Abstract
Background
Anaemia is a major global health problem, especially in developing countries. Postpartum anaemia hurts both maternal and newborn baby health. Anaemia in pregnancy is sufficiently emphasized; however, very little attention has been paid to postpartum anaemia in Ethiopia. Therefore, this study aimed to investigate the proportion of immediate postpartum anaemia and associated factors among postpartum mothers in Debre Markos Referral Hospital.
Methods
Institutional-based cross-sectional study was conducted among 424 study participants from August 1st to October 30th, 2019. A systematic random sampling technique was employed to select the study participants. Data were collected through both face-to-face interview and maternal chart review by using a pretested questionnaire. Data were cleaned, coded, and entered using Epi Data version 4.6.0.0 and then exported to SPSS version 24 for analysis. First, binary logistic regression was applied to identify candidate variables for multivariable regression. Then, variables at p value <0.2 were entered into a multivariable logistic regression to control possible confounders. Finally, variables at p value <0.05 were considered as statistically significant.
Results
The proportion of immediate postpartum anaemia was 24.3%. Frequency of antenatal care (ANC) visits <4 times [AOR = 2.40; 95% CI (1.29, 4.43)], antepartum haemorrhage (APH) [AOR = 5.08; 95% CI (1.91, 13.55)], postpartum haemorrhage (PPH) [AOR = 4.47; 95% CI (2.25, 8.88)], giving birth assisted by instruments (vacuum or forceps) [AOR = 3.99; 95% CI (1.42, 11.23)], poor adherence to iron and folic acid (IFA) [AOR = 2.52; 95% CI (1.06, 6.04)], and midupper arm circumference (MUAC) <23 cm [AOR = 3.25; 95% CI (1.87, 5.65)] were the predictors.
Conclusion
The proportion of immediate postpartum anaemia was a moderate public health concern. ANC, APH, PPH, mode of delivery, adherence to IFA supplementation, and MUAC measurement were the factors affecting the magnitude of anaemia. Therefore, interventions that would address the above mentioned factors need to be implemented.
1. Introduction
The postnatal period is a critical phase in the lives of mothers and newborn babies. Even though most maternal and infant deaths occur during this time, this is the most neglected period for the provision of quality of care, especially in low resource setting countries [1].
There is no consensus on the definition of postpartum anaemia. Nevertheless, as it can be inferred from the definition given by different scholars, postpartum anaemia (PPA) occurs when haemoglobin level <11 gm/dl at 1 week and <12 gm/dl at 8 weeks of the postpartum period [2, 3]. Accordingly, haemoglobin level 10–10.9 gm/dl is categorized as mild anaemia, 7–9.9 gm/dl and <7 gm/dl are categorized as moderate and severe anaemia, respectively [4]. Furthermore, even if there is no clear agreement as to the right time to determine the postpartum haemoglobin level, it is usually recommended to check on the first postpartum day [5, 6].
The prevalence of anaemia among postnatal mothers in developed countries ranges from 10% to 30% and in developing countries 50% to 80% [2, 6]. In Ethiopia despite of the 2020 anaemia reduction plan, postpartum anaemia among lactating women increased from 18% in 2011 to 28.6% in 2016 [7, 8]. Anaemia is an indirect cause of maternal morbidity and mortality which accounts for 2% of total maternal mortality in Ethiopia [8, 9]. Blood loss and anaemia were interrelated causes of maternal complications. Due to bleeding, postnatal mothers lose a significant amount of iron during labour and delivery [10, 11]. PPA is also strongly associated with poor quality of life, palpitation, increase maternal infection, fatigue, reduced cognitive ability, emotional instability, and postpartum depression. These outcomes may, in turn, result in poor mother-child bonding, inability to provide care and breastfeeding, or slow infant development [2, 12, 13].
The studies conducted in different parts of the world revealed that factors like young maternal age (<21 years) [14], low educational status of the mother [15], rural residence [16], cesarean mode delivery, episiotomy assisted delivery, prenatal anaemia [17–19], antepartum haemorrhage (APH), postpartum haemorrhage (PPH) [20], antenatal care (ANC) visit less than four [21], reactive (positive) in Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) test, malaria [15, 16, 22], and poor adherence of iron and folic acid (IFA) intake during pregnancy [15, 23–25] were some of the independent factors significantly associated with the occurrence of postpartum anaemia. The reduction of postpartum anaemia is a component of target 2 (50% anaemia reduction plan) of World Health Organizations' (WHO) to achieve sustainable development goals.
Some studies were conducted in Ethiopia on anaemia during pregnancy, yet postpartum anaemia screening is the least emphasized illness in the postpartum period. Early diagnosis and identifying the possible risk factors are helpful to manage PPA on time before further complications developed. This study might provide insight into postpartum anaemia to health care providers to propose targeted screening and intervention measures for those whose haemoglobin level <11 gm/dl. Furthermore, researchers might also be benefited by using this result as baseline data to conduct further community-based studies covering a wide area. Several factors contribute to PPA and it can be difficult to generalize the causes for all mothers who reside in a different area. Therefore, this study was intended to assess the proportion and associated factors of anaemia among immediate postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia.
2. Material and Methods
2.1. Study Setting, Design, and Period
This study was conducted in Debre Markos Referral Hospital which is located in East Gojjam Zone, Northwest Ethiopia, and 299 km far from Addis Ababa, the capital city of Ethiopia. This hospital serves more than 3.5 million people who reside in the town and neighbouring areas. In the maternity department, a total of 7 gynaecologists, 1 emergency surgeon, 1 MSc clinical midwife, 14 general practitioners, and 36 midwives work as health care providers. This department has 60 beds for inpatient clients to serve high-risk mothers, gynecologic case-patients, and postnatal mothers. The annual delivery report showed that 6017 mothers gave childbirth in this hospital. The study was conducted by using an institutional-based cross-sectional study design method and data was collected from August 1st to October 30th, 2019.
2.2. Source and Study Population
All postnatal mothers who gave birth in Debre Markos Referral Hospital and mothers who gave birth somewhere else but came to the hospital within 24 hours of the postpartum period were considered as source population. Mothers who fulfilled the criteria to be a source population and avail themselves during the data collection period were the study population.
2.3. Inclusion and Exclusion Criteria
All postnatal mothers who gave birth in Debre Markos Referral Hospital and mothers who gave birth somewhere else but arrived at the hospital within 24 hours during the data collection period were included in this study. Mothers who were anaemic before conception and/or during pregnancy were excluded in this study.
2.4. Sample Size Determination
Sample size calculation was based on single population proportion formula by using the following assumption: 50% proportion since there were no the same previous studies, 95% confidence level, and 5% margin of error, and then the calculated sample size was 385. Finally, by adding a 10% nonresponse rate, the final sample size was 424. Sample size determination by using the second objective (statistically significant factors) was calculated by Epi info version 7.2.1, and the maximum sample size was 312. Therefore, the sample calculated by the first objective was larger than the sample size determined by the second objective. Therefore, the final sample size for this study was 424.
2.5. Sampling Technique and Procedure
Systematic random sampling was employed to select the study participants. Based on 3 consecutive previous month's average number of delivery data (497), bed numbers in maternity unit (60), and sample size (424); interval was computed. Beds occupied by postnatal mothers were selected with a systematic random sampling technique every 8th bed interval. After written informed consent was taken, data were collected through interviews and chart reviews. Blood samples were collected and processed using the standard procedures for haemoglobin determination at 24 hours before mothers have discharged from the hospital.
2.6. Study Variables
2.6.1. Dependent Variable
Dependent variable is immediate postpartum anaemia.
2.6.2. Independent Variables
Sociodemographic related variables include age, maternal education level, maternal occupation, religion, residence, marital status, husband education level, husband occupation, and estimated average monthly income. Obstetrical related variables include parity, antepartum haemorrhage, multiple pregnancies, abortion, interpregnancy interval, antenatal care visit, frequency of antenatal care visit, gestational age at initial (first) ANC visit, place of delivery, mode of delivery, duration of second-stage labour, episiotomy, perineal tear, the weight of newborn, and postpartum haemorrhage. Coexisting infections related variables include helminths infestation, malaria infection, medical disease during pregnancy, HIV/AIDS, syphilis, and urinary tract infection. Dietary and micronutrient uptake related variables include hot drink (tea, coffee, or milk) when she has taken iron, meal frequency per day, adherence of IFA, and midupper arm circumference (MUAC).
2.7. Operational Definitions
Immediate postpartum period is the first 24 hours after childbirth [26].
Postpartum anaemia is when the haemoglobin level is less than 11 gm/dl at 24 hours of the postpartum period [19, 27].
Adherence of iron and folic acid supplementation means women who had taken iron folate supplements ≥90 days during the most recent pregnancy [8].
2.8. Data Collection Tools and Procedures
The questionnaire was developed after reviewing different pieces of literature [20, 21, 28] conducted in different parts of the world. First, the questionnaire was prepared in English and translated into a local language Amharic and then backto English to keep consistency. Two BSc midwives and one master midwife were recruited for data collection and supervision, respectively. After taking written informed consent, data were collected through both face-to-face interview and retrospective maternal chart review by using a semistructured pretested questionnaire. MUAC was measured via tape measures on the nondominant hand, mostly left hand. The result was interpreted to the United Nation International Children's Emergency Fund (UNICEF) and WHO recommendations of cutoff point <23 cm as undernourished and ≥23 cm as well nourished. About 1-2 milliliter of venous blood was collected from each study participant aseptically for haemoglobin estimation. Then, haemoglobin was determined using automated blood analyzer Cell-Dyne 1800 (Abbot Laboratories Diagnostic Division, USA) by experienced laboratory technologist. Finally, the level of haemoglobin was collected and attached to their respective charts. At the end, anaemic mothers were managed with iron and folate or transfused blood based on their haemoglobin levels and advised on iron-rich diets intake.
2.9. Data Quality Control
The training was provided for both data collectors and a supervisor. The questionnaire was pretested at Debre Berhan referral hospital with 5% of the sample size. Completeness, clarity, and appropriateness of the questionnaire were modified accordingly after the pretest. Completeness of data was checked daily.
2.10. Data Processing and Analysis
Collected data were checked for completeness, consistency, clarity, and missed values. Then, it was cleaned, coded, and entered using Epi Data version 4.6.0.0 and then exported to SPSS (Statistical Package for Social Science) version 24 for analysis. Descriptive analysis: frequency, proportion, mean, median, and standard deviation were computed and presented with texts, tables, and graphs. The normality assumption was checked with a histogram and box plot. Multicollinearity and chi-square assumptions were done. Then, binary logistic regression was performed and variables at p value <0.2 were entered into a multivariable logistic regression to control possible confounders. Crude and adjusted odds ratios with 95% confidence interval were computed. Finally, variables at p value <0.05 were considered as statistically significant.
3. Results
3.1. Sociodemographic Characteristics
Four hundred twenty-four immediate postnatal mothers were involved in this study. The mean age of the study population was 27.79 years with a standard deviation of ±5.12. Based on the age category, 241 (56.8%) study participants were with the age range of 25–34 years. More than one-third (35%) of mothers were unable to read and write and two hundred seventy-seven (65.3%) of mothers were housewives by their occupation. Almost all (95.5%) were orthodox Christian religion followers. More than half (55.7%) of the participants were from urban areas. More than one-third (40.9%) of husbands were farmers. Most of the participants' husband might be predominantly involved in farming activities and urban farming is also a common practice these days. Regarding estimated average monthly income, 158 (39.2%) of the respondents' income was within the range of 1000–3000 birr with a median income of 3500 birr and IQR ± 3500 birr. (Table 1).
Table 1.
Sociodemographic related characteristics of postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019 (n = 424).
| Variables | Category | Frequency | Percent (%) |
|---|---|---|---|
| Age | 15–24 | 121 | 28.6 |
| 25–34 | 241 | 56.8 | |
| 35–49 | 62 | 14.6 | |
|
| |||
| Maternal education status | Unable to read and write | 148 | 35.0 |
| Able to read and write | 49 | 11.6 | |
| Primary class completed | 55 | 13.0 | |
| Secondary class completed | 81 | 19.1 | |
| Diploma and above | 90 | 21.3 | |
|
| |||
| Occupation of respondent | Housewife | 277 | 65.3 |
| Government employee | 73 | 17.2 | |
| Private employee | 25 | 5.9 | |
| Merchant | 38 | 9 | |
| Others∗ | 11 | 2.6 | |
|
| |||
| Religion | Orthodox | 405 | 95.5 |
| Muslim | 14 | 3.3 | |
| Protestant | 5 | 1.2 | |
|
| |||
| Residence | Rural | 188 | 44.3 |
| Urban | 236 | 55.7 | |
|
| |||
| Marital status | Married | 408 | 96.2 |
| Unmarried | 16 | 3.8 | |
|
| |||
| Husband education level | Unable to read and write | 133 | 32.3 |
| Able to read and write | 31 | 7.5 | |
| Primary class completed | 55 | 13.4 | |
| Secondary class completed | 80 | 19.4 | |
| Diploma and above | 113 | 27.4 | |
|
| |||
| Husband occupation | Government employee | 104 | 25.5 |
| Private employee | 68 | 16.7 | |
| Merchant | 49 | 12 | |
| Farmer | 167 | 40.9 | |
| Others∗ | 20 | 4.9 | |
|
| |||
| Monthly income (ET birr) | <1000 | 18 | 4.5 |
| 1000–3000 | 158 | 39.2 | |
| 3001–5000 | 102 | 25.3 | |
| ≥5001 | 125 | 31.0 | |
∗Others represent daily labourers and students for both occupation of study participants (mothers) and husbands.
3.2. Obstetrical Related Factors
Among the total 424 study participants, 218 (51.5%) were primipara mothers. Fifty-six (25%) of the mothers had a short interpregnancy interval which was less than two years. A majority (88.4%) of the study participants had antenatal care follow-up during the most recent pregnancy. From those, more than half (60.6%) of the mothers had ≥4 ANC visits, and two hundred fifty-nine (69.1%) of them started the follow-up before 16 weeks of gestation. Of the total study participants, 27 (6.4%) of them had an antepartum haemorrhage in the latest pregnancy. Almost all (95.0%) of the study participants gave birth in health institutions and more than half (52.6%) of the study participants gave childbirth through cesarean section, and 89.5% of the operations were emergency/unplanned. (Table 2).
Table 2.
Obstetrical related characteristics of postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019 (n = 424).
| Variables | Category | Frequency | Percent (%) |
|---|---|---|---|
| Parity | Primipara | 218 | 51.5 |
| Para 2–4 | 171 | 40.2 | |
| Grand multiparous | 35 | 8.3 | |
|
| |||
| History of abortion | Yes | 71 | 31.6 |
| No | 154 | 68.4 | |
|
| |||
| Interpregnancy interval in years | <2 | 56 | 25.0 |
| ≥2 | 168 | 75.0 | |
|
| |||
| Attend ANC during this pregnancy | Yes | 375 | 88.4 |
| No | 49 | 11.6 | |
|
| |||
| GA when ANC visit initiated in weeks | <16 | 259 | 69.1 |
| 16–24 | 69 | 18.4 | |
| 25–30 | 45 | 12.0 | |
| 31–34 | 2 | 0.5 | |
|
| |||
| Frequency of ANC visits (times) | <4 | 148 | 39.4 |
| ≥4 | 227 | 60.6 | |
|
| |||
| Antepartum haemorrhage | Yes | 27 | 6.4 |
| No | 397 | 93.6 | |
|
| |||
| Multiple pregnancies | Yes | 23 | 5.4 |
| No | 401 | 94.6 | |
|
| |||
| Place of birth | Health institution | 403 | 95.0 |
| Home | 21 | 5.0 | |
|
| |||
| Mode of delivery | SVD | 176 | 41.5 |
| IAVD | 25 | 5.9 | |
| C/S | 223 | 52.6 | |
|
| |||
| Type of C/S | Elective | 25 | 10.5 |
| Emergency | 198 | 89.5 | |
|
| |||
| Manual removal of placenta | Yes | 20 | 4.7 |
| No | 404 | 95.3 | |
|
| |||
| Episiotomy | Yes | 19 | 4.5 |
| No | 405 | 95.5 | |
|
| |||
| Prolonged second stage | Yes | 32 | 7.5 |
| No | 392 | 92.5 | |
|
| |||
| Perineal tear | Yes | 41 | 9.7 |
| No | 383 | 90.3 | |
|
| |||
| Weight of newborn in gram | <2500 | 72 | 17.0 |
| 2500–3999 | 348 | 82.1 | |
| ≥4000 | 4 | 0.9 | |
|
| |||
| Postpartum haemorrhage | Yes | 60 | 14.2 |
| No | 364 | 85.8 | |
ANC: antenatal care, GA: gestational age, IAVD: instrumental assisted vaginal delivery, SVD: spontaneous vaginal delivery, and C/S: cesarean section.
3.3. Coexisting Infection-Related Factors
One hundred twelve (26.5%) of the respondents had clinically confirmed medical and parasitic illness before or/and during the latest pregnancy. Among these, preeclampsia was the most frequent complaint (33.60%), whereas tuberculosis was the least one (2.64%). (Figure 1).
Figure 1.
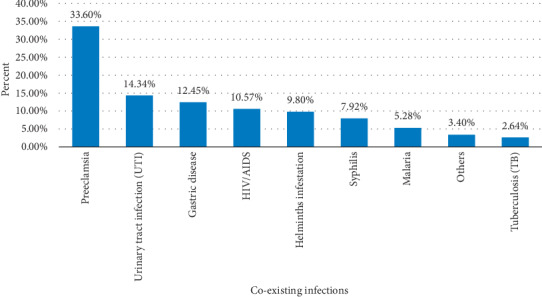
Magnitude of coexisting infections among postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019. ∗Others include liver disease, heart disease, and pneumonia.
3.4. Dietary and Micronutrients Utilization Related Factors
Three hundred sixty-eight of the study participants were started on IFA tablets during the most recent pregnancy. Among these, 176 (51.5%) were started before 16 weeks of gestation, whereas 45.9% and 2.6% were started during second- and third-trimester pregnancy, respectively. Among those mothers who took IFA tablets, only 93 (27.3%) of them had good adherence. Among IFA tablets supplied mothers, 135 (39.5%) participants drank hot drink when they took iron. More than two-thirds of 322 (76.1%) of the mothers ate three or fewer times per day during pregnancy. Close to half (46%) of the mothers' midupper arm circumference was less than 23 cm. (Table 3).
Table 3.
Dietary and micronutrient uptake characteristics of postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019 (n = 424).
| Variables | Category | Frequency | Percent (%) |
|---|---|---|---|
| IFA tablet took during pregnancy | Yes | 368 | 86.8 |
| No | 56 | 13.2 | |
|
| |||
| GA when IFA tablet started | <16 weeks | 176 | 51.5 |
| 20–24 weeks | 90 | 26.3 | |
| 26–30 weeks | 67 | 19.6 | |
| 30–34 weeks | 9 | 2.6 | |
|
| |||
| Adherence to iron and folic acid supplementation | Poor adherence | 248 | 72.7 |
| Good adherence | 93 | 27.3 | |
|
| |||
| Hot drink while taking iron | Yes | 135 | 39.5 |
| No | 207 | 60.5 | |
|
| |||
| Frequency of meal per day | ≤3 | 322 | 76.1 |
| >3 | 101 | 23.9 | |
|
| |||
| Midupper arm circumference in centimeter (cm) | <23 | 195 | 46.0 |
| ≥23 | 229 | 54.0 | |
IFA: iron and folic acid, GA: gestational age.
3.5. Proportion and Associated Factors of Immediate Postpartum Anaemia
3.5.1. The Proportion of Immediate Postpartum Anaemia
Postpartum anaemia was observed among 103 (24.3%) mothers. Postpartum haemoglobin concentrations of study participants ranged from 6.70 gm/dl to 17.50 gm/dl with a mean value of 12.4 gm/dl and SD ± 1.88 gm/dl. From the total 24.3% anaemic mothers, 13.4%, 10.2%, and 0.7% of them were categorized as mild, moderate, and severe anaemia, respectively (Figure 2).
Figure 2.
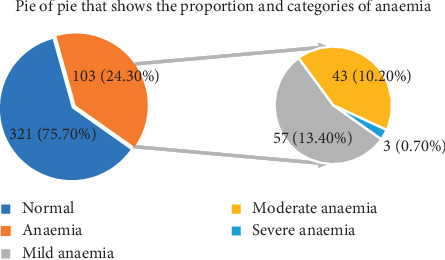
Categories of immediate postpartum anaemia among postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019 (n = 424).
3.5.2. Associated Factors of Postpartum Anaemia
In binary logistic regression analysis, maternal educational level (being unable to read and write and able to read and write), rural residence, home delivery, preterm delivery, and less frequent meal per day were variables associated with immediate PPA with p value <0.2, but all these variables were eliminated by backward multivariate regression. In multivariable logistic regression, antenatal care visit <4 times, antepartum haemorrhage, instrumental delivery, postpartum haemorrhage, poor adherence to IFA supplementation, and MUAC <23 cm were independent variables significantly associated with p value <0.05.
The odds of postpartum anaemia were higher among postnatal women who had <4 antenatal care visits [AOR = 2.40; 95% CI (1.29, 4.43)]. The higher likelihood of postpartum anaemia was observed among postnatal women who were experienced APH and PPH compared to their counterparts [AOR = 5.08; 95% CI (1.91, 13.55)] and [AOR = 4.47; 95% CI (2.25, 8.88)], respectively. Mothers who gave birth by instrumental assisted vaginal delivery were almost 4 times more likely to be anaemic compared to those who gave birth through spontaneous vaginal delivery [AOR = 3.99; 95% CI (1.42, 11.23)]. Mothers who poorly adhere to IFA supplementation were 2.5 times more likely to develop postpartum anaemia compared to those who had good adherence [AOR = 2.52; 95% CI (1.06, 6.04)]. Increased odds of anaemia were noted among postpartum women with MUAC measurements of <23 cm [AOR = 3.25; 95% CI (1.87, 5.65)] (Table 4).
Table 4.
Logistic regression showing factors associated with immediate postpartum anaemia among postnatal mothers in Debre Markos Referral Hospital, Northwest Ethiopia, 2019 (n = 424).
| Independent variables | Postpartum anaemia | COR 95% CI | AOR 95%CI | |
|---|---|---|---|---|
| Yes | No | |||
| Maternal education status | ||||
| Unable to read and write | 55 | 93 | 5.32 (2.48–11.44) | 2.00 (0.81–4.88) |
| Able to read and write | 14 | 35 | 3.60 (1.43–9.09) | 2.05 (0.71–5.87) |
| Primary school class | 10 | 45 | 2.00 (0.76–5.23) | 1.66 (0.57–4.80) |
| Secondary school class | 15 | 66 | 2.04 (0.85–4.98) | 1.09 (0.40–2.99) |
| Diploma and above | 9 | 81 | 1 | 1 |
|
| ||||
| Residence | ||||
| Rural | 59 | 129 | 2.00 (1.27–3.13) | 0.63 (0.31–1.29) |
| Urban | 44 | 192 | 1 | 1 |
|
| ||||
| Frequency of ANC visits | ||||
| <4 times | 46 | 102 | 3.10 (1.84–5.25) | 2.40 (1.29–4.43)∗ |
| ≥4 times | 29 | 198 | 1 | 1 |
|
| ||||
| Antepartum haemorrhage | ||||
| Yes | 17 | 10 | 6.15 (2.71–13.9) | 5.08 (1.91–13.55)∗ |
| No | 86 | 311 | 1 | 1 |
|
| ||||
| GA at delivery | ||||
| Preterm | 31 | 58 | 1.96 (1.18–3.25) | 1.41 (0.73–2.71) |
| Term | 69 | 255 | 1 | 1 |
|
| ||||
| Place of birth | ||||
| Health institution | 92 | 311 | 1 | 1 |
| Home | 11 | 10 | 3.72 (1.61–9.00) | 1.92 (0.60–6.19) |
|
| ||||
| Mode of delivery | ||||
| SVD | 39 | 137 | 1 | 1 |
| IAVD | 13 | 12 | 3.81 (1.57–8.77) | 3.99 (1.42–11.23)∗ |
| Cesarean section | 51 | 172 | 1.04 (0.65–1.67) | 1.06 (0.59–1.88) |
|
| ||||
| Postpartum haemorrhage | ||||
| Yes | 36 | 24 | 6.65 (3.72–11.89) | 4.47 (2.25–8.88)∗ |
| No | 67 | 297 | 1 | 1 |
|
| ||||
| Adherence of IFA supplementation | ||||
| Poor adherence | 53 | 195 | 2.88 (1.31–6.33) | 2.52 (1.06–6.04)∗ |
| Good adherence | 8 | 85 | 1 | 1 |
|
| ||||
| Frequency of meal per day | ||||
| ≤3 | 87 | 235 | 1.97 (1.09–3.54) | 1.21 (0.59–2.48) |
| >3 | 16 | 85 | 1 | 1 |
|
| ||||
| Midupper arm circumference | ||||
| <23 cm | 71 | 124 | 3.53 (2.12–5.66) | 3.25 (1.87–5.65)∗ |
| ≥23 cm | 32 | 197 | 1 | 1 |
GA: gestational age; SVD: spontaneous vaginal delivery; IAVD: instrumental assisted vaginal delivery; IFA: iron and folic acid; AOR: adjusted odds ratio; COR: crude odds ratio; 1: reference and ∗significantly associated with p value <0.05.
4. Discussion
This study assessed the proportion and factors associated with immediate postpartum anaemia among postnatal mothers at 24-hour postpartum period in Debre Markos Referral Hospital. The proportion of immediate postpartum anaemia was 24.3%.
This value was in line with the study done in Germany (22%), Jimma (28.7%), Costal Karnataka (26.5%), and Mekelle (24.2%), respectively [20, 21, 24, 29]. But, haemoglobin cutoff points to define anaemia and the time (postpartum period) to diagnose anaemia were different.
The magnitude of immediate postpartum anaemia in this study was lower than the study done in Uganda (30%) [17]; Madrid, Spain (29%) [30]; Mancha Centro hospital, Spain (45%) [28]; Tamil Nadu, India (47.3%) [23]; Pakistan (47.9%) [15]; and Myanmar 73.8% [27]. The possible reason for this variation might be due to anaemic mothers in preconception and pregnancy period being excluded from this study, use of different cutoff points to define postpartum anaemia, and difference in postpartum time of screening. Due to a lack of consensus on the definition of PPA, different scholars use different cutoff points, like Hgb<12 mg/dl at 10 weeks in Uganda, Hgb <11 mg/dl at 24 hours postpartum in Mancha Centro hospital, Spain, and Hgb <12 mg/dl among all lactating in Myanmar. Geographical difference might be also another factor for the abovementioned variation. Additionally, a staple food in Asian countries is rice, which lacks iron content. Ethiopians are eating cereals, “teff injera” (ferment teff flour), and fruits that do have high iron content [21].
However, this proportion was higher than the Amhara region DHS data report (17.2%) [6]; the study was done in Kenya (16.4%) [8] and Ghana (16%) [31]. The variations might be because the above studies cover a wide range of areas and include all breastfeeding mothers who were far from the immediate postpartum period or close to 6-week and 6-month postpartum period. This shows that when the time of the postpartum period extends, mothers will have enough time to recover from anaemia or haemoglobin level will be increased [32].
Independent factors significantly associated with immediate postpartum anaemia were frequency of ANC visits <4 times in the most recent pregnancy, having antepartum haemorrhage during most recent pregnancy, instrumental assisted vaginal delivery, having the experience of primary postpartum haemorrhage during the most recent birth, poor adherence to IFA supplementation during the latest pregnancy, and MUAC <23 cm at the time of interview.
A majority (88.4%) of the study participants had ANC follow-up and 39% of them had <4 times of visits. The odds of postnatal mothers who had <4 times of antenatal care visits were about 2.4-fold higher to develop postpartum anaemia than those who had ≥4 ANC visits. This finding was supported by the studies conducted in Jimma, Tigray, and Ethiopia DHS data [21, 33, 34]. The possible explanations are as follows: mothers who had <4 ANC visits might not get enough IFA supplements, have poor adherence to IFA supplementation, and not screened/detected the risk factors early and treat on time.
Among all study participants, 27 (6.4%) of them had an antepartum haemorrhage. Mothers who had antepartum haemorrhage were 5 times more likely to be anaemic in the immediate postpartum period, compared to their counterparts. This finding was consistent with the finding from Germany [20]. The possible explanation might be due to the loss of iron stores during pregnancy and blood loss during delivery could be the complications of APH.
The odds of anaemia among postpartum mothers who experienced massive postpartum blood loss were 4.5 times higher than the odds of anaemia among postnatal mothers who did not develop postpartum haemorrhage. The similar findings were reported in Germany, Saudi Arabia, and Tamil Nadu, India [14, 20, 35]. Excessive bleeding after birth decreases the red blood cell component called haemoglobin. In every milliliter blood loss, a half milligram of iron will be reduced in the blood [11].
Mothers who gave birth by instrumental (vacuum or forceps) assisted mode of delivery were almost 4 times more likely to be anaemic in the postpartum period when compared to those who gave birth through spontaneous vaginal delivery. This finding agreed with the studies done in Spain (two studies) and Saudi Arabia [11, 18, 35]. It might be due to the fact that instrumental assisted vaginal delivery increases the risk of episiotomy, spontaneous perineal, or/and cervical tear, and this tear may be also extended to the uterus. Clinicians are usually misdiagnosing the tears and repairing after mothers bleed a lot.
The odds of PPA were 2.5-fold higher among postpartum mothers who had poor adherence to IFA supplementation compared to their counterparts. This finding was in agreement with the studies carried out in Uganda, Pakistan, and Tanzania [15, 23, 25]. The possible explanation might be due to the depleting of stored maternal iron since physiologic requirements of iron during pregnancy and labour are high. Therefore, not taking IFA based on the right order could reduce iron store and results in anaemia even with minimal blood loss during childbirth.
Increased odds of anaemia were noted among postnatal mothers whose MUAC measurements <23 cm compared to those whose MUAC measurements ≥23 cm. This study is supported by the study done in Jimma, Myanmar, and Tanzania [21, 25, 36]. The most likely explanation might be iron deficiency anaemia usually related to nutritional deficiency. MUAC measurement <23 cm indicates that poor muscle mass lacks adequate energy intake. Haemoglobin concentration and maternal MUAC had a linear relationship which was also another explanation [16].
4.1. Limitation of the Study
Since the interview was about the past nine months' activity of mothers, recall bias was one of the limitations. Besides, environmental and behavioural factors were not assessed. The study was facility based and difficult to generalize for the entire community. As the study design was a cross-sectional, a causal relationship could not be established.
5. Conclusions
The proportion of postpartum anaemia was a moderate public health problem in Debre Markos Referral Hospital. Antepartum haemorrhage, instrumental assisted vaginal delivery, frequency of antenatal care visit <4 times, postpartum haemorrhage, poor adherence to IFA supplementation, and MUAC less than 23 cm were independent factors significantly associated with immediate postpartum anaemia. Therefore, interventions that would address the abovementioned factors need to be implemented, and further researches that will address this study limitation should be also considered.
Acknowledgments
The authors are grateful to Debre Markos Referral Hospital and the midwives working therein for their unreserved guidance at the time of the interview and for their help to access maternal charts easily. The authors would also like to thank the Amhara Regional Health Bureau for their financial support for data collectors and to buy materials. Last but not least, the authors extend their special thanks to data collectors and study participants. This study was funded by the Amhara Regional Health Bureau.
Abbreviations
- ANC:
Antenatal care
- AOR:
Adjusted odds ratio
- APH:
Antepartum haemorrhage
- COR:
Cruds odds ratio
- CS:
Cesarean section
- DHS:
Demographic health survey
- GA:
Gestation age
- HIV:
Human immune virus
- IAVD:
Instrumental assisted vaginal delivery
- IFA:
Iron and folic acid
- MUAC:
Midupper arm circumference
- OR:
Odds ratio
- PPA:
Postpartum anaemia
- PPH:
Postpartum haemorrhage
- SPSS:
Statistical package for social science
- SVD:
Spontaneous vaginal delivery
- TB:
Tuberculosis
- VDRL:
Venereal disease research history
- WHO:
World Health Organization.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
Ethical approval was obtained from the University of Gondar, College of Medicine and Health Science, School of Midwifery Ethical Clearance Committee. A permission letter was obtained from the Debre Markos Referral Hospital administrator before the starting of the study. Each study participant was informed about the benefits and objectives of the study.
Consent
Written informed consent was obtained from all the study participants. The willingness nature of participation and the right to refuse or withdraw at any time was emphasized. Confidentiality was maintained during and after data collection.
Disclosure
The funder had no role in the study design, analysis, interpretation, writing, or decision to publish this manuscript.
Conflicts of Interest
The authors report no conflicts of interest in this work.
Authors' Contributions
All the authors contributed in conception and design, data entry, analysis, interpretation, writing of the report, and critically revising the paper. They approved the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: World Health Organization; 2013. https://www.who.int/maternal_child_adolescent/documents/postnatal-care-recommendations/en/ [PubMed] [Google Scholar]
- 2.Milman N. Postpartum anemia: definition, prevalence, causes, and consequences. Annals of Hematology. 2011;90(11):1247–1253. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 3.FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: iron deficiency anemia in pregnancy. International Journal of Gynecology & Obstetrics. 2019;144(3):322–324. doi: 10.1002/ijgo.12740. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Hemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization; 2011. http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 5.Api O., Breyman C., Cetiner M., Demir C., Ecder T. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. Journal of Turkish Society of Obstetrics and Gynecology. 2015;12(3):173–181. doi: 10.4274/tjod.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rukiya A., Gachuno O., Machoki J. M. Determinants of post-partum anaemia–a cross sectional study. Journal of Obstetrics and Gynecology of Eastern and Central Africa. 2015;27(1):33–82. [Google Scholar]
- 7.Federal Democratic Republic of Ethiopia. Goverment of Ethiopia National Nutritional Program, 2016–2020. Addis Ababa, Ethiopia: Federal Democratic Republic of Ethiopia; 2016. [Google Scholar]
- 8.Central Statistical Agency (CSA), ICF. Demographic and Health Survey 2016. Addis Ababa, Ethiopia: CSA and ICF; 2017. [Google Scholar]
- 9.Ethiopian Public Health Institute. Emergency Obstetric and Newborn Care (EmONC) Assessment 2016. Addis Ababa, Ethiopia: Ethiopian Public Health Institute; 2017. [Google Scholar]
- 10.World Health Organization. Nutritional Anaemias Tools for Effective Prevention and Control. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 11.Rathod S., Samal S. K., Samal S., Mahapatra P. C. A revolution in the treatment of postpartum anemia in Indian women. International Journal of Applied and Basic Medical Research. 2015;5(1):p. 25. doi: 10.4103/2229-516x.149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas J. D., Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. The Journal of Nutrition. 2001;131(2):676S–690S. doi: 10.1093/jn/131.2.676s. [DOI] [PubMed] [Google Scholar]
- 13.Wassef A., Nguyen Q. D., St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. Journal of Psychosomatic Obstetrics & Gynecology. 2018;40(1):19–28. doi: 10.1080/0167482x.2018.1427725. [DOI] [PubMed] [Google Scholar]
- 14.Rakesh P. S., Gopichandran V., Jamkhandi D., Manjunath K., George K., Prasad J. Determinants of postpartum anemia among women from a rural population in southern India. International Journal of Women’s Health. 2014;6:395–400. doi: 10.2147/ijwh.s58355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Innocent N. H., Mugyenyi G. R., Ssalongo W. G. M., et al. Incidence and factors associated with postpartum anemia at mbarara regional referral hospital. Journal of Health, Medicine and Nursing. 2016;23:2422–8419. [Google Scholar]
- 16.Feleke B. E., Feleke T. E. Pregnant mothers are more anemic than lactating mothers, a comparative cross-sectional study, Bahir Dar, Ethiopia. BMC Hematology. 2018;18(1):p. 2. doi: 10.1186/s12878-018-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio-Álvarez A., Molina-Alarcón M., Hernández-Martínez A. Incidence of postpartum anaemia and risk factors associated with vaginal birth. Women and Birth. 2018;31(3):158–165. doi: 10.1016/j.wombi.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Brichs X. U., Carballeira M. R., Fernández A. G., Picanol E. P. Anaemia in pregnancy and in the immediate postpartum period. Prevalence and risk factors in pregnancy and childbirth. Medicina Clínica. 2016;146(10):429–435. doi: 10.1016/j.medcli.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Infante-Torres N., Molina-Alarcón M., Rubio-Álvarez A., Rodríguez-Almagro J., Hernández-Martínez A. Relationship between duration of second stage of labour and postpartum anaemia. Women and Birth. 2018;31(5):318–324. doi: 10.1016/j.wombi.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann R. L., Richter R., Bergmann K. E., Dudenhausen J. W. Prevalence and risk factors for early postpartum anemia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2010;150(2):126–131. doi: 10.1016/j.ejogrb.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Alemayehu M. Factors associated with anemia among lactating mothers in subsistence farming households from selected districts of Jimma zone, south western Ethiopia: a community based cross-sectional study. Journal of Nutrition & Food Sciences. 2017;7(3):p. 595. doi: 10.4172/2155-9600.1000595. [DOI] [Google Scholar]
- 22.Emegoakor C. F., Iyoke C. A., Ezegwui H. U., Umeora O. U., Lawani L. O., Madu A. J. Rates and determinants of peripartum and puerperal anemia in Enugu, Nigeria. Nigerian Journal of Clinical Practice. 2016;19(6):709–714. doi: 10.4103/1119-3077.178912. [DOI] [PubMed] [Google Scholar]
- 23.Rabia S., Jalil N., Feroze S., et al. Frequency and determinants of maternal anaemia in early postpartum period. Annals of Abbasi Shaheed Hospital & Karachi Medical & Dental College. 2018;23(1) [Google Scholar]
- 24.Bhagwan D., Kumar A., Rao C. R., Kamath A. Prevalence of anaemia among postnatal mothers in coastal Karnataka. Journal of Clinical and Diagnostic research. 2016;10(1) doi: 10.7860/jcdr/2016/14534.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petraro P., Duggan C., Urassa W., et al. Determinants of anemia in postpartum HIV-negative women in Dar es Salaam, Tanzania. European Journal of Clinical Nutrition. 2013;67(7):708–717. doi: 10.1038/ejcn.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. WHO Technical Consultation on Postpartum and Postnatal Care. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 27.Dündar B., Çakmak B. D. The prevalence and analysis of risk factors for postpartum anemia in women without prepartum anemia. Haydarpaşa Numune Training and Research Hospital Medical Journal. 2019;59(2):165–170. doi: 10.14744/hnhj.2019.75436. [DOI] [Google Scholar]
- 28.Garrido C. M., León J., Vidal A. R. Maternal anaemia after delivery: prevalence and risk factors. Journal of Obstetrics and Gynaecology. 2018;38(1):55–59. doi: 10.1080/01443615.2017.1328669. [DOI] [PubMed] [Google Scholar]
- 29.Fanta G. A., Zelelow Y. B. Prevalence and associated risk factors of immediate postpartum anemia in two teaching hospitals in Mekelle. Ethiopian Journal of Reproductive Health. 2020;12(1) [Google Scholar]
- 30.Zhao A., Cao S. J., Gao H. C., Xiao Q. Y., Win N. N., Zhang Y. M. Anemia among lactating mothers in Kokang, Myanmar. The Southeast Asian Journal of Tropical Medicine and Public Health. 2016;47(6):1298–1305. [PubMed] [Google Scholar]
- 31.Kofie P., Tarkang E. E., Manu E., et al. Prevalence and associated risk factors of anaemia among women attending antenatal and post-natal clinics at a public health facility in Ghana. BMC Nutrition. 2019;5(1):p. 40. doi: 10.1186/s40795-019-0303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinlaja O. Hematological changes in pregnancy-The preparation for intrapartum blood loss. Obstetrics & Gynecology International Journal. 2016;4(3):p. 109. doi: 10.15406/ogij.2016.04.00109. [DOI] [Google Scholar]
- 33.Haileslassie K., Mulugeta A., Girma M. Feeding practices, nutritional status and associated factors of lactating women in Samre Woreda, South Eastern Zone of Tigray, Ethiopia. Nutrition Journal. 2013;12(1):p. 28. doi: 10.1186/1475-2891-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakew Y., Biadgilign S., Haile D. Anaemia prevalence and associated factors among lactating mothers in Ethiopia: evidence from the 2005 and 2011 demographic and health surveys. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006001.e006001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattar G., Alsahafi N., Shami B., Abulkhair S., Alhazmi N., Alsaleh R. Incidence of postpartum anemia among postpartum patients in East Jeddah Hospital. International Journal of Pharma and Bio Sciences. 2019;9(2):39–46. doi: 10.22376/ijpbs/lpr.2019.9.2.p39-46. [DOI] [Google Scholar]
- 36.Zhao A., Gao H., Zhang Y., et al. Prevalence of anemia and its risk factors among lactating mothers in Myanmar. The American Journal of Tropical Medicine and Hygiene. 2014;90(5):963–967. doi: 10.4269/ajtmh.13-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


