Abstract
A primary aorto-duodenal fistula (ADF), a rare, spontaneous development of a communication between the aorta and duodenum, is a disastrous complication of an abdominal aortic aneurysm. A 73-year-old patient with primary ADF underwent emergent endovascular aneurysm repair (EVAR), followed by staged omentopexy, without removing a stent graft (SG). The patient received long-term treatment with antibiotics, and there has been no evidence of infection during a follow-up period of three years. Emergency EVAR coupled with omentopexy, may be a treatment option for primary ADF, even when it means leaving the SG in a potentially infectious site.
Keywords: primary aorto-duodenal fistula, abdominal aortic aneurysm, endovascular aneurysm repair
Introduction
Primary aorto-enteric fistula (AEF) is a rare, but serious, complication of abdominal aortic aneurysm (AAA), which is a communication between the aorta and the enteric tract with no previous vascular intervention. Despite advances in imaging and endoscopic technology, diagnosing and treating primary AEF are difficult because of its nonspecific and subtle clinical presentation. A mortality rate of operative repair for AEF has been reported to be extremely high. Although AEF is a potentially infectious disease, an aorto-duodenal fistula (ADF) seems to be less infectious than a colon fistula. Here, we report a case of primary ADF treated by successful endovascular aneurysm repair (EVAR) to control hemorrhage rapidly, followed by staged omentopexy without removal of a stent graft (SG), and long-term antibiotic therapy.
Case Report
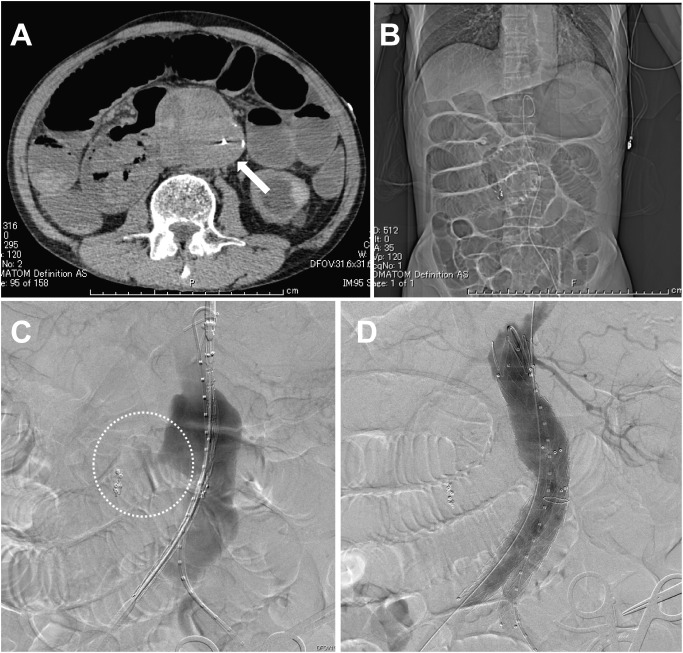
A 73-year-old man presenting with massive hematemesis and loss of consciousness was transferred to our hospital by ambulance. The patient’s hemodynamics had been collapsed, and blood tests at the time of arrival revealed severe anemia, renal dysfunction, and disseminated intravascular coagulation [hemoglobin 6.4 g/dL, creatinine 7.0 mg/dL, platelet count 6.0×109/L, fibrin/fibrinogen degradation products (FDP) 60 µg/mL, fibrinogen 104 mg/dL, prothrombin ratio and international normalized ratio (PT-INR) 1.43]. His condition was temporarily stabilized with a rapid blood transfusion. Emergency endoscopy revealed no gastric bleeding, but the duodenum was filled from continuous bleeding. Endoscopic hemostasis was abandoned, and the patient was transferred to the interventional radiology suite. Selective celiac and superior mesenteric arterial angiography revealed no abnormal findings that would cause duodenal bleeding. Computed tomography (CT) scanning had revealed an 80-mm AAA, which was adjacent to the third portion of the duodenum, but failed to provide evidence of contrast leakage into the intestinal tract (Fig. 1A). Digital subtraction angiography (DSA) showed possible slight blood leakage from the AAA into the duodenum (Fig. 1B). Based on these findings, primary ADF was comprehensively suspected. Considering the patient’s general condition, including chronic renal failure requiring hemodialysis, emergency EVAR was chosen, instead of a conventional open repair, to seal the bleeding site.
Fig. 1 Computed tomography (CT) and digital subtraction angiography (DSA) taken in an interventional radiology suite. (A) CT image shows an 80-mm infrarenal abdominal aortic aneurysm (AAA) (white arrow) and the storage of intestinal gas, leading to suspicion of ileus. The definitive diagnosis of aorto-duodenal fistula cannot be made. (B) DSA revealed AAA ruptured into the duodenum (dotted circle). (C) After deployment of the stent graft system, DSA shows the successful exclusion of ruptured AAA.
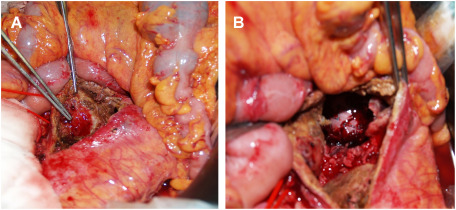
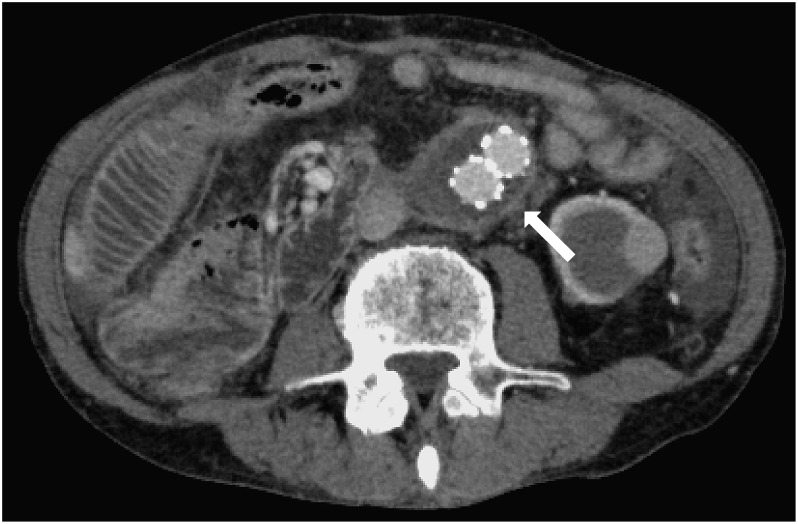
Under general anesthesia, bilateral common femoral arteries were exposed. Heparin was not injected because of collapsed coagulopathy due to massive bleeding. An Endurant SG (Medtronic Cardiovascular, Santa Rosa, CA, USA; ETBF2516C166EJ) was implanted from a distal level of the left renal artery to the right common iliac artery. The contralateral leg with ETLW1613C124EJ was deployed to the left common iliac artery. Post-deployment DSA showed the bleeding site was sealed successfully and there were type II endoleaks related to the bilateral L3 lumbar arteries (Fig. 1C). A laparotomy for direct duodenal fistula closure and omentopexy were planned for the next day since his hemodynamics improved after EVAR. The aneurysm wall was incised after managing the type II endoleaks by clipping the L3 lumbar arteries. Removal of the possibly infectious aneurysmal wall, including large amount of thrombus, revealed the SG inside the aneurysm. The SG was macroscopically isolated from potentially contaminated intestinal fluid. There were two 3-mm wide fistulas between the anterior wall of AAA and the third part of the duodenum (Fig. 2). The duodenal defect was suture-closed with 4-0 PDS, and then the SG was covered with an omental flap. The patient began to receive broad spectrum antibiotics of Meropenem 3 g and Vancomycin 500 mg from the second day. The blood cultures revealed growth of coagulase-negative staphylococci (CNS), H. parainfluenza and actinomyces on postoperative day 5. The appropriate intravenous antibiotics of Sulbactam/Ampicillin 2 g were continued for six weeks, followed by Amoxicillin 500 mg orally for a further six months. The patient was discharged home on postoperative day 48 after an uneventful hospitalization, and there has been no evidence of infection during a follow-up period of 3 years (Fig. 3).
Fig. 2 Intraoperative pictures during the staged laparotomy. (A) Two 3-mm wide fistulas between the anterior wall of the abdominal aortic aneurysm and the third part of the duodenum are identified inside the aneurysm. (B) A deployed stent graft is seen inside the aneurysm, which is isolated and uncontaminated with intestinal fluid.
Fig. 3 Follow-up computed tomography scanning three years after endovascular aneurysm repair. It shows a shrinkage of abdominal aortic aneurysm (white arrow) and no infectious signs around the aneurysm covered with the omentum.
Discussion
Primary AEF is a direct communication between the native aorta and any portion of the gastrointestinal tract. Its incidence is reportedly 0.04% to 0.07%,1) whereas secondary AEF is 0.36% to 1.6%.2) Secondary AEF is a late anastomotic complication of prosthetic aortic vascular grafts or an EVAR-related complication caused by an enlarged AAA, and is a well-known complication after intraoperative radiotherapy for pancreatic adenocarcinoma.3)
Literature reports that even primary AEFs are often fatal, with a total mortality rate of 80–100% without surgical intervention.1,2) The precise etiology of primary AEF formation is unclear, but atherosclerotic AAA is discovered in 83% of cases, which indicates that the repetitive mechanical stress from the aneurysm’s repeated pulsation is a primary AEF feature.4)
Primary AEFs frequently occur at the third portion of the duodenum (54%) because of its retroperitoneal location overlying the aorta, followed by the esophagus (28%), the jejunum and ileum (12%), colon (3%) and then stomach (2%).5) Despite advances in imaging and endoscopic technology, diagnosing and treating ADF are still difficult because of its nonspecific and subtle clinical presentation. CT scanning has been advocated as the preferred initial diagnostic method for patients with ADF, with a reported sensitivity of up to 93%.6) Endoscopy has less sensitivity than CT scanning, with reported findings in 25% to 80%.1,2) Although angiography is helpful as a diagnostic method, it should be a secondary choice because it is rather time-consuming during an emergency.
ADF management goals have been to maintain distal perfusion after controlling the hemorrhage and preventing infection recurrence. For stable patients with minimal comorbidities and significant life expectancies, open ADF repair is optimal management. Whereas, like the present case, patients with hemodynamic instability have a higher risk for open repair. EVAR provides patients a less invasive alternative to seal the fistula and control bleeding rapidly. EVAR’s important benefits include avoiding large incisions, aortic cross clamping, respiratory function interference, and significant blood loss. Baril et al. reported that EVAR for primary ADF had lower incidences of perioperative complications and shorter hospital stays than surgical treatment.7)
EVAR effectiveness for ruptured AAA has been reported8); however, there is a concern about conversion to open repair due to perioperative complications. One reason for open conversion after EVAR includes persistent type II endoleaks, leading to abdominal compartment syndrome.9) Similarly, for a primary ADF, there continuous duodenal bleeding through the lumbar arteries as type II endoleaks is possible after a simple EVAR. In this case, lumbar artery-derived T2Es, confirmed by CT, were treated by direct clipping during staged open surgery.
It may be controversial, but the SG was preserved without removal from the possibly infectious intra-AAA sac because contamination of the surgical field was localized for small fistulas, and the identified CNS on blood culture was a broad type of antibiotic-sensitive bacteria. Infection control is often difficult when treating ADF with EVAR because the newly implanted SG can be at risk for persistent infection. Some authors state that lifelong suppressive antibiotic therapy is mandatory, whereas many others suggest a 6–8 week course of oral antibiotics. In this case, the patient has been doing well, with no symptoms of infection, for three years after EVAR following treatment with intravenous antibiotics for six weeks, followed by oral administration for a further six months.
Other department experts, including acute physicians who are likely to have initial contact with AEF patients, must be aware of several options as initial treatment for AEF because comprehensive, long-term treatment strategies are essential.
Conclusion
We reported a case of successful emergency EVAR, followed by staged direct duodenal repair, omentopexy, and long-term antibiotic therapy for primary ADF. Although EVAR is basically contraindicated in a potentially infectious site, it may be an optional ADF treatment for limited patients.
Disclosure Statement
All authors declare no conflict of interest.
Author Contributions
Study conception: SM, YK
Data collection: SM, KU
Investigation: SM, YK, KU
Writing: SM
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all author
References
- 1).Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92: 143-52. [DOI] [PubMed] [Google Scholar]
- 2).Hallett JW Jr, Marshall DM, Petterson TM, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg 1997; 25: 277-84; discussion, 285-6. [DOI] [PubMed] [Google Scholar]
- 3).Liu B, Howard JM. Vascular-enteric fistulas associated with radiation therapy in patients with pancreatic adenocarcinoma. HPB (Oxford) 2002; 4: 83-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Keunen B, Houthoofd S, Daenens K, et al. Case of primary aortoenteric fistula: review of therapeutic challenges. Ann Vasc Surg 2016; 33: 230.e5-13. [DOI] [PubMed] [Google Scholar]
- 5).Saers SJF, Scheltinga MRM. Primary aortoenteric fistula. Br J Surg 2005; 92: 143-52. [DOI] [PubMed] [Google Scholar]
- 6).Armstrong PA, Back MR, Wilson JS, et al. Improved outcomes in the recent management of secondary aortoenteric fistula. J Vasc Surg 2005; 42: 660-6. [DOI] [PubMed] [Google Scholar]
- 7).Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44: 250-7. [DOI] [PubMed] [Google Scholar]
- 8).Karkos CD, Harkin DW, Giannakou A, et al. Mortality after endovascular repair of ruptured abdominal aortic aneurysms; a systematic review and meta-analysis. Arch Surg 2009; 144: 770-8. [DOI] [PubMed] [Google Scholar]
- 9).Chaar CI, Eid R, Park T, et al. Delayed open conversions after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2012; 55: 1562-69.e1. [DOI] [PubMed] [Google Scholar]