Highlights
-
•
Treatment with SGLT2 inhibitors reduces the incidence of cardiovascular death and heart failure hospitalization in patients with and without diabetes.
-
•
This review discusses the potential mechanisms by which SGLT2 inhibitors exert their beneficial effects, including beneficial effects on cardiac energy metabolism, reducing inflammation, improving kidney function, and increasing erythropoiesis.
-
•
Future studies are required to clarify how SGLT2 inhibitors exert their impressive cardiovascular effects, which will allow for a more specific targeting of heart failure therapy.
Key Words: erythropoetin, inflammation, ketones, renal function, sympathetic nervous system
Abbreviations and Acronyms: EPO, erythropoietin; LV, left ventricular; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3; ROS, reactive oxygen species; SGLT, sodium glucose co-transporter; SNS, sympathetic nervous system; T2DM, type 2 diabetes mellitus
Summary
Recent clinical trials have shown that sodium glucose co-transport 2 (SGLT2) inhibitors have dramatic beneficial cardiovascular outcomes. These include a reduced incidence of cardiovascular death and heart failure hospitalization in people with and without diabetes, and those with and without prevalent heart failure. The actual mechanism(s) responsible for these beneficial effects are not completely clear. Several potential theses have been proposed to explain the cardioprotective effects of SGLT2 inhibition, which include diuresis/natriuresis, blood pressure reduction, erythropoiesis, improved cardiac energy metabolism, inflammation reduction, inhibition of the sympathetic nervous system, prevention of adverse cardiac remodeling, prevention of ischemia/reperfusion injury, inhibition of the Na+/H+-exchanger, inhibition of SGLT1, reduction in hyperuricemia, increasing autophagy and lysosomal degradation, decreasing epicardial fat mass, increasing erythropoietin levels, increasing circulating pro-vascular progenitor cells, decreasing oxidative stress, and improving vascular function. The strengths and weaknesses of these proposed mechanisms are reviewed in an effort to try to synthesize and prioritize the mechanisms as they relate to clinical event reduction.
Central Illustration
One of the most serious health concerns in the world is heart failure, with over 50 million people worldwide being afflicted with this disease (1). Despite the advances made in the treatment of heart failure, patients diagnosed with heart failure still have a very poor prognosis and quality of life. It also remains the most common reason for hospitalization in older individuals (1). Patients with type 2 diabetes mellitus (T2DM) are particularly susceptible to developing heart failure, which is the major cause of morbidity and mortality in these individuals (2, 3, 4). It is therefore critical to develop new therapies and approaches to prevent and treat heart failure.
A number of sodium glucose co-transporter 2 (SGLT2) inhibitors have been developed to treat hyperglycemia in T2DM, which act by inhibiting glucose reabsorption in the proximal tubule of the kidney (5). A number of large clinical trials have been conducted to evaluate the safety and efficacy of SGLT2 inhibitors in patients with diabetes (with established vascular disease, multiple cardiovascular risk factors, or renal insufficiency) and in those with established heart failure and reduced ejection fraction (with and without type 2 diabetes) (6, 7, 8, 9, 10, 11, 12). The remarkable results from the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcomes Event Trial in Type 2 Diabetes Mellitus Patients—Removing Excess Glucose) demonstrated that T2DM patients who were at high risk of cardiovascular disease had an early reduction in major cardiovascular and renal outcomes (8). This included a marked reduction in cardiovascular death and hospitalization for heart failure in patients treated with empagliflozin. Subsequent large trials with other SGLT2 inhibitors, such as canagliflozin (CANVAS [Canagliflozin Cardiovascular Assessment Study] [9] and CREDENCE [Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy] trials [10]) and dapagliflozin (DECLARE-TIMI 58 [Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes] trial (11), confirmed these observations in a broader population of primary and secondary prevention patients (see Zelniker et al. [12] and Verma et al. [13] for discussions of trials).
Whereas the aforementioned trials provided robust evidence to suggest that SGLT2 inhibitors can prevent incident heart failure, 2 important questions remained unanswered. First, could these therapies also be used in the treatment of prevalent heart failure, and second, is the benefit seen in people without type 2 diabetes? Importantly, in this regard, the recently completed DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, which enrolled 4,744 patients with heart failure and reduced ejection fraction demonstrated a marked reduction on worsening heart failure or cardiovascular death on top of excellent heart failure standard-of-care therapy (6). Furthermore, this benefit was similar in those with and without type 2 diabetes and was consistent across the spectrum of A1c evaluated either categorically or continuously.
Potential Mechanisms By Which SGLT2 Inhibition is Cardioprotective
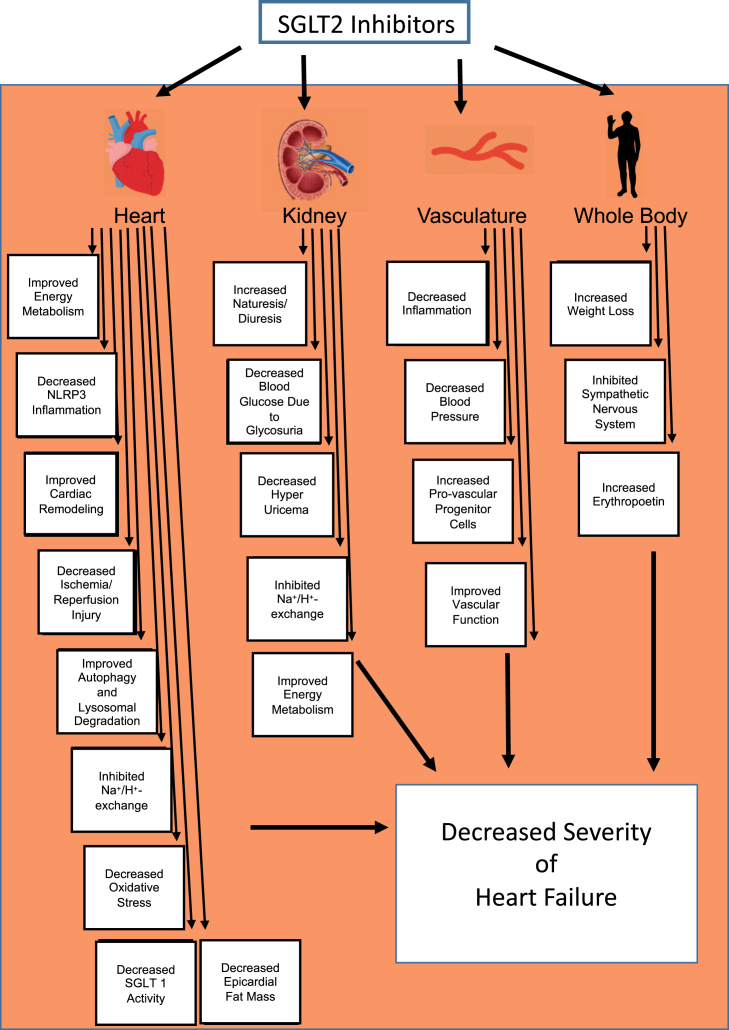
A substantial number of theories have been proposed to explain the beneficial effects of SGLT2 inhibitors (14, 15, 16, 17, 18). These include beneficial effects of SGLT2 inhibition on the following: 1) blood pressure lowering; 2) increasing diuresis/natriuresis; 3) improving cardiac energy metabolism; 4) preventing inflammation; 5) weight loss; 6) improving glucose control; 7) inhibiting the sympathetic nervous system; 8) preventing adverse cardiac remodeling; 9) preventing ischemia/reperfusion injury; 10) inhibiting the cardiac Na+/H+ exchanger; 11) inhibiting SGLT1; 12) reducing hyperuricemia; 13) increasing autophagy and lysosomal degradation; 14) decreasing epicardial fat mass; 15) increasing erythropoietin (EPO) levels; 16) increasing circulating provascular progenitor cells; 17) decreasing oxidative stress; and 18) improving vascular function (Figure 1). In the sections that follow, we provide a summary of these proposed mechanisms and a synthesis of what mechanism(s) are likely most important in terms of the observed clinical results observed.
Figure 1.
Possible Mechanisms by Which SGLT2 Inhibitors Decrease the Severity of Heart Failure
Improved cardiac energetics with SGLT2 inhibition. NLRP3 = nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3; SGLT2 = sodium glucose co-transporter 2.
Blood pressure lowering
Hypertension is a prevalent modifiable risk factor for the development of heart failure. Because SGLT2 inhibitors lower blood pressure (19), some of the beneficial effects of SGLT2 inhibitors in the setting of heart failure have been suggested to be related to this blood pressure improved cardiac energetics with SGLT2 inhibition lowering effect. Although the exact mechanism(s) for the antihypertensive effects of SGLT2 inhibition are not fully understood, they are probably mediated by the osmotic and diuresis effects of SGLT2 inhibitors as a result of an inhibition of sodium reabsorption in the proximal tubules of the kidney. SGLT2 inhibition can result in a 30% to 60% increase in urinary sodium excretion (20). The antihypertensive effect of SGLT2 inhibition is greater than that of the thiazide diuretics when used in combination with ß-blockers or calcium antagonists (21,22). By lowering blood pressure, SGLT2 inhibitors may lower cardiac afterload, with resultant improvement in ventricular arterial coupling and cardiac efficiency. This would be expected to benefit the failing heart. However, the blood pressure–lowering effects of SGLT2 inhibition are modest and are unlikely to completely explain the beneficial cardiovascular and kidney effects of these drugs. In addition, blood pressure lowering would be anticipated to have a greater effect on stroke rates compared with other cardiovascular outcomes, which was not observed in the EMPA-REG OUTCOME trial (8). Finally, in the DAPA-HF trial, the reductions in blood pressure were quite modest and unlikely to be related to the large reduction in failure events (6).
Diuresis and natriuresis
SGLT2 inhibitors have been shown to promote natriuresis and glucosuria, and it has been suggested that the resultant osmotic diuresis may improve heart failure outcomes. In fact, mediation analyses from the EMPA-REG OUTCOME trial suggested that hemoconcentration (presumed to be secondary to volume contraction) accounted for about 50% of the cardiovascular benefit observed (8). It is difficult to explain the benefits of SGLT2 inhibitors purely based on diuresis, because other diuretic strategies per se have not been associated with an improved event reduction in heart failure studies. It has been suggested that SGLT2 inhibitors may differ somewhat from classical diuretics. In a study comparing dapagliflozin and hydrochlorothiazide, for example, a reduction in plasma volume and increase in erythrocyte mass was observed with dapagliflozin but not with hydrochlorothiazide (23). When compared with a loop diuretic (bumetanide), dapagliflozin was associated with more reduction in interstitial versus intravascular volume (24). It has therefore been speculated that SGLT2 inhibition may afford a differential effect in regulating interstitial fluid (vs. intravascular volume), which may limit the reflex neurohumoral stimulation that occurs in response to intravascular volume contraction with traditional diuretics.
Improved cardiac energy metabolism
Dramatic changes in energy metabolism occur in the failing heart. As heart failure progresses, a continual decline in mitochondrial oxidative metabolism occurs, and the heart becomes more reliant on glycolysis as a source of energy (25). Mitochondrial glucose oxidation decreases in the failing heart (26, 27, 28), leading to a decrease in energy production and a fuel-starved heart (29). The uncoupling between glycolysis and glucose oxidation in the failing heart also leads to increased proton production that leads to a decrease in cardiac efficiency (cardiac work / O2 consumed) (25, 26, 27, 28). This decrease in cardiac efficiency is not confined to patients with heart failure and reduced ejection fraction, but also occurs in patients with heart failure and preserved ejection fraction with left ventricular (LV) hypertrophy who also have a reduced LV mechanical efficiency (30).
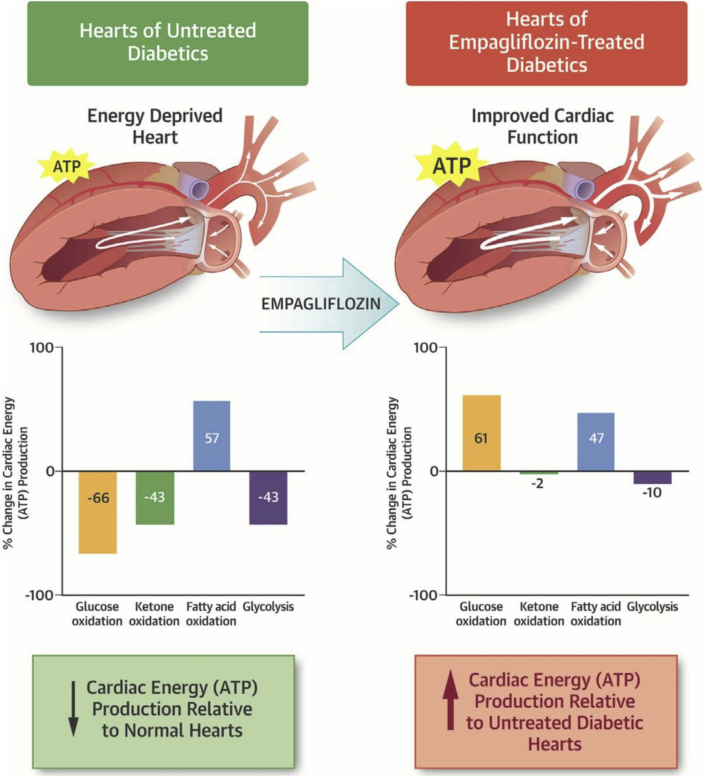
It has been proposed that the beneficial effects of SGLT2 inhibitors in heart failure can occur by improving cardiac energetics and improving cardiac efficiency. SGLT2 inhibitors increase circulating ketone levels, secondary to mobilizing adipose tissue fatty acids, which are then used by the liver for ketogenesis (31, 32, 33). Circulating ketone levels can increase following SGLT2 inhibitor treatment even in the absence of diabetes (33). These ketones have been proposed to improve cardiac energetics and cardiac efficiency by being a “thrifty” fuel for the heart (34,35). However, we have shown that ketones are not a more efficient source of fuel for the heart, but they are an additional source of fuel for the failing heart (36,37). The failing heart is “energy starved,” due primarily to a decrease in mitochondrial oxidative metabolism (25,29). Ketone oxidation is increased in the failing heart, which has been proposed to be an adaptive metabolic process (37, 38, 39, 40). Increasing plasma ketone levels in the blood due to SGLT2 inhibition does increase cardiac ketone oxidation rates and therefore improves energy supply to the “starving” failing heart (41). In diabetic cardiomyopathic mice, empagliflozin-induced increases in cardiac ketone oxidation provide an additional source of fuel for the heart, which is associated with an improvement in cardiac performance (Figure 2) (41). In support of this, Santos-Gallego et al. (42) have shown that empagliflozin can decrease adverse remodeling and heart failure in a porcine model of heart failure, by improving cardiac energetics. It has also been shown that SGLT2 inhibitors can improve mitochondrial respiratory function in diabetic rats (43), which also may contribute to improving energy production in the heart. These changes in mitochondrial respiration have been proposed to be partially mediated due to favorable alterations in energy supply to the heart. Ketone infusions into patients with heart failure is also associated with an improvement in contractile performance (44). Of interest, this ketone-induced improvement in contractile performance is also not associated with an increase in cardiac efficiency. This makes sense, as the oxidation of ketones, compared with the oxidation of glucose, are not a more efficient source of energy. As a result, some of the beneficial effects of SGLT2 inhibition in heart failure may occur secondary to increasing fuel supply to the failing heart, as opposed to supplying the heart with a more efficient source of fuel. Additionally, the increase in cardiac ketone oxidation in the failing heart is not associated with a decrease in either glucose or fatty acid oxidation, resulting in an overall increase in adenosine triphosphate production (37,41). Therefore, whereas SGLT2 inhibitors may not increase cardiac efficiency in the failing heart, they may supply the heart with an extra source of fuel, which may be particularly beneficial for the energy-compromised failing heart.
Figure 2.
SGLT2 Inhibition Increases Cardiac Energy Production
SGLT2 inhibitors can increase cardiac energy metabolism. Reproduced with permission from Verma et al. (41). ATP = adenosine triphosphate; SGLT2 = sodium glucose co-transporter 2.
Reduction in inflammation
Inflammation is an important contributor to heart failure severity, and proinflammatory biomarkers are elevated in patients with heart failure and correlate with the severity of the disease (45,46). This association between heart failure and markers of inflammation is evident in patients both with reduced and with preserved ejection fraction (47). Inflammatory cytokines not only cause endothelial dysfunction, they also can increase extracellular matrix turnover and increase fibrosis. The SGLT2 inhibitors, empagliflozin (48), canagliflozin (49), and dapagliflozin (50) have been shown to attenuate or ameliorate the inflammatory profile in patients with diabetes. This decrease in anti-inflammatory properties of SGLT2 inhibitors has the potential to decrease molecular processes related to inflammation, such as extracellular matrix turnover and fibrosis (51). In support of this, dapagliflozin has been shown to have marked antifibrotic effects in the post-infarct rat heart by suppressing collagen synthesis (51). Empagliflozin also significantly attenuates cell-mediated extracellular matrix collagen remodeling (52).
How SGLT2 inhibitors modify the inflammatory process is not exactly clear. Decreasing glucose levels with SGLT2 inhibitors may decrease macrophage inflammatory response, as macrophages preferentially utilize glucose from glycolysis as an energy source (53). Alternatively, SGLT2 inhibitors may directly target the inflammatory pathways independent of glucose lowering per se. The nucleotide-binding domain-like receptor protein (specifically, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3 [NLRP3]) inflammasome plays an important role in mediating inflammation. The NLRP3 inflammasome also contributes to chronic inflammation in heart failure, thereby increasing heart failure severity (54). Recent evidence in the kidney (55), liver (56), macrophages (57), vasculature (58), and heart (59,60) suggests that empagliflozin can inhibit the NLRP3 inflammasome and that this can occur independent of glucose lowering per se. Whether this is a direct or indirect effect of SGLT2 inhibitors on NLRP3 is not clear. The ketone ß-hydroxybutyrate is an effective blocker of the NLRP3 inflammasome-mediated inflammatory process (61). Because SGLT2 inhibitors increase circulating ß-hydroxybutyrate levels, it is possible that some of the beneficial effects of SLT2 inhibition could occur secondary to ketone inhibition of the NLRP3 inflammasome.
Weight loss
The excretion of glucose by the kidney with SGLT2 inhibitor treatment results in a loss of calories. As a result of this, there is a subsequent decrease in body weight as fatty acids are mobilized from adipose tissue stores. Clinical studies have consistently shown body weight reduction in patients treated with SGLT2 inhibitors (62). Whereas this weight loss may contribute to the beneficial effects of SGLT2 inhibition, other mechanisms must also be involved, as weight loss strategies have been much less effective in decreasing heart failure severity compared with SGLT2 inhibition, therefore this is unlikely to be an important mechanism of the heart failure benefit observed. For example, in the DAPA-HF trial, whereas there was a modest numeric reduction in weight observed, the magnitude of which appeared to be greater in those with diabetes (63). Furthermore, the effects of SGLT2 inhibition on weight loss are moderate and diminish with time, due in part to counter-regulatory mechanisms (such as increased energy intake) being activated to attempt to maintain weight (64).
Improving glucose control
Whereas SGLT2 inhibitors are effective glucose-lowering agents, the efficacy on heart failure is unlikely related to improvements in glucose lowering per se. Hyperglycemia itself has been shown to be a weak risk factor for cardiovascular disease (65). In addition, the rapid efficacy noted (within days of treatment initiation) is difficult to reconcile with a glucose-lowering effect. Furthermore, the differences in glycemic control in the cardiovascular outcome trials were small (in an effort to fulfill the glycemic equipoise principle of the trials), and post hoc analyses from trials suggested that baseline A1c or changes in A1c were not associated with any treatment modification with SGLT2 inhibitors (65). Definitive proof of this concept emerged from the DAPA-HF trial wherein the efficacy of dapagliflozin was entirely consistent in those with and without diabetes. Even in those without diabetes, efficacy was similar in those with pre-diabetes or impaired glucose tolerance compared with in those who were truly euglycemic. When studied continuously, using fractional polynomial analyses, baseline A1c was unrelated to the efficacy of dapagliflozin to reduce heart failure and mortality in DAPA-HF. In addition, in experimental models of heart failure, the benefit of SGLT2 inhibition has been observed independent of diabetes or hyperglycemia (60,66,67).
Inhibiting the SNS
The observation that SGLT2 inhibitors reduce blood pressure in the absence of increasing heart rate suggests, indirectly, that these agents may be associated with a reduction in sympathetic nervous system (SNS) activity. In fact, accumulating data indicate that SGLT2 inhibition may lead to a reduction in sympathetic nerve activity, inhibit norepinephrine turnover in brown adipose tissue, and reduce tyrosine hydroxylase production (68, 69, 70, 71, 72). These sympathoinhibitory effects appear to be observed in both animal models of diabetes as well as those with obesity (without diabetes) (68, 69, 70, 71, 72). It has also been postulated that the effects of SGLT2 inhibition to reduce SNS activity may be secondary to a reduction in renal stress with resultant inhibition of renal afferent sympathetic activation (73).
Preventing adverse cardiac remodeling
Adverse cardiac remodeling is an important contributor to heart failure severity. This includes the development of cardiac hypertrophy, fibrosis, inflammation, and cardiomyocyte cell death. Several experimental and human studies have demonstrated beneficial effects of SGLT2 inhibition on cardiac remodeling (23,67,74, 75, 76, 77). In a randomized trial, people with type 2 diabetes and a history of coronary artery disease were treated with empagliflozin versus placebo for 6 months (76). The primary outcome—change in LV mass index (evaluated by cardiac magnetic resonance imaging) was significantly lower in those treated with empagliflozin versus in those who received placebo. Although these data do not provide insight about the exact mechanism of action, they do suggest that even short-term exposure to SGLT2 inhibitors can promote cardiac reverse remodeling (Figure 3). Inhibition of the mammalian target of rapamycin pathway, a major pathway involved in cardiac hypertrophy, by SGLT2 inhibition may also be involved (76). Prevention of adverse remodeling with SGLT2 inhibition is also associated with a decreased fibrosis (52,78), which may in part be mediated by the anti-inflammatory actions of SGLT2 inhibition (see reduction in inflammation discussion). As a result, SGLT2 inhibition may reverse the cardiac remodeling seen in heart failure, thereby reducing LV wall stress and improving cardiac function.
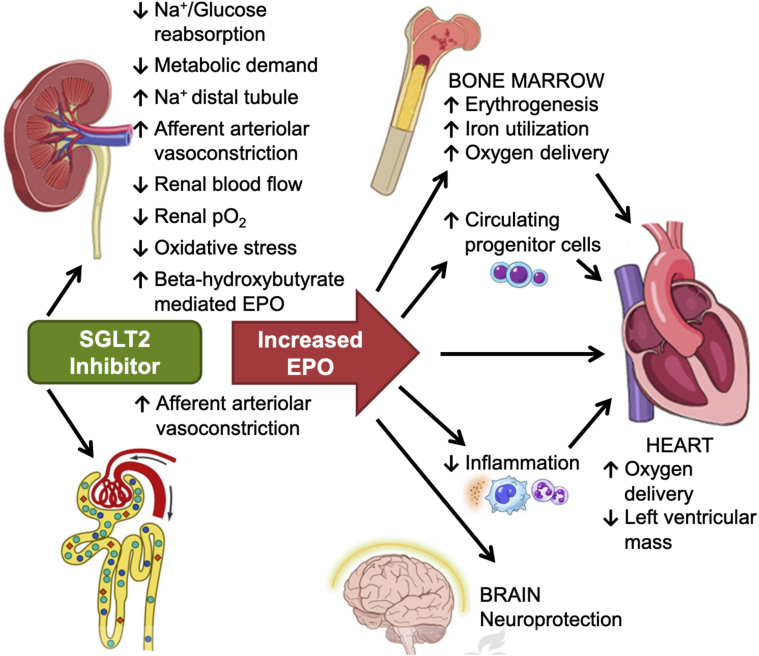
Figure 3.
Multiple Sites for the Beneficial Effects of SGLT2 Inhibition
Proposed renal mechanisms for increased erythropoietin (EPO) with sodium glucose co-transporter 2 (SGLT2) inhibitors.
Reproduced with permission from Mazer et al. (95).
Preventing ischemia/reperfusion injury
Ischemia/reperfusion injury can promote cardiomyocyte cell death and heart failure. Recent experimental evidence suggests that SGLT2 inhibition has a cardioprotective effect against ischemia/reperfusion injury in both diabetic and nondiabetic rats (79). This beneficial effect of SGLT2 inhibition on ischemia/reperfusion injury is associated with a decrease in calmodulin kinase II activity, resulting in improved sarcoplasmic reticulum Ca2+ flux and increased contractility. However, whether this effect occurs in humans remains unclear.
Inhibiting the cardiac Na+/H+ exchanger
The Na+/H+ exchanger is increased in the failing heart and can lead to Na+ and Ca2+ overload in the heart (reviewed by Wakabayashi et al. [80]). Inhibition of the Na+/H+ exchanger has also been demonstrated to protect the heart in several experimental models of heart failure (see Wakabayashi et al. [80] for review). Inhibition of the Na+/H+ exchanger has been proposed to explain the beneficial effects of SGLT2 inhibitors in heart failure (81, 82, 83, 84, 85). The cardiac Na+/H+ exchanger is inhibited by SGLT2 inhibitors, which can lower myocardial Na+ levels (81). However, it is not clear whether direct inhibition of the cardiac Na+/H+ exchanger occurs at clinically relevant concentrations of SGLT2 inhibitors. In addition, the development of Na+/H+ exchanger inhibitors has not been shown to be beneficial in the clinical setting of heart failure (85). Therefore, it is not clear whether some of the beneficial effects of SGLT2 inhibition in the setting of heart failure may occur secondary to direct inhibition of the cardiac Na+/H+ exchanger.
Inhibiting SGLT1
Although the heart does not express SGLT2, it does express some SGLT1. Inhibition of SGLT1 in the heart has the potential to decrease myocardial Na+ and glucose uptake and decrease hyperglycemia-induced generation of reactive oxygen species (ROS) (86). However, whereas some of the SGLT2 inhibitors will decrease SGLT1, such as canagliflozin, the concentration necessary to inhibit SGLT1 is much higher than the plasma concentrations seen with the clinical use of SGLT2 inhibitors. In addition, the use of dual inhibitors of SGLT2 and SGLT1 can exacerbate cardiac dysfunction post-myocardial infarction in rats (87). As a result, it is unlikely that the beneficial effects of SGLT2 inhibitors can be explained by any secondary effect on SGLT1 inhibition.
Reducing hyperuricemia
SGLT2 inhibitors decrease plasma uric acid, which adversely affects the prognosis of heart failure (88). Small reductions in uric acid levels have been seen with SGLT2 inhibitor treatment (89). This may be attributed to the increased glycosuria in the proximal tubules due to SGLT2 inhibition, which stimulates uric acid secretion (90). However, whether a reduction in hyperuricemia by SGLT2 inhibition is a marker or plays a causal role remains unknown.
Increasing autophagy and lysosomal degradation
Cardiac autophagy and lysosomal degradation can be impaired in diabetes and heart failure (77,91). By driving catabolic rates due to constant glycosuria, it has been proposed that SGLT2 inhibition can promote autophagy and lysosomal degradation, thereby improving mitochondrial morphology and function (77). An SGLT2-mediated inhibition of mammalian target of rapamycin may also stimulate autophagy and lysosomal degradation, leading to the enhanced degradation of dysfunctional organelles. It therefore cannot be ruled out that some of the benefit of SGLT2 inhibition in heart failure may be secondary to their effects on stimulating autophagy.
Decreasing epicardial fat mass
High epicardial adipose tissue attenuation on computed tomography is associated with an increased risk of cardiovascular events (92). Epicardial adipose tissue can produce a number of bioactive molecules that can negatively affect heart function and contribute to coronary artery disease. In addition, SGLT2 inhibitors reduce the accumulation and inflammation of peri-vascular adipose tissue, thus minimizing the secretion of leptin and its paracrine actions on the heart to promote fibrosis (84). In patients with diabetes who also have coronary artery disease, SGLT2 inhibition reduces epicardial adipose tissue mass, as well as the levels of bioactive molecules such as tumor necrosis factor-α and plasminogen activator inhibitor-1 (93). This may contribute to a decreased adverse remodeling of the failing heart.
Increasing EPO levels
The fact that SGLT2 inhibitors raise the hematocrit (94), even in those without diabetes (as seen in DAPA-HF), has led to the suggestion that these agents may promote erythropoiesis via enhanced EPO secretion by the kidney (95). Such an increase in EPO may serve to favorably influence cardiomyocyte mitochondrial function, angiogenesis, cell proliferation, and inflammation, in addition to directly enhancing myocardial tissue oxygen delivery (95). Mazer et al. (95) recently evaluated this in the EMPA-Heart CardioLink-6 randomized clinical trial and demonstrated that EPO levels increased significantly after 1 month of empagliflozin treatment in people with type 2 diabetes and coronary artery disease accompanied by an increase in hematocrit, reduced ferritin and red blood cell hemoglobin concentration (Figure 3). Whether EPO levels go up in people without type 2 diabetes remains unknown.
Increasing Circulating Provascular Progenitor Cells
Preliminary evidence in humans points toward an effect of SGLT2 inhibitors on the restoration of provascular progenitor cells in people with type 2 diabetes. In one such completed study, Hess et al. (96) observed that empagliflozin treatment was associated with a reduction in the number proinflammatory M1 cells while increasing the number of M2 polarized, anti-inflammatory cells. By using the Aldeflour assay (STEMCELL Technologies, Cambridge, Massachusetts), the investigators found that SGLT2 inhibition reduced systemic granulocyte burden in individuals with T2DM, increased circulating ALDHhiSSCmid monocytes and induced a transition from M1 to M2 polarization—all of which is consistent with maturation of collateral vessels during arteriogenesis. The investigators concluded that SGLT2 inhibition may uniquely serve as a strategy to promote the recovery of circulating provascular cells in T2DM. Whether this occurs in people without T2DM is unknown.
Decreasing oxidative stress
Excessive cardiac mitochondrial ROS production is an important contributor to contractile dysfunction in human heart failure and in animal models of heart failure (for review see Zhou and Tian [97]). During the development of heart failure, increased oxidative stress can result in mitochondrial dysfunction. This increase in ROS production may occur due to a stimulation of mitochondrial respiration for adenosine triphosphate production and an increased electron transport chain activity. In diabetic mice, increasing glycemic control with SGLT2 inhibition can decrease myocardial ROS production and cardiac fibrosis (97). In human coronary arterial endothelial cells, SGLT2 inhibition can also decrease ROS generation (98). How SGLT2 inhibition decreases ROS production is not clear, although this may occur secondary to favorable effects on the inflammatory process (see reduction in inflammation discussion), cardiac mitochondrial oxidative metabolism (see improved cardiac energy metabolism discussion), or decreasing the potential for cardiac glucotoxicity (which can promote ROS production). Furthermore, there is a paucity of data on SGLT2 inhibition on ROS production in the absence of type 2 diabetes.
Improving vascular function
Vascular smooth muscle and endothelial dysfunction contributes to the pathophysiology of heart failure (99,100). Its existence in patients with heart failure increases morbidity and mortality. SGLT2 inhibition has been shown to improve vascular function by attenuating endothelial cell activation, inducing direct vasorelaxation, reducing endothelial cell dysfunction and molecular changes associated with early atherogenesis decreasing arterial wall stiffness, and decreasing vascular resistance (101, 102, 103, 104). A number of underlying mechanisms for the beneficial actions of SGLT2 inhibition may occur, including beneficial effects of SGLT2 inhibition on inhibiting the inflammatory pathways and improving mitochondrial function (101). Induction of vasodilation via activation of protein kinase G and voltage-gated potassium channels by SGLT2 inhibitors has also been proposed (105). The direct effects of SGLT2 inhibition on the vascular, combined with the natriuresis effects of SGLT2 inhibition, may contribute to the desirable hemodynamic effects seen with SGLT2 inhibition.
From a List To Synthesis: What Mechanisms Most Likely Explain The Benefits Observed?
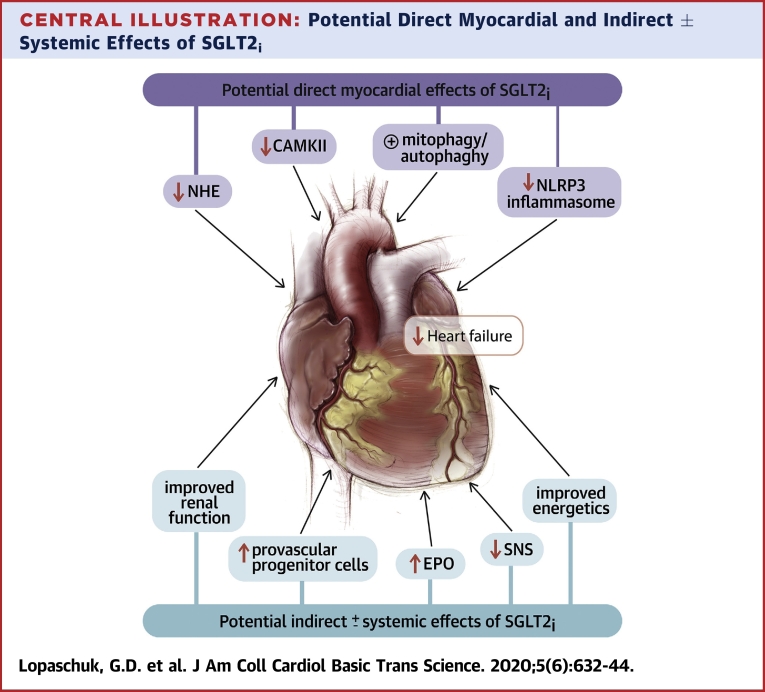
A number of clinical trials have demonstrated that SGLT2 inhibitors have impressive beneficial cardiovascular effects in both patients with diabetes and patients who do not have diabetes, but do have heart failure. As a result, SGLT2 inhibitors are a new weapon that can be added to the arsenal of weapons used to treat heart failure. However, it remains unclear exactly how SGLT2 inhibitors produce their impressive clinical benefits in patients with heart failure (Central Illustration). It is clear that its classical actions of lowering blood glucose cannot fully explain these benefits. The early onset of the beneficial effects of SGLT2 inhibitors in clinical trials suggests that these benefits are not occurring as a result of slowing the atherosclerotic process. Whereas the published reports are replete with numerous proposed mechanisms linking SGLT2 inhibition to cardiovascular protection, how do we synthesize and prioritize these to help explain the observed clinical benefits? Taking a bedside-to-bench approach, the clinical data would suggest that the mechanism(s) involved must account for the following key elements: 1) efficacy in the treatment and prevention of heart failure; 2) efficacy on top of excellent background therapy, including neprilysin inhibition; 3) rapid onset of the benefit; 4) efficacy independent of glycemic status; and 5) association with renal protection. We opine that the renal effects of SGLT2 inhibitors are an important mechanism of action and that some of the cardiovascular benefits are secondary to this. According to this theory, SGLT2 inhibition result in an early hemodynamic effect at the level of the proximal renal tubule. This in turn promotes sodium and water loss while also, through tubule-glomerular feedback, promoting afferent arteriolar constriction. The ensuring reduction in intraglomerular pressure leads to renal protection. Improving renal function and/or reducing renal stress can indirectly improve cardiac function through various pathways, including a reduction in afferent SNS activation, reduction in inflammation, and ROS generation. We propose that future studies are required to examine these possibilities. We also argue that the renal hemodynamic effects are observed independent of glycemia, because in the DAPA-HF trial an initial drop in estimated glomerular filtration rate was observed in people both with and without diabetes. The increase in EPO production may also be secondary to an improvement in renal health and may explain why the hematocrit is increased to a similar extent in people both with and without diabetes in DAPA-HF. Additional intriguing mechanisms for the benefits of SGLT2 inhibition in heart failure include improving cardiac energy metabolism and decreasing cardiac inflammation. Further studies are needed to examine SGLT2 inhibition impacts on these pathways in the setting of heart failure, as well as the potential inter-relationship between these 2 pathways (because ketones can inhibit the inflammatory pathway). Further studies should help elucidate exactly how SGLT2 inhibitors exert these impressive cardiovascular effects.
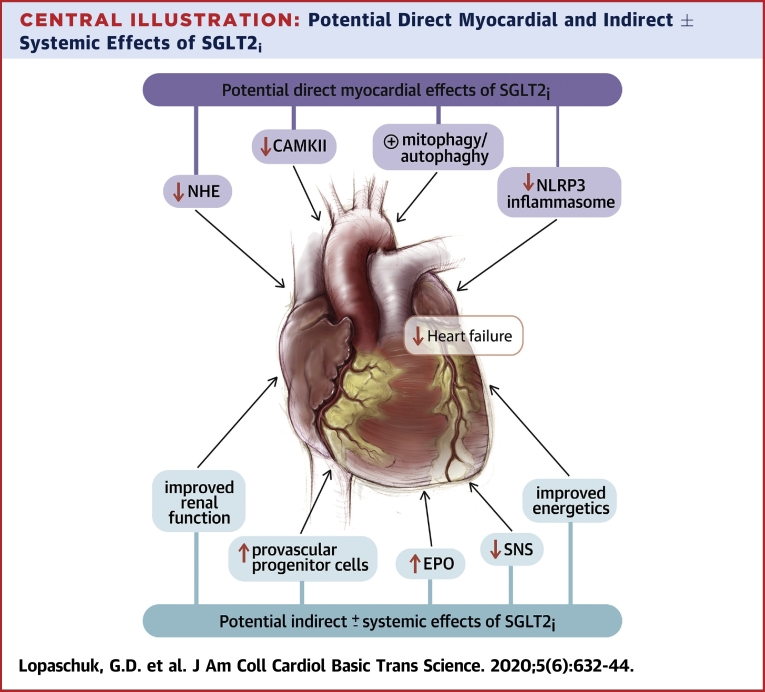
Central Illustration.
Potential Direct Myocardial and Indirect ± Systemic Effects of SGLT2i
CAMKII = calmodulin-dependent protein kinase II; EPO = erythropoietin; NHE = sodium/hydrogen exchanger; NLRP3 = nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3; SGLT2i = sodium glucose co-transporter 1(2) inhibitor; SNS = sympathetic nervous system.
Footnotes
Dr. Lopaschuk has received speaking honoraria from AstraZeneca; has served as a consultant for Boehringer Ingelheim and Servier; and is a shareholder in Metabolic Modulators Research Ltd. Dr. Verma has received research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd., Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group; is a Tier 1 Canada Research Chair in cardiovascular surgery; and is the president of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 2.Khan S.S., Butler J., Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311:2379–2380. doi: 10.1001/jama.2014.4115. [DOI] [PubMed] [Google Scholar]
- 3.Swoboda P.P., McDiarmid A.K., Erhayiem B. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Cardiovascular disease and risk management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S103–S123. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 5.Gallo L.A., Wright E.M., Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray J.J.V., Solomon S.D., Inzucchi S.E., for the DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381 doi: 10.1056/NEJMoa1911303. 1995–200. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt D.L., Verma S., Braunwald E. The DAP-HF trial: a momentous victory in the war against heart failure. Cell Metab. 2019;30:847–849. doi: 10.1016/j.cmet.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B., Wanner C., Lachin J.M., for the EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Neal B., Perkovic V., Mahaffey K.W., for the CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V., Jardine M.J., Neal B., for CREDENCE Trial Investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott S.D., Raz I., Bonaca M.P., for the DECLARE-TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380 doi: 10.1056/NEJMc1902837. 380:347–357. [DOI] [PubMed] [Google Scholar]
- 12.Zelniker T.A., Wiviott S.D., Raz I. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 13.Verma S., Juni P., Mazer C.D. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet. 2019;393:3–5. doi: 10.1016/S0140-6736(18)32824-1. [DOI] [PubMed] [Google Scholar]
- 14.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 15.Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Cardiol. 2017;120:S28–S36. doi: 10.1016/j.amjcard.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Butler J., Handelsman Y., Bakris G., Verma S. Use of sodium-glucose co-transport inhibitors in patients with and without type 2 diabetes: implication for incident and prevalent heart failure. Eur J Heart Fail. 2020 Jan 11 doi: 10.1002/ejhf.1708. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Filippatos T.D., Liontos A., Papakitsou I., Elisaf M.S. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med. 2019;131:82–88. doi: 10.1080/00325481.2019.1581971. [DOI] [PubMed] [Google Scholar]
- 18.Lam C.S.P., Chandramouli C., Ahooja V., Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazidi M., Rezaie P., Gao H.K., Kengne A.P. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrannini E., Baldi S., Frascerra S. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40:771–776. doi: 10.2337/dc16-2724. [DOI] [PubMed] [Google Scholar]
- 21.Weber M.A., Mansfield T.A., Cain V.A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 22.Kario K., Bohm M., Mahfoud F. Twenty-four-hour ambulatory blood pressure reduction patterns after renal denervation in the SPYRAL HTN-OFF MED Trial. Circulation. 2018;138:1602–1604. doi: 10.1161/CIRCULATIONAHA.118.035588. [DOI] [PubMed] [Google Scholar]
- 23.Lambers Heerspink H.J., de Zeeuw D., Wie L., Leslie B., List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallow K.M., Helmlinger G., Greasley P.J., McMurray J.J.V., Boulton D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 25.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Jaswal J., Ussher J.R. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Zhang L., Battiprolu P.K. Malonyl CoA decarboxylase inhibition improves cardiac function post-myocardial infarction. J Am Coll Cardiol Basic Trans Science. 2019;4:385–400. doi: 10.1016/j.jacbts.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori J., Basu R., McLean B.A. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 30.AbouEzziddine O.F., Kemp B.J., Borlaug B.A. Energetics in heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrannini E., Muscelli E., Frascerra S. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Jobori H., Daniele G., Adams J. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 2017;19:809–813. doi: 10.1111/dom.12881. [DOI] [PubMed] [Google Scholar]
- 33.Ferrannini E., Baldi S., Frascerra S. Shift to fatty substrates utilization in response to sodium-glucose co-transporter-2 inhibition in nondiabetic subjects and type 2 diabetic patients. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 34.Mudaliar S., Alloju S., Henry R.R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini F., Mark M., Mayoux E. CV Protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 36.Lopaschuk G.D., Verma S. Empagliflozin’s fuel hypothesis: not so soon. Cell Metab. 2016;24:200–202. doi: 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Ho K.L., Zhang L., Wagg C. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovas Res. 2019;115:1606–1616. doi: 10.1093/cvr/cvz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedi K.C., Snyder N.W., Brandimarto J. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aubert G., Martin O.J., Horton J.L. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton J.L., Davidson M.T., Kurishima C. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4 doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma S., Rawat S., Ho K.L. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. J Am Coll Cardiol Basic Trans Science. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos-Gallego C.G., Requena-Ibanez J.A., San Antonio R. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 43.Shao Q., Meng L., Lee S. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. doi: 10.1186/s12933-019-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen R., Moller N., Gormsen L.C. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dick S.A., Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 46.Mehta J.L., Pothineni N.V. Inflammation in heart failure: the holy grail? Hypertension. 2016;68:27–29. doi: 10.1161/HYPERTENSIONAHA.116.07307. [DOI] [PubMed] [Google Scholar]
- 47.Briasoulis A., Androulakis E., Christophides T., Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21:169–176. doi: 10.1007/s10741-016-9533-z. [DOI] [PubMed] [Google Scholar]
- 48.Iannantuoni F., de Marañon A.M., Diaz-Morales N. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med. 2019;8:1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heerspink H.J.L., Perco P., Mulder S. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–1166. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leng W., Wu M., Pan H. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann Transl Med. 2019;7:429. doi: 10.21037/atm.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee T.-M., Chang N.-C., Lion S.-Z. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 52.Kang S., Verma S., Hassanabad A.F. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA-REG OUTCOME results. Can J Cardiol. 2019 Aug 29 doi: 10.1016/j.cjca.2019.08.033. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Rotkvić P.G., Berković M.C., Bulj N., Rotkvić L. Minireview: are SGLT2 inhibitors heart savers in diabetes? Heart Fail Rev. 2019 Aug 13 doi: 10.1007/s10741-019-09849-3. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Butts B., Gary R.A., Dunbar S.B., Butler J. The importance of NLRP3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benetti E., Mastrocola R., Vitarelli G. Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther. 2016;359:45–53. doi: 10.1124/jpet.116.235069. [DOI] [PubMed] [Google Scholar]
- 56.Tahara A., Kurosaki E., Yokono M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y.-H., Kim S.R., Han D.H. Senescent T cells predict the development of hyperglycemia in humans. Diabetes. 2019;68:156–162. doi: 10.2337/db17-1218. [DOI] [PubMed] [Google Scholar]
- 58.Leng W., Ouyang X., Lei X. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE−/− mice. Mediators Inflamm. 2016;2016:6305735. doi: 10.1155/2016/6305735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye Y., Jia X., Bajaj M., Birnbaum Y. Dapagliflozin attenuates Na+/H+exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Therap. 2018;32:553–558. doi: 10.1007/s10557-018-6837-3. [DOI] [PubMed] [Google Scholar]
- 60.Byrne N.J., Matsumura N., Maayah Z.H. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 61.Youm Y.H., Nguyen K.Y., Trant R.W. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai X., Yang W., Gao X. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring) 2018;26:70–80. doi: 10.1002/oby.22066. [DOI] [PubMed] [Google Scholar]
- 63.Pereira M.J., Eriksson J.W. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79:219–230. doi: 10.1007/s40265-019-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee P.C., Ganguly S., Goh S.Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19:1630–1641. doi: 10.1111/obr.12755. [DOI] [PubMed] [Google Scholar]
- 65.Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia. 2013;56:686–695. doi: 10.1007/s00125-012-2817-5. [DOI] [PubMed] [Google Scholar]
- 66.Cannon C.P., Perkovic V., Agarwal R. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE trial. Circulation. 2019;141:407–410. doi: 10.1161/CIRCULATIONAHA.119.044359. [DOI] [PubMed] [Google Scholar]
- 67.Connelly K.A., Zhang Y., Vistram A. Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Trans Science. 2019;4:27–37. doi: 10.1016/j.jacbts.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiba Y., Yamada T., Tsukita S. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0150756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshikawa T., Kishi T., Shinohara K. Arterial pressure lability is improved by sodium-glucose cotransporter 2 inhibitor in streptozotocin-induced diabetic rats. Hypertens Res. 2017;40:646–651. doi: 10.1038/hr.2017.14. [DOI] [PubMed] [Google Scholar]
- 70.Matthews V.B., Elliot R.H., Rudnicka C., Hricova J., Herat L., Schlaich M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–2068. doi: 10.1097/HJH.0000000000001434. [DOI] [PubMed] [Google Scholar]
- 71.Kimmerly D.S., Shoemaker J.K. Hypovolemia and neurovascular control during orthostatic stress. Am J Physiol. 2002;282:H645–H655. doi: 10.1152/ajpheart.00535.2001. [DOI] [PubMed] [Google Scholar]
- 72.Jordan J., Tank J., Heusser K. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:604–612. doi: 10.1016/j.jash.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Byrne N.J., Parajuli N., Levasseur J.L. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. J Am Coll Cardiol Basic Trans Science. 2017;2:347–354. doi: 10.1016/j.jacbts.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi L., Zhu D., Wang S. Dapagliflozin attenuates cardiac remodeling in mice model of cardiac pressure overload. Am J Hypertens. 2019;32:452–459. doi: 10.1093/ajh/hpz016. [DOI] [PubMed] [Google Scholar]
- 76.Verma S., Garg A., Yan A.T. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 77.Esterline R.L., Vaag A., Oscarsson J., Vora J. SGLT2 inhibitors: clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. 2018;178:R113–R125. doi: 10.1530/EJE-17-0832. [DOI] [PubMed] [Google Scholar]
- 78.Lee H.C., Shiou Y.L., Jhuo S.J. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45. doi: 10.1186/s12933-019-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim V.G., Bell R.M., Arjun S., Kolatsi-Joannou M., Long D.A., Yellon D.M. SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. J Am Coll Cardiol Basic Trans Science. 2019;4:15–26. doi: 10.1016/j.jacbts.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wakabayashi S., Hisamitsu T., Nakamura T.Y. Regulation of the cardiac Na⁺/H⁺ exchanger in health and disease. J Mol Cell Cardiol. 2013;61:68–76. doi: 10.1016/j.yjmcc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Baartscheer A., Schumacher C.A., Wust C.A. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uthman L., Baartscheer A., Schumacher C.A. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018;9:1575. doi: 10.3389/fphys.2018.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Packer M. Do sodium-glucose co-transporter-2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes Metab. 2018;20:1361–1366. doi: 10.1111/dom.13229. [DOI] [PubMed] [Google Scholar]
- 84.Iborra-Egea O., Santiago-Vacas E., Yurista S.R. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. J Am Coll Cardiol Basic Trans Science. 2019;4:831–840. doi: 10.1016/j.jacbts.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mentzer R.M., Bartels C., Bolli R., for the EXPEDITION Study Investigators Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 86.Bell R.M., Yellon D.M. SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol. 2017;6:435–437. doi: 10.1016/S2213-8587(17)30314-5. [DOI] [PubMed] [Google Scholar]
- 87.Connelly K.A., Zhang Y., Desjardins J.F., Thai K., Gilbert R.E. Dual inhibition of sodium-glucose linked cotransporters 1 and 2 exacerbates cardiac dysfunction following experimental myocardial infarction. Cardiovasc Diabetol. 2018;17:99. doi: 10.1186/s12933-018-0741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hare J.M., Johnson R.J. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation. 2003;107:1951–1953. doi: 10.1161/01.CIR.0000066420.36123.35. [DOI] [PubMed] [Google Scholar]
- 89.Schernthaner G., Gross J.L., Rosenstock J. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chino Y., Samukawa Y., Sakai S. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391–404. doi: 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nussenzweig S.C., Verma S., Finkel T. The role of autophagy in vascular biology. Circ Res. 2015;116:480–488. doi: 10.1161/CIRCRESAHA.116.303805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raggi P., Gadiyaram V., Zhang C., Chen Z., Lopaschuk G., Stillman A.E. Statins reduce epicardial adipose tissue attenuation independent of lipid lowering: a potential pleiotropic effect. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato T., Aizawa Y., Yuasa S. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sano M., Takei M., Shiraishi Y., Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazer C.D., Connelly P.W., Gilbert R.E. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation. 2019 Nov 11 doi: 10.1161/CIRCULATIONAHA.119.044235. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Hess D.A., Terenzi D.C., Trac J.Z. SGLT2 Inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metab. 2019;30:609–613. doi: 10.1016/j.cmet.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 97.Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C., Zhang J., Xue M. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uthman L., Homayr A., Juni R.P. Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell Physiol Biochem. 2019;53:865–886. doi: 10.33594/000000178. [DOI] [PubMed] [Google Scholar]
- 100.Endemann D.H., Schiffrin E.L. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 101.Patel A.R., Kuvin J.T., Pandian N.G. Heart failure etiology affects peripheral vascular endothelial function after cardiac transplantation. J Am Coll Cardiol. 2001;37:195–200. doi: 10.1016/s0735-1097(00)01057-3. [DOI] [PubMed] [Google Scholar]
- 102.Gaspari T., Spizzo I., Liu H. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: a potential mechanism for inhibition of atherogenesis. Diabetes Vasc Dis Res. 2017;15:64–73. doi: 10.1177/1479164117733626. [DOI] [PubMed] [Google Scholar]
- 103.Mancini S.J., Boyd D., Katwan O.J. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018;8:5276. doi: 10.1038/s41598-018-23420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Juni R.P., Kuster D.W.D., Goebel M. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. J Am Coll Cardiol Basic Trans Science. 2019;4:575–591. doi: 10.1016/j.jacbts.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li H., Shin S.E., Seo M.S. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018;197:46–55. doi: 10.1016/j.lfs.2018.01.032. [DOI] [PubMed] [Google Scholar]