Abstract
Subacute myelo-optico-neuropathy (SMON) is caused by the ingestion of clioquinol (5-chloro-7-iodo-8-hydroxyquinoline), which is an intestinal antibacterial drug. Patients with SMON typically suffer from abnormal dysesthesia in the lower limbs, which cannot explain the mechanism only in pathology and electrophysiology. Neuromodulation therapies are increasingly being investigated as a means of alleviating abnormal sensory disturbances. We report here the response to repetitive transcranial magnetic stimulation (rTMS) for dysesthesia in a patient with SMON. The patient underwent rTMS treatment once per week for 12 weeks. rTMS was administered at 10 Hz, 90% of the resting motor threshold over the bilateral primary motor cortex foot area, for a total of 1,500 stimuli per day. After the treatment had finished at 12 weeks, the abnormal dysesthesia gradually declined. At first, there were improvements only in the area with a feeling of adherence. Later, this sensation was eliminated. Three months following the application, most of the feeling of adherence had disappeared and the feeling of tightness was slightly reduced. In contrast, the throbbing feeling had not changed during this period. Dysesthesia may indicate a process of central sensitization, which would contribute to chronic neuromuscular dysfunction. This case suggests that rTMS is a promising therapeutic application for dysesthesia.
Keywords: Transcranial magnetic stimulation, Subacute myelo-optico-neuropathy, Dysesthesia, Neuromodulation
Introduction
Subacute myelo-optico-neuropathy (SMON) is caused by the ingestion of clioquinol (5-chloro-7-iodo-8-hydroxyquinoline), which is an intestinal antibacterial drug. The condition was discovered in Japan between the 1950s and 1960s. Although all prescriptions of clioquinol were stopped over 50 years ago, approximately 30% of patients' symptoms worsened or were unchanged [1]. The atypical dysesthesia is present in a large proportion of patients with SMON, and it has a significantly negative impact on quality of life. This strange dysesthesia, which cannot explain the mechanism only in pathology, electrophysiology, and neurological examination, is one of the causes of the patients' suffering. In recent years, some authors have hypothesized that the pain and dysesthesia could be due to abnormal neural activation changes [2, 3].
Repetitive transcranial magnetic stimulation (rTMS) is a variant of noninvasive neuromodulation, after which persistent plastic changes are believed to be induced [4, 5]. For administering TMS, the magnetic pulses from the coil travel through the skull toward the cortical area, resulting in brain network modulation. The parameters of rTMS influence its modulatory effect, especially the frequency. The parameters are classified into high-frequency stimulation (HF-rTMS, ≥5 Hz) and low-frequency stimulation (<5 Hz). HF-rTMS usually facilitates enhanced cortical excitability, and this technique is increasingly being investigated as a means of alleviating sensory disturbances such as neurological pain. Moreover, the clinical changes induced by neuromodulation could provide useful insights into a mechanism-based approach to dysesthesia.
Case Report
We present the case of a 69-year-old woman who first noted a numbness in both lower limbs at 17 years of age, after having had gastroenteritis with 1 month of clioquinol treatment. After the cessation of clioquinol administration, her numbness gradually subsided. At 19 years of age, she was again prescribed clioquinol for 1 year, and numbness and weakness in the lower limbs as well as visual disturbances appeared. The numbness spread to the trunk, and she developed a gait disturbance. The numbness was associated with peculiar symptoms such as feeling of tingling ache, of tight ankles, and something adhering to the sole of her foot. The symptoms gradually worsened after age 40 years. She had refused to take any medications because of a fear of side effects. She visited our hospital at 69 years of age.
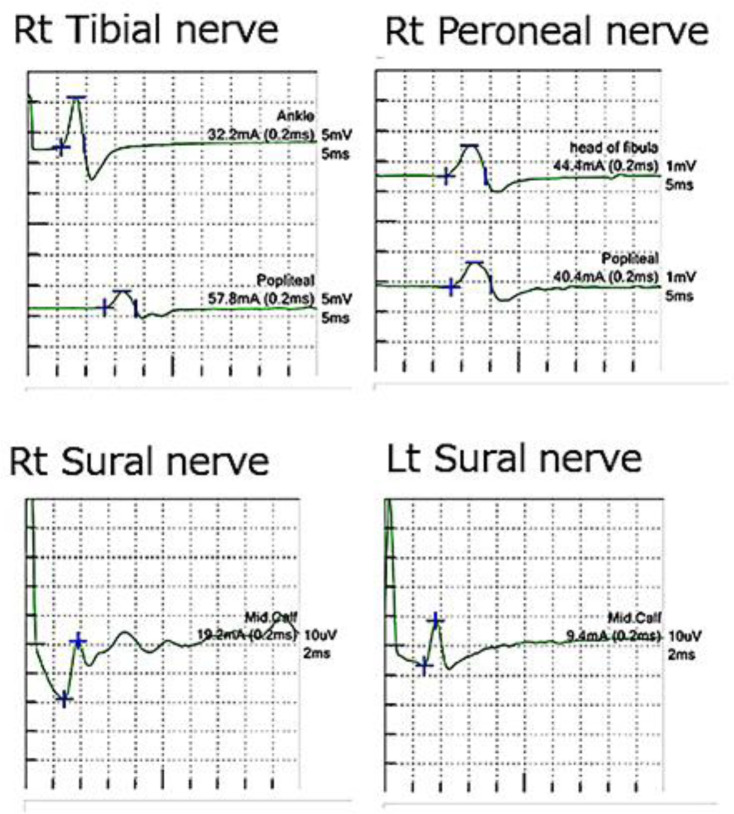
Neurological examination at admission revealed that she was alert and well oriented. She was completely blind and could not count fingers, but the other cranial nerves were intact. She had pronounced dysbasia and could not stand alone. Moderate weakness was diffusely present bilaterally in the lower extremities. Muscle atrophy was not evident on inspection. Her grip strength measurements were 16 and 18 kg for the right and left sides, respectively. A sensory deficit was noted and accentuated distally in both lower extremities. Light touch and pain sensations were moderately impaired, and vibration and joint sensations were remarkably reduced in the distal portions of the lower limbs. She demonstrated a positive Romberg sign. With regard to dysesthesia, she felt throbbing in her lower legs, a feeling that something was adhering to the entire soles of the feet, and a tight feeling in the ankles and metacarpophalangeal joints. Hand tremors were not noted. Her deep tendon reflexes were reduced in the upper extremities and entirely absent in the lower limbs. The plantar response was flexor. Nerve conduction studies were performed and the findings were within the normal range; large fiber sensory neuropathy was not evident (Fig. 1). Magnetic resonance imaging revealed no important abnormality in the spinal cord or brain. However, the brain was observed to be slightly atrophied in the occipital lobe. The Hamilton Depression Rating Scale revealed a total score of 6/37, with impairment in work and activities, hypochondriasis orientation, and late insomnia.
Fig. 1.
The findings of nerve conduction studies were within the normal range; sensory and motor neuropathy were not evident. Rt, right; Lt, left.
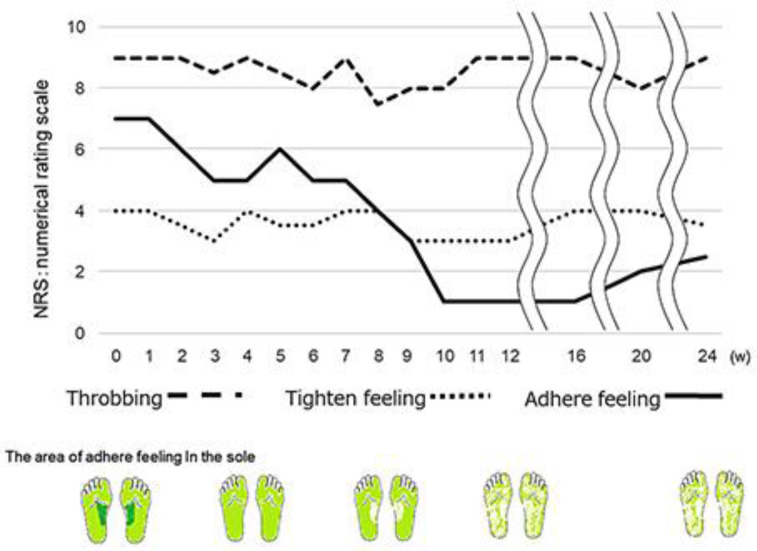
The patient underwent rTMS using the MagPro X100 (MagVenture, Farum, Denmark), which was connected to a 70-mm diameter figure-eight coil. rTMS was administered at 10 Hz for 5 s with 25 s between trains and at 90% of the resting motor threshold over the bilateral primary motor cortex foot area, angled tangentially to the head. A total of 1,500 biphasic pulses were given per session. There was 1 session per week for 12 weeks. We evaluated the dysesthesia using a numerical rating scale in an oral examination. Figure 2 shows the changing symptoms of dysesthesia. The dysesthesia was gradually reduced by the stimulation. At 2 weeks, there were improvements only in the area with the feeling of adherence. At 10 weeks, the majority of the feeling of adherence had disappeared and the feeling of tightness had declined slightly. In contrast, the throbbing feeling had not changed. During this period, she had no severalty adverse event (no skin problems at the site of coil placement, nor headache or seizure) and the treatment was well tolerated. The Hamilton Depression Rating Scale revealed a total score of 4/37 at 10 weeks. Neuropsychological test revealed slightly changed hypochondriasis orientation and insomnia. The patient maintained the improvements in the dysesthesia following the treatment for at least 3 months.
Fig. 2.
The changing symptoms of dysesthesia. The feeling of adherence was gradually reduced by the stimulation.
Discussion
We report here for the first time a patient with SMON and dysesthesia who had a good response to rTMS. SMON is characterized by subacute onset of neurological disturbances between the lower extremities and trunk as well as visual impairments [2]. The pathological features are characterized by a systemic degeneration of the long tracts of the spinal cord combined with polyneuropathy and optic nerve involvement. Sensory disturbances, including dysesthesia, usually appear in both lower extremities in patients with SMON. Although objective sensory examination reveals that abnormal, tactile, pain, and vibration sensations are reduced or increased, the parts of dysesthesia are present that do not accord to the objective neurological deficits. Although all prescriptions of clioquinol were stopped over 50 years ago, about 30% of patients' symptoms worsened or were unchanged [5]. Treatment of dysesthesia remains a challenge for clinicians because there is no accepted algorithm. Pharmacotherapy is commonly considered to be the first-line therapy, while there are some adverse effects.
rTMS is a noninvasive brain stimulation technique. It can modulate brain activity in specific, distributed cortical networks, thus modulating the activity of circuits involved in chronic processing at the cortical level. The underlying biological mechanisms by which rTMS works are only partially known, although they appear to be related to the phenomena of long-term potentiation. The efficacy of HF-rTMS in the primary motor cortex for patients with chronic neuropathic pain has been demonstrated in previous studies [6]. The beneficial effect observed with rTMS is supported by previous clinical experience, even though the mechanism has yet to be completely clarified [7]. In some reports, neuropathic pain and itch have been suggested to be associated with central sensitization [8, 9]. Central sensitization is a cause of novel inputs to nociceptive pathways, including those that do not normally drive them, such as chronic low-threshold mechanoreceptor. It also produces hypersensitivity in noninflamed tissue by changing the sensory response elicited by normal inputs and increases sensitivity long after the initiating cause may have disappeared and when no peripheral pathology may be present [10]. rTMS modulates the excitability of neuronal activity and facilitates neural network changes. The mechanism for the relief of pain and itch are based on the modification of neuronal activity involved in the neural circuits responsible for processing and perception [11]. This current change in dysesthesia after rTMS probably means that it has a similar pathogenesis as neuropathic pain. In this case, the feeling of adherence, throbbing, and tightness might have appeared due to central sensitization. A recent study reported a throbbing feeling produced by transmission of arterial pulsation from blood vessels [12]. On the other hand, the adherence and tightness feeling have assessed the underlying mechanisms. We suspect that the adherence and tightness feelings are based on a deafferentation sensory disturbance, such that the sensory neurons below the level of the deafferentation cannot exert their normal inhibitory influences due to the development of aberrant connections [13].
In conclusion, this initial case study provides research opportunities for the therapeutic use of rTMS in patients with SMON. Our results showed effects for a minimum of 3 months after stimulation treatment. Dysesthesia appears not only in patients with SMON, but also in other neuromuscular and demyelinating diseases, such as movement disorders, spinal cord disorders, and neuropathies. The dysesthesia sometimes continues even after successful treatment of the underlying disease. The dysesthesia could indicate a process of central sensitization, which might contribute to neuromuscular disease chronicity. This case suggests that the neuromodulation technique is a promising therapeutic application for dysesthesia. On the other hand, the results in this case could be due to the placebo effect, thus future placebo-controlled cohort studies are warranted.
Statement of Ethics
After a full explanation of the study procedures, written informed consent for publication of this case report was obtained from the patient in accordance with the Declaration of Helsinki. The Ethics Committee of Osaka University Hospital approved the study protocol.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by a Grant-in-Aid for Research on Intractable Diseases (the SMON Research Committee) from the Ministry of Health, Labor, and Welfare of Japan, and the Strategic Research Program for Brain Sciences from MEXT and AMED of Japan.
Author Contributions
T. Mano examined the patient, contributed to data collection, and wrote the manuscript. S. Kuru reviewed the manuscript.
Acknowledgement
The authors wish to thank the Department of Neuromodulation and Neurosurgery, Osaka University Graduate School of Medicine.
References
- 1.Konagaya M, Matsumoto A, Takase S, Mizutani T, Sobue G, Konishi T, et al. Clinical analysis of longstanding subacute myelo-optico-neuropathy: sequelae of clioquinol at 32 years after its ban. J Neurol Sci. 2004 Mar;218((1-2)):85–90. doi: 10.1016/j.jns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Sobue I, Aoki K, Ohtani M. Prognosis of SMON patients. Jpn J Med Sci Biol. 1975;28(Suppl):203–17. [PubMed] [Google Scholar]
- 3.Tateishi J. Subacute myelo-optico-neuropathy: clioquinol intoxication in humans and animals. Neuropathology. 2000 Sep;20((s1Suppl)):S20–4. doi: 10.1046/j.1440-1789.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 4.Maniglia M, Trotter Y, Aedo-Jury F. TMS reveals inhibitory extrastriate cortico-cortical feedback modulation of V1 activity in humans. Brain Struct Funct. 2019 Dec;224((9)):3399–408. doi: 10.1007/s00429-019-01964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoe M, Mano T, Maruo T, Hosomi K, Shimokawa T, Kishima H, et al. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson's disease: A double-blind crossover pilot study. J Clin Neurosci. 2018 Jan;47:72–8. doi: 10.1016/j.jocn.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, et al. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain. 2013 Jul;154((7)):1065–72. doi: 10.1016/j.pain.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: A review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009 Aug;2((3)):339–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen JP, Dixneuf V, Esnaut J, Moreno AS, Malineau C, Nizard J, et al. The Value of High-Frequency Repetitive Transcranial Magnetic Stimulation of the Motor Cortex to Treat Central Pain Sensitization Associated With Knee Osteoarthritis. Front Neurosci. 2019 Apr;13:388. doi: 10.3389/fnins.2019.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Laarhoven AI, Marker JB, Elberling J, Yosipovitch G, Arendt-Nielsen L, Andersen HH. Itch sensitization? A systematic review of studies using quantitative sensory testing in patients with chronic itch. Pain. 2019 Dec;160((12)):2661–78. doi: 10.1097/j.pain.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000 Jun;288((5472)):1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 11.Ma SM, Ni JX, Li XY, Yang LQ, Guo YN, Tang YZ. High-Frequency Repetitive Transcranial Magnetic Stimulation Reduces Pain in Postherpetic Neuralgia. Pain Med. 2015 Nov;16((11)):2162–70. doi: 10.1111/pme.12832. [DOI] [PubMed] [Google Scholar]
- 12.Ahn AH. On the temporal relationship between throbbing migraine pain and arterial pulse. Headache. 2010 Oct;50((9)):1507–10. doi: 10.1111/j.1526-4610.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993 Mar;78((3)):393–401. doi: 10.3171/jns.1993.78.3.0393. [DOI] [PubMed] [Google Scholar]