microorganism, test media were inoculated using three different microbial cell counts. Media were inoculated with 1, 10, and 100 cfu of test organisms in 100 µL of TSB.
Determination of Detection Limits
The most probable number (MPN) method was used to determine the detection limits of the BacT/ALERT 3D Dual T system [19]. Media were inoculated with microbial counts of 100 cfu followed by a 5-fold dilution series under different incubation conditions. The limit of detection (LOD) was determined as the inverse of the MPN values, and confidence intervals were calculated using the MPN method.
Statistical Analysis
For descriptive purposes, arithmetic mean and standard deviation were calculated, as appropriate. Categorical and continuous variables were analyzed using either the Student ttest or nonparametric tests, as appropriate. Statistical analyses were performed using SPSS Statistics version 21.0 (IBM Corp, Armonk, NY, USA).
Results
Growth Promotion Test
The growth-promoting properties of the culture method and media were analyzed by inoculating at least four representative units of media with less than 100 cfu of each selected microorganism. All required bacterial test organisms were detected in time periods of less than 3 days, while both fungal test strains were detected in less than 5 days with the BacT/ALERT 3D Dual T system using aerobic and anaerobic media as required by the EP and the USP (data not shown).
The Influence of the Inoculum Cell Count on Detection Time
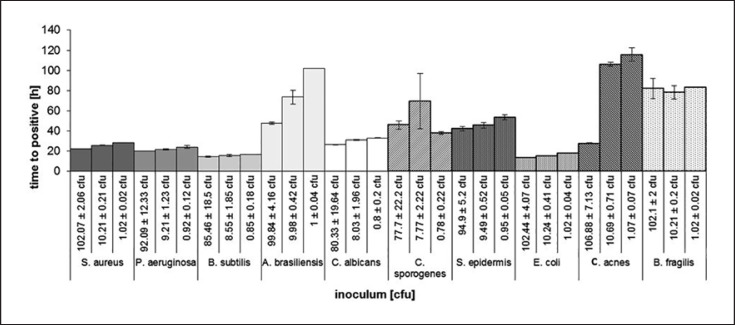
The detection times for most of the microorganisms were largely independent of the inoculated cell counts. Only A. brasiliensis and C. acnes showed a relevant increase in the time to positivity. For Aspergillus, the detection times were 47.7 ± 1 h for 100 ± 4 cfu versus 102.1 h for 1 ± 0.1 cfu, and for C. acnes the times were 27.6 ± 0.5 h for 106.8 ± 7 cfu versus 115.7 h for 1.1 ± 0.1 cfu. The other tested microorganisms showed only marginal differences in time to detection in the tested inoculum ranges between 1 and 100 cfu (Fig. 1).
Fig. 1.
Detection time (h) of different microbiological contaminants (Staphylococcus aureus; Pseudomonas aeruginosa; Bacillus subtilis; Aspergillus brasiliensis; Candida albicans; Clostridium sporogenes; Staphylococcus epidermidis; Escherichia coli; Cutibacterium acnes; Bacteroides fragilis) depending on varying contamination levels from approximately 1 to 100 cfu. The results indicate mean time to positivity of three experiments per condition and the associated standard deviation.
The Influence of Incubation Temperature and Presence of Oxygen on the LOD
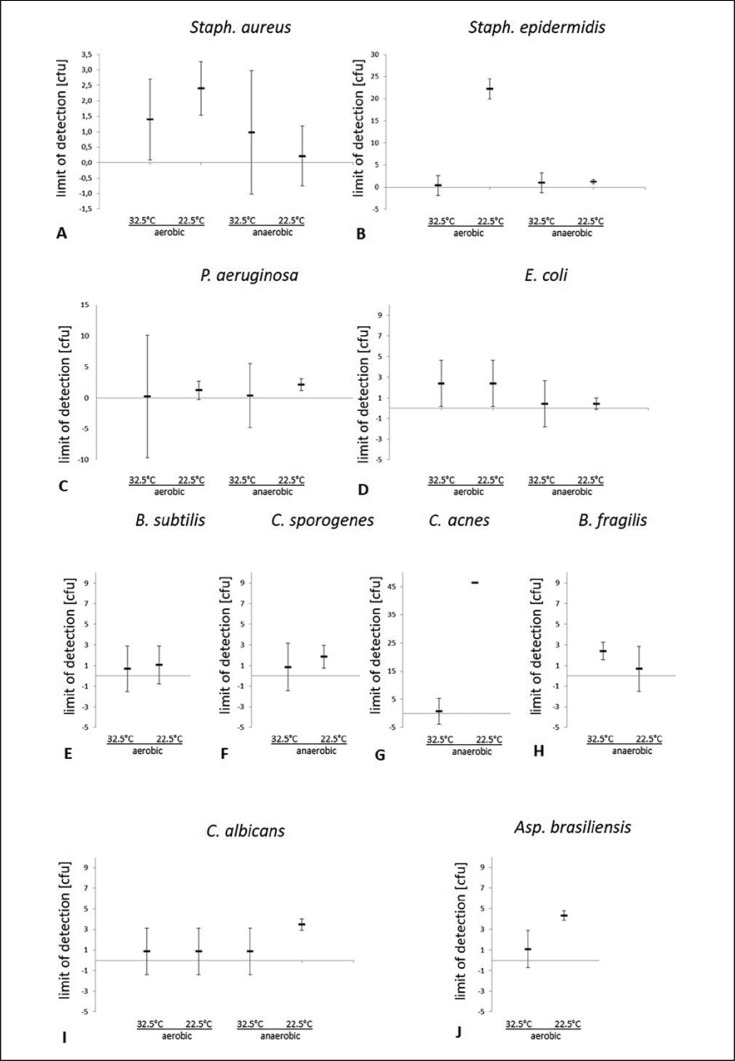
The test procedure showed a significantly increased LOD for S. epidermidis of 22.2 ± 0.1 cfu in test runs under aerobic conditions compared to test runs under anaerobic conditions at 22.5°C (1.3 ± 1.4 cfu), or the test runs at 32.5°C (1.3 ± 1.3 cfu aerobic; 0.98 ± 2.0 cfu anaerobic) (Fig. 2B). S. aureus also showed an increased LOD under aerobic conditions (2.4 ± 0.9 cfu) versus anaerobic conditions (0.2 ± 1.0 cfu) at 22.5°C; however, this did not reach the level of significance (Fig. 2A). The LOD for P. aeruginosa (Fig. 2C) and E. coli (Fig. 2D) showed no significant differences between aerobic and anaerobic conditions, but the limits of detection for E. coliwere slightly reduced in anaerobic test runs for both tested incubation temperatures. The validated test system also did not show significant temperature-dependent differences in the LOD for B. subtilis under aerobic conditions (Fig. 2E) or for C. sporogenes (Fig. 2F) and B. fragilis (Fig. 2H) under anaerobic conditions. The LOD for C. acnes (Fig. 2G) was drastically elevated at 22.5°C (46.4 ± 4.0 cfu) compared to incubation at 32.5°C (0.73 ± 2.0 cfu). Regarding the fungal test isolates, the LOD of C. albicans was significantly increased at 22.5°C compared to 32.5°C under anaerobic conditions (3.5 ± 0.5 cfu at 22.5°C; 0.9 ± 2.2 cfu at 32.5°C) (Fig. 2I) and for A. brasiliensisat 22.5°C compared to 32.5°C under aerobic conditions (4.3 ± 0.4 cfu at 22.5°C; 1.1 ± 1.5 cfu at 32.5°C) (Fig. 2J).
Fig. 2.
Limit of detection (cfu) of the automated BacT/ALERT 3D Dual T culture system depending on different microbiological contaminants (Staphylococcus aureus (A), Staphylococcus epidermidis (B); Pseudomonas aeruginosa (C); Escherichia coli (D); Bacillus subtilis (E); Clostridium sporogenes (F); Cutibacterium acnes (G); Bacteroides fragilis (H); Candida albicans (I); Aspergillus brasiliensis (J)), incubation temperature (32.5°C and 22.5°C) and aerobic vs. anaerobic incubation conditions. Depicted are the results of 5-fold dilution series in triplicates. The limit of detection was determined as reciprocal of most probable number (MPN) values, and confidence intervals were calculated using the MPN method.
The Influence of Incubation Temperature and Oxygen on Detection Time
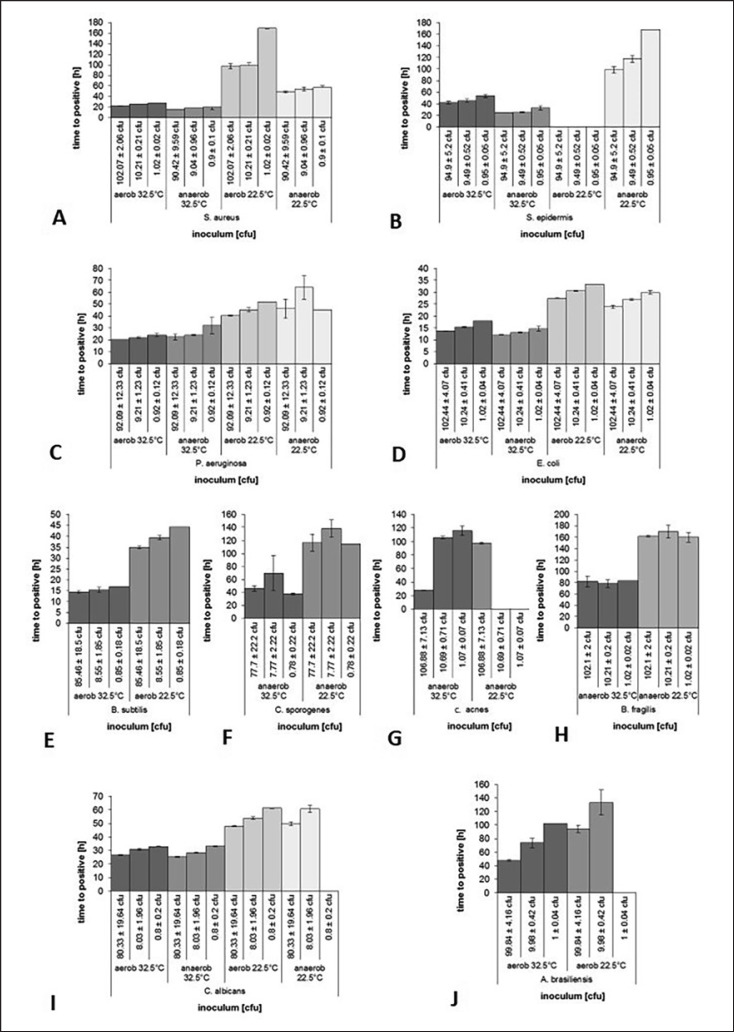
A low incubation temperature of 22.5°C led to a significantly prolonged detection time for each tested microorganism. S. epidermidis was not detected by the test system at inoculum counts of up to 100 cfu at an incubation temperature of 22.5°C under aerobic conditions (Fig. 3B). C. acnes was not detected by the system in low inoculum counts of 10 cfu or fewer under anaerobic conditions at 22.5°C (Fig. 3G). A. brasiliensis (Fig. 3J) and C. albicans(Fig. 3I) were not detected at inoculum counts of 1.0 ± 0.04 or 0.8 ± 0.2 cfu and aerobic conditions or anaerobic conditions at 22.5°C, respectively. The 32.5°C incubation conditions yielded better detection performance of the BacT/ALERT 3D Dual T culture system for all tested conditions and microorganisms.
Fig. 3.
Detection time (h) of varying contamination levels of different microbiological contaminants (Staphylococcus aureus (A), Staphylococcus epidermidis (B); Pseudomonas aeruginosa (C); Escherichia coli (D); Bacillus subtilis (E); Clostridium sporogenes (F); Cutibacterium acnes (G); Bacteroides fragilis (H); Candida albicans (I); Aspergillus brasiliensis (J)) depending on incubation temperature (32.5°C and 22.5°C) and aerobic vs. anaerobic incubation conditions. The results indicate mean time to positivity of three experiments per condition and the associated standard deviation.
The Influence of PBC Presence on Detection of Microbial Contaminants
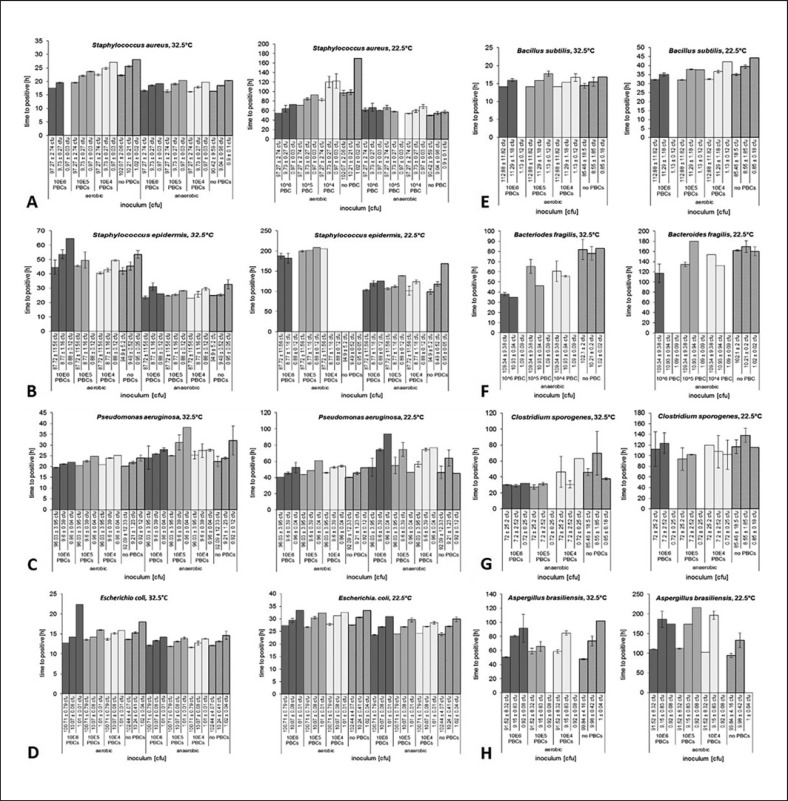
The presence of between 104 and 106 PBCs in culture media affected the performance of the system to detect S. aureus (Fig. 4A), S. epidermidis (Fig. 4B), B. fragilis(Fig. 4G), C. sporogenes (Fig. 4H), B. subtilis (Fig. 4F), and A. brasiliensis (Fig. 4I). However, only the detection of very low microbial cell counts of approximately 1 cfu was affected by the presence of PBCs (Fig. 4G). The presence of 106 PBCs only yielded a detection failure for higher cell counts of contaminating B. fragilis(10.9 ± 0.9 cfu) at 22.5°C under anaerobic conditions, while B. fragiliscontamination by 10.9 ± 0.9 cfu was detected in the presence of 106 PBCs at 32.5°C and thus was unaffected by the presence of PBCs (Fig. 4G). However, P. aeruginosa (Fig. 4C) and E. coli(Fig. 4D) were generally not affected by the presence of between 104 and 106 PBCs in culture media. During the test runs, there was no accumulation of false positive results in the BacT/ALERT 3D Dual T culture system due to the presence of PBCs.
Fig. 4.
Detection time (h) of different microbiological contaminants (Staphylococcus aureus (A), Staphylococcus epidermidis (B); Pseudomonas aeruginosa (C); Escherichia coli (D); Bacillus subtilis (E); Bacteroides fragilis (F); Clostridium sporogenes (G); Aspergillus brasiliensis (H)) depending on the presence of cellular matrices represented by peripheral blood mononuclear cells from 104 to 106 cells per sample under varying incubation temperatures and aerobic vs. anaerobic incubation conditions. The results indicate mean time to positivity of three experiments per condition and the associated standard deviation.
Discussion
Proving the microbiological safety of cellular products poses a significant problem for blood banks, stem cell laboratories, and manufacturers of cell therapeutics. This is partly because microbiological control of cellular products is frequently associated with considerable sample and procedural difficulties. An additional problem is often the short time period between sampling for quality control and actual application of the product as well as low sample volumes or low-level contamination below the detection limit of the applied test systems. Sterility testing, however, by definition, is limited in ensuring the absolute sterility of a product with regard to method sensitivity, sample size, and characteristics. Therefore, the accurate evaluation and conformity check of all properties of alternative test systems, also based on existing regulations, is required. In addition, new methods for the quality and microbiological control of cells have to provide reliable results and a high level of robustness.
Importantly, because of liability issues EP-compliant methods need to produce results on a high level of confidence. Of note, this study was not designed to verify EP compliance of the evaluated test system but rather aimed to analyze the importance of different influence factors on the detection performance of an automated culture system.
In a previous study, we showed that there are no inferiorities of the BacT/ALERT 3D DualT system compared to the conventional direct inoculation method regarding the system's ability to detect microbial contamination in the context of sterility testing. Furthermore, the sterility test results obtained with this automated technique showed less statistical variance than results obtained by the direct inoculation method [20].
The testing of biological samples, injectable and blood products by the conventional BacT/ALERT 3D system, based on the processing of diagnostic blood cultures, has previously been analyzed by other groups [2, 9, 13, 15, 17, 21, 22, 23, 24]. However, these studies involved high incubation temperatures adapted to primarily human pathogens and no process contaminants caused by handling and processing of the product. In addition, the scope of validation and the suitability of the nutrient media are not readily transferable from diagnostic purposes to sterility testing or microbiological control of cellular products. In contrast, our study focused on the evaluation of an automated sterility testing system of cellular products using dual temperature incubation with anaerobic incubation at 31–34°C and aerobic incubation at 21–24°C, for hypothetically advanced detection performance, especially for environmental contaminants, which occasionally prefer or even require low growth temperatures, because studies have suggested that the incubation of inoculated media between 35 and 37°C limits the spectrum of detectable microorganisms [25].
Various studies of automated culture methods for the control of cellular products using different incubation systems have been published in recent years. The BacT/ALERT 3D Dual T system, as well as the composition of iAST in the iNST medium, are a good approximation of the current requirements of the pharmacopoeia and were therefore selected as a test system in our study. A comparative evaluation of the BACTEC FX (Becton Dickinson) systems with the compendial USP <71> method for the detection of Product Sterility Testing Contaminants was recently published and highlighted the better performance of the BacT/ALERT 3D Dual T system [26].
Conventional culture methods can lead to difficulties in growth evaluation due to cloudiness of the nutrient medium caused by the product or complete lack of evaluability due to the presence of red blood cells. However, automated procedures may also be associated with problems in the investigation of microbial contamination. One example is the influence of metabolically active cells contained in the product to be tested on carbon dioxide-based readout of automated culture processes. Against this background, the quality of the evaluation software is of crucial importance for distinguishing between microbial growth and metabolism of the cell matrix. Notably, in our study, there was no increase in false positive results caused by the presence of metabolically active blood cells.
Independent of the aforementioned automated culture techniques, new rapid methods for the detection of microbial contaminants in cellular products are currently available and are used for the quality control of platelet concentrates; however, these methods are currently less sensitive than growth-based methods [27]. Growth-based procedures therefore continue to be the gold standard for sterility and microbiological control. In addition, growth-based techniques offer supplementary possibilities for further analysis of the cultivated contaminants by means of resistance testing and molecular analysis, which are not available for rapid methods but represent an added value.
Looking at the effect of culture conditions on the detection performance of the system on the tested microorganisms, no advantages of low-temperature incubation at 22.5°C could be detected. In addition, the kinetics of S. aureus and S. epidermidis detection improved under anaerobic conditions in the evaluated method. In summary, a combined aerobic and anaerobic approach to the microbiological control of cellular products makes sense. However, the need for low-temperature incubation found elsewhere could not be confirmed in our approach. Here, a generally standardized incubation temperature of 32°C, lowered in comparison to the incubation temperatures of blood cultures, could make sense to detect psychrophilic process contaminants as well. However, it can be assumed that different incubation temperatures may result in altered detection performance. The presence of cellular constituents may interfere with the detection of contaminants. In this context, our study also showed advantages of higher incubation temperatures at 32.5°C and the combination of aerobic and anaerobic cultures. In addition, an incubation period analogous to the sterility test over a period of 14 days does not seem necessary. A relatively sufficient end result could be expected even after 5 days of incubation for the constellations examined in our study.
Conclusion
The automated culture technique using the BacT/ALERT 3D DualT method represents a sufficient approach for the microbiological control of cellular products. The automated culture technology offers some advantages in comparison to microbiological control by conventional culture. These advantages include, in particular, the lower hands-on effort, the lower susceptibility to interference, in particular by the human factor, and the better standardizability, together with comparable sensitivity and a short time to positivity. Interference from differences in microbial contaminants and cellular matrices was effectively addressed in our study by a medium incubation temperature of 32.5°C and the use of both aerobic and anaerobic culture conditions. The availability and selection of variable culture conditions can therefore be useful depending on the expected contaminant spectrum and the product matrix to be examined. Taken together, our results suggest that the use of this flexible automated method may be extremely promising for the microbiological control of cellular products. However, the product-specific application must be validated and tested as part of a comprehensive product-related validation process.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that they have no financial or nonfinancial competing interests.
Funding Sources
This study was financially supported by bioMérieux Deutschland GmbH, Nürtingen, Germany. bioMérieux Deutschland GmbH was not involved in conducting the experiments, the interpretation of the results, or the writing of the manuscript.
Author Contributions
S.-K.G. contributed to the design of the study, interpreted the data, and drafted the manuscript. C.G. conducted the experiments and drafted the manuscript. S.J.K. conducted the experiments and drafted the manuscript. N.T.M. contributed to the design of the study and wrote parts of the manuscript. F.G. designed the study, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.European Directorate for the Quality of Medicines & HealthCare (EDQM) CoE 2.6.1 Sterility: European Pharmacopoeia 81. 2014 [Google Scholar]
- 2.Bugno A, Lira RS, Oliveira WA, Almodovar AA, Saes DP, de Jesus Andreoli Pinto T. Application of the BacT/ALERT(R) 3D system for sterility testing of injectable products. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology] 2015;46:743–747. doi: 10.1590/S1517-838246320140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman FW. The sterility testing of pharmaceuticals. J Pharm Sci. 1969 Nov;58((11)):1301–8. doi: 10.1002/jps.2600581102. [DOI] [PubMed] [Google Scholar]
- 4.Van Doorne H, Van Kampen BJ, Van der Lee RW, Rummenie L, Van der Veen AJ, De Vries WJ. Industrial manufacture of parenteral products in The Netherlands. A survey of eight years of media fills and sterility testing. PDA journal of pharmaceutical science and technology / PDA. 1998;52:159–164. [PubMed] [Google Scholar]
- 5.Brecher ME, Means N, Jere CS, Heath D, Rothenberg S, Stutzman LC. Evaluation of an automated culture system for detecting bacterial contamination of platelets: an analysis with 15 contaminating organisms. Transfusion. 2001 Apr;41((4)):477–82. doi: 10.1046/j.1537-2995.2001.41040477.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunne WM, Jr, Case LK, Isgriggs L, Lublin DM. In-house validation of the BACTEC 9240 blood culture system for detection of bacterial contamination in platelet concentrates. Transfusion. 2005 Jul;45((7)):1138–42. doi: 10.1111/j.1537-2995.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- 7.McDonald CP, Rogers A, Cox M, Smith R, Roy A, Robbins S, et al. Evaluation of the 3D BacT/ALERT automated culture system for the detection of microbial contamination of platelet concentrates. Transfus Med. 2002 Oct;12((5)):303–9. doi: 10.1046/j.1365-3148.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 8.McDonald CP, Roy A, Lowe P, Robbins S, Hartley S, Barbara JA. Evaluation of the BacT/Alert automated blood culture system for detecting bacteria and measuring their growth kinetics in leucodepleted and non-leucodepleted platelet concentrates. Vox Sang. 2001 Oct;81((3)):154–60. doi: 10.1046/j.0042-9007.2001.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Mastronardi C, Perkins H, Derksen P, denAdmirant M, Ramirez-Arcos S. Evaluation of the BacT/ALERT 3D system for the implementation of in-house quality control sterility testing at Canadian Blood Services. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2010;48:1179–1187. doi: 10.1515/CCLM.2010.240. [DOI] [PubMed] [Google Scholar]
- 10.Tokuno O, Hayakawa I, Hashimoto M, Nakamura M, Sugimoto T, Minami H. Evaluation with the BacT/ALERT microbial detection system of bacterial contamination in autologous blood donation and transfusion. Transfus Med. 2012 Feb;22((1)):73–4. doi: 10.1111/j.1365-3148.2011.01116.x. [DOI] [PubMed] [Google Scholar]
- 11.Kielpinski G, Prinzi S, Duguid J, du Moulin G. Roadmap to approval: use of an automated sterility test method as a lot release test for Carticel, autologous cultured chondrocytes. Cytotherapy. 2005;7((6)):531–41. doi: 10.1080/14653240500361079. [DOI] [PubMed] [Google Scholar]
- 12.Moldenhauer J, Sutton SV. Towards an improved sterility test. PDA journal of pharmaceutical science and technology / PDA. 2004;58:284–286. [PubMed] [Google Scholar]
- 13.Jimenez L, Rana N, Amalraj J, Walker K, Travers K. Validation of the BacT/ALERT(R) 3D System for Rapid Sterility Testing of Biopharmaceutical Samples. PDA journal of pharmaceutical science and technology / PDA. 2012;66:38–54. doi: 10.5731/pdajpst.2012.00790. [DOI] [PubMed] [Google Scholar]
- 14.Tokuno O, Hayakawa A, Yanai T, Mori T, Ohnuma K, Tani A, et al. Sterility Testing of Stem Cell Products by Broad-Range Bacterial 16S Ribosomal DNA Polymerase Chain Reaction. Lab Med. 2015;46((1)):34–41. doi: 10.1309/LMKT4P9FFI2BBSIU. [DOI] [PubMed] [Google Scholar]
- 15.Walther-Wenke G, Doerner R, Montag T, Greiss O, Hornei B, Knels R, et al. Working party on Bacteria Safety in Transfusion Medicine of the Advisory Board of the German Ministry of Health (Arbeitskreis Blut), Berlin, Germany Bacterial contamination of platelet concentrates prepared by different methods: results of standardized sterility testing in Germany. Vox Sang. 2006 Apr;90((3)):177–82. doi: 10.1111/j.1423-0410.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 16.Thorpe TC, Wilson ML, Turner JE, DiGuiseppi JL, Willert M, Mirrett S, et al. BacT/Alert: an automated colorimetric microbial detection system. J Clin Microbiol. 1990 Jul;28((7)):1608–12. doi: 10.1128/jcm.28.7.1608-1612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray L, McGowan N, Fleming J, Bailey L. Use of the BacT/alert system for rapid detection of microbial contamination in a pilot study using pancreatic islet cell products. J Clin Microbiol. 2014 Oct;52((10)):3769–71. doi: 10.1128/JCM.00447-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Convention US. [{LT}]1058[{GT}] Analytical Instrument Qualification. United States Pharmacopeial Convention : USP 38, 2015 [Google Scholar]
- 19.Weenk GH. Microbiological assessment of culture media: comparison and statistical evaluation of methods. Int J Food Microbiol. 1992 Oct;17((2)):159–81. doi: 10.1016/0168-1605(92)90113-h. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser SJ, Mutters NT, Backhaus J, Frank U, Gunther F. Sterility Testing of Injectable Products: Evaluation of the Growth-based BacT/ALERT(R) 3D Dual T Culture System. PDA journal of pharmaceutical science and technology / PDA. 2016;70:568–576. doi: 10.5731/pdajpst.2016.006460. [DOI] [PubMed] [Google Scholar]
- 21.Bourbeau P, Riley J, Heiter BJ, Master R, Young C, Pierson C. Use of the BacT/Alert blood culture system for culture of sterile body fluids other than blood. J Clin Microbiol. 1998 Nov;36((11)):3273–7. doi: 10.1128/jcm.36.11.3273-3277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuu HM, Stock F, McGann M, Carter CS, Atkins JW, Murray PR, et al. Comparison of automated culture systems with a CFR/USP-compliant method for sterility testing of cell-therapy products. Cytotherapy. 2004;6((3)):183–95. doi: 10.1080/14653240410005997. [DOI] [PubMed] [Google Scholar]
- 23.Parveen S, Kaur S, David SA, Kenney JL, McCormick WM, Gupta RK. Evaluation of growth based rapid microbiological methods for sterility testing of vaccines and other biological products. Vaccine. 2011 Oct;29((45)):8012–23. doi: 10.1016/j.vaccine.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 24.Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002 Nov;44((3)):235–40. doi: 10.1016/s0732-8893(02)00451-0. [DOI] [PubMed] [Google Scholar]
- 25.Montag-Lessing T, Störmer M, Schurig U, Brachert J, Bubenzer M, Sicker U, et al. [Problems in microbial safety of advanced therapy medicinal products. Squaring the circle] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010 Jan;53((1)):45–51. doi: 10.1007/s00103-009-0993-3. [DOI] [PubMed] [Google Scholar]
- 26.England MR, Stock F, Gebo JE, Frank KM, Lau AF. Comprehensive Evaluation of Compendial USP[{LT}]71[{GT}], BacT/Alert Dual-T, and Bactec FX for Detection of Product Sterility Testing Contaminants. J Clin Microbiol. 2019 Jan;57((2)):57. doi: 10.1128/JCM.01548-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sireis W, Rüster B, Daiss C, Hourfar MK, Capalbo G, Pfeiffer HU, et al. Extension of platelet shelf life from 4 to 5 days by implementation of a new screening strategy in Germany. Vox Sang. 2011 Oct;101((3)):191–9. doi: 10.1111/j.1423-0410.2011.01485.x. [DOI] [PubMed] [Google Scholar]