Abstract
Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of non-small-cell lung cancer, which is resistant to the conventional chemotherapy and radiotherapy with a poor prognosis. Limited case reports have showed good response to the immunotherapy in PSC patients with high PD-L1 expression generally. Herein, we report a case of rapid recurrence of PSC during postoperative adjuvant chemotherapy in a 62-year-old male ex-smoker. The patient had high PD-L1 expression (tumor proportion score: 90%) and KRAS exon 2 mutation. Nivolumab combined with anlotinib was administered synchronously. Clinical symptoms gradually relieved and response evaluation on imaging revealed a partial response after 8 weeks. This case suggests immunotherapy combined with antiangiogenic agent anlotinib may be a potential promising strategy to treat PSC patients.
Keywords: Anlotinib, Immunotherapy, Nivolumab, Pulmonary sarcomatoid carcinoma, PD-L1
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare and aggressive subtype of poorly differentiated non-small-cell lung cancer (NSCLC) representing less than 1% of primary lung cancers [1, 2]. PSC is more likely to relapse after radical surgery and typically resistant to conventional chemotherapy and radiotherapy with a poor prognosis. The median overall survival (OS) of surgically resected PSC was significantly shorter than that of contemporaneously surgically resected NSCLC (24 vs. 42 months) [3]. And for advanced or metastatic PSC receiving first-line chemotherapy, median progression-free survival and OS was 2 and 6.3 months [4].
Although clinically available molecular targets such as EGFR mutation, ALK rearrangement, and MET exon 14 mutation have been reported to respond to corresponding targeted therapy, the prevalence of total driver gene targeted by available targeted drugs is relatively low in PSC patients [5, 6]. Immunotherapy with immune checkpoint inhibitors (ICIs) has been the recommended therapeutic schedule in NSCLC showing better survival benefit. Because of the high PD-L1 expression and tumor mutational burden generally, the successful treatment of immunotherapy in PSC patients has been reported in limited case reports [7]. Furthermore, some clinical trials have showed encouraging antitumor activity of immunotherapy combined with antiangiogenic therapy [8]. And in the 2019 World Conference on Lung Cancer (WCLC), preliminary results of a phase I clinical trial showed the objective response rate (ORR) was 72.7% in 22 NSCLC patients receiving first-line treatment of immunotherapy with sintilimab combined with oral antiangiogenic agent anlotinib.
In the present study, we report the successful treatment of a PSC patient with nivolumab combined with anlotinib.
Case Report
A 62-year-old male with a 30-pack-year smoking history was hospitalized in a local medical institution due to the symptoms of cough and hemoptysis in December 2018. After complete inspection and evaluation, left total pneumonectomy, partial pericardiectomy, and lymph node dissection were performed via thoracoscope. He was diagnosed as PSC (pT4N1M0 stage IIIA) after surgery. The lung cancer-related gene test through next-generation sequencing showed KRAS exon 2 mutation. From January to April in 2019, he was treated with four cycles of docetaxel and cisplatin. During the period of postoperative adjuvant chemotherapy, he found one subcutaneous nodule gradually increasing in the left upper posterior arm in February, resulting in edema and pain of the total left upper arm. Subsequently in May, he again found a painless subcutaneous nodule in the lower abdomen. Excisional biopsy of the subcutaneous nodule in the left upper arm and needle biopsy of the abdominal subcutaneous nodule both showed sarcomatoid carcinoma.
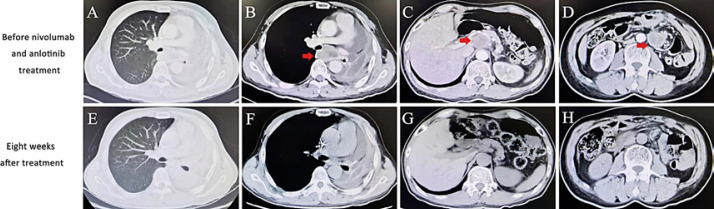
In June 2019, he was referred to the hospital where I work for further treatment. PD-L1 expression had a tumor proportion score (TPS) of 90% using the anti-PD-L1 antibody clone 22C3. Imaging examination showed local recurrence in the thorax and multiple distant metastases involving hilum area, retroperitoneum, mesentery, abdominal wall, and so on (Fig. 1A–D). Since June, he has been treated with nivolumab (180 mg, q2w) and anlotinib (12 mg p.o. qd, days 1–14, 21 days as a cycle). A partial response (Response Evaluation Criteria in Solid Tumors ver. 1.1) was confirmed 8 weeks after first combination therapy by computed tomography (Fig. 1E–H). Meanwhile, stomachache and edema and pain of left upper arm relieved. The subcutaneous nodule almost regressed. But he discontinued the treatment of anlotinib in November 2019 because of mild oral mucositis. Now he continued the immunotherapy with nivolumab on schedule.
Fig. 1.
Imaging results before and after the treatment of nivolumab combined with anlotinib. A–D Chest and abdomen computed tomography (CT) showed local recurrence in the thorax and multiple metastases in the abdomen. E–H Chest and abdomen CT showed significant shrinkage of tumors at the time of first assessment after combination therapy.
Discussion
According to the current 2015 WHO classification, PSC is defined as a poorly differentiated NSCLC containing at least 10% spindle cells and/or giant cells or a carcinoma with spindle and giant cells exclusively, which has five histological subtypes including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma [9]. PSC is more prevalent in current or former male smokers.
In our case, the patient harbored KRAS gene mutation. Some previous studies have analyzed the mutational profile of PSC using high-throughput sequencing technology. Fallet et al. [10] found the most frequent mutations were KRAS (27.2%), EGFR (22.2%), and TP53 (22.2%) after testing surgical biopsies from 81 patients. Besides, Lococo et al. [11] discovered 55% of total 49 patients had TP53 mutations and 39% had KRAS mutations. Despite the limited cases and different sequencing panel, the mutations in TP53 and KRAS are prevalent in PSC patients and the presence of KRAS mutations tends to predict a poor prognosis [12]. The present patient with KRAS mutation relapsed rapidly during adjuvant chemotherapy indicating the strong aggressiveness. Although no clinical therapeutic option to target KRAS mutation is available, several novel inhibitors targeting KRAS mutation are now in clinical trials indicating molecular targeted therapy is a potential strategy in KRAS mutant PSC patients in the future [13].
In the era of immunotherapy, ICIs targeting PD-1/PD-L1 have been the standard and preferential therapeutic strategy in NSCLC. Furthermore, the expression of PD-L1 in the tumor tissue has been the common and optimal biomarker to predict the efficacy of immunotherapy. Yang et al. [14] reported 36.5% of 148 PSC patients were positive for PD-L1 with a definition of positive as TPS ≥1%. Naito et al. [15] also showed about 91% of 35 PSC patients had a positive PD-L1 expression (TPS ≥1%) and 60% had a high PD-L1 expression (TPS ≥50%). A French study showed only 1 was negative for PD-L1 expression (TPS ≤5%) in 19 available PSC samples. Meanwhile, tumor mutational burden (TMB), another predictive biomarker, was high in 7/8 PSC patients (TMB ≥10 mutations/megabase). Tumor-infiltrating lymphocytes in the tumor microenvironment also affect the response to immunotherapy. Vieira et al. [16] revealed immune-cell infiltration including CD3+ T cells and CD163+ macrophages were higher in PSC than in NSCLC. In addition, this study showed PD-L1 expression of PSC was higher than that of NSCLC (53 vs. 20% of cases). But the prevalence of PD-L1 expression could reach to 56% with a ≥1% cutoff in 731 NSCLC patients [17]. The variety of PD-L1 expression in different studies could be attributed to different antibody clones and thresholds of positivity and tumor heterogeneity. The PD-L1 expression in our case was very high (TPS = 90%).
During the period of conventional chemoradiotherapy, a study utilizing the National Cancer Data Base showed that patients with PSC have inferior survival outcomes relative to other subtypes of NSCLC [18]. From this large database the median OS was 16.9 months in stage I-II PSC, 5.8 months in stage III, and 5.4 months in stage IV. Maneenil et al. [19] also revealed median OS of 127 analyzed PSC patients with stage I to IV was 9.9 months, among which 25 patients received palliative chemotherapy with an ORR of 8%. From these early studies, PSC is resistant to chemotherapy with a poor prognosis. But for the PSC patients with negative driver gene targeted by available clinical drugs, limited case reports had shown successful treatment and long-term response with ICIs despite the rarity of PSC [20, 21, 22]. In a study of 37 PSC patients with immunotherapy in second line or beyond, the ORR was 40.5% and median OS was 12.7 months [23]. But one-third of patients including high PD-L1 expression or KRAS mutation subgroup exhibited early progression within 2 months. In the present case, on the basis of immunotherapy with nivolumab, anlotinib was administered synchronously. VEGFR signaling could modulate the immune response by reducing tumor T-cell infiltration and increasing suppressive immune cells such as regulatory cells and myeloid derived suppressor cells, ultimately leading to the immunosuppressive tumor microenvironment [8]. Anlotinib as a novel multitarget antiangiogenic agent mainly targeting VEGFR, EGFR, and PDGFR has been approved for the third-line treatment of advanced NSCLC by the Chinese Food and Drug Administration. Therefore, anlotinib combined with immunotherapy could synergistically act on the tumor and improve the efficacy of patients. Just in our case, the PSC patient receiving combination treatment had an early partial response sustaining until now.
Conclusions
In conclusion, to our knowledge, this is the first report to show the good response to the treatment of nivolumab combined with anlotinib in PSC. Immunotherapy combined with antiangiogenic agent anlotinib may be a potential promising strategy to treat PSC patients. But further studies in more cases will be required to confirm the efficacy and safety of immunotherapy combined with anlotinib for PSC.
Statement of Ethics
The patient provided consent of the use of information for publication.
Disclosure Statement
The authors report no conflicts of interest in this work.
Funding Sources
No funding sources.
Author Contributions
Caibao Jin was responsible for writing the manuscript. Bin Yang mainly participated in manuscript revision.
References
- 1.Weissferdt A. Pulmonary Sarcomatoid Carcinomas: A Review. Adv Anat Pathol. 2018;25((5)):304–13. doi: 10.1097/PAP.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 2.Shum E, Stuart M, Borczuk A, Wang F, Cheng H, Halmos B. Recent advances in the management of pulmonary sarcomatoid carcinoma. Expert Rev Respir Med. 2016;10((4)):1–10. doi: 10.1586/17476348.2016.1157475. [DOI] [PubMed] [Google Scholar]
- 3.Rahouma M, Kamel M, Narula N, Nasar A, Harrison S, Lee B, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg. 2018;53((4)):828–34. doi: 10.1093/ejcts/ezx417. [DOI] [PubMed] [Google Scholar]
- 4.Vieira T, Girard N, Ung M, Monnet I, Cazes A, Bonnette P, et al. Efficacy of First-Line Chemotherapy in Patients with Advanced Lung Sarcomatoid Carcinoma. J Thorac Oncol. 2013;8((12)):1574–7. doi: 10.1097/01.JTO.0000437008.00554.90. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Zhang Y, Lu J, Xu C, Liang J, Wang F, et al. Pulmonary Sarcomatoid Carcinoma with ALK Rearrangement: Frequency, Clinical-Pathologic Characteristics, and Response to ALK Inhibitor. Transl Oncol. 2017;10((2)):115–20. doi: 10.1016/j.tranon.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer − A systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. doi: 10.1016/j.lungcan.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Sukrithan V, Sandler J, Gucalp R, Gralla R, Halmos B. Immune Checkpoint Blockade Is Associated with Durable Responses in Pulmonary Sarcomatoid Carcinoma. Clin Lung Cancer. 2019;20((3)):e242–6. doi: 10.1016/j.cllc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res. 2019;11:7707–19. doi: 10.2147/CMAR.S212238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10((9)):1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 10.Fallet V, Saffroy R, Girard N, Mazieres J, Lantuejoul S, Vieira T, et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta™ Panel: exploring therapeutic targets. Ann Oncol. 2015;26((8)):1748–53. doi: 10.1093/annonc/mdv232. [DOI] [PubMed] [Google Scholar]
- 11.Lococo F, Gandolfi G, Rossi G, Pinto C, Rapicetta C, Cavazza A, et al. Deep Sequencing Analysis Reveals That KRAS Mutation Is a Marker of Poor Prognosis in Patients with Pulmonary Sarcomatoid Carcinoma. J Thorac Oncol. 2016;11((8)):1282–92. doi: 10.1016/j.jtho.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Mehrad M, Roy S, LaFramboise WA, Petrosko P, Miller C, Incharoen P, et al. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology. 2018;73((2)):207––14. doi: 10.1111/his.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020;84:101974. doi: 10.1016/j.ctrv.2020.101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Xu J, Li R, Gao Y, He J. PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol. 2019;145((12)):3055–65. doi: 10.1007/s00432-019-03023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naito M, Tamiya A, Takeda M, Taniguchi Y, Saijo N, Naoki Y, et al. A High PD-L1 Expression in Pulmonary Pleomorphic Carcinoma Correlates with Parietal-Pleural Invasion and Might Predict a Poor Prognosis. Intern Med. 2019;58((7)):921–7. doi: 10.2169/internalmedicine.1462-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira T, Antoine M, Hamard C, Fallet V, Duruisseaux M, Rabbe N, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer. 2016;98:51–8. doi: 10.1016/j.lungcan.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Chan AWH, Tong JHM, Kwan JSH, Chow C, Chung LY, Chau SL, et al. Assessment of programmed cell death ligand-1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non-small cell lung carcinoma. Mod Pathol. 2018;31((9)):1381–90. doi: 10.1038/s41379-018-0053-3. [DOI] [PubMed] [Google Scholar]
- 18.Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, et al. Pulmonary Sarcomatoid Carcinoma: An Analysis of the National Cancer Data Base. Clin Lung Cancer. 2017;18((3)):286–92. doi: 10.1016/j.cllc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Maneenil K, Xue Z, Liu M, Boland J, Wu F, Stoddard SM, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer. 2018;19((3)):e323–33. doi: 10.1016/j.cllc.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Miura T, Horiuchi H, Usui K. The Successful Treatment of Pulmonary Pleomorphic Carcinoma with Pembrolizumab: A Case Report. Case Rep Oncol. 2017;10((2)):752–7. doi: 10.1159/000479552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorozuya T, Taya T, Yasuda K, Nagano Y, Shioya M, Chiba H, et al. Long-term response with durvalumab after chemoradiotherapy for pulmonary pleomorphic carcinoma: a case report. Thorac Cancer. 2020;11((4)):1090–−3. doi: 10.1111/1759-7714.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senoo S, Ninomiya T, Makimoto G, Nishii K, Kano H, Watanabe H, et al. Rapid and Long-term Response of Pulmonary Pleomorphic Carcinoma to Nivolumab. Intern Med. 2019;58((7)):985–9. doi: 10.2169/internalmedicine.0890-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domblides C, Leroy K, Monnet I, Mazières J, Barlesi F, Gounant V, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol. 2020 doi: 10.1016/j.jtho.2020.01.014. [DOI] [PubMed] [Google Scholar]