Visual Abstract
Key Words: atherosclerosis, IL-1β, NLRP3 inflammasome, sex
Abbreviations and Acronyms: ACVD, atherosclerotic cardiovascular disease; BM, bone marrow; CAS, castration; ER, estrogen receptor; HFD, high-fat diet; FLICA, fluorescent labeled inhibitors of caspases; IL, interleukin; NLRP3, nucleotide-binding domain and leucine-rich repeat containing (NLR) protein3; OVX, ovariectomy
Highlights
-
•
In this study we observed sex-specific effects of the NLRP3 inflammasome on atherogenesis in LDLR-deficient mice, with NLRP3 inflammasome playing a more prominent role in atherosclerosis in female mice than in males.
-
•
Sex hormones may be involved in NLRP3 inflammasome–mediated atherogenesis and may underlie differential responses to anti-NLRP3 therapy between males and females.
-
•
Testosterone may play an inhibitory role by blocking NLRP3 inflammasome and inflammation in atherogenesis, whereas female sex hormones may promote NLRP3 inflammasome–mediated atherosclerosis.
-
•
The results of the present study may help design future clinical trials, with the objective to personalize cardiovascular care for men and women.
Summary
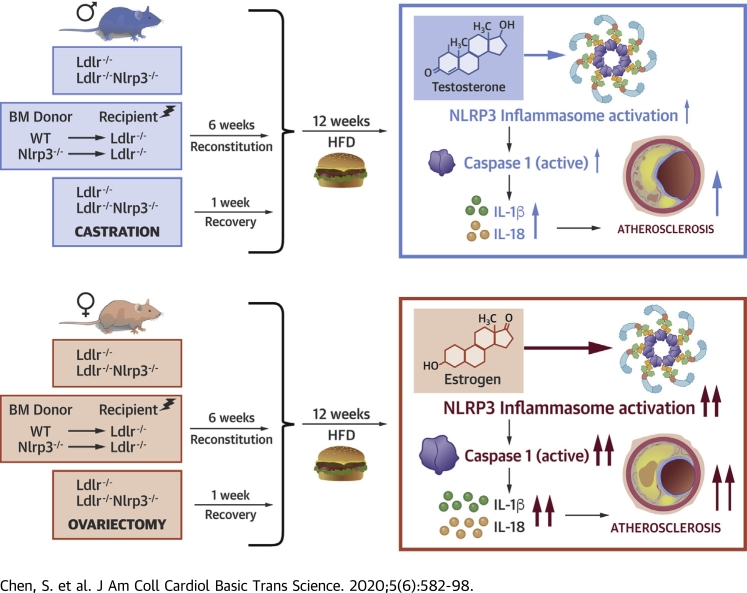
In the Ldlr-/- mouse model of atherosclerosis, female Nlrp3-/- bone marrow chimera and Nlrp3-/- mice developed significantly smaller lesions in the aortic sinus and decreased lipid content in aorta en face, but a similar protection was not observed in males. Ovariectomized female mice lost protection from atherosclerosis in the setting of NLRP3 deficiency, whereas atherosclerosis showed a greater dependency on NLRP3 in castrated males. Thus, castration increased the dependency of atherosclerosis on the NLRP3 inflammasome, suggesting that testosterone may block inflammation in atherogenesis. Conversely, ovariectomy reduced the dependency on NLRP3 inflammasome components for atherogenesis, suggesting that estrogen may promote inflammasome-mediated atherosclerosis.
Atherosclerotic cardiovascular disease (ACVD) is the leading cause of morbidity and mortality globally in men and women (1). To date, most clinical studies on ACVD have primarily included men, and the knowledge about ACVD in women has been largely based on extrapolation. Although more men than women die from ACVD, and men develop disease at a younger age (40 to 60 years of age) (2,3), women have higher mortality trends in ACVD (4,5), and experience more complications, such as bleeding and coronary vascular injury (6). Plaque erosion, the cause of coronary thrombosis and acute myocardial infarction, occurs at a higher frequency in women than in men (7,8). Recent evidence highlighted ACVD risk factors exclusive to women (9), including common disorders of pregnancy, such as gestational hypertension and diabetes, and frequently occurring endocrine disorders in women of reproductive age (e.g., polycystic ovary syndrome and early menopause) (10,11) caused by hormonal dysregulation. In addition, women with autoimmune disease are at an increased risk of developing ACVD (12).
Inflammation contributes to all stages of atherosclerosis, from plaque formation to instability and final plaque rupture (13). Multiple studies have highlighted the prominent role of the nucleotide-binding domain and leucine-rich repeat (NLR) pyrin domain containing protein3 (NLRP3) inflammasome and interleukin (IL)-1 cytokines in atherogenesis (14, 15, 16, 17), and IL-1α and IL-1β have been observed in human atherosclerotic plaques (18). However, the role of the NLRP3 inflammasome pathway in diet-induced acceleration of atherosclerosis is still controversial, with 2 main groups reporting contrasting results in experimental mouse models. Although Duewell et al. (19) demonstrated a proatherogenic role for the NLRP3 inflammasome activation in response to cholesterol in Ldlr-/- mice, Menu et al. (20) reported no differences in atherosclerosis progression in mice with genetic deletion of key inflammasome components. The latter study used ApoE -/- mice and 8-fold higher cholesterol in the diet compared with the former study (21). However, another key difference between these 2 experimental studies with that whereas Duewell et al. (19) clearly described the use of female mice, Menu et al. (20) did not state the sex of the mice used. Emerging evidence has shown that estrogen can act as an inflammatory protective factor to suppress NLRP3-mediated neuroinflammation in the hippocampus (22,23). However, the relationship between NLRP3 and estrogen in ACVD has not been elucidated.
Several studies strongly suggest that the key differences in the immune-inflammatory processes and resulting inflammatory infiltrate between men and women with ACVD may be driven by sex hormones (24). Current dogma holds that estrogen has anti-inflammatory effects, whereas testosterone promotes inflammation (24). Indeed, the finding that the incidence of ACVD increases in women as estrogen declines with age and following menopause could be interpreted to indicate a protective role for estrogen in the heart (24). However, in clinical studies hormone-replacement therapy has failed to decrease ACVD events (25, 26, 27), emphasizing the complexity of the relationship between vascular biology and estrogen hormones. Indeed, the role of estrogen signaling on expression of IL-1β seems to differ depending on cell type (22,28, 29, 30). Similarly, although in general testosterone is believed to promote innate immune cell activation and production of proinflammatory cytokines, there are many conflicting studies (31). Many studies now suggest that testosterone inhibits atherosclerosis (32, 33, 34, 35, 36), and that testosterone deficiency increases the risk of atherosclerotic events (37, 38, 39). The reason for these conflicting findings may be that the understanding of the effect of sex hormones on immune cells is derived mainly from cell culture and animal studies of normal, healthy cells, rather than disease contexts. Importantly, mechanistic studies examining sex differences in inflammation during atherosclerosis have, for the most part, not yet been conducted.
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) recently demonstrated modest but significant therapeutic benefit of treatment with a monoclonal antibody targeting only IL-1β (canakinumab) in patients with previous myocardial infarction (40). A secondary analysis showed that subgroups of women and men achieved similar clinical efficacy with canakinumab (41), despite only 26% of the participants being female, indicating that a smaller sample size was needed for females to achieve the same clinical benefit as males. These results suggest a sex-specific difference in the therapeutic responses to IL-1β inhibition, where females may be more responsive than males. Although the results of the CANTOS trial are a milestone in cardiovascular medicine, the safety concerns and potentially prohibitive cost make it unlikely that canakinumab will ultimately be used for secondary prevention. Therefore, finding ways to identify subsets of patients who derive maximum benefits from canakinumab (or other anti-inflammatory agents) is critical. Here, we investigated the role of sex in NLRP3 inflammasome–mediated inflammation in atherosclerosis as a first step toward identifying these patient subsets.
Methods
Animal studies
All animal experiments were performed according to the guidelines and approved protocols (Protocol #8299) of the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee. Cedars-Sinai Medical Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and abides by all applicable laws governing the use of laboratory animals. Laboratory animals are maintained in accordance with the applicable portions of the Animal Welfare Act and the guidelines prescribed in the U.S. Department of Health and Human Services publication, Guide for the Care and Use of Laboratory Animals.
Mice
All mice were on the C57BL/6 background for these studies. Both male and female Nlrp3−/−Ldlr−/− and Ldlr−/− mice were used (42). For bone marrow (BM) transplantation, BM from wild-type, and Nlrp3-/- mice was transplanted into irradiated Ldlr-/- mice. After recovery (6 weeks), chimeric mice were placed on a high-fat diet (HFD) containing 0.15% cholesterol (Harlan Teklad) for 12 weeks (42,43).
Castration
One week before HFD, Nlrp3−/−Ldlr−/− and Ldlr−/− male mice of 8 weeks of age underwent castration (CAS) or sham-surgery. Mice were maintained on inhalation anesthesia (1.5% isoflurane) via nose-cone. Before the start of the surgery, carprofen (5 mg/kg body weight) was administered subcutaneously. The area between the penis and the anus was shaved and cleaned with betadine followed by alcohol to disinfect the scrotum. The area between the penis and the anus was lifted to make a small 1-mm horizontal cut. To remove the testes, a small 1-mm cut into the inner skin membrane enclosing the testicles was made and the testicles were exteriorized. Testicular arteries were tied off using resorbable Vicryl sutures before removing the testes. Once the testes were removed, the wound was sealed with 2 nylon sutures. Following spontaneous movement, buprenorphine (0.5 mg/kg body weight) and 300 μl of warm saline were administered subcutaneously. In sham-operated mice, both the skin and inner skin membrane between the penis and anus were incised. The testes were drawn out and placed back and the wound was sealed with interrupted nylon sutures.
Ovariectomy
One week before HFD, Nlrp3−/−Ldlr−/− and Ldlr−/− female mice of 8 weeks of age underwent ovariectomy (OVX) or sham-surgery. Mice were maintained on inhalation anesthesia (1.5% isoflurane) via nose-cone. Before the start of the surgery, carprofen (5 mg/kg body weight) was administered subcutaneously. The area below the ribs was shaved and cleaned with betadine followed by alcohol. This area was then lifted with forceps to make a small 2-cm horizontal cut. Resorbable Vicryl sutures were used to clamp the horn beneath the ovary and each ovary was removed using forceps and scissors. The uterine horns were then placed back into the body and the peritoneal cavity was closed using interrupted resorbable Vicryl sutures. The skin was closed with interrupted nylon sutures. Following spontaneous movement, buprenorphine (0.5 mg/kg body weight) and 300 μl of warm saline were administered subcutaneously. In sham-operated mice, both the skin and inner skin membrane were incised. The ovaries were externalized and returned to the abdominal cavity and the wound was sealed with interrupted nylon sutures.
Assessment of atherosclerotic lesions in the aorta and aortic sinus
The aortas were dissected and the adherent (adventitial) fat was gently removed. Whole aortas were opened longitudinally from the aortic arch to the iliac bifurcation, mounted en face, and stained for lipids with oil red O. Hearts were embedded in optimum cutting temperature compound (OCT) (Tissue-Tek, Sakura, Torrance, California) and serial 7-μm-thick cryosections from the aortic sinus were mounted and stained with oil red O and hematoxylin. Six frozen aortic root cross sections for oil red O stain or hematoxylin were captured with BZ-X710 microscope (Keyence, Itasca, Illinois) digital camera. Image analysis was performed by a trained observer blinded to the genotype of the mice. Lesion areas were quantified with image analysis software using a BZ-X710 microscope (Keyence). The lesion area in the aorta en face preparations was expressed as a percent of the aortic surface area, as previously reported (44). Necrotic core was measured by hematoxylin-eosin staining and quantified with image analysis software.
Meso scale discovery
IL-1β in mouse plasma samples was measured using the U-PLEX Mouse IL-1β Assay (Meso Scale Diagnostics, Rockville, Maryland) per the manufacturer’s instructions. The samples were read and analyzed by Meso Scale Discovery QuickPlex SQ120 instrumentation and Workbench 4.0 Software (Meso Scale Diagnostics).
Cytokine assay
IL-18 in mouse plasma samples was measured using enzyme-linked immunosorbent assay kits for murine IL-18 (Abcam, Cambridge, Massachusetts). Cell culture supernatants were assayed using commercially available enzyme-linked immunosorbent assay kits for murine IL-1β and tumor necrosis factor-α (eBioscience, San Diego, California) according to the manufacturer’s instructions.
Immunofluorescence staining and image acquisition
For immunohistochemical staining of frozen sections, fixing and antigen blocking were performed using immunoglobulin from the species of the secondary antibodies. Next, the sections were incubated with primary antibodies overnight at 4°C, followed by incubation with the appropriate secondary antibodies conjugated with fluorescent dyes. For assessment of macrophage content, cells were detected using anti-MOMA-2 antibody and for colocalization staining, nuclei were counterstained with DAPI. Caspase-1 activity was detected by fluorescent labeled inhibitors of caspases (FLICA) staining. Images (3 sections per animal) were captured using the BZ-9000 microscope (Keyence) and analyzed by BZ analyzer software.
Serum lipid profiles
Mice were sacrificed and sera from mice were obtained at the end of experiments and after an overnight fast. Total cholesterol concentrations and lipid profiles were determined in duplicate by using a colorimetric assay (infinity cholesterol reagent, Sigma Diagnostics, St. Louis, Missouri) as described earlier (43).
Statistical analysis
Results are reported as mean ± SEM. All data were analyzed with the GraphPad Prism statistical software version 7 (GraphPad Software, Inc., San Diego, California). Statistical differences between 2 groups were assessed using a 2-sided Student's t-test. Values of p < 0.05 were considered significant.
Sample size and power calculations
We followed the American Heart Association scientific statement on the recommendation of design and execution and reporting of animal atherosclerosis studies as published in 2017 by the American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology and Council on Basic Cardiovascular Sciences (45). The sample sizes needed in each experimental group were based on 80% power and 2-sided tests for 5% level of significance. Based on our prior experience, given expected experimental variance within a treatment group of up to 20%, we calculated that a minimum of 8 to 10 animals per group was required. To account for any additional variability or mortality during the experiments, we calculated a priori that 10 to 12 mice were used in each group. Both male and female mice were used and analyzed separately because this was the main focus of this study.
Results
NLRP3 inflammasome plays a greater role in HFD-induced atherosclerosis in female compared with male Ldlr-/- mice
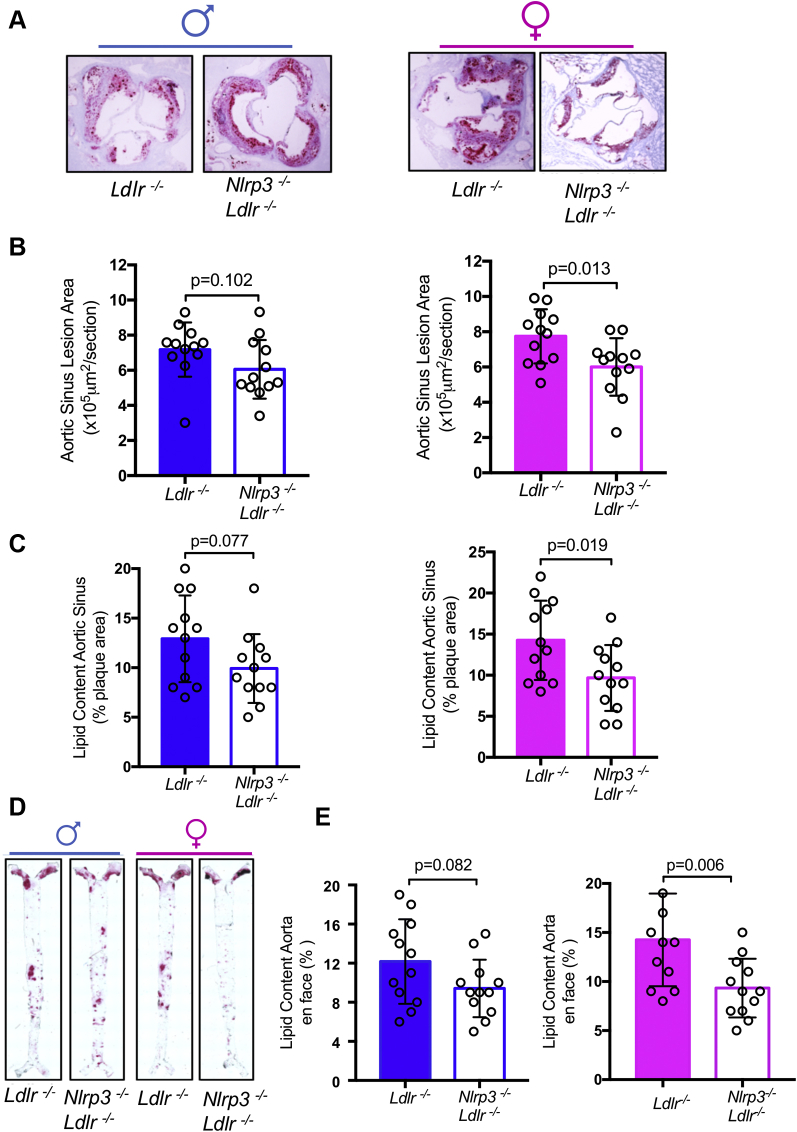
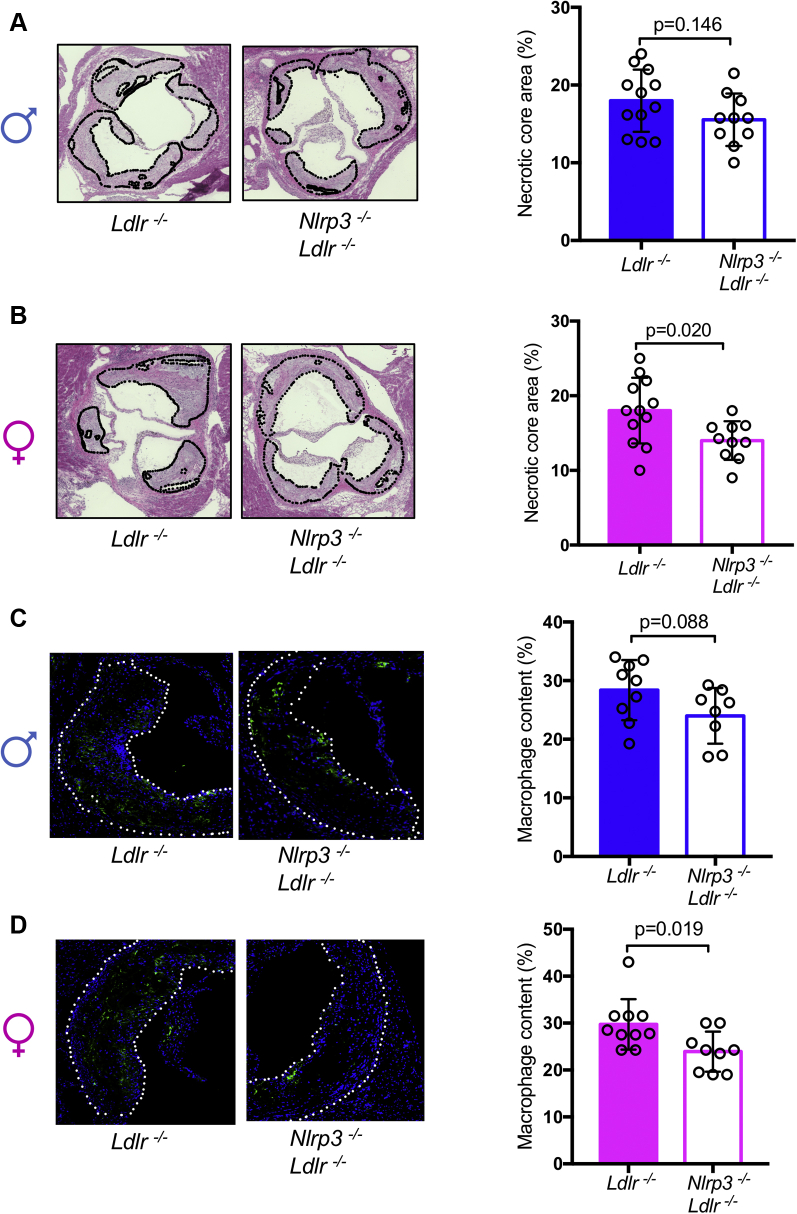
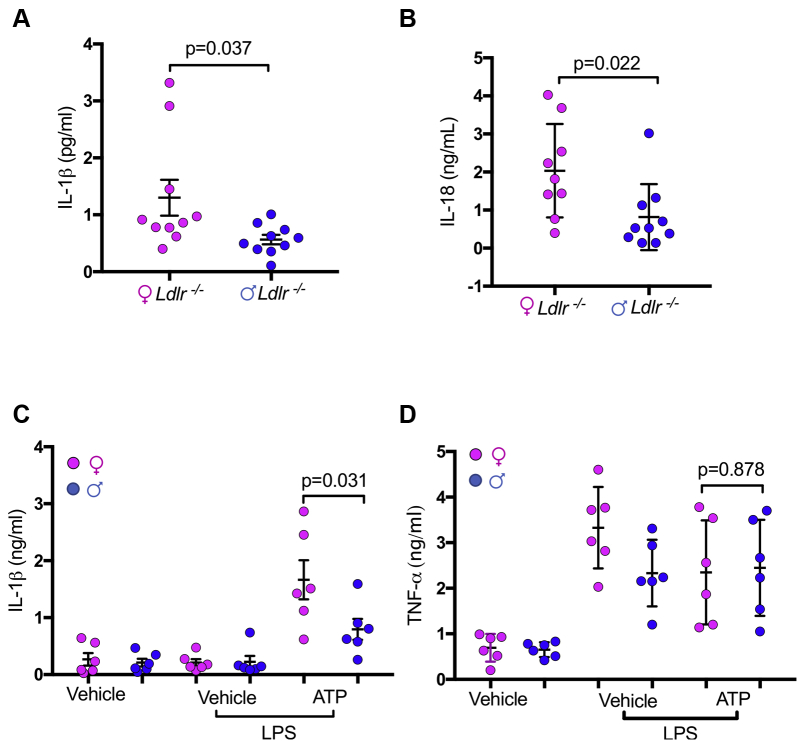
To assess sex differences in inflammasome-mediated acceleration of atherosclerosis, we generated Nlrp3-/-Ldlr-/- mice and fed them an HFD for 12 weeks. Despite similar blood cholesterol levels and lipid profile (Table 1), in female mice, NLRP3 deficiency resulted in a 30% decrease in plaque size in the aortic root compared with Ldlr-/- alone, whereas this difference was not significant in males (Figures 1A and 1B). DKO females also exhibited 32% less lipid content in aortic root plaque (Figure 1C) and 38% less lipid coverage in aortic en face compared with control animals, whereas in males there was no significant difference between DKO and control animals (Figures 1D and 1E). In HFD-fed male mice, NLRP3 deficiency did not affect necrotic core size (Figure 2A) or macrophage content (Figure 2C), whereas these parameters were significantly reduced in NLRP3-deficient females compared with control animals (Figures 2B and 2D). Lipid accumulation and cell death within the lesions contribute to activation of inflammatory cells that release proinflammatory and proatherogenic mediators into the serum (46,47). We measured IL-1β and IL-18 in the serum and found significantly higher concentrations of IL-1β and IL-18 in female Ldlr−/− mice compared with male mice (Figures 3A and 3B). Additionally, peritoneal macrophages were isolated from female and male Ldlr−/− mice after 12 weeks HFD. Cells were pretreated with lipopolysaccharide and then stimulated with NLRP3 activator ATP. Female macrophages secreted significantly more IL-1β, but not tumor necrosis factor-α, compared with male macrophages (Figures 3C and 3D).
Table 1.
Total Cholesterol Level, Lipoprotein Profile and Triglyceride Concentrations in Serum (mg/dl) of Mice
| Groups | TC | HDL | LDL | TG |
|---|---|---|---|---|
| Ldlr-/- M | 1,041 ± 90 | 51 ± 16.1 | 118 ± 15.3 | 148 ± 17.6 |
| Nlrp3-/-Ldlr-/- M | 1,100 ± 58 | 49 ± 13.5 | 109 ± 16.9 | 129 ± 17.1 |
| Ldlr-/- F | 987 ± 82 | 59 ± 16.8 | 102 ± 19.1 | 135 ± 19.5 |
| Nlrp3-/-Ldlr-/- F | 1,029 ± 59 | 62 ± 17.1 | 115 ± 16.9 | 141 ± 21.5 |
Values mean ± SEM.
F = female; HDL = high-density lipoprotein; LDL = low-density lipoprotein; M = male; TC = total cholesterol; TG = triglyceride.
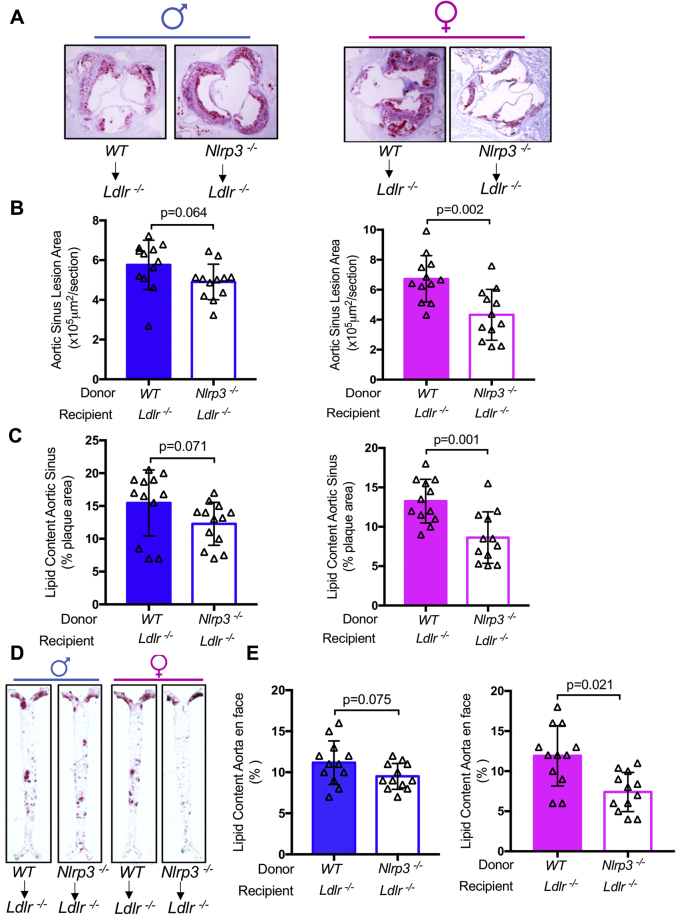
Figure 1.
NLRP3 Deficiency Reduces Diet-Induced Atherosclerosis Development in Female But Not Male Mice
(A) Representative oil red O staining of aortic sinus plaque in Ldlr-/- and Nlrp3-/-Ldlr-/- mice (n = 12). (B) Quantification of area of aortic sinus plaques. (C) Quantification of lipid content of aortic sinus. (D) Representative oil red O staining of aortic en face Ldlr-/- and Nlrp3-/-Ldlr-/- mice (n = 12). (E) Quantification of aortic lesion coverage. Data are presented as mean value ± standard error of the mean. Statistical significance was determined using Student’s t-test.
Figure 2.
Comparison of Necrotic Core and Macrophage Content in Diet-Induced Atherosclerosis Lesion of Female Versus Male Mice
(A) Necrotic core area (hematoxylin-eosin) in male aortic root (n = 10 to 12). (B) Necrotic core area (hematoxylin-eosin) in female aortic root (n = 10 to 12). (C) Macrophage content in male aortic root by MOMA-2 staining and DAPI (for nucleus) (n = 8 to 11). (D) Macrophage content in female aortic root by MOMA-2 and DAPI (for nucleus) staining (n = 8 to 10). Data are presented as mean ± SEM. Statistical significance was determined using Student’s t-test.
Figure 3.
IL-1β and IL-18 Secretion Are Higher in Female Ldlr-/- Mice Compared With Males
(A) Female and male Ldlr−/− mice were fed 12 weeks high-fat diet, plasma concentrations of IL-1β were measured by Meso Scale Discovery. (B) Plasma concentrations of IL-18 were measured by enzyme-linked immunosorbent assay. (C and D) Peritoneal macrophages were isolated from female and male Ldlr−/− mice after 12 weeks high-fat diet. Four-hour lipopolysaccharide-primed peritoneal macrophages from female or male Ldlr-/- mice were stimulated with 5 mM ATP (1 h). IL-1β and TNF-α concentrations in the culture supernatant were determined by enzyme-linked immunosorbent assay. Vehicle: 0.01% dimethyl sulfoxide in PRMI1640 medium. All data are mean ± SEM and representative of 3 independent experiments in triplicate. Statistical significance was determined using Student’s t-test. IL = interleukin; LPS = lipopolysaccharide; TNF = tumor necrosis factor.
NLRP3 action in hematopoietic cells modulates atherosclerosis in females
The previously discussed results suggest that the NLRP3 inflammasome plays a greater role in lesion development in female mice compared with male mice. We next used a BM chimera (donor → irradiated recipient: wild-type→Ldlr-/-; Nlrp3-/-→Ldlr-/-) approach to determine the role of NLRP3 in BM-derived cells in the sex difference in inflammasome-mediated acceleration of atherosclerosis. After 12 weeks on HFD, despite similar blood cholesterol levels and lipid profile (Table 2), female recipients of Nlrp3-/- BM developed significantly smaller lesions in the aortic sinus and lower lipid content in aortic root (Figures 4A to 4C) and less aorta en face lipid coverage (Figures 4D and 4E) than did female recipients of wild-type BM. However, this protection was not observed in males (Figures 4A to 4E). These data confirmed our previous results and further suggest that the NLRP3 inflammasome in hematopoietic cells plays a greater role in HFD-mediated atherosclerosis in females than in males in the Ldlr-/- model.
Table 2.
Total Cholesterol Level, Lipoprotein Profile and Triglyceride Concentrations in Serum (mg/dl) of Mice
| Donor (BM) to Recipient | TC | HDL | LDL | TG |
|---|---|---|---|---|
| WT to Ldlr-/- M | 987 ± 78 | 59 ± 15.7 | 110 ± 19.2 | 134 ± 28 |
| Nlrp3-/- to Ldlr-/- M | 901 ± 48 | 62 ± 12.5 | 104 ± 21 | 128 ± 20.1 |
| WT to Ldlr-/- F | 1,012 ± 77 | 52 ± 15.3 | 112 ± 17.8 | 150 ± 13.2 |
| Nlrp3-/- to Ldlr-/- F | 978 ± 81 | 61 ± 17.4 | 117 ± 13.9 | 136 ± 16.4 |
Values are mean ± SEM.
BM = bone marrow; WT = wild-type; other abbreviations as in Table 1.
Figure 4.
Nlrp3 Deficiency in Bone Marrow Cells Reduces Diet-Induced Atherosclerosis Development in Female But Not Male Mice
All mice were on Ldlr-/- background. Irradiated Ldlr-/- mice received wild-type or Nlrp3-/- bone marrow cells. After 8 weeks reconstitution, the mice were fed high-fat diet for 12 weeks. (A) Representative oil red O staining of aortic sinus plaque in mice (n = 12). (B) Quantification of area of aortic sinus plaques. (C) Quantification of lipid content of aortic sinus. (D) Representative oil red O staining of aortic en face (n = 12). (E) Quantification of aortic lesion coverage. Data are presented as mean ± SEM. Statistical significance was determined using Student’s t-test. WT = wild-type.
Sex hormones modulate NLRP3-mediated atherosclerosis
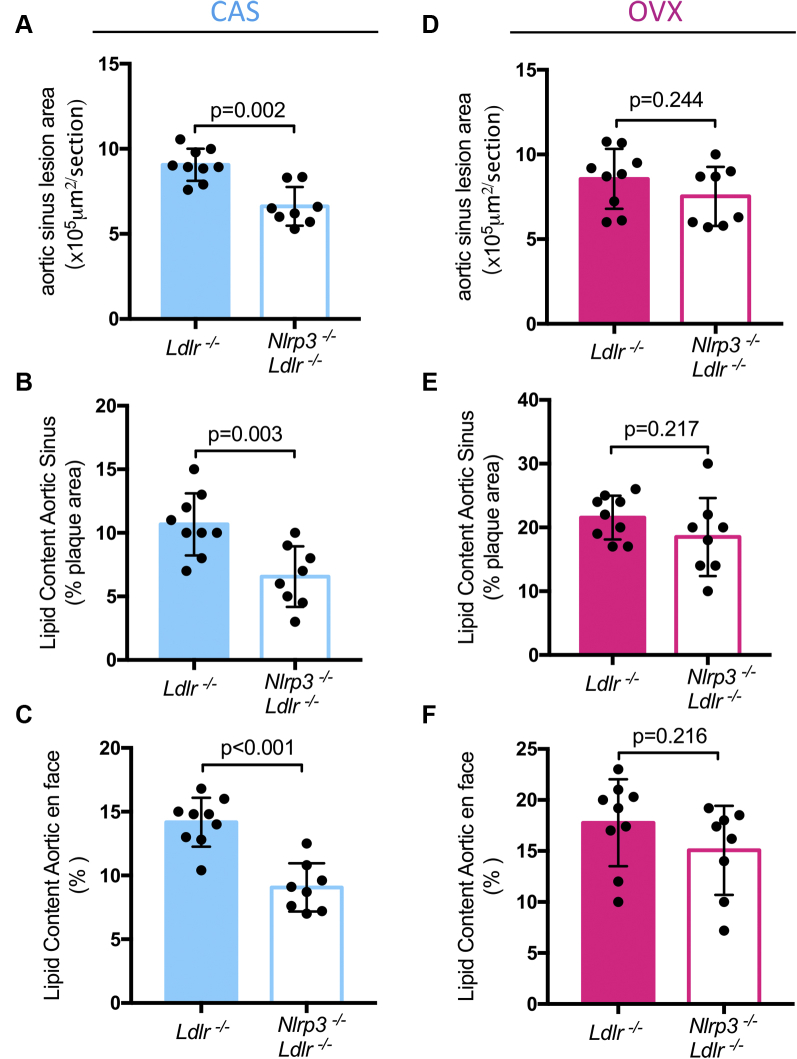
To determine whether the differences we observed between sexes were mediated by sex hormones, we performed CAS in male Ldlr-/- and Nlrp3-/-Ldlr-/- mice; sham-surgeries in male Ldlr-/- and Nlrp3-/-Ldlr-/- mice were done to control and rule out any effects that may be caused by the surgery itself (48). After 1 week of recovery, all mice were fed HFD for 12 weeks before sacrifice. As expected based on our previous data, in sham-operated male mice, there was no difference in aortic lesion size between genotypes (Supplemental Figure 1A), but in the CAS group, DKO mice now showed significant protection (Figure 5A). Similar protection in CAS DKO mice was observed in terms of lipid content in the aortic sinus (Figure 5B), and in aortic en face (Figure 5C), but not in sham-operated male DKO mice (Supplemental Figures 1B and 1C), suggesting that testosterone may suppress the role of the inflammasome in atherosclerosis development. The blood cholesterol levels and lipid profiles were similar in each group (Table 5), and so was their weight gain (Table 4). We next performed sham-surgery or OVX in female Ldlr-/- and Nlrp3-/-Ldlr-/- mice. As expected, sham DKO female mice had smaller aortic root lesions size (Supplemental Figure 1D), lipid content (Supplemental Figure 1E), and lipid content in aortic en face (Supplemental Figure 1F) than sham Ldlr-/- mice. However, these differences were lost in the OVX group (Figures 5D to 5F) supporting a role for estrogen or progesterone in the effect of the inflammasome. Notably, the blood cholesterol levels and lipid profiles were similar in each group (Table 5), and so was their weight gain (Table 4). Taken together, these data suggest that testosterone suppresses inflammasome-mediated atherosclerosis, whereas estrogen or progesterone promotes inflammasome-mediated atherosclerosis development.
Table 3.
Total Cholesterol Level, Lipoprotein Profile and Triglyceride Concentrations in Serum (mg/dl) of Mice
| Groups (Males) | TC | HDL | LDL | TG |
|---|---|---|---|---|
| Ldlr-/- sham | 942 ± 89 | 51 ± 16.3 | 121 ± 16.4 | 141 ± 17.6 |
| Nlrp3-/-Ldlr-/- sham | 1,001 ± 77 | 62 ± 17.6 | 116 ± 18.2 | 136 ± 15.1 |
| Ldlr-/- CAS | 923 ± 85 | 64 ± 15.5 | 109 ± 20 | 125 ± 19.1 |
| Nlrp3-/-Ldlr-/- CAS | 988 ± 78 | 59 ± 18.2 | 117 ± 16.9 | 131 ± 22.3 |
Values are mean ± SEM.
CAS = castration; other abbreviations as in Table 1.
Figure 5.
Comparison of Aortic Sinus Lesion Size in Nlrp3 Ldlr DKO in Male CAS and Female OVX Mice
Oil red O staining of aortic root (A), aortic root lipid content (B), and aortic en face (C) in male CAS mice (n = 8 to 11). Oil red O staining of aortic root (D), aortic root lipid content (E), and aortic en face (F) in female OVX mice (n = 8 to 10). Data are presented as mean ± SEM. Statistical significance was determined using Student’s t-test. CAS = castration; OVX = ovariectomy.
Table 5.
Total Cholesterol Level, Lipoprotein Profile and Triglyceride Concentrations in Serum (mg/dl) of Mice
| Groups (Females) | TC | HDL | LDL | TG |
|---|---|---|---|---|
| Ldlr-/- sham | 965 ± 71 | 58 ± 15.4 | 104 ± 15.4 | 145 ± 17.6 |
| Nlrp3-/-Ldlr-/- sham | 1,011 ± 69 | 63 ± 18.1 | 117 ± 16.7 | 139 ± 15.1 |
| Ldlr-/- OVX | 914 ± 78 | 60 ± 16.6 | 111 ± 19.8 | 141 ± 19.6 |
| Nlrp3-/-Ldlr-/- OVX | 960 ± 88 | 55 ± 17.1 | 102 ± 17.9 | 136 ± 19.3 |
Table 4.
Weight Gain of Sham and Surgery Mice
| Groups (Males) | Weight Gain (g) | Groups (Females) | Weight Gain (g) |
|---|---|---|---|
| Ldlr-/- sham | 13.2 ± 6.6 | Ldlr-/- sham | 16.8 ± 8.2 |
| Nlrp3-/-Ldlr-/- sham | 15.4 ± 4.7 | Nlrp3-/-Ldlr-/- sham | 13.2 ± 5.3 |
| Ldlr-/- CAS | 14.3 ± 4.2 | Ldlr-/- OVX | 14.6 ± 6.2 |
| Nlrp3-/-Ldlr-/- CAS | 13.6 ± 5.6 | Nlrp3-/-Ldlr-/- OVX | 12.7 ± 5.9 |
Values are mean ± SEM.
OVX = ovariectomy; other abbreviations as in Table 3.
Testosterone limits macrophage inflammasome activity in atherosclerosis lesion
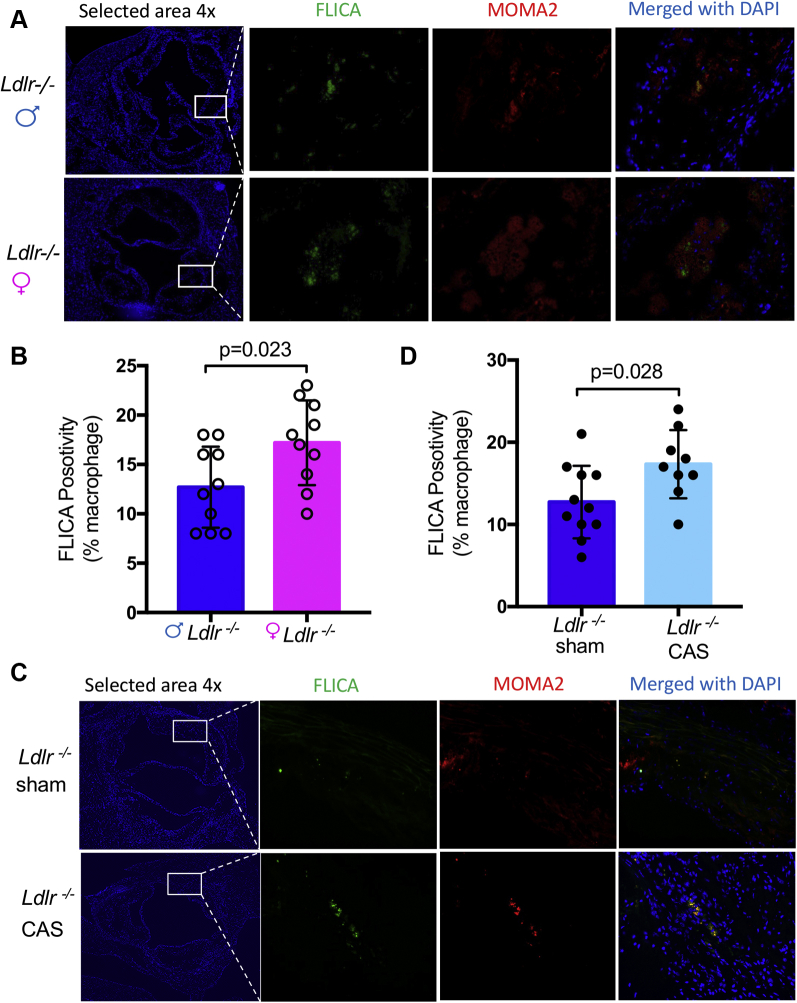
Abnormal inflammasome activation and the subsequent increase in circulating IL-1β and IL-18 correlate with enhanced macrophage recruitment to lesions (49). Therefore, we next measured caspase-1 activity, a read-out of inflammasome activation, in lesion macrophages in aortic roots using FLICA. Plaque macrophages from Ldlr-/- male mice had significantly less caspase-1 activity compared with those from Ldlr-/- female mice (Figures 6A and 6B). Interestingly, CAS significantly increased FLICA positivity in the aortic roots compared with sham-operated males (Figures 6C and 6D); however, OVX did not alter FLICA positivity in aortic roots compared with sham-operated females (Supplemental Figure 2). These findings indicate that at baseline, female mice have more NLRP3 inflammasome activation in plaque macrophages than males, which may drive the enhanced macrophage accumulation. Additionally, in agreement with the effect on plaque formation, loss of testosterone caused by CAS exacerbates inflammasome activity.
Figure 6.
Comparison of Active Caspase-1 Macrophages in Diet-Induced Atherosclerosis Lesion
(A) Representative images for caspase-1 positivity in lesion macrophages of male versus female Ldlr-/- mice. Caspase-1 activity was assessed by fluorescent-labeled inhibitors of caspases (green) in macrophages (MOMA-2) (red) in atherosclerotic lesions of Ldlr−/− mice fed high-fat diet for 12 weeks. (B) Quantification of active caspase-1+ cells in lesion macrophages (n = 10). (C) Representative images for caspase-1 positivity in lesion macrophages of male sham versus CAS Ldlr-/- mice (n = 9 to 11). (D) Quantification of active caspase-1+ cells in lesion macrophages. Data are presented as mean value ± standard error of the mean. Statistical significance was determined using Student’s t-test. FLICA = fluorescent labeled inhibitors of caspases; other abbreviation as in Figure 5.
Discussion
Inflammation plays an important role in atherogenesis, plaque rupture, and subsequent thrombosis leading to acute ischemic syndromes (13). Recent studies have suggested key roles for the proinflammatory cytokines IL-1β and IL-1α in atherosclerosis (46,50, 51, 52, 53). Notably, genetic deficiency of IL-1α, even when restricted to BM-derived cells, mitigates atherosclerotic burden in a mouse model (52), and this protective effect is even more pronounced when combined with depletion of IL-1β (52).
The NLRP3 inflammasome is a multicomponent complex that tightly regulates the maturation and secretion of IL-1β, IL-1α, and IL-18 (54,55). Given that the NLRP3 inflammasome regulates multiple cytokines, some researchers have suggested that targeting NLRP3 or caspase-1 may yield better outcomes in inflammatory disease than targeting the IL-1β alone or other IL-1 cytokines in isolation (16). In this study, we found that Nlrp3 deficiency decreased lesion development and aortic lipid accumulation in HFD-fed Ldlr−/− female mice, but although the trend was evident in male mice, this protection was not significant, suggesting that female mice may have greater sensitivity to NLRP3 inflammasome compared with male mice, whereas in both genders NLRP3 plays a role. Furthermore, we showed that this protection was related to inflammasome activity in hematopoietic cells, because BM chimera females that received NLRP3-deficient BM showed a similar reduction in atherosclerosis. Interestingly, this difference was lost on OVX, suggesting a role for estrogen and/or progesterone in the effect. In contrast, in male Ldlr-deficient mice, CAS conferred significant protection from lesion development and lipid accumulation. Taken together, these data suggest that sex hormones play a role in inflammasome-mediated atherogenesis and thus may influence the response to inhibitors of IL-1β, IL-1α, and IL-18.
A role for sex in modulating atherogenesis is supported by early work in mouse models, which demonstrated that atherosclerotic lesions in the aortic root were larger in female mice (56, 57, 58), although this observation has not been a consistent finding across studies (59). The general understanding of how sex hormones influence the immune system is that estrogens have immune-enhancing effects, whereas progesterone and androgens, such as testosterone and dihydrotestosterone, exert mainly immunosuppressive effects (60). Consistent with this paradigm, estrogen markedly enhances lipopolysaccharide-induced IL-1β promotor activity in the murine macrophage RAW cell line (28,29), and macrophages from female rats secrete more IL-1β and IL-6 in response to lipopolysaccharide (28). Females typically develop a more vigorous innate and adaptive immune response to antigen challenges (61,62), which can accelerate pathogen clearance but can also lead to increased immune-related pathology, such as autoimmune or inflammatory diseases (12,63). Androgens exert an overall inhibitory effect on Th1 differentiation (64) and suppress inflammatory immune cells, such as dendritic cells and macrophages (65). Of interest, CAS changed the protection provided by NLRP3 deficiency on atherosclerosis and under these conditions NLRP3 deficiency provided protection from atherosclerosis. It is possible that testosterone acts like an independent proatherogenic factor, which in the mouse model masks the otherwise NLRP3 protective function. Alternatively, there could be a direct connection between testosterone and NLRP3 inflammasome activation, which needs to be further studied in the future, because our current data cannot rule out this possibility.
However, contradictions to this dogma have been demonstrated, revealing complexities that challenge this paradigm. For example, androgen and estrogen signaling have been shown to enhance alternative macrophage polarization (66). Notably, the regulatory effect of estrogen and estrogen receptor (ER) signaling on the NLRP3 inflammasome seems to be context dependent. For example, in hepatocellular and endometrial carcinoma cells, estrogen upregulates the NLRP3 inflammasome via ERβ (30,67). But in the brain and in fibroblast-like synoviocytes, estrogen inhibits activation of the NLRP3 inflammasome (22). ERα and ERβ are NOD-like receptors (NLRs) transcription regulation factors, because they both regulate NLR expression and promote inflammasome colocalization, and a selective ERα antagonist significantly inhibits NLRP3 expression and inflammasome activity (68). Furthermore, the role of inflammasome activation in inflammatory pathways may also be context-specific, because in contrast to our findings, gene expression studies suggest that the inflammasome plays a more central role in abdominal aortic aneurysms in males than in females (69). Our finding of a role for female sex hormones in driving inflammasome-mediated atherogenesis in mice could be interpreted as contradictory to clinical studies, because menopause (a state of estrogen deficiency) is associated with higher ACVD risk in human females. However, the effect of estrogen deficiency on inflammasome activity in human subjects is unknown, and the enhanced risk of ACVD in post-menopausal females may also be related to advancing age and age-related alterations in inflammatory responses that are independent of inflammasome activity.
Despite the well-established link between inflammation and atherosclerosis, clinical data demonstrating a direct benefit of targeting inflammation had been absent until the CANTOS trial showed the potential for using anti-inflammatory therapy (anti-IL-1β), confirming that IL1β is an important potential therapeutic target for human atherosclerosis and related complications (40). However, for this approach to be clinically useful, it is critical to identify subsets of patients who will derive maximum benefits from canakinumab (or other anti-inflammatory agents) and are at low risk for serious infection. In the CANTOS trial, risk reduction with anti-IL1β therapy was observed in both men and women; however, women formed only 26% of the cohort, indicating that women may be more responsive than men to this therapy (41). Furthermore, the fact that males in the CANTOS trial demonstrated benefit from the anti-IL1β therapy does conflict with the findings reported here, because NLRP3 inhibition could affect several pathways beyond IL-1β, including IL-1α and IL-18 as stated previously. Indeed, there are still important gaps in the understanding of the role of the NLRP3 inflammasome and caspase-1 in atherosclerosis. Of interest, a recent study linked increased NLRP3 expression in human carotid plaques to pathological features, such as vascular inflammation, plaque composition, and vulnerability (16). The authors highlighted the importance of NLRP3 inflammasome and caspase-1-driven IL-1α and IL-1β production in atherosclerotic carotid plaques, supporting the view that release of both of these IL-1 isoforms is determined by the NLRP3-caspase-1 pathway in atherosclerotic plaques (16).
A novel, common, and powerful cardiovascular risk factor has recently emerged: clonal hematopoiesis of indeterminate potential, which arises from somatic mutations in hematopoietic stem cells (17). Studies have shown that individuals who acquire somatic clonal hematopoiesis of indeterminate potential mutations with age have a 40% increase in cardiovascular risk, independent of traditional risk factors (70). Most cases of clonal hematopoiesis of indeterminate potential are caused by mutations in only a handful of genes, including TET2 (17,70, 71, 72). Ldlr-/- mice engineered to bear the TET2 loss of function that is similar to clonal hematopoiesis and increased cardiovascular disease risk in humans (17) had activated NLRP3 inflammasome in myeloid cells, enhanced IL-1β production, and developed accelerated atherosclerosis (73,74). Even though male-to-female mice comparisons were not reported, it is of interest that, in both of these experimental studies the recipient mice that developed increased NLRP3-induced accelerated atherosclerosis were female (73,74).
Women have higher death rates following myocardial infarction than men (75,76), because of differences in the pathogenesis of atherosclerosis, differential efficacy of drugs (77,78), and because vascular assist devices may fit men better than women (79,80). As Clayton and Tannenbaum have argued (81), failure to analyze for male and female clinical trial participants separately may mask important differences in the effects of interventions, toxicity, symptoms, or adverse effects. To date, most clinical studies in this area contain a majority of male subjects, and no trial that we are aware of prespecified analysis of differences by sex. Therefore, understanding how the immune response differs in men and women in the context of atherosclerosis may improve the treatment of cardiovascular disease.
In summary, there is a need for additional investigation on the role of estrogens, progesterone, and testosterone on the NLRP3 inflammasome and IL-1β as well as IL-1α signaling in atherogenesis as it relates to the biologic mechanisms underlying pathophysiological processes in males and females. Our data add weight and a sense of urgency to the efforts of Robinet et al. and the Council on Arteriosclerosis, Thrombosis and Vascular Biology to encourage preclinical arterial pathology researchers to consider sex as a biologic variable when designing and reporting experiments (1), which will improve the design of clinical trials, and help optimize cardiovascular care for men and women.
Conclusions
The present study suggests that loss of NLRP3 inflammasome components leads to more significant reductions in atherosclerotic plaque size and lipid content in female mice than in male mice. Furthermore, CAS increases dependency on NLRP3 inflammasome components for atherogenesis, and increases inflammasome activity, suggesting that testosterone plays an inhibitory role, blocking inflammation in atherogenesis. OVX reduces dependency of atherogenesis on NLRP3 inflammasome components, suggesting that female sex hormones sensitize inflammation in atherogenesis. Our data provide biologic insights into the clinical merit of anti-NLRP3-directed therapies, and the biologic mechanisms underlying pathophysiological processes in males versus females as they pertain to atherosclerosis and the NLRP3 inflammasome, which could help inform the design of future clinical trials.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The CANTOS trial suggested that IL-1β-directed therapy with a neutralizing monoclonal antibody moderately reduces recurrent ischemic events and cardiovascular death among patients with coronary artery disease and elevated C-reactive protein. Therefore, IL1β, which is under the control of the NLRP3 inflammasome and caspase-1, is an important potential therapeutic target for human atherosclerosis and related complications. However, NLRP3 inflammasome also controls secretion of IL-1α and IL-18, inflammatory cytokines that have also been implicated in development of atherosclerosis, and some researchers have suggested that targeting NLRP3-caspase-1 may yield better outcomes than targeting the IL-1β alone or IL-1 isoforms in isolation. Herein, we show that sex hormones may be involved in NLRP3 inflammasome–mediated atherogenesis and may lead to differential responses to anti-NLRP3 therapy between males and females. In a mouse model of atherosclerosis, females with global Nlrp3 deletion or those receiving Nlrp3 -/- BM developed significantly fewer lesions in the aortic sinus and decreased lipid content in aorta, but Nlrp3 deficiency did not confer similar protection in males. Ovariectomized female mice lost protection mediated by NLRP3 deficiency, whereas castrated males showed stronger correlations between NLRP3 inflammasome and atherosclerosis. Overall, the findings of present study suggest that testosterone may play an inhibitory role by blocking NLRP3 inflammasome and inflammation in atherogenesis, whereas female sex hormones may promote NLRP3 inflammasome–mediated atherosclerosis.
TRANSLATIONAL OUTLOOK: The specific role and underlying mechanisms of inflammasome activation and inflammation in atherogenesis are topics of active research. The role of the NLRP3 inflammasome pathway in diet-induced atherosclerosis is still controversial, and the impact of sex hormones has not been explored. In this study we observed sex-specific effects of the NLRP3 inflammasome on atherogenesis in LDLR-deficient mice, with NLRP3 inflammasome playing a more prominent role in atherosclerosis in female mice than in males. The CANTOS study demonstrated modest therapeutic benefit of a monoclonal antibody targeting IL-1β (canakinumab) in male and female patients with previous myocardial infarction, indicating that IL-1β is an important therapeutic target. However, the NLRP3 inflammasome controls not only IL-1β secretion, but also IL-1α and IL-18, leading some researchers to advocate that targeting NLRP3-caspase-1 may yield better outcomes. Furthermore, a secondary analysis of the CANTOS trial revealed that whereas women and men showed similar clinical efficacy with canakinumab, only 26% of the participants were female, suggesting that a smaller sample size was needed for females to achieve the same clinical benefit. Therefore, finding ways to identify subsets of patients who will derive maximum benefits from canakinumab (or other anti-inflammatory agents) is crucial, and it is critically important to understand the role of sex in NLRP3 inflammasome–mediated IL-1β and IL-1α-driven inflammation in atherosclerosis. The present study lends support to the impact of estrogens and testosterone on the inflammasome in atherogenesis and yields important information on biologic mechanisms underlying pathophysiological processes in atherosclerosis in both sexes. The results of the present study may help design future clinical trials, with the objective to personalize cardiovascular care for men and women.
Acknowledgments
The authors thank Wenxuan Zhang, Ganghua Huang, and P. Sun for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants HL66436-05 (to Dr. Arditi) and HL111483 (to Dr. Chen). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures, please see the online version of this paper.
Contributor Information
Prediman K. Shah, Email: PredimanKrishan.Shah@cshs.org.
Moshe Arditi, Email: moshe.arditi@cshs.org.
Appendix
References
- 1.Maas A.H., van der Schouw Y.T., Regitz-Zagrosek V. Red alert for women's heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. doi: 10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 2.Shaw L.J., Bugiardini R., Merz C.N. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosamond W., Flegal K., Friday G. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V., Abramson J.L., Veledar E., Weintraub W.S. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–1181. doi: 10.1161/hc1002.105133. [DOI] [PubMed] [Google Scholar]
- 6.Argulian E., Patel A.D., Abramson J.L. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Arbustini E., Dal Bello B., Morbini P. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82:269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell I.C., Suever J.D., Timmins L.H. Biomechanics and inflammation in atherosclerotic plaque erosion and plaque rupture: implications for cardiovascular events in women. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sattar N., Greer I.A. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veltman-Verhulst S.M., van Rijn B.B., Westerveld H.E. Polycystic ovary syndrome and early-onset preeclampsia: reproductive manifestations of increased cardiovascular risk. Menopause. 2010;17:990–996. doi: 10.1097/gme.0b013e3181ddf705. [DOI] [PubMed] [Google Scholar]
- 11.Ray J.G., Vermeulen M.J., Schull M.J., Redelmeier D.A. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather D., Petri M.A., Coronado M.J., Cooper L.T. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8:269–284. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller A.M., McInnes I.B. Cytokines as therapeutic targets to reduce cardiovascular risk in chronic inflammation. Curr Pharm Des. 2011;17:1–8. doi: 10.2174/138161211795049796. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura E., Lopez L.R., Shoenfeld Y., Ames P.R. beta2-glycoprotein I and oxidative inflammation in early atherogenesis: a progression from innate to adaptive immunity? Autoimmun Rev. 2012;12:241–249. doi: 10.1016/j.autrev.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Sollberger G., Strittmatter G.E., Garstkiewicz M., Sand J., Beer H.D. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20:115–125. doi: 10.1177/1753425913484374. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X., Wang F., Wang Y. Inflammasome-driven interleukin-1alpha and interleukin-1beta production in atherosclerotic plaques relates to hyperlipidemia and plaque complexity. J Am Coll Cardiol Basic Trans Science. 2019;4:304–317. doi: 10.1016/j.jacbts.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal S., Fontanillas P., Flannick J. Age-related clonal hematopoiesis ASSO standard error of the mean cited with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltrami-Moreira M., Vromman A., Sukhova G.K., Folco E.J., Libby P. Redundancy of IL-1 isoform signaling and its implications for arterial remodeling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duewell P., Kono H., Rayner K.J. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menu P., Pellegrin M., Aubert J.F. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Nardo D., Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y.J., Sheng H., Bao Q.Y., Wang Y.J., Lu J.Q., Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Thakkar R., Wang R., Sareddy G. NLRP3 inflammasome activation in the brain after global cerebral ischemia and regulation by 17beta-estradiol. Oxid Med Cell Longev. 2016:8309031. doi: 10.1155/2016/8309031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8:49–59. doi: 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossouw J.E., Anderson G.L., Prentice R.L. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 26.Hulley S., Grady D., Bush T. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 27.Wilson P.W., Garrison R.J., Castelli W.P. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N Engl J Med. 1985;313:1038–1043. doi: 10.1056/NEJM198510243131702. [DOI] [PubMed] [Google Scholar]
- 28.Ruh M.F., Bi Y.H., D'Alonzo R., Bellone C.J. Effect of estrogens on IL-1 beta promoter activity. J Steroid Biochem. 1998;66:203–210. doi: 10.1016/s0960-0760(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 29.Ruh M.F., Bi Y.H., Cox L., Berk D., Howlett A.C., Bellone C.J. Effect of environmental estrogens on IL-1 beta promoter activity in a macrophage cell line. Endocrine. 1998;9:207–211. doi: 10.1385/ENDO:9:2:207. [DOI] [PubMed] [Google Scholar]
- 30.Wei Q., Guo P.B., Mu K. Estrogen suppresses hepatocellular carcinoma cells through ER beta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804–816. doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- 31.Rettew J.A., Huet Y.M., Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- 32.Bruck B., Brehme U., Gugel N. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1997;17:2192–2199. doi: 10.1161/01.atv.17.10.2192. [DOI] [PubMed] [Google Scholar]
- 33.Hanke H., Lenz C., Hess B., Spindler K.D., Weidemann W. Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation. 2001;103:1382–1385. doi: 10.1161/01.cir.103.10.1382. [DOI] [PubMed] [Google Scholar]
- 34.Alexandersen P., Haarbo J., Byrjalsen I., Lawaetz H., Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 35.Gordon G.B., Bush D.E., Weisman H.F. Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest. 1988;82:712–720. doi: 10.1172/JCI113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan L., Shi W., Dinh H. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98:3589–3593. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oskui P.M., French W.J., Herring M.J., Mayeda G.S., Burstein S., Kloner R.A. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herring M.J., Oskui P.M., Hale S.L., Kloner R.A. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrohon J.A., Jessup W., Handelsman D.J., Celermajer D.S. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation. 1999;99:2317–2322. doi: 10.1161/01.cir.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 40.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P.M., MacFadyen J.G., Everett B.M. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 42.Tumurkhuu G., Dagvadorj J., Porritt R.A. Chlamydia pneumoniae hijacks a host autoregulatory IL-1 beta loop to drive foam cell formation and accelerate atherosclerosis. Cell Metab. 2018;28:432–448. doi: 10.1016/j.cmet.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S., Shimada K., Crother T.R., Erbay E., Shah P.K., Arditi M. Chlamydia and lipids engage a common signaling pathway that promotes atherogenesis. J Am Coll Cardiol. 2018;71:1553–1570. doi: 10.1016/j.jacc.2018.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tumurkhuu G., Shimada K., Dagvadorj J. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ Res. 2016;119:e76–e90. doi: 10.1161/CIRCRESAHA.116.308362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daugherty A., Tall A.R., Daemen M. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. doi: 10.1161/ATV.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 46.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 47.Kleemann R., Zadelaar S., Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coronado M.J., Bruno K.A., Blauwet L.A. Elevated sera sST2 is associated with heart failure in men <= 50 years old with myocarditis. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins G.R., Wen H., Ting J.P. Inflammasomes and metabolic disorders: old genes in modern diseases. Mol Cell. 2014;54:297–308. doi: 10.1016/j.molcel.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirii H., Niwa T., Yamada Y. Lack of interleukin-1 beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscl Throm Vas. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 51.Gardner S.E., Humphry M., Bennett M.R., Clarke M.C. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1alpha-dependent senescence-associated secretory phenotype. Arterioscler Thromb Vasc Biol. 2015;35:1963–1974. doi: 10.1161/ATVBAHA.115.305896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freigang S., Ampenberger F., Weiss A. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 53.Sheedy F.J., Moore K.J. IL-1 signaling in atherosclerosis: sibling rivalry. Nat Immunol. 2013;14:1030–1032. doi: 10.1038/ni.2711. [DOI] [PubMed] [Google Scholar]
- 54.Gross O., Yazdi A.S., Thomas C.J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 56.Paigen B., Holmes P.A., Mitchell D., Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987;64:215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 57.Smith J.D., Trogan E., Ginsberg M., Grigaux C., Tian J., Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tangirala R.K., Rubin E.M., Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 59.Marsh M.M., Walker V.R., Curtiss L.K., Banka C.L. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999;40:893–900. [PubMed] [Google Scholar]
- 60.Giefing-Kroll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beagley K.W., Gockel C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 62.Bhatia A., Sekhon H.K., Kaur G. Sex hormones and immune dimorphism. Scientific World Journal. 2014:159150. doi: 10.1155/2014/159150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klein S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 64.Rupp M.R.G., Jorgensen T.N. Androgen-induced immunosuppression. Front Immunol. 2018;9:794. doi: 10.3389/fimmu.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trigunaite A., Dimo J., Jorgensen T.N. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Becerra-Diaz M., Strickland A.B., Keselman A., Heller N.M. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol. 2018;201:2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S.G., Wu X.X., Hua T. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. Onco Targets Ther. 2019;12:6927–6936. doi: 10.2147/OTT.S218240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan W., Gao X., Ding C. Estrogen receptors participate in carcinogenesis signaling pathways by directly regulating NOD-like receptors. Biochem Biophys Res Commun. 2019;511:468–475. doi: 10.1016/j.bbrc.2019.02.085. [DOI] [PubMed] [Google Scholar]
- 69.Wu X.Y., Cakmak S., Wortmann M. Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Molecular Medicine. 2016;22:508–518. doi: 10.2119/molmed.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaiswal S., Natarajan P., Ebert B.L. Clonal hematopoiesis and atherosclerosis. N Engl J Med. 2017;377:1401–1402. doi: 10.1056/NEJMc1710381. [DOI] [PubMed] [Google Scholar]
- 71.Sano S., Oshima K., Wang Y., Katanasaka Y., Sano M., Walsh K. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123:335–341. doi: 10.1161/CIRCRESAHA.118.313225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sano S., Oshima K., Wang Y. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuster J.J., MacLauchlan S., Zuriaga M.A. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villablanca A.C., Jayachandran M., Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci. 2010;119:493–513. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- 76.Shlipak M.G., Angeja B.G., Go A.S. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. doi: 10.1161/hc4401.98414. [DOI] [PubMed] [Google Scholar]
- 77.Miller V.M., Kararigas G., Seeland U. Integrating topics of sex and gender into medical curricula-lessons from the international community. Biol Sex Differ. 2016;7(Suppl):44. doi: 10.1186/s13293-016-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seeland U., Regitz-Zagrosek V. Sex and gender differences in cardiovascular drug therapy. Handb Exp Pharmacol. 2012;2014:211–236. doi: 10.1007/978-3-642-30726-3_11. [DOI] [PubMed] [Google Scholar]
- 79.Potapov E., Schweiger M., Lehmkuhl E. Gender differences during mechanical circulatory support. ASAIO J. 2012;58:320–325. doi: 10.1097/MAT.0b013e318251cdf9. [DOI] [PubMed] [Google Scholar]
- 80.Magnussen C., Bernhardt A.M., Ojeda F.M. Gender differences and outcomes in left ventricular assist device support: the European Registry for Patients with Mechanical Circulatory Support. J Heart Lung Transplant. 2018;37:61–70. doi: 10.1016/j.healun.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 81.Clayton J.A., Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA. 2016;316:1863–1864. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.