Abstract
Mutations in the X-linked gene IQSEC2 are associated with multiple cases of epilepsy, epileptic encephalopathy, intellectual disability and autism spectrum disorder, the mechanistic understanding and successful treatment of which remain a significant challenge in IQSEC2 and related neurodevelopmental genetic diseases. To investigate disease etiology, we studied behaviors and synaptic function in IQSEC2 deficient mice. Hemizygous Iqsec2 null males exhibit growth deficits, hyperambulation and hyperanxiety phenotypes. Adult hemizygotes experience lethal spontaneous seizures, but paradoxically have a significantly increased threshold to electrically induced limbic seizures and relative resistance to chemically induced seizures. Although there are no gross defects in brain morphology, hemizygotes exhibit stark hippocampal reactive astrogliosis. Electrophysiological recordings of hippocampal neurons reveal increased excitatory drive specifically onto interneurons, and significant alterations in intrinsic electrical properties specific to the interneuron population. As they age, hemizygotes also develop an increased abundance of parvalbumin-positive interneurons in the hippocampus, neurons in which IQSEC2 is expressed in addition to the excitatory neurons. These findings point to a novel role of IQSEC2 in hippocampal interneuron synaptic function and development with implications for a class of intractable neurodevelopmental diseases.
Keywords: IQSEC2, knockout mouse model, epileptic encephalopathy, seizure, synaptic signaling, interneurons, behavior
1. Introduction
Mutations in the IQSEC2 gene on Chromosome Xp11.22 are implicated in multiple cases of X-linked epileptic encephalopathy (XLEE) and X-linked intellectual disability (XLID). IQSEC2 encodes a large 1488 amino acid protein with multiple functionally important domains such as the catalytic Sec 7 domain for loading GTP onto the small GTPase Arf6, a pleckstrin homology (PH) domain, a putative calmodulin binding IQ-like motif, a coiled-coiled (CC) motif and a PDZ protein binding domain (Murphy et al. 2006; Sanda et al. 2009). The IQSEC guanine exchange factor (GEF) family consists of three members - IQSEC1, IQSEC2 and IQSEC3 - proteins with non-overlapping functions. While IQSEC1 and IQSEC2 are expressed exclusively at excitatory synapses, IQSEC3 is a part of the inhibitory postsynapse (Um 2017). The IQSEC2 protein is highly enriched in the postsynaptic density (PSD) of excitatory glutamatergic synapses where it has been shown to form protein-protein interactions with postsynaptic density (PSD) proteins such as PSD-95 (Sakagami et al. 2008), IRSp53 (Sanda et al. 2009) CamkII (Baucum et al. 2015), SAP-97 (Baucum et al. 2015) and NMDARs (Elagabani et al. 2016). Functionally, at excitatory synapses, it has been shown to carry out activity dependent removal of the AMPAR subunit GluA1 (Myers et al. 2012), insertion of AMPAR subunit GluA2 (Brown et al. 2016), mediate long term depression (Brown et al.,2016), regulate synaptic NMDAR expression (Elagabani et al. 2016) and dendritic spine development (Hinze et al. 2017). Therefore, it can be safely hypothesized that glutamatergic synapse alterations due to mutations in IQSEC2 would underlie associated behavioral and cognitive perturbations.
IQSEC2 was first linked to epileptic encephalopathy in a patient with X autosome translocation causing disruption of the IQSEC2 gene (Morleo et al. 2008). Shortly after, a large X chromosome resequencing study revealed IQSEC2 mutations in patients with non-syndromic X-linked intellectual disability (XLID) (Shoubridge, et al. 2010). Functional assays revealed that the identified mutations affecting conserved amino acids in the functional Sec 7 and IQ-like motif domains all suppressed IQSEC2 catalytic activity (Shoubridge, Walikonis, et al. 2010) Since then, there has been a surge in the number of identified clinical IQSEC2 cases. To date, over 70 pathogenic variants have been recorded , and around 136 patients harboring IQSEC2 mutations reported (Mignot et al. 2018; Shoubridge, Harvey, and Dudding-Byth 2019; Radley et al. 2019; Zerem et al. 2016). Commonly diagnosed IQSEC2 variant phenotype includes epilepsy, intellectual disability, developmental delay, developmental regression, autism , speech and language deficits. (Mignot et al. 2018; Zerem et al. 2016; Radley et al. 2019; Shoubridge, Harvey, and Dudding-Byth 2019). Lennox-Gastaut syndrome and Rett Syndrome-like phenotypes are also reported co-morbidities in a few patients (Olson et al. 2015; Srivastava et al. 2018; Radley et al. 2019; Zerem et al. 2016). The seizure types range from spasms to generalized tonic-clonic, and epilepsy associated with epileptic encephalopathy is pharmacoresistant (Zerem et al. 2016; Shoubridge et al. 2019; Mignot et al. 2018).
Interestingly, Mignot and colleagues (Mignot et al. 2018) report that a significant fraction of IQSEC2 pathogenic variants identified in their screen of 42 patients, 37 of which were previously undiagnosed, are predicted to end in early termination codons. These loss of function variants primarily linked to de novo mutations, affect both sexes and are linked to a spectrum of phenotypic severity spanning from severe intellectual disability, epileptic encephalopathy, global developmental delay, loss of speech and autism.
Despite the steady increase in these reported clinical cases, the research in animal models remains at a nascent stage. The first published report of the Iqsec2 knockout mouse model was in 2017 (Hinze et al. 2017) in which they used hippocampal neuronal cultures from the null mice to examine IQSEC2’s role in regulation of dendritic branching and morphogenesis. Earlier this year, Rogers and colleagues (Rogers et al. 2019) reported on the IQSEC2 animal model harboring a disease-causing missense mutation (A350V) in the IQ-like domain. This mutation exhibits reduced levels of AMPA receptors and autism-like behavioral perturbations. While our manuscript was in preparation, Jackson and colleagues (Jackson et al. 2019) published on the IQSEC2 mouse model which was earlier examined in the 2017 study. Here they carried out behavior and seizure phenotyping primarily of the Iqsec2 heterozygous knockout female mice and showed that downstream Arf6 signaling pathway is altered in Iqsec2 mutants. Additionally, they also reported a novel IQSEC2 nonsense variant C.566C > A, p.(S189*) in an elderly female patient.
Here we describe the impact of IQSEC2 deficiency on disease etiology using the Iqsec2 knockout mouse, modeling the majority of disease variants. We observed altered seizure susceptibility, developmental and behavioral abnormalities, hippocampus-specific reactive gliosis, increased excitatory transmission onto hippocampal interneurons, and atypical expression of hippocampal fast-spiking parvalbumin interneurons. Importantly, these results outline IQSEC2 as an important regulator of in vivo network homeostasis, highlight a novel role in hippocampal interneuron function and suggest new pathophysiological signature in this and related childhood neurological disease.
2. Methods
2.1. Mice and genotyping:
Iqsec2 knockout mice were generated using CRISPR/Cas9 at The Jackson Laboratory (Bar Harbor, ME). Briefly, a guide RNA 5’-AGCGACTCATTGAAGCTTTCAGG-3’ was injected into single-cell mouse embryos of the C57BL/6NJ (B6NJ) inbred strain. Of 9 founder mice, one founder male carrying a 1 nt deletion in approximately 50% of somatic DNA, was bred to wildtype B6NJ females, from which the mutant colony was expanded. For optimizing fecundity and maternal care, mice were maintained on a mixed genetic background of C3HeB/FeJ x B6NJ. Male mice were used for experiments, unless stated otherwise, to avoid the confounding effects of X-inactivation in females. Genotyping was by PCR amplification and Sanger sequencing using primer pairs: Iqsec2 F: ATG GGT GTG TCA GTG GTT TGT GTA GAG, Iqsec2 R: TTG GAA CTC ACT ACA TAG ACC AGT CTG. The protein nomenclature for the frameshift mutation is p.Phe860SerfsTer8.
2.2. RNA isolation and qRT-PCR:
Forebrain, cortex and hippocampus from either young or adult animals were used for RNA analysis. Isolation of total RNA with Trizol (Invitrogen, USA) was operated according to the manufacturer’s protocol with minor modifications and qRT-PCR performed with an Applied Biosystems - Quant Studio5.
RT-PCR primers
Rpl13
F: AGCCGGAATGGCATGATACTG
R: TATCTCACTGTAGGGCACCTC
Pvalb
F: TGTCGATGACAGACGTGCTC
R: TTCTTCAACCCCAATCTTGC
Sst
F: ACCGGGAAACAGGAACTGG
R: TTGCTGGGTTCGAGTTGGC
Iqsec2
5’ CTTCATCCTGGAGAGGAAAGG 3’
5’ CACTGGGGCTGTACATATCG 3’
Iqsec1
F: AGTCCAGACCACTACGAGCA
R: TGCGTTCTAGCATTTCCACCT
Iqsec3
F: CACCATTCAAACCGCTTTTCG
R: CACCAGACTCTCCGCTGTG
2.3. Western blotting:
To evaluate protein levels, protein extracts were prepared as described from dissected forebrain and hippocampal tissue (Murphy et al., 2006). Primary antibodies: IQSEC2 (N-terminus antibody- 1:100; antiserum UCT 88, a gift from Dr. Randall Walikonis recognizes residues 10-198 of human IQSEC2) and AB_2718685 (C-terminus antibody 1:50 ; Invitrogen recognizes residues 1380 - 1430 of human IQSEC2), guinea pig anti-parvalbumin-α (1:1000; Synaptic Systems, Cat# 195004) , rabbit anti-IQSEC1 (1:500 Biorybt, cat# orb183807), rabbit anti-IQSEC3( 1:500, Thermo Fisher, cat# PA5-25866) anti-glial fibrillary acid protein (GFAP; 1:750; Sigma-Aldrich, Cat# G3893), mouse anti-tubulin (1:2000; Biolegend, Cat# 903401). Signal was detected with corresponding HRP-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP substrate (Millipore).
2.4. Immunofluorescence:
Two methods were used for IHC since the IQSEC2 serum antibodies do not work with perfused tissue: Flash-frozen sections (IQSEC2, PV together); PFA perfused sections (PV only). Primary antibodies: rabbit anti-IQSEC2 [1:100; antiserum UCT 88,(Murphy, Jensen, and Walikonis 2006)], chicken anti-IQSEC2 [1:50; antiserum UCT C3(Murphy, Jensen, and Walikonis 2006),guinea pig anti-parvalbumin-α [1:1000; Synaptic Systems, Cat# 195004], and anti-glial fibrillary acid protein (GFAP) antibody (1:750; Sigma-Aldrich, Cat# G3893). Secondary antibodies: Alexa Fluor 488-conjugated goat anti-mouse, 568-conjugated goat anti-rabbit, and Alexa Fluor 647-conjugated goat anti-chicken IgG (1:500 in blocking buffer; Invitrogen) and Alexa Fluor 555-conjugated goat anti-guinea pig (1:500 in blocking buffer; Abcam)
Parvalbumin immunoreactive neurons were imaged using an automated slide-scanner (Zeiss LSM-800 confocal microscope and Zen v2.3). In a blind study, total counts of parvalbumin immunoreactive cells were made from 3 sections per animal from the left hemisphere using ImageJ (v1.46), from dorsal hippocampus separated into the CA1, CA3 and DG regions. The total counts for the hippocampus included the subiculum. Regions were delineated using clearly visible landmarks and predefined boundaries according to the Allen Brain Atlas.
Dentate gyrus measurement:
We used Cavalieri's principle, which allows obtaining an estimated volume of an object of arbitrary shape and size to measure thickness of the granule cell layer of the supra pyramidal blade. The following formula was used:
where V is the volume, T the distance between parallel sections, A the calculated area of a section, and n the total number of sections. 3 region matching sections between the genotypes were measured every 15μm.
2.5. Cell culture for electrophysiology:
To generate astrocyte feeder layers, cortical hemispheres from postnatal day 0-1 (PND0-1) wildtype C57BL/6J mice of either sex were dissected in cold HBSS (Gibco). For primary neuron culture, hippocampi from PND1-2 mice were dissected in cold HBSS and cell culture carried out as described (Weston, Chen, and Swann 2014b). The day after plating, approximately 4 × 1010 genome copies of AAV8-CaMKII-GFP virus (UNC Vector Core) was added to each well.
2.6. Electrophysiology:
Whole-cell recordings were performed with patch-clamp amplifiers (MultiClamp 700B amplifier; Molecular Devices) under the control of Clampex 10.3 or 10.5 (Molecular Devices, pClamp, RRID:SCR_011323). Data were acquired at 10 kHz and low-pass filtered at 6 kHz. The series resistance was compensated at 70%, and only cells with series resistances maintained at less than 15 MΩ were analyzed. Patch electrodes were pulled from 1.5-mm o.d. thin-walled glass capillaries (Sutter Instruments) in five stages on a Flaming–Brown micropipette puller (model P-97; Sutter Instruments). Internal solution contained the following: 136 mM KCl, 17.8 mM HEPES, 1 mM EGTA, 0.6 mM MgCl2, 4 mM ATP, 0.3 mM GTP, 12 mM creatine phosphate, and 50 U/ml phosphocreatine kinase. The pipette resistance was between 2 and 4 MΩ. Standard extracellular solution contained the following (in mM): 140 NaCl, 2.4 KCl, 10 HEPES, 10 glucose, 4 MgCl2, and 2 CaCl2 (pH 7.3, 305 mOsm). All experiments were performed at room temperature (22–23°C). Whole-cell recordings were performed on neurons from Iqsec2−/Y and wildtype groups in parallel on the same day (day 12–15 in vitro). On each day, and for each group, an attempt was made to record from equal numbers of glutamatergic (GFP+) and GABAergic (GFP−) neurons. All electrophysiology experiments were performed by two independent investigators blinded to the genotypes, and all data were analyzed offline with AxoGraph X software (AxoGraph Scientific, RRID:SCR_014284). From a total of five cultures, we recorded from a total of 52 glutamatergic and 53 GABAergic wildtype neurons, and 54 glutamatergic and 56 GABAergic mutant neurons. From all five cultures, we recorded from a total of 34 wildtype E-I neuron pairs, and 32 mutant E-I neuron pairs.
For voltage-clamp experiments, neurons were held at −70 mV. Miniature synaptic potentials were recorded for 70–90 s in 500 nM tetrodotoxin (TTX, Enzo Life Sciences) to block AP-evoked release, and either the GABAA receptor antagonist bicuculine methiodide (30 μM; hello bio) to isolate mEPSCs, or the AMPA receptor antagonist NBQX disodium salt (10 μM; TOCRIS Bioscience) to isolate mIPSCs. Data were filtered at 1 kHz and analyzed using template-based miniature event detection algorithms implemented in the AxoGraph X 1.6 software. The threshold for detection was set at 3.5 times the baseline SD from a template of 0.5 ms rise time and 3 ms decay for mEPSCs, and 20 ms decay for mIPSCs. For paired neuron recordings, action potential (AP)-evoked EPSCs and IPSCs were triggered by a 2 ms somatic depolarization to 0 mV at 0.1 Hz. The shape of the evoked response and the effect of receptor antagonists (bicuculline and NBQX) were analyzed to verify the glutamatergic or GABAergic identities of the responses.
For current-clamp experiments, intrinsic electrophysiological properties of neurons were tested by injecting 500-ms square current pulses incrementing in 20 pA steps, starting at −100 pA. Resting membrane potential (Vm) was calculated from a 50 ms average before current injection. The membrane time constant (τ) was calculated from an exponential fit of current stimulus offset. Input resistance was calculated from the steady state of the voltage responses to the hyperpolarizing current steps. Membrane capacitance was calculated by dividing the time constant by the input resistance. APs were evoked with 500 ms, 20 pA depolarizing current steps, and the rheobase was defined as the minimum current required to evoke an AP during the 500 ms of sustained somatic current injections. The AP threshold was defined as the membrane potential at the inflection point of the rising phase of the AP. The AP amplitude was defined as the difference in membrane potential between the AP peak and the threshold, and the afterhyperpolarization was the difference between AP threshold and the lowest point of hyperpolarization. The AP half-width was defined as the width of the AP at half-maximal amplitude. The membrane potential values were not corrected for the liquid junction potential.
2.7. Seizure testing:
For the PTZ test, adult mice between 9–12 weeks of age were injected subcutaneously with a threshold dose (50 mg/kg) of PTZ (Sigma-Aldrich, Co), placed onto clean bedding in a clear plastic box, and observed for 30 min. The incidence and latency to seizure endpoint standards (Racine, 1972) were recorded, and the group mean latency to tonic-clonic seizures was determined. The 6 Hz electroconvulsive threshold (ECT) test was as described previously with minor modifications (Frankel et al. 2001), was performed once daily with increasing stimulus until a partial seizure was observed. Group means were calculated to determine threshold. For video-EEG, adult mice were anesthetized and electrode implant surgery was performed as recently described (57).
2.8. Pup developmental milestones:
Developmental milestones were assessed every other day starting at PND4 through PND10 (Yang et al. 2012). To avoid circadian rhythm-induced variability, each test litter was examined during the same 1 hr window. To assess physical development, pups were weighed; for righting reflex, the pups were turned on their back and given 30 sec to right themselves. To observe isolation-induced USV, pups were removed from their nest and isolated from mother and littermates. Pups were placed gently into a small isolation container made of plastic, containing fresh bedding material and USVs recorded using UltraSoundGate Condenser Microphone CM 16 (Avisoft Bioacoustics, Berlin, Germany). Each recording was carried out for 3 min and the number of calls emitted by the pups counted by an operator blinded to genotype.
2.9. Adult behavior:
For the open field test, adult Iqsec2 mice (8–12 weeks) were placed in the center of an acryliopen-field box and allowed to freely explore the environment for 60 min under the light condition of 10 lux Ethovision XT software (Noldus) was used to determine distinct features of locomotor activity. For the elevated plus-maze test, the elevated plus-maze system by Med Associates Inc (Fairfax, VT) was used. Light conditions for the test was 30 lux. In the test, mice were introduced to the center region of the elevated plus-maze and allowed to explore freely for 5 min. Male-female social interactions were evaluated in a 5-min test session as previously described (Murphy, Jensen, and Walikonis 2006)
2.10. Statistics:
Statistical analysis was performed using GraphPad Prism 7 software. Unpaired two-tailed Student’s t-test for parametric data and Mann–Whitney test for non-parametric data was used to detect genotype differences in immunofluorescence intensity, mRNA and protein expression, elevated maze plus times, adult USV counts and 6 Hz test thresholds. 2-way ANOVA with Sidak’s multiple comparison test as a post-hoc analysis was used to analyze developmental milestones, pup ultrasonic vocalizations, righting reflex across days and adult open field data across 1 hr recording time. A log-rank Mantel-Cox test was performed to compare the Kaplan–Meier analysis for adult mortality and the fisher’s exact test for PTZ seizure latency. For electrophysiology data, we used generalized estimating equations (GEE) in SPSS V24 (IBM, RRID:SCR_002865), allowing for within-subject correlations and the specification of the most appropriate distribution for the data. All data distributions were assessed with the Shapiro-Wilk test. Datasets that were significantly different from the normal distribution (p < 0.05) were fit with models using the gamma distribution and a log link. Normal datasets were fit with models using a linear distribution and identity link. We used the model-based estimator for the covariance matrix and an exchangeable structure for the working correlation matrix Goodness of fit was determined using the corrected quasi likelihood under independence model criterion and by the visual assessment of residuals. Because neurons and animals from the same culture are not independent measurements, culture was used as the subject variable, and animals and neurons were considered within-subject measurements. All values reported in the text are estimated marginal means +/− standard error.
3. Results
3.1. Validation of the Iqsec2 knockout model
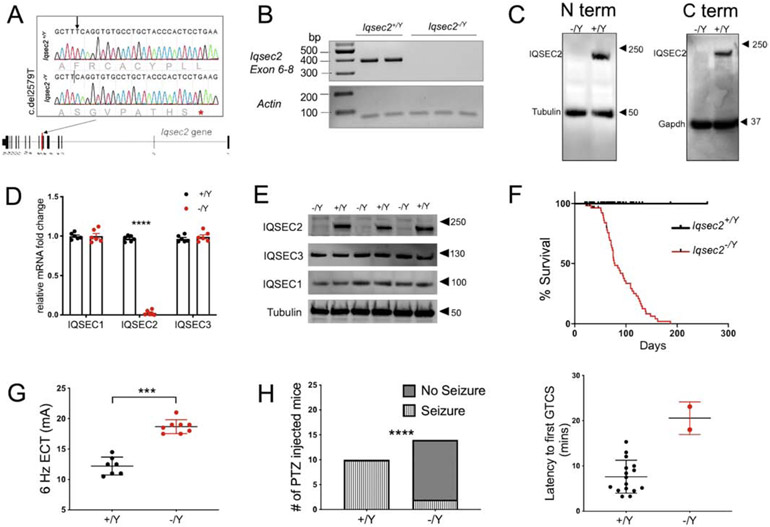
To model loss-of-function IQSEC2 disease, we created an Iqsec2 knockout mouse on the C57BL/6NJ (B6NJ) strain background using CRISPR/Cas9, resulting in a single nucleotide deletion and translational frameshift (p.Phe860SerfsTer8) in Iqsec2 exon 7 (Figure 1A). Because hemizygous Iqsec2−/Y males do not mate well, and on the B6NJ background heterozygous females are inattentive to pups, all studies were performed on a mixed hybrid background involving the C3HeB/FeJ and C57BL/6NJ strains. To detect Iqsec2 gene expression, RNA primers spanning exon 6 to exon 8 were designed. RT-PCR showed lack of amplification in the Iqsec2−/Y animals compared to the wildtype littermates (Figure 1B). To investigate protein expression, we used forebrain homogenates from postnatal day (PND) 21 animals and probed for IQSEC2 using a N-terminus antibody (UCT 88 was developed against residues 10-198 of human IQSEC2 (Murphy, Jensen, and Walikonis 2006) and a C-terminus antibody (AB_2718685 recognizes residues 1380-1430 of human IQSEC2). Western blotting showed lack of protein expression and no evidence of truncated protein expression with either antibody (Figure 1C). This evidence suggests that the premature stop codon in exon 7 subjects the mutant Iqsec2 mRNA to nonsense mediated decay. The N-terminus antibody was used for all subsequent experiments and data presented.
Figure 1: Generation and characterization of Iqsec2 mutant mice.
A. Illustration describing the location of the point mutation on exon 7 of the longest coding Iqsec2 isoform NM_0011111125. An asterisk denotes the stop codon introduced by a frameshift due to deletion of the T nucleotide at position 2579. B. Semi quantitative RT-PCR analysis shows no amplification of exon 6-8 in the Iqsec2+/Y animals. C. Western blot showing staining for IQSEC2 and tubulin using 50 micrograms of whole brain lysate from P21 animals from each genotype using a N-terminus and C-terminus antibody shows a lack of IQSEC2 protein expression in the null animals. D. Quantitative analysis of the Iqsec1 and Iqsec2 mRNA transcript expression from whole brain of P14 Iqsec2+/Y and Iqsec1−/Y animals. Iqsec1 transcript expression is unaltered between Iqsec2+/Y (0.977± 0.015) and Iqsec2−/Y (0.993± 0.64) animals, Iqsec3 transcript expression is unaltered between Iqsec2+/Y (0.965± 0.017) and Iqsec2−/Y (0.990± 0.065) animals , whereas Iqsec2 transcript expression is significantly reduced in Iqsec2−/Y (0.046± 0.023) animals compared to control Iqsec2+/Y (0.963±0.20) animals, 5 animals per genotype, p<0.0001 E. Western blot showing staining for IQSEC1, IQSEC2 (N-terminus antibody), and IQSEC3 using 50 micrograms of whole brain lysate from P21 animals from each genotype (n=3 for each genotype) shows no difference in IQSEC1 and IQSEC3 expression in Iqsec2−/Y animals . F. Monitoring survival of littermate mice revealed a markedly shortened lifespan of Iqsec2−/Y (n=62; median survival = 95 days) compared to Iqsec2+/Y (n=51) mice, log-rank Mantel Cox test, p <0.0001 G. Seizure thresholds are significantly different for the 6 Hz electroconvulsive test between the Iqsec2+/Y (n=7) and Iqsec2−/Y (n=8) animals (12.21± 0.5548 mA; 18.69±0.4002 mA, respectively); Mann Whitney U test, *** p=0.0002; error bars indicate ± SEM. H. Significantly decreased GTCS incidence in Iqsec2−/Y mice (n=14) compared to Iqsec2+/Y mice (n=10) after 50 mg/kg PTZ, p<0.0001 by contingency χ2 analysis. The Iqsec2−/Y mice that exhibited GTCS on PTZ administration had a longer latency compared to the Iqsec2+/Y mice.
To examine whether the Iqsec2 point mutation impacts expression of IQSEC family members, namely IQSEC1, IQSEC2, and IQSEC3 we evaluated respective mRNA transcripts from forebrain samples of PND14 mutants and controls by RT-qPCR and protein levels using western blot at PND21. Compared to wildtype littermates, hemizygous males showed an approximate 40-fold decrease in Iqsec2 mRNA expression with no change in either Iqsec1 or Iqsec3 mRNA expression (Figure 1D). Western blotting confirmed unchanged protein expression for IQSEC1 and IQSEC3 in Iqsec2−/Y animals and complete absence of IQSEC2 protein (Figure 1E), confirming specificity of Iqsec2 targeting and importantly that the hemizygous mutant males are null for the Iqsec2 allele.
3.2. Increased adulthood mortality in Iqsec2−/Y animals
Although Iqsec2 heterozygotes and hemizygotes were born in the expected Mendelian ratio and had no overt signs of neurological deficits, they had increased mortality compared to wildtype littermates. Iqsec2−/Y mice had a median lifespan of 95 days and did not survive past 187 days (Figure 1F). Despite showing no evidence of seizure activity during regular handling, post-mortem examination of all Iqsec2−/Y animals revealed retracted forelimbs and extended hindlimbs, suggestive of a maximal tonic seizure. Generalized tonic-clonic and maximal tonic seizures were captured live in several mice during long-term video monitoring, with hemizygotes observed suddenly breaking into a wild run followed by a terminal seizure.
3.3. Decreased susceptibility to induced seizures in Iqsec2−/Y animals
To check for the presence of non-convulsive seizures or interictal epileptiform activity, continuous 48 hr video-EEG was recorded from three Iqsec2−/Y and two littermate control 9-week old adult mice (Supplementary Figure 1B). We also evaluated longer-term recordings in four Iqsec2−/Y mice between 60 and 110 days of age for a total of 96 hr. No indication of any epileptiform activity was detected in these 7 mice. We do note, however, that during the final stages of preparation of our manuscript, a new publication by Jackson and colleagues reported recording spontaneous seizure activity in some adult Iqsec2−/Y animals during continuous live-video surveillance (Jackson et al. 2019). We suspect that differences in the respective strain background, or in the animal care environment, impact the manifestation of recurrent (non-lethal) spontaneous seizures.
To gain further insight into network excitability, we examined threshold to induced seizures using the 6 Hz electroconvulsive threshold (ECT) test and the pentylenetetrazole (PTZ) chemoconvulsion test. The 6 Hz ECT induces partial or “psychomotor” seizures and in particular is considered to be useful for studying pharmacoresistant epilepsy of limbic origin. Surprisingly and counterintuitively, compared wildtype (12.21 ± 0.5548 mA, n=7), Iqsec2−/Y animals had a significantly elevated threshold to 6 Hz ECT (18.69 ± 0.4002 mA, n=8, p=0.0002; Figure 1G). Even heterozygous Iqsec2 mutant females had an elevated 6 Hz threshold (15.39 ± 0.535 mA, n=14), in between the threshold for the wildtype controls and knockout animals (Supplementary Figure 1A), consistent with what might be expected from random Chr X inactivation. Similarly, in the chemoconvulsion test, using a dose of PTZ at the threshold for generalized tonic-clonic seizures (GTCS) in this mixed strain background, compared to all of the control animals (n=10), only 2 of the 14 Iqsec2−/Y animals experienced GTCS (p<0.0001). Furthermore, those 2 Iqsec2−/Y mice showed increased latency to GTCS compared to the wildtype (Figure 1H). Together, these results indicate that the susceptibility to some forms of induced seizures is actually suppressed in Iqsec2−/Y mice.
3.4. Developmental and adult behavioral deficits in Iqsec2−/Y animals
Patients harboring IQSEC2 variants present a wide spectrum of phenotypes beyond seizures, including developmental regression, language development delay, intellectual disability and ASD. To test related functionality in Iqsec2 null mice, we performed a battery of neurobehavioral tests to assess development trajectory and adult behavior.
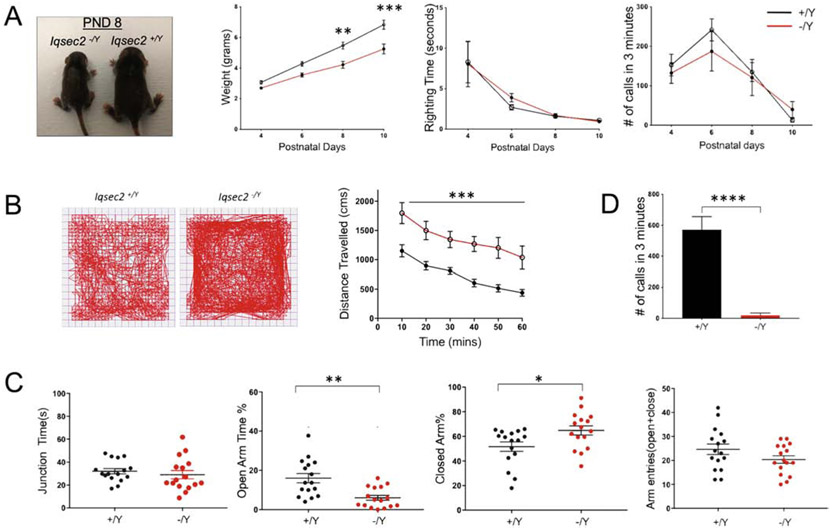
For developmental milestones (Figure 2A), Iqsec2+/Y (n=16) and Iqsec2−/Y (n=10) pups were assessed, with observer blind to genotype, every other day from PND4–10 for weight gain and neurological reflex including righting reflex and maternal separation-induced ultrasonic vocalization (USV). An overall genotype difference was observed in pup growth (F(1, 24)=12.89, p=0.0015), whereby Iqsec2−/Y pups grew more slowly than wildtype littermates, first apparent at PND8 (PND4 p=0.6495, PND6 p=0.08, PND8 p=0.001, PND10 p<0.0001). No significant difference between genotypes was found for righting reflex (F(1, 24)=0.03721, p=0.8487) or for separation-induced USV (F(1, 24)=0.02588, p=0.8754). Additionally, the Iqsec2−/Y adult animal growth never catches up to wildtype littermates (Supplementary Figure 2A), although the weight difference becomes less statistically significant with age (PND21 p<0.0001, PND60 p=0.09).
Figure 2: Mouse pup and adult behavioral phenotyping of Iqsec2−/Y animals.
A. Panels from left to right show comparison of Iqsec2 pups at PND8 and graphs for developmental milestones. Analysis of developmental milestone markers revealed genotype differences on measures of body weight but not righting reflex Number of ultrasonic vocalizations emitted by pups separated from the nest did not differ significantly among genotypes but were fewer in Iqsec2−/Y mice (n=10) compared to Iqsec2+/Y mice (n=16); Two-way ANOVA, Sidak’s multiple comparison test, **p<0.01, ***p< 0.001; error bars indicate ± SEM. Adult IQSEC2 animals are hyperambulatory (B), hyperanxious (C) and display defects in social interaction (D). B. Representative tracks of Iqsec2+/Y and Iqsec2−/Y mice during a 60-min open field session and graph showing total distance traveled (cms) measured in 10-min time bins across a 60-min session in an open field box, Iqsec2+/Y (n=16) and Iqsec2−/Y (n=14), Two-way ANOVA, ***p < 0.001; error bars indicate ± SEM. C. Time spent in junction, percent time spent in open arms, percent time spent in closed arms and total number of open and closed arm entries by Iqsec2+/Y (n=16) and Iqsec2−/Y (n=14) mice during 5 min exploration in elevated plus maze. Mann Whitney U test *p< 0.05, **p < 0.01; error bars indicate ± SEM. D. Total number of USV calls emitted by Iqsec2+/Y (n=10) and Iqsec2−/Y (n=12) mice when introduced to a novel estrous female during a social interaction session; Mann Whitney U test, ****p < 0.0001; error bars indicate ± SEM.
For adult behaviors, 8–12 week-old Iqsec2−/Y and wildtype male littermates were assessed in the open field arena (Figure 2B), revealing a significant increase in ambulatory activity as measured by total distance travelled over a 60 min period (F(1, 28)=13.77, p=0.001), but no difference was observed in time spent in the center (Supplementary Figure 3) (F(1,28)=2.173, p=0.1516).
We also tested the animals for anxiety-related behavior using the elevated plus-maze test (Figure 2C). Overall, Iqsec2−/Y mice spent significantly more time in the closed arms (p=0.024) and reciprocally, less time in the open arms (p=0.001) than wildtype littermates. These behaviors suggest anxiety in mature Iqsec2 null mice. Adult males typically display USV upon initiating courtship or marking their territory. Despite having viable sperm (based on successful cryopreservation), adult Iqsec2−/Y mice did not successfully mate in our hands. Therefore, we measured female-induced USV in male hemizygotes at 9–11 weeks of age, as an analogous approach to assess social and verbal communication skills (Figure 2D). Similar measurement techniques have been frequently deployed in study of animal models with neurological deficits such as ASD (Yang et al. 2012). For this test, male Iqsec2−/Y and wildtype littermates were paired with unfamiliar estrous B6NJ females in a testing cage for a total of 5 min and the total number of calls emitted by the male mice quantified. Compared to wildtype counterparts, Iqsec2−/Y males emitted significantly fewer ultrasonic vocalizations (p<0.0001), strongly suggesting that loss of Iqsec2 impairs social male-female interaction.
3.5. IQSEC2 protein expression and reactive astrogliosis in Iqsec2−/Y animals
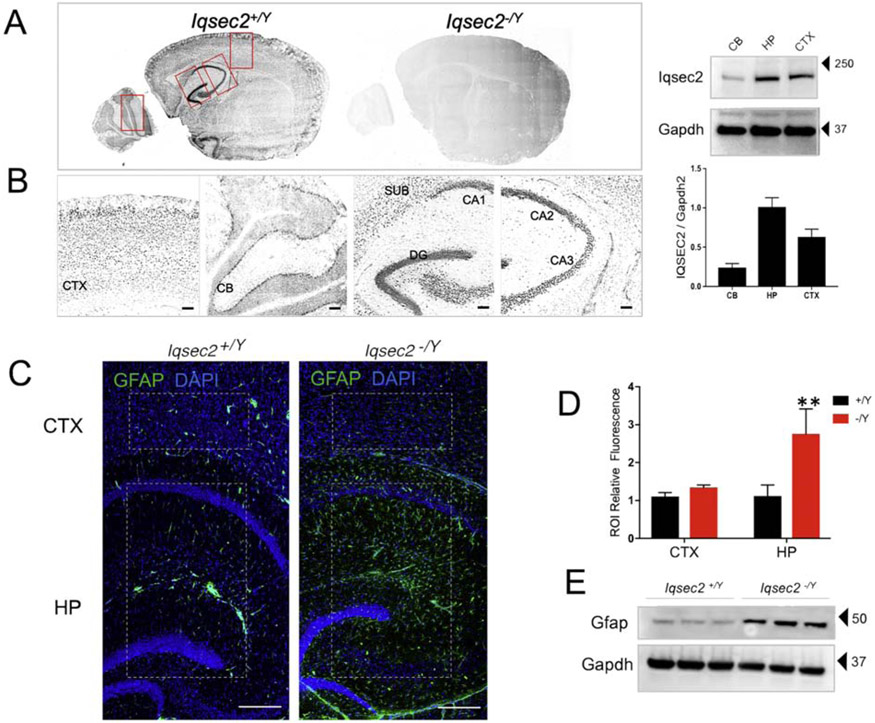
Although evidence for Iqsec2 mRNA localization within the brain exists (Sanda et al. 2009), protein had not been previously examined in situ. Temporal expression patterns demonstrate that the IQSEC2 protein is first detected at PND7 and reaches peak stable expression at PND14 (Elagabani et al. 2016b). Therefore, to obtain a snapshot of spatial protein expression we probed sagittal sections from Iqsec2+/Y and Iqsec2−/Y animals at PND16 with a serum antibody against IQSEC2 (Murphy 2006). Consistent with previously reported forebrain mRNA expression (Sanda et al. 2009) IQSEC2 expression was highest in the hippocampal formation (Figure 3A-B), suggesting functional importance in the region. To note, staining was completely absent from sections from Iqsec2−/Y animals, confirming antibody specificity.
Figure 3: IQSEC2 expression and reactive gliosis in the hippocampus.
A. Sagittal sections of PND21 mice stained with antibody against IQSEC2 (antiserum UCT88). Staining is present in Iqsec2+/Y (n=5 animals) sections and absent from Iqsec2−/Y (n=4 animals) sections confirming lack of protein expression. B. The boxed regions in panel A were zoomed in to show staining in the cortex, cerebellum and hippocampus. Expression pattern revealed increased staining in the hippocampus including CA1, CA2, CA3, dentate gyrus and subiculum Scale bar is 20 μm. Western blot run with 50 micrograms of protein loaded from cerebellar, hippocampal and cortical lysates from a PND28 Iqsec2+/Y brain and stained against IQSEC2 and loading control GAPDH. Graph shows IQSEC2 expression normalized to input, n=3; Error bars represent standard deviation of the mean value. C. Representative sections show that astrogliosis is absent in adult Iqsec2+/Y sections (n=5 mice) but present and confined to the hippocampus in Iqsec2−/Y sections (n=7 mice). Scale bars: 200 μm Boxed regions in Panel A indicate region of interest (ROI) used for immunofluorescence quantification. D. Graph shows quantification of ROI-specific GFAP fluorescence for cortex and hippocampus compared between the genotypes. Mann Whitney U test, **p< 0.01; error bars indicate ± SEM E. Western blot shows 50 ug of hippocampal lysate from 3 individual animals from each genotype (PND45–60) probed against GFAP and loading control GAPDH.
Characterization of general brain architecture by Nissl staining did not reveal obvious abnormalities nor were any differences detected in brain to body weight ratio in the Iqsec2−/Y mice (Supplementary Figure 2B-E). Double immunostaining for cleaved caspase 3, GFAP, and in parallel FluoroJade C (FJC) was performed to ascertain degenerating cells or overt cell death – both were negative. However, there was significantly increased GFAP immunoreactivity, a proxy for reactive astrogliosis, in Iqsec2−/Y hippocampal region compared to control in PND28 animals (Iqsec2−/Y N=5, Iqsec2+/Y N=7; Figure 3C-E). Quantification by western blot confirmed the increase in GFAP protein expression in Iqsec2−/Y hippocampal lysate (N=3 for each genotype); p<0.05; Figure 3E). In this study, we confined our examinations to the hippocampus since high protein expression and region-specific reactive astrogliosis in the knockout mice underscored both the functional relevance and therefore, the vulnerability of this region to IQSEC2 loss.
3.6. Loss of Iqsec2 enhances spontaneous and evoked glutamatergic strength onto GABAergic hippocampal neurons
Given the localization of IQSEC2 at the excitatory postsynaptic density, it is not surprising that previous studies showed that it regulates glutamatergic synaptic transmission. More specifically, one group showed that Iqsec2 knockdown reduces, and Iqsec2 overexpression increases, evoked excitatory postsynaptic current (eEPSC) amplitude onto CA1 pyramidal neurons of PND5-PND6 rats (Brown et al. 2016), whereas another reported that Iqsec2 knockdown reduces the frequency of miniature excitatory postsynaptic currents (mEPSCs) onto CA1 pyramidal neurons of PND16 mice (Elagabani et al. 2016). To determine whether IQSEC2 genetic loss alters synaptic transmission in hippocampal neurons, we generated dissociated hippocampal neuron cultures from PND1–PND2 Iqsec2−/Y and wildtype littermate pups. The cultured neurons were transfected with an adeno-associated virus expressing an enhanced green fluorescent protein driven by a calcium/calmodulin-dependent kinase II promoter (AAV-CamKII-EGFP) to better distinguish between glutamatergic and GABAergic neurons. Between 12–15 days in vitro (DIV), we performed whole-cell patch-clamp electrophysiology to assess alterations in synaptic transmission.
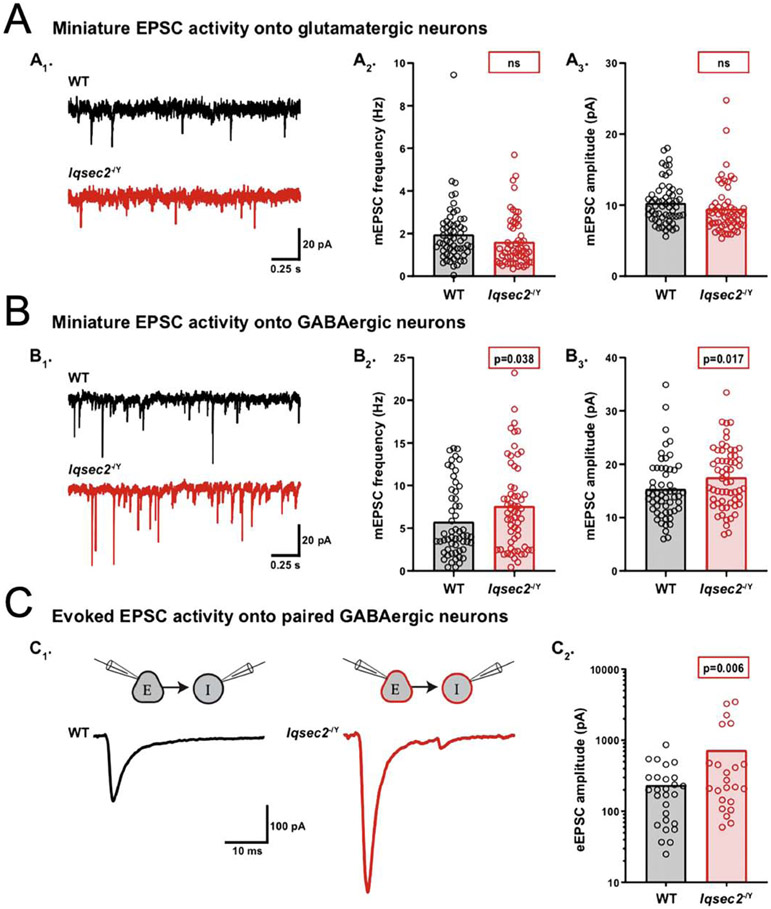
First, we performed voltage-clamp recordings to analyze spontaneous miniature activity onto glutamatergic and GABAergic neurons from each group. To obtain miniature activity, we recorded from neurons in a solution containing tetrodotoxin (TTX; 500 nM) and either bicuculine (30 μM) to isolate mEPSCs, or NBQX (10 μM) to isolate miniature inhibitory postsynaptic currents (mIPSCs). In contrast to previous reports, we observed no statistically significant difference in mEPSC frequency or amplitude onto Iqsec2−/Y glutamatergic neurons compared with wildtype (WT: 1.96 ± 0.18 Hz, Iqsec2−/Y : 1.63 ± 0.15 Hz, p = 0.17; Figure 4A1,2 and WT: 10.2 ± 0.4 pA, Iqsec2−/Y: 9.4 ± 0.4 pA, p = 0.19; Figure 4A1,3). However, there were significant increases in mEPSC frequency and amplitude onto Iqsec2−/Y GABAergic neurons compared with wildtype (WT: 5.74 ± 0.5 Hz, Iqsec2−/Y : 7.58 ± 0.7 Hz, p = 0.038; Figure 4B1,2 and WT: 15.3 ± 0.6 pA, Iqsec2−/Y: 17.3 ± 0.7 pA, p = 0.017; Figure 4B1,3). There were no genotype-dependent differences in mIPSCs onto glutamatergic (WT: 2.38 ± 0.2 Hz, Iqsec2−/Y: 2.26 ± 0.2 Hz, p = 0.65; Supplementary Figure 4A1,2 and WT: 17.8 ± 1.4 pA, Iqsec2−/Y : 16.9 ± 1.3 pA, p = 0.45; Supplementary Figure 4A1,3) or GABAergic neurons (WT: 2.72 ± 0.2 Hz, Iqsec2−/Y: 3.10 ± 0.3 Hz, p = 0.25; Supplementary Figure 4B1,2 and WT: 18.8 ± 1.5 pA, Iqsec2−/Y: 18.3 ± 1.4 pA, p = 0.71; Supplementary Figure 4B1,3).
Figure 4: IQSEC2 loss increases excitatory drive onto GABAergic neurons.
A-C. Using whole-cell, voltage-clamp electrophysiology, spontaneous miniature and evoked activity was recorded from glutamatergic and GABAergic hippocampal neurons derived from wildtype (WT) and Iqsec2−/Y mice at days 12–15 in vitro. Example traces of miniature EPSC activity onto wildtype (WT) (black) and Iqsec2−/Y (red) glutamatergic neurons (A1) and GABAergic neurons (B1). Bar graph with overlaid dot plots show that the mEPSC frequency and amplitude onto glutamatergic neurons were both unaltered (A2 and A3), whereas onto GABAergic neurons they were both increased (B2 and B3), by Iqsec2 loss. C1. Example traces of evoked EPSCs onto paired wildtype (WT) (black) and Iqsec2−/Y (red) GABAergic neurons. C2. Bar graphs with overlaid dot plots show that the eEPSC amplitude onto GABAergic neurons was increased by loss of Iqsec2. For all graphs, the bars indicate the mean of the data set and each dot represents the mean response from one neuron. All p-values were calculated using Generalized Estimating Equations, and significant p-values are indicated in red boxes for each graph (ns indicates p>0.05).
To determine whether the enhanced spontaneous glutamatergic transmission onto GABAergic neurons also occurs upon evoked release, we performed paired recordings of glutamatergic and GABAergic neurons from Iqsec2−/Y and wildtype hippocampal neuron cultures (DIV 12–15). For each pair, we stimulated a glutamatergic neuron and recorded the eEPSC onto a paired GABAergic neuron (Figure 4C1), or we stimulated a GABAergic neuron and recorded the eIPSC onto a paired glutamatergic neuron (Supplementary Figure 4C1). Similar to the mEPSC data, the average amplitude of the EPSCs evoked onto GABAergic neurons was increased in the Iqsec2−/Y pairs relative to that of the wildtype pairs (WT: 236 ± 53 pA, Iqsec2−/Y : 762 ± 184 pA, p = 0.006; Figure 4 C1,2). Also, in agreement with the mIPSC data, there was no genotype-dependent difference detected in the eIPSCs onto glutamatergic neurons (WT: 1514 ± 316 pA, Iqsec2−/Y 1081 ± 225 pA, p = 0.27; Supplementary Figure 4C1,2). Taken together, these data identify an increase in excitatory synaptic input specifically onto interneurons downstream of IQSEC2 loss, suggesting neuron subtype-specific roles for this PSD protein.
3.7. Loss of Iqsec2 alters the intrinsic electrical properties of GABAergic hippocampal neurons
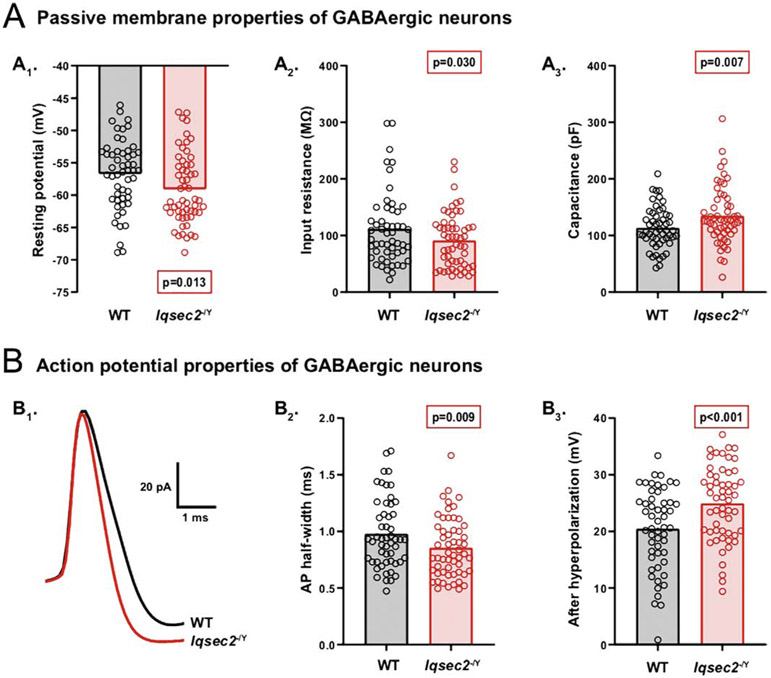
To determine whether the alterations in synaptic transmission are accompanied by alterations in the intrinsic electrical properties, we performed current-clamp recordings and analyzed passive and active membrane characteristics of glutamatergic and GABAergic neurons from Iqsec2−/Y and wildtype hippocampal neuron cultures (DIV12–15). Iqsec2−/Y glutamatergic neurons showed no differences in passive or active membrane properties relative to those of wildtype (Supplementary Table 1). However, Iqsec2−/Y GABAergic neurons had a more hyperpolarized resting membrane potential, and a lower input resistance with a higher membrane capacitance (WT: −56.7 ± 0.7 mV, Iqsec2−/Y: −59.1 ± 0.7 mV, p = 0.013; Figure 5A1, WT: 111.2 ± 7.2 MΩ, Iqsec2−Y: 91.1 ± 5.8 MΩ, p = 0.030; Figure 5A2, and WT: 113.3 ± 5.2 pF, Iqsec2−/Y : 134.6 ± 6.0 pF, p = 0.007; Figure 5A3). Frequently, neurons with a hyperpolarized resting membrane potential and lower input resistance also have an increased rheobase, which is the amount of current necessary to stimulate the firing of an action potential (AP). Thus, we next injected increasing amounts of current into the neurons to determine their rheobase. Although the average rheobase of Iqsec2−/Y GABAergic neurons appeared to show the predicted increase relative to that of wildtype, the difference was not significant (WT: 245.3 ± 27.4 pA, Iqsec2−/Y 319.3 ± 34.6 pA, p = 0.094). Moreover, the AP threshold and amplitude were unaltered by Iqsec2 loss (WT: −35.5 ± 0.6 mV, Iqsec2−/Y: −36.1 ± 0.6 mV, p = 0.440; WT: 63.6 ± 1.4 mV, Iqsec2−/Y : 64.3 ± 1.4 mV, p = 0.733). However, the Iqsec2−/Y GABAergic neurons fired APs with narrower half-widths and larger afterhyperpolarizations (AHPs) than wildtype (WT: 0.98 ± 0.04 ms, Iqsec2−/Y : 0.85 ± 0.03 ms, p = 0.009; Figure 5B1,2, WT: 20.4 ± 0.8 mV, Iqsec2−/Y: 24.9 ± 0.8 mV, p < 0.001; Figure 5B1,3). Taken together, these data demonstrate profound alterations in multiple intrinsic electrical properties specifically in GABAergic neurons following Iqsec2 loss, further supporting neuron subtype-specific roles for IQSEC2. Interestingly, several of the observed alterations in Iqsec2−/Y GABAergic neurons, including the lower input resistance, narrower AP half-widths, and larger AHPs, are hallmark features of fast-spiking (FS) interneurons, suggesting either an enrichment of FS interneurons, or perhaps, simply an enhancement of an FS-like phenotype due to Iqsec2 loss
Figure 5: IQSEC2 loss alters multiple intrinsic electrical properties of GABAergic neurons.
A–B. Using whole-cell, current-clamp electrophysiology, intrinsic electrical properties of GABAergic hippocampal neurons derived from wildtype and Iqsec2−/Y mice were assessed at days 12–15 in vitro. A. Bar graphs with overlaid dot plots illustrate alterations in passive membrane properties of Iqsec2−/Y GABAergic neurons relative to those of wildtype (WT), including a more hyperpolarized resting membrane potential (A1), a lower input resistance (A2), and a higher membrane capacitance (A3). B. Action potential properties of GABAergic neurons were also altered by IQSEC2 loss. B1. Representative AP traces recorded from wildtype (WT) (black) and Iqsec2−/Y (red) GABAergic neurons illustrate the narrower AP half-width (B2, bar graph with overlaid dot plot) and larger AP afterhyperpolarization (B3, bar graph with overlaid dot plot) of Iqsec2−/Y GABAergic neurons relative to those of wildtype (WT). For all graphs, the bars indicate the mean of the data set and each dot represents the mean response from one neuron. All p-values were calculated using Generalized Estimating Equations, and significant p-values are indicated in red boxes for each graph.
3.8. Loss of Iqsec2 results in an increase in PV interneurons in adult hippocampus
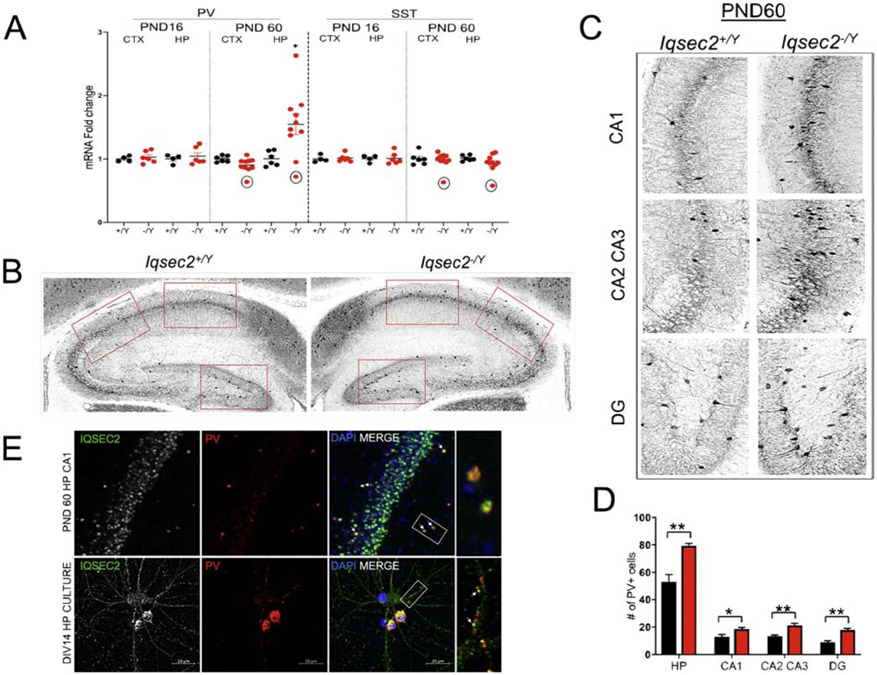
Together, parvalbumin (PV)- and somatostatin (SST)-expressing interneurons constitute the largest non-overlapping groups of interneurons in cortex and hippocampus. Interneuron maturation is dictated closely by excitatory input during development (Pelkey et al. 2017). Since GABAergic interneurons are impacted upon IQSEC2 loss, we examined expression of these inhibitory cell markers, PV and SST, both temporally at PND16 and PND60, and spatially in cortex and hippocampus. Beginning with mRNA expression we observed a significant increase in PV mRNA expression in the hippocampus of PND60 Iqsec2−/Y animals compared with control (Iqsec2+/Y: 1.005±0.047, Iqsec2−/Y: 1.547±0.1643, p=0.0225, Figure 6A) while the expression of SST across the timepoints and genotypes remained unaltered (Figure 6A). Since an increase in PV mRNA expression can stem from either a cell intrinsic increase in expression as a response to synaptic activity (Turner et al. 2010) or an increase in number of PV-expressing neurons themselves, we examined PND60 brain sections for PV interneuron expression and found an increased number of PV-expressing cells in the hippocampus of Iqsec2−/Y animals (Figure 6B-D), suggesting that the increase in PV mRNA expression is likely a consequence of an increased number of PV-expressing interneurons. Interestingly, the hippocampus of an outlier PND60 animal (marked in the graphs) showed a robust decrease in PV and SST mRNA expression in both the hippocampus and cortex. Additionally, we carried out morphometric analysis of the dentate gyrus thickness of the PND60 animals and found no difference between the genotypes (Supplementary Figure 2E), suggesting that the increase in PV cell number occurs without an increase in overall dentate gyrus cell number in adult Iqsec2−/Y animals.
Figure 6: PV expression is altered in the hippocampus of adult Iqsec2−/Y mice.
A. RT-qPCR values from cortical and hippocampal samples of PND14 and PND60 mice representing parvalbumin (Pvalb) and somatostatin (Sst) mRNA levels. Signals were normalized to Rpl13 mRNA levels and expressed as fold change compared to Iqsec2+/Y mice. Note: the outlier PND60 Iqsec2−/Y animal (circled in black in the graphs) with almost a 40% decrease in hippocampal Pvalb and Sst mRNA compared to control animals showed a similar decrease in Pvalb and Sst mRNA in the cortex. B. Representative PV immunofluorescence images from hippocampal coronal sections of PND60 Iqsec2+/Y and Iqsec2−/Y animals. C. Zoomed-in sections indicated by the red boxES in (B) showing PV cells in the CA1, CA2/CA3 and dentate gyrus, respectively. D. Graph shows stereological counts of PV-positive cells in the hippocampus of PND60 animals (4 animals from each genotype, 3 sections each). Student’s t test, *p < 0.05, **p< 0.01; error bars indicate ± SEM. E. Confocal images showing double labeling of IQSEC2 and PV in a hippocampal section of a PND60 animal and in dissociated hippocampal cultures at DIV 14. In the hippocampal panel, white arrows in the third section indicate cells which co-labeled for IQSEC2 and PV, while the last section shows a zoomed-in image of the co-labeled cells enclosed by the white box In the neuron panel, the first section shows endogenous IQSEC2 expression in an excitatory neuron and in PV-expressing interneurons. IQSEC2 is expressed through the entire cell body including protrusions but highly enriched at the postsynaptic sites , evident by the punctate staining expression along the dendrites. Last section shows IQSEC2 and PV co-labeling along dendrites in PV/IQSEC2 positive-interneurons (evident by the co-labeling in the soma) indicating that the two proteins could be co-localized to the same postsynaptic compartments. Scale bar is 20 μm.
Although IQSEC2 is known to densely localize in spines of excitatory neurons, its expression in interneurons was heretofore not described. To further clarify our findings, we also examined IQSEC2 expression in PV-positive cells. Importantly, using DIV14 dissociated hippocampal neuronal cultures, we identified that IQSEC2 is highly expressed in PV-positive interneurons (Figure 6E). Additionally, PV-positive neurons in brain slices of adult Iqsec2+/Y animals were also reactive against the IQSEC2 antibody (Figure 6E). These findings suggest that developmental loss of IQSEC2 from these FS interneurons may be responsible for alterations in their intrinsic properties.
Discussion
De novo and inherited mutations in IQSEC2 have been identified in multiple patients diagnosed with autism spectrum disorder, intellectual disability, developmental delay, epilepsy and/or epileptic encephalopathy (Mignot et al. 2018; Shoubridge, Harvey, and Dudding-Byth 2019; Radley et al. 2019). Accordingly, Iqsec2 knockout mice have several neurobehavioral phenotypes anticipated from human clinical features, including spontaneous seizures, developmental delay, hyperactivity and deficits in adult male-female social interactions. In our study we focused on male mice to minimize phenotypic dispersion that results from mosaicism due to random X-inactivation in heterozygous females. We note that several in vivo phenotypes are consistent with those reported very recently in an independent Iqsec2 model that was published while our study was in preparation (Jackson et al. 2019)
Probing further, we noticed several unusual phenotypic features, including relative resistance to both 6 Hz electrically-induced partial or limbic seizures and to pentylenetetrazole-induced tonic-clonic seizures. While these findings are counterintuitive to the occurrence of severe spontaneous seizures, they are nevertheless striking and unique characteristics of the knockout mice offering clues to the underlying cellular and molecular etiology of IQSEC2 loss, in particular by suggesting a focus on the hippocampus and on inhibitory neurotransmission , respectively. Although IQSEC2 is known to be expressed at excitatory synapses and regulates transmission onto excitatory neurons (Murphy 2006; Brown et al. 2016; Myers et al. 2012), our detailed electrophysiological investigation of hippocampal synaptic transmission showed that loss of IQSEC2 affects excitatory transmission onto GABAergic neurons in a fundamentally different way, and that IQSEC2 is also strongly expressed in parvalbumin-positive hippocampal interneurons - the most abundant type of hippocampal interneuron and tight regulators of excitatory neuron output (Booker and Vida 2018). Concomitant histological and molecular analysis further compelled our investigations. Specifically in the hippocampus of IQSEC2 deficient mice we observe reactive astrogliosis without overt cell loss. We also observe an age-dependent increase in parvalbumin expression and an increase in the number of parvalbumin-expressing neurons.
Electrophysiological examination of dissociated hippocampal neurons revealed significant changes at the excitatory synapses onto GABAergic interneurons (but not onto excitatory neurons), namely, an increase in excitatory drive as measured through both miniature and evoked events. Also, only GABAergic hippocampal neurons displayed significant alterations in intrinsic properties. It is known from other studies in vivo and in vitro that shRNA-mediated loss of IQSEC2 results in increased excitatory cell dendritic branching and spine density (Hinze et al. 2017), factors that could result in an increased dendritic territory for the number of excitatory presynaptic contacts onto interneurons. Our finding that the membrane capacitance, and presumably total membrane area, is increased in Iqsec2−/Y GABAergic neurons supports this possibility. The results also suggest the hypothesis that in a hippocampal circuit, the functional consequence of an increased excitatory drive onto interneurons would be a predominantly hyperinhibited network. This hypothesis is consistent with data from Rogers and colleagues (Rogers et al. 2019) who very recently showed a significant decrease in hippocampal synaptic transmission in mutant mice that carry an Iqsec2 missense mutation.
The medial ganglionic eminence (MGE) produces approximately 60% of all neocortical and hippocampal interneurons and is comprised chiefly of functionally divergent cells types broadly identified by parvalbumin and somatostatin expression (Tricoire et al. 2011; Pleasure et al. 2000; Wonders and Anderson 2006; Butt et al. 2005). Importantly, the excitatory postsynaptic densities of these MGE derived interneurons differ in molecular composition from the excitatory postsynaptic densities of excitatory and caudal gang lionic eminence (CGE) derived interneurons in that they express calcium permeable GluA1 containing AMPARs but lack receptors containing GluA2 (Akgül and McBain 2016). Published studies show that while IQSEC2 aids GluA1 internalization (Myers et al. 2012), its overexpression increases GluA2 surface insertion (Brown et al. 2016). Since our results show that loss of IQSEC2 selectively affects excitatory synapses onto GABAergic neurons but not onto excitatory neurons, we hypothesize that the increase in miniature EPSCs upon IQSEC2 loss is being driven by increase in GluA1 expression in MGE derived interneurons. Furthermore, we speculate that even within the MGE population, there may be cell subtypes more vulnerable to IQSEC2 loss. Our electrophysiological recordings show that some altered cell intrinsic properties of GABAergic cells such as lower input resistance, narrower AP half-widths, and larger AHPs resemble features of FS parvalbumin positive cells and our histological data shows a clear increase in the abundance of parvalbumin positive cells in the hippocampus of adult mice. In future studies a conditional knockout approach will help to clarify the cell autonomous role of IQSEC2 in different neuronal subtypes.
It is presently unclear whether the membrane excitability changes in GABAergic neurons are secondary to the increased glutamatergic input or primary drivers of the increased glutamatergic input, or both. The known role of IQSEC2 at the postsynapse suggests that the synaptic changes drive the excitability changes. Other neurodevelopmental disease-causing gene disruptions have also been shown to simultaneously affect membrane excitability and synaptic input suggesting that convergent changes at membranes and synapses (Yi et al. 2016; Tai et al. 2014; Weston, Chen, and Swann 2014) or disruptions in the balance may underlie many disease phenotypes (Antoine et al. 2019). Nevertheless, the data overall strongly implicate inhibitory neurons in the hippocampus as important players of pathogenesis associated with IQSEC2 deficiency.
Several of our observations are consistent with other models of childhood neurodevelopmental diseases in the ASD, ID and epileptic encephalopathy spectrum. Developmentally increased parvalbumin cell number and parvalbumin mRNA expression was reported in mouse models of Rett Syndrome caused by genetic ablation of Cdkl5 and Mecp2 (Zhu and Xiong 2019; Patrizi et al. n.d.; Rudenko et al. 2015; Morello et al. 2018). Excitatory synaptogenesis closely dictates interneuron development (Tremblay, Lee, and Rudy 2016; Hu et al. 2017). Overall, parvalbumin expressing cells, compared to other interneuron types, receive over 10-fold more excitatory than inhibitory inputs (Buhl et al. 1996). Additionally, parvalbumin is a calcium-binding albumin protein whose expression in cells is closely regulated by synaptic calcium influx (44). At the excitatory synapse, IQSEC2, which has a calmodulin-binding domain, regulates NMDAR signaling and AMPAR subunit expression, thereby effectively regulating calcium entry into the cell (Rogers et al. 2019; Myers et al. 2012; Brown et al. 2016; Elagabani et al. 2016). IQSEC2 loss might therefore, upset excitatory synaptic activity-induced calcium entry, providing an alternative explanation for the increased parvalbumin expression. Therefore, based on our data, we suggest that a persistent increase in excitatory synaptic input onto these interneurons during development along with compensation for altered calcium dynamics likely results in higher parvalbumin cell expression upon IQSEC2 loss. A small percentage of somatostatin (SST)-producing non-FS cells is also known to express some PV (Maccaferri and McBain 1995). However, since we did not detect any difference in SST mRNA between the genotypes and timepoints, we conclude that the increased parvalbumin mRNA expression is being driven by an increase in parvalbumin-expressing FS cells.
Fast spiking parvalbumin interneurons mediate feedforward inhibition in the hippocampal and cortical circuits. Since this form of inhibition is strongly effective in preventing the spread of epileptic activity beyond the generation locus, these interneurons are regarded as one of the main obstacles on the pathway to the generation of convulsions (Andreae 2018). Thus, the sheer increase in number along with an increased excitatory input onto these interneurons may explain the unexpected increased resistance to induced seizures in the mutant animals.
In light of our cellular and electrophysiological observations, an obvious puzzle is how would a hypoexcitable network result in seizure activity? Since the Iqsec2−/ Y mice die of a lethal seizure, it is tempting to speculate that the abnormally increased excitatory drive onto interneurons may play a role in the preictal transition to seizure activity in a mutant hippocampus. The rationale is that repeated excitation of inhibitory neurons such as that seen with seizure activity would result in switched signs of chloride ion reversal potential, turning GABA release depolarizing from hyperpolarizing (Mackenzie and Maguire 2015). We also find evidence of hippocampal reactive gliosis in Iqsec2−/Y mice suggestive of a region under stress. Although, we did not find any evidence of cell death in adult mice, the increased excitatory drive onto interneurons during development could result in excitotoxicity mediated interneuron cell-death shifting balance to increased excitation. In fact, one knockout animal in our adult mRNA expression profiling group displayed an almost 30% decrease in PV and SST mRNA expression in both the hippocampus and cortex (Figure 6A). Based on this observation, we predict that recruitment of the cortex by the hippocampus, driven by interneuron cell death, may be the harbinger for seizure activity in these animals.
Conclusion
The skewing of E/I balance towards a decreased E/I ratio is indeed increasingly observed in multiple loss-of-function neurodevelopmental disorders in genes encoding neuroligin, β-neurexin, SHANK3, fragile X mental retardation protein (FMRP), and Rett syndrome-associated MeCP2 (Gatto and Broadie 2010). Importantly, almost all of these genetic models report spontaneous seizure activity. Such a convergence of diverse genetic factors on a few key common features suggests a few impactful mechanisms to maintain status quo. Given the pointed anomalies in hippocampal PV+ interneurons observed here, we expect that continued study of the IQSEC2 model will not only assist in design of therapeutic intervention for IQSEC2 but possibly also in related neurodevelopmental disorders with overlapping pathophysiological signatures.
Supplementary Material
Supplementary Figure 1: Seizure phenotyping of mutant Iqsec2 mice: A. Seizure thresholds are significantly different for the 6 Hz electroconvulsive test between the Iqsec2+/Y (n=7), Iqsec2−/X (15.39 ± 0.535 mA, n=14) and Iqsec2−/Y (n=8) animals (12.21± 0.5548 mA; 18.69±0.4002 mA, respectively); Mann Whitney U test, *** p=0.0002; error bars indicate ± SEM. B. Representative baseline EEG data from control and Iqsec2−/Y mice demonstrates normal EEG pattern with no trace of abnormal epileptiform activity
Supplementary Figure 2: No gross differences in brain morphology in Iqsec2−/Y mice A. Iqsec2−/Y mice weigh less than their wildtype counterparts at PND21 (p<0.001, n=10 for each genotype) and PND60 (p=0.09, n=15 for each genotype) B. No significant difference in brain volume of Iqsec2−/Y mice at PND21 (p=0.6234, n=6 for each genotype) compared to their wildtype counterparts. C. Nissl staining of coronal sections from PND21 animals revealed no gross morphological differences between the genotypes (n=6 for each genotype). Scale bar is 1000 μm D. No significant difference in cortical layer thickness of Iqsec2−/Y mice compared to their wildtype counterparts (n=6 for each genotype) E. Dentate gyrus morphometry shows no difference in granule cell layer thickness between the genotypes at PND60 (p=0.8702, n=15 slices from 5 animals for each genotype)
Supplementary Figure 3: Center time in open field assay: No significant difference was noted in time spent in the center between the genotypes (p=0.1516)
Supplementary Figure 4: IQSEC2 loss does not alter spontaneous or evoked IPSC activity. A-C. Using whole-cell, voltage-clamp electrophysiology, spontaneous miniature and evoked IPSC activity was recorded from glutamatergic and GABAergic hippocampal neurons derived from wildtype (WT) and Iqsec2−/Y mice at days 12–15 in vitro. Example traces of miniature IPSC activity onto wildtype (WT) (black) and Iqsec2−/Y (red) glutamatergic neurons (A1) and GABAergic neurons (B1). Bar graph with overlaid dot plots show that the mIPSC frequency and amplitude onto glutamatergic (A2 and A3) and GABAergic (B2 and B3) neurons were unaltered by Iqsec2 loss. C1. Example traces of evoked IPSCs onto paired wildtype (WT) (black) and Iqsec2−/Y (red) glutamatergic neurons. C2. Bar graphs with overlaid dot plots show that the eIPSC amplitude onto glutamatergic neurons was unaltered by loss of Iqsec2. For all graphs, the bars indicate the mean of the data set and each dot represents the mean response from one neuron. All p-values were calculated using Generalized Estimating Equations, and ns indicates p>0.05.
Supplementary Table 1. Intrinsic electrical properties of glutamatergic hippocampal neurons. Summary of intrinsic electrical properties of cultured glutamatergic neurons (days 12–15 in vitro) from the hippocampi of WT and Iqsec2−/Y mice. The membrane resting potential (Vrest), input resistance (Rin), time constant (τm), and capacitance (Cm), of each neuron was measured immediately following break-in, and then APs were evoked with 500 ms, 20 pA depolarizing current steps to measure AP parameters. Data are represented as mean ± SEM, and n is the number of glutamatergic neurons in each group. The p-values were calculated using Generalized Estimating Equations.
Highlights.
Iqsec2 knockout mice display growth and adult behavior deficits.
Iqsec2 knockout mice exhibit increased adulthood mortality due to lethal seizure but are relatively resistant to induced seizures.
IQSEC2 expression is strongest in the hippocampus and loss results in hippocampus specific gliosis.
IQSEC2 loss results in aberrant excitatory input onto interneurons and in altered intrinsic properties of interneurons.
IQSEC2 is expressed in parvalbumin interneurons, and IQSEC2 loss results in an increase in parvalbumin interneurons in adult hippocampus.
Acknowledgements
We would like to thank Dr. Randall Walikonis for his generous gift of the IQSEC2 antibody.
Funding
This research was supported by NIH grant R37 NS031348 to Dr. Wayne Frankel at Columbia University Medical Center and NIH grant R01 NS110945 to Matthew Weston at the University of Vermont.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akgül Gülcan, and McBain Chris J.. 2016. “Diverse Roles for Ionotropic Glutamate Receptors on Inhibitory Interneurons in Developing and Adult Brain.” The Journal of Physiology 594 (19): 5471–90. 10.1113/JP271764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae Laura C. 2018. “Not Just Uninhibited: Interneurons and Seizure Onset.” Science Translational Medicine 10 (468): eaav9143 10.1126/scitranslmed.aav9143. [DOI] [Google Scholar]
- Antoine Michelle W., Langberg Tomer, Schnepel Philipp, and Feldman Daniel E.. 2019. “Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models.” Neuron 101 (4): 648–661.e4. https://doi.Org/10.1016/j.neuron.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum Anthony J., Shonesy Brian C., Rose Kristie L., and Colbran Roger J.. 2015. “Quantitative Proteomics Analysis of CaMKII Phosphorylation and the CaMKII Interactome in the Mouse Forebrain.” ACS Chemical Neuroscience 6 (4): 615–31. 10.1021/cn500337u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker Sam A., and Vida Imre. 2018. “Morphological Diversity and Connectivity of Hippocampal Interneurons.” Cell and Tissue Research 373 (3): 619–41. 10.1007/s00441-018-2882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Joshua C., Petersen Amber, Zhong Ling, Himelright Miranda L., Murphy Jessica A., Walikonis Randall S., and Gerges Nashaat Z.. 2016a. “Bidirectional Regulation of Synaptic Transmission by BRAG1/IQSEC2 and Its Requirement in Long-Term Depression.” Nature Communications 7 (March): 11080 10.1038/ncomms11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Szilágyi T, Halasy K, and Somogyi P. 1996. “Physiological Properties of Anatomically Identified Basket and Bistratified Cells in the CA1 Area of the Rat Hippocampus in Vitro.” Hippocampus 6 (3): 294–305. https://doi.Org/10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Butt Simon J. B., Fuccillo Marc, Nery Susana, Noctor Steven, Kriegstein Arnold, Corbin Joshua G., and Fishell Gord. 2005. “The Temporal and Spatial Origins of Cortical Interneurons Predict Their Physiological Subtype.” Neuron 48 (4): 591–604. https://doi.Org/10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Elagabani Mohammad Nael, Briševac Dušica, Kintscher Michael, Pohle Jörg, Köhr Georg, Schmitz Dietmar, and Kornau Hans-Christian. 2016a. “Subunit-Selective N-Methyl-d-Aspartate (NMDA) Receptor Signaling through Brefeldin A-Resistant Arf Guanine Nucleotide Exchange Factors BRAG1 and BRAG2 during Synapse Maturation.” The Journal of Biological Chemistry 291 (17): 9105–18. 10.1074/jbc.M115.691717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN, Taylor L, Beyer B, Tempel BL, and White HS. 2001. “Electroconvulsive Thresholds of Inbred Mouse Strains.” Genomics 74 (3): 306–12. 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- Gatto Cheryl L., and Broadie Kendal. 2010. “Genetic Controls Balancing Excitatory and Inhibitory Synaptogenesis in Neurodevelopmental Disorder Models.” Frontiers in Synaptic Neuroscience 2 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze SJ, Jackson MR, Lie S, Jolly L, Field M, Barry SC, Harvey RJ, and Shoubridge C. 2017a. “Incorrect Dosage of IQSEC2, a Known Intellectual Disability and Epilepsy Gene, Disrupts Dendritic Spine Morphogenesis.” Translational Psychiatry 7 (5): e1110 10.1038/tp.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Jia Sheng, Vogt Daniel, Sandberg Magnus, and Rubenstein John L.. 2017. “Cortical Interneuron Development: A Tale of Time and Space.” Development 144 (21): 3867–78. 10.1242/dev.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Matilda R., Loring Karagh E., Homan Claire C., Hn Thai Monica, Määttänen Laura, Arvio Maria, Jarvela Irma, et al. 2019. “Heterozygous Loss of Function of IQSEC2/Iqsec2 Leads to Increased Activated Arf6 and Severe Neurocognitive Seizure Phenotype in Females.” Life Science Alliance 2 (4). 10.26508/lsa.201900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, and McBain CJ. 1995. “Passive Propagation of LTD to Stratum Oriens-Alveus Inhibitory Neurons Modulates the Temporoammonic Input to the Hippocampal CA1 Region.” Neuron 15 (1): 137–45. 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Mackenzie Gerardo G., and Maguire Jamie L.. 2015. “Chronic Stress Shifts the GABA Reversal Potential in the Hippocampus and Increases Seizure Susceptibility.” Epilepsy Research 109: 13–27. 10.1016/j.eplepsyres.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot Cyril, McMahon Aoife C., Bar Claire, Campeau Philippe M., Davidson Claire, Buratti Julien, Nava Caroline, et al. 2018a. “IQSEC2-Related Encephalopathy in Males and Females: A Comparative Study Including 37 Novel Patients.” Genetics in Medicine: Official Journal of the American College of Medical Genetics, September 10.1038/s41436-018-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello Noemi, Schina Riccardo, Pilotto Federica, Phillips Mary, Melani Riccardo, Plicato Ornella, Pizzorusso Tommaso, Pozzo-Miller Lucas, and Giustetto Maurizio. 2018. “Loss of Mecp2 Causes Atypical Synaptic and Molecular Plasticity of Parvalbumin-Expressing Interneurons Reflecting Rett Syndrome–Like Sensorimotor Defects.” ENeuro 5 (5). https://doi.Org/10.1523/ENEURO.0086-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morleo Manuela, Iaconis Daniela, Chitayat David, Peluso Ivana, Marzella Rosalia, Renieri Alessandra, Mari Francesca, and Franco Brunella. 2008. “Disruption of the IQSEC2 Transcript in a Female with X;Autosome Translocation t(X;20)(P11.2;Q11.2) and a Phenotype Resembling X-Linked Infantile Spasms (ISSX) Syndrome.” Molecular Medicine Reports 1 (1): 33–39. [PubMed] [Google Scholar]
- Murphy Jessica A. 2006. “BRAG1, a Sec7 Domain-Containing Protein, Is a Component of the Postsynaptic Density of Excitatory Synapses.” Brain Research 1120 (1): 35–45. https://doi.Org/10.1016/j.brainres.2006.08.096. [DOI] [PubMed] [Google Scholar]
- Murphy Jessica A., Jensen Ole N., and Walikonis Randall S.. 2006. “BRAG1, a Sec7 Domain-Containing Protein, Is a Component of the Postsynaptic Density of Excitatory Synapses.” Brain Research 1120 (1): 35–45. https://doi.Org/10.1016/j.brainres.2006.08.096. [DOI] [PubMed] [Google Scholar]
- Myers Kenneth R, Wang Guangfu Sheng Yanghui Conger Kathryn K., Casanova James E., and Zhu J. Julius. 2012. “Arf6-GEF BRAG1 Regulates JNK-Mediated Synaptic Removal of GluA1-Containing AMPA Receptors: A New Mechanism for Nonsyndromic X-Linked Mental Disorder.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32 (34): 11716–26. 10.1523/JNEUROSCI.1942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson Heather E., Tambunan Dimira, LaCoursiere Christopher, Goldenberg Marti Pinsky Rebecca, Martin Emilie, Ho Eugenia, Khwaja Omar, Kaufmann Walter E., and Poduri Annapurna. 2015. “Mutations in Epilepsy and Intellectual Disability Genes in Patients with Features of Rett Syndrome.” American Journal of Medical Genetics. Part A 167A (9): 2017–25. https://doi.Org/10.1002/ajmg.a.37132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi Annarita, Awad Patricia N., Chattopadhyaya Bidisha, Li Chloe, Di Cristo Graziella, and Fagiolini Michela. n.d. “Accelerated Hyper-Maturation of Parvalbumin Circuits in the Absence of MeCP2.” Cerebral Cortex. Accessed May 21, 2019 10.1093/cercor/bhz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey Kenneth A., Chittajallu Ramesh, Craig Michael T., Tricoire Ludovic, Wester Jason C., and McBain Chris J.. 2017. “Hippocampal GABAergic Inhibitory Interneurons.” Physiological Reviews 97 (4): 1619–1747. 10.1152/physrev.00007.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, and Rubenstein JL. 2000. “Cell Migration from the Ganglionic Eminences Is Required for the Development of Hippocampal GABAergic Interneurons.” Neuron 28 (3): 727–40. 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Racine RJ 1972. “Modification of Seizure Activity by Electrical Stimulation. II. Motor Seizure.” Electroencephalography and Clinical Neurophysiology 32 (3): 281–94. 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Radley Jessica A., O’Sullivan Rory B. G., Turton Sarah E., Cox Helen, Vogt Julie, Morton Jenny, Jones Elizabeth, et al. 2019. “Deep Phenotyping of Fourteen New Patients with IQSEC2 Variants, Including Monozygotic Twins of Discordant Phenotype.” Clinical Genetics, January 10.1111/cge.13507. [DOI] [PubMed] [Google Scholar]
- Rogers Eli J., Jada Reem, Schragenheim-Rozales Kinneret, Sah Megha, Cortes Marisol, Florence Matthew, Levy Nina S., et al. 2019. “An IQSEC2 Mutation Associated With Intellectual Disability and Autism Results in Decreased Surface AMPA Receptors.” Frontiers in Molecular Neuroscience 12: 43 10.3389/fnmol.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko Andrii, Seo Jinsoo, Hu Ji, Su Susan C., de Anda Froylan Calderon, Durak Omer, Ericsson Maria, Carlén Marie, and Tsai Li-Huei. 2015. “Loss of Cyclin-Dependent Kinase 5 from Parvalbumin Interneurons Leads to Hyperinhibition, Decreased Anxiety, and Memory Impairment.” The Journal of Neuroscience 35 (6): 2372–83. 10.1523/JNEUROSCI.0969-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami Hiroyuki, Sanda Masashi, Fukaya Masahiro, Miyazaki Taisuke, Sukegawa Jun, Yanagisawa Teruyuki, Suzuki Tatsuo, Fukunaga Kohji, Watanabe Masahiko, and Kondo Hisatake. 2008. “IQ-ArfGEF/BRAG1 Is a Guanine Nucleotide Exchange Factor for Arf6 That Interacts with PSD-95 at Postsynaptic Density of Excitatory Synapses.” Neuroscience Research 60 (2): 199–212. https://doi.Org/10.1016/j.neures.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Sanda Masashi, Kamata Akifumi, Katsumata Osamu, Fukunaga Kohji, Watanabe Masahiko, Kondo Hisatake, and Sakagami Hiroyuki. 2009. “The Postsynaptic Density Protein, IQ-ArfGEF/BRAG1, Can Interact with IRSp53 through Its Proline-Rich Sequence.” Brain Research 1251 (January): 7–15. https://doi.Org/10.1016/j.brainres.2008.11.061. [DOI] [PubMed] [Google Scholar]
- Shoubridge Cheryl, Harvey Robert J., and Dudding-Byth Tracy. 2019a. “IQSEC2 Mutation Update and Review of the Female-Specific Phenotype Spectrum Including Intellectual Disability and Epilepsy.” Human Mutation 40 (1): 5–24. 10.1002/humu.23670. [DOI] [PubMed] [Google Scholar]
- Shoubridge Cheryl, Tarpey Patrick S., Abidi Fatima, Ramsden Sarah L., Rujirabanjerd Sinitdhorn, Murphy Jessica A., Boyle Jackie, et al. 2010. “Mutations in the Guanine Nucleotide Exchange Factor Gene IQSEC2 Cause Nonsyndromic Intellectual Disability.” Nature Genetics 42 (6): 486–88. 10.1038/ng.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge Cheryl, Walikonis Randall S., Gécz Jozef, and Harvey Robert J.. 2010. “Subtle Functional Defects in the Arf-Specific Guanine Nucleotide Exchange Factor IQSEC2 Cause Non-Syndromic X-Linked Intellectual Disability.” Small GTPases 1 (2): 98–103. 10.4161/sgtp.1.2.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava Siddharth, Desai Sonal, Cohen Julie, Smith-Hicks Constance, Barañano Kristin, Fatemi Ali, and Naidu SakkuBai. 2018. “Monogenic Disorders That Mimic the Phenotype of Rett Syndrome.” Neurogenetics 19 (1): 41–47. 10.1007/s10048-017-0535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Chao, Abe Yasuyuki, Westenbroek Ruth E., Scheuer Todd, and Catterall William A.. 2014. “Impaired Excitability of Somatostatin- and Parvalbumin-Expressing Cortical Interneurons in a Mouse Model of Dravet Syndrome.” Proceedings of the National Academy of Sciences of the United States of America 111 (30): E3139–3148. 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay Robin, Lee Soohyun, and Rudy Bernardo. 2016. “GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits.” Neuron 91 (2): 260–92. https://doi.Org/10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire Ludovic, Pelkey Kenneth A., Erkkila Brian E., Jeffries Brian W., Yuan Xiaoqing, and McBain Chris J.. 2011. “A Blueprint for the Spatiotemporal Origins of Mouse Hippocampal Interneuron Diversity.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 31 (30): 10948–70. 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner Christopher Paul, DeBenedetto Danielle, Ware Emily, Stowe Robert, Lee Andrew, Swanson John, Walburg Caroline et al. 2010. “Postnatal Exposure to MK801 Induces Selective Changes in GAD67 or Parvalbumin.” Experimental Brain Research 201 (3): 479–88. 10.1007/s00221-009-2059-z. [DOI] [PubMed] [Google Scholar]
- Um Ji Won. 2017. “Synaptic Functions of the IQSEC Family of ADP-Ribosylation Factor Guanine Nucleotide Exchange Factors.” Neuroscience Research, Central synapse, neural circuit and brain function, 116 (March): 54–59. https://doi.Org/10.1016/j.neures.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Weston Matthew C., Chen Hongmei, and Swann John W.. 2014a. “Loss of MTOR Repressors Tsc1 or Pten Has Divergent Effects on Excitatory and Inhibitory Synaptic Transmission in Single Hippocampal Neuron Cultures.” Frontiers in Molecular Neuroscience 7: 1 10.3389/fnmol.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders Carl P., and Anderson Stewart A.. 2006. “The Origin and Specification of Cortical Interneurons.” Nature Reviews. Neuroscience 7 (9): 687–96. 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Mu Yang, Bozdagi Ozlem, Luisa Scattoni Maria, Wöhr Markus, Roullet Florence I., Katz Adam M., Abrams Danielle N., et al. 2012. “Reduced Excitatory Neurotransmission and Mild Autism-Relevant Phenotypes in Adolescent Shank3 Null Mutant Mice.” Journal of Neuroscience 32 (19): 6525–41. 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Fei, Danko Tamas, Calado Botelho Salome, Patzke Christopher, Pak ChangHui, Wernig Marius and Südhof Thomas C. 2016. “Autism-Associated SHANK3 Haploinsufficiency Causes Ih Channelopathy in Human Neurons.” Science (New York, N.Y.) 352 (6286): aaf2669 10.1126/science.aaf2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerem Ayelet, Hagjnoya Kazuhiro, Lev Dorit, Blumkin Lubov, Kivity Sara, Linder Ilan, Shoubridge Cheryl, et al. 2016a. “The Molecular and Phenotypic Spectrum of IQSEC2-Related Epilepsy.” Epilepsia 57 (11): 1858–69. https://doi.Org/10.1111/epi.13560. [DOI] [PubMed] [Google Scholar]
- Zhu Yong-Chuan, and Xiong Zhi-Qi. 2019. “Molecular and Synaptic Bases of CDKL5 Disorder.” Developmental Neurobiology 79 (1): 8–19. 10.1002/dneu.22639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Seizure phenotyping of mutant Iqsec2 mice: A. Seizure thresholds are significantly different for the 6 Hz electroconvulsive test between the Iqsec2+/Y (n=7), Iqsec2−/X (15.39 ± 0.535 mA, n=14) and Iqsec2−/Y (n=8) animals (12.21± 0.5548 mA; 18.69±0.4002 mA, respectively); Mann Whitney U test, *** p=0.0002; error bars indicate ± SEM. B. Representative baseline EEG data from control and Iqsec2−/Y mice demonstrates normal EEG pattern with no trace of abnormal epileptiform activity
Supplementary Figure 2: No gross differences in brain morphology in Iqsec2−/Y mice A. Iqsec2−/Y mice weigh less than their wildtype counterparts at PND21 (p<0.001, n=10 for each genotype) and PND60 (p=0.09, n=15 for each genotype) B. No significant difference in brain volume of Iqsec2−/Y mice at PND21 (p=0.6234, n=6 for each genotype) compared to their wildtype counterparts. C. Nissl staining of coronal sections from PND21 animals revealed no gross morphological differences between the genotypes (n=6 for each genotype). Scale bar is 1000 μm D. No significant difference in cortical layer thickness of Iqsec2−/Y mice compared to their wildtype counterparts (n=6 for each genotype) E. Dentate gyrus morphometry shows no difference in granule cell layer thickness between the genotypes at PND60 (p=0.8702, n=15 slices from 5 animals for each genotype)
Supplementary Figure 3: Center time in open field assay: No significant difference was noted in time spent in the center between the genotypes (p=0.1516)
Supplementary Figure 4: IQSEC2 loss does not alter spontaneous or evoked IPSC activity. A-C. Using whole-cell, voltage-clamp electrophysiology, spontaneous miniature and evoked IPSC activity was recorded from glutamatergic and GABAergic hippocampal neurons derived from wildtype (WT) and Iqsec2−/Y mice at days 12–15 in vitro. Example traces of miniature IPSC activity onto wildtype (WT) (black) and Iqsec2−/Y (red) glutamatergic neurons (A1) and GABAergic neurons (B1). Bar graph with overlaid dot plots show that the mIPSC frequency and amplitude onto glutamatergic (A2 and A3) and GABAergic (B2 and B3) neurons were unaltered by Iqsec2 loss. C1. Example traces of evoked IPSCs onto paired wildtype (WT) (black) and Iqsec2−/Y (red) glutamatergic neurons. C2. Bar graphs with overlaid dot plots show that the eIPSC amplitude onto glutamatergic neurons was unaltered by loss of Iqsec2. For all graphs, the bars indicate the mean of the data set and each dot represents the mean response from one neuron. All p-values were calculated using Generalized Estimating Equations, and ns indicates p>0.05.
Supplementary Table 1. Intrinsic electrical properties of glutamatergic hippocampal neurons. Summary of intrinsic electrical properties of cultured glutamatergic neurons (days 12–15 in vitro) from the hippocampi of WT and Iqsec2−/Y mice. The membrane resting potential (Vrest), input resistance (Rin), time constant (τm), and capacitance (Cm), of each neuron was measured immediately following break-in, and then APs were evoked with 500 ms, 20 pA depolarizing current steps to measure AP parameters. Data are represented as mean ± SEM, and n is the number of glutamatergic neurons in each group. The p-values were calculated using Generalized Estimating Equations.