Pharyngitis is an inflammation of the mucous membranes and underlying structures of the throat. Acute pharyngitis is one of the most common illnesses for which children in the United States visit primary care physicians; pediatricians make the diagnosis of acute pharyngitis, acute tonsillitis, or streptococcal sore throat more than 7 million times annually.1
Many viruses and bacteria can cause acute pharyngitis, either as a separate entity or as part of a more generalized illness. A partial list of the more common microorganisms that can cause acute pharyngitis is presented in Table 29-1 . Most cases of acute pharyngitis in children and adolescents are caused by viruses and are benign and self-limited. Group A beta-hemolytic streptococci (GAS) (Streptococcus pyogenes) is the most important of the bacterial causes of acute pharyngitis. Strategies for the diagnosis and treatment of pharyngitis in children and adolescents are directed at distinguishing the large group of patients with viral pharyngitis who would not benefit from antimicrobial therapy from the much smaller group of patients with GAS pharyngitis for whom antimicrobial therapy would be beneficial. Making this distinction is extremely important in attempting to minimize the unnecessary use of antimicrobial agents in children and adolescents.
TABLE 29-1.

Etiology of Acute Pharyngitis
Rights were not granted to include this table in electronic media. Please refer to the printed book.
Modified from Bisno AL, Gerber MA, Gwaltney JM, et al. Practice guideline for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113–125, with permission.
© 2008
ETIOLOGY
Viruses are the most common cause of acute pharyngitis in children and adolescents. Respiratory viruses (e.g., influenza virus, parainfluenza virus, rhinovirus, coronavirus, adenovirus, and respiratory syncytial virus) are frequent causes of acute pharyngitis. Other viruses that frequently cause acute pharyngitis include coxsackievirus, echovirus, herpes simplex virus (HSV), and Epstein–Barr virus (EBV). The acute pharyngitis produced by EBV is often accompanied by other clinical findings of infectious mononucleosis (e.g., splenomegaly, generalized lymphadenopathy). Systemic infections with other viruses (e.g., cytomegalovirus, rubella virus, and measles virus) can be associated with acute pharyngitis.
GAS is the most common bacterial cause of acute pharyngitis, accounting for 15% to 30% of the cases of acute pharyngitis in children. Other bacteria that can cause acute pharyngitis include groups C and G beta-hemolytic streptococci and Corynebacterium diphtheriae. Arcanobacterium haemolyticum is a rare cause of acute pharyngitis in adolescents and Neisseria gonorrhoeae can cause acute pharyngitis in sexually active adolescents. Other bacteria such as Francisella tularensis and Yersinia enterocolitica as well as mixed infections with anaerobic bacteria (e.g., Vincent angina) are rare causes of acute pharyngitis. Chlamydophila pneumoniae and Mycoplasma pneumoniae have been implicated as rare causes of acute pharyngitis, particularly in adults. Although other bacteria such as Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae are frequently isolated from throat cultures of children and adolescents with acute pharyngitis, their etiologic role in this illness has not been clearly established.
EPIDEMIOLOGY
Most cases of acute pharyngitis occur during the colder months of the year when respiratory viruses (e.g., rhinovirus, coronavirus, influenza virus, and adenovirus) are prevalent. Spread among family members in the home is a prominent feature of the epidemiology of most of these agents, with children being the major reservoir of infection. GAS pharyngitis is primarily a disease of children 5 to 15 years of age, and, in temperate climates, its prevalence is highest in the winter and early spring.
The incidence of gonococcal pharyngitis is highest among sexually active adolescents and young adults. The usual route of infection is orogenital sexual contact with an infected sexual partner. Sexual abuse must be strongly considered when N. gonorrhoeae is isolated from the pharynx of a prepubertal child. Widespread immunization with diphtheria toxoid has made diphtheria a rare disease in the United States, with fewer than 5 cases reported annually in recent years.
Both group C and group G beta-hemolytic streptococci can cause acute pharyngitis with clinical features similar to those of GAS pharyngitis. Group C streptococcus is a relatively common cause of acute pharyngitis among college students and among adults who come to an emergency department.2, 3 Group C streptococci can also cause epidemic, foodborne pharyngitis. Outbreaks of group C streptococcal pharyngitis related to ingestion of contaminated food products such as unpasteurized cow milk have been reported in families and schools.4 Although there have been several well-documented foodborne outbreaks of group G streptococcal pharyngitis, the etiologic role of group G streptococci in acute, endemic pharyngitis remains unclear. A community-wide, respiratory outbreak of group G streptococcal pharyngitis in a pediatric population was described in which group G streptococcus was isolated from 56 of 222 (25%) consecutive children with acute pharyngitis seen in a private pediatric office.5 Results of DNA fingerprinting of the group G streptococcal isolates suggested that 75% of them were the same strain.
The role of group C and group G streptococci in acute pharyngitis may be underestimated for several reasons. In the clinical laboratory, anaerobic incubation increases the yield of these organisms, but many laboratories do not routinely use anaerobic incubation for throat cultures. In addition, because laboratories may only report bacitracin-susceptible streptococci (consistent with GAS) and many group C and group G streptococci are bacitracin-resistant, group C and group G streptococci would be missed. Finally, many clinicians are no longer performing throat cultures but relying solely on rapid antigen detection tests (RADTs), and group C and group G streptococci would not be identified by an RADT for GAS.6
CLINICAL MANIFESTATION
The presence of certain clinical and epidemiological findings suggests GAS as the cause of an episode of acute pharyngitis (Box 29-1 ). Patients with GAS pharyngitis commonly present with sore throat (usually of sudden onset), severe pain on swallowing, and fever. Headache, nausea, vomiting, and abdominal pain can also be present. Examination typically reveals tonsillopharyngeal erythema with or without exudates, and tender, enlarged anterior cervical lymph nodes. Other findings may include a beefy, red, swollen uvula; petechiae on the palate; excoriated nares (especially in infants); and a scarlitiniform rash. However, none of these findings is specific for GAS pharyngitis. Many patients with GAS pharyngitis exhibit signs and symptoms that are milder than a “classic” case of this illness. Some of these patients have bona fide GAS pharyngitis (i.e., have a rise in antistreptococcal antibodies), whereas others are merely colonized with GAS and have pharyngitis due to an intercurrent viral infection.
BOX 29-1.

Clinical and Epidemiologic Characteristics of Group A Beta-Hemolytic Streptococci (GAS) and Viral Pharyngitis
Rights were not granted to include this box in electronic media. Please refer to the printed book.
Modified from Bisno AL, Gerber MA, Gwaltney JM, et al. Practice guideline for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113–125, with permission.
© 2008
Scarlet fever is an upper respiratory tract infection associated with a characteristic rash, that is caused by a pyrogenic exotoxin (erythrogenic toxin)-producing GAS in individuals who do not have antitoxin antibodies. Scarlet fever is encountered less often and is less virulent than in the past, but its incidence is cyclical, depending on both the prevalence of toxin-producing strains of GAS and the immune status of the population. The modes of transmission, age distribution, and other epidemiologic features are otherwise similar to those of GAS pharyngitis.
The rash of scarlet fever appears within 24 to 48 hours of the onset of signs and symptoms, although it can appear with the first signs of illness. The rash often begins around the neck and then spreads over the trunk and extremities. It is a diffuse, finely papular, erythematous eruption producing a bright red discoloration of the skin that blanches with pressure. Involvement is often more intense along the creases in the antecubital area, axillae, and groin. The involved skin has a goose-pimple appearance and feels rough. The face is usually spared, although the cheeks may be erythematous with pallor around the mouth (Figure 29-1 ). After 3 to 4 days, the rash begins to fade and is followed by desquamation, first on the face, progressing downward, and often resembling that following mild sunburn. Occasionally, sheet-like desquamation may occur around the free margins of the fingernails, the palms, and the soles. Examination of the pharynx of a patient with scarlet fever reveals essentially the same findings as with GAS pharyngitis. In addition, the tongue is usually coated and the papillae are swollen. After desquamation, the reddened papillae are prominent, giving the tongue a strawberry appearance.
Figure 29-1.
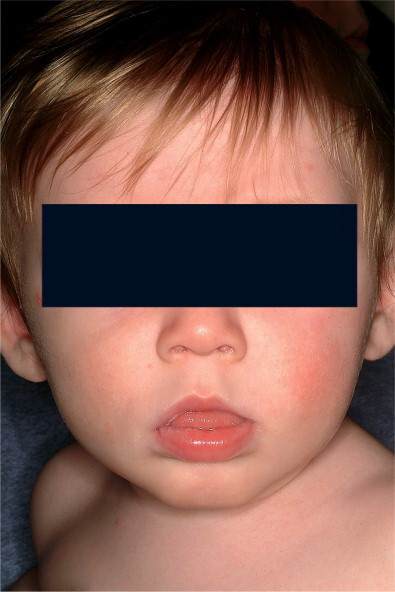
Child has group A streptococcal pharyngitis and scarlatiniform rash, with characteristic circumoral pallor.
(Courtesy of J.H. Brien.©)
In contrast, the presence of certain clinical findings (e.g., conjunctivitis, cough, hoarseness, coryza, anterior stomatitis, discrete ulcerative lesions, viral exanthema, myalgia and diarrhea) suggests a virus rather than GAS as the cause of an episode of acute pharyngitis (see Box 29-1).
Acute pharyngitis caused by adenovirus is typically associated with fever, erythema of the pharynx, enlarged tonsils with exudate, and enlarged cervical lymph nodes. Adenoviral pharyngitis can be associated with conjunctivitis, and, when it is, it is referred to as pharyngoconjunctival fever. The pharyngitis of pharyngoconjunctival fever can persist for up to 7 days, the conjunctivitis for up to 14 days, and both resolve spontaneously. Outbreaks of pharyngoconjunctival fever have been associated with transmission in swimming pools; widespread epidemics and sporadic cases also occur.
Enteroviruses (coxsackievirus, echovirus, and newer enteroviruses) can cause acute pharyngitis, especially during the summer and early fall. The pharynx may be erythematous but tonsillar exudate and cervical adenopathy are unusual. Fever may be prominent. Resolution usually occurs within a few days. Herpangina is a specific syndrome caused by coxsackie A or B virus or echoviruses and is characterized by fever and painful, discrete, gray-white papulovesicular lesions on an erythematous base in the posterior oropharynx. These lesions become ulcerative and usually resolve within 7 days. Hand, foot, and mouth disease is a specific syndrome caused by coxsackievirus A16 virus. It is characterized by painful vesicles and ulcers throughout the oropharynx associated with vesicles on the palms, soles, and sometimes on the trunk or extremities. These lesions usually resolve within 7 days.
Primary oral HSV infections usually occur in young children and typically produce acute gingivostomatitis associated with ulcerating vesicular lesions throughout the anterior mouth including the lips, but sparing the posterior pharynx. The gingivostomatitis can last up to 2 weeks and is often associated with high fever. The pain may be intense and the oral intake of fluids may be impaired, leading to dehydration. In adolescents and adults HSV can also produce a mild pharyngitis that may or may not be associated with typical vesicular, ulcerating lesions.
Acute pharyngitis is a common finding in adolescents and young adults with infectious mononucleosis caused by EBV. The pharyngitis of infectious mononucleosis can be severe with clinical findings identical to those of GAS pharyngitis (Figure 29-2A ). However, generalized lymphadenopathy and hepatosplenomegaly can also be present. Posterior cervical lymphadenopathy, presternal and periorbital edema, and palatal petechiae are distinctive if present (Figure 29-2B). If amoxicillin has been given, an intense maculopapular rash is expected (Figure 29-3 ). Fever and pharyngitis typically last 1 to 3 weeks, whereas the lymphadenopathy and hepatosplenomegaly resolve over 3 to 6 weeks. Laboratory findings include the presence of atypical lymphocytosis (Figure 29-4 ), heterophile antibodies, and specific antibodies to EBV antigens.
Figure 29-2.

Epstein–Barr virus mononucleosis in an adolescent girl. Tonsillar erythema and exudates (A) and periorbital edema (B) are clues to the diagnosis.
(Courtesy of J.H. Brien.©)
Figure 29-3.

Adolescents (A, B) with Epstein–Barr virus mononucleosis who received amoxicillin and developed diffuse erythematous raised rashes. Note predominance on trunk, and coalescence (B).
(Courtesy of J.H. Brien.©)
Figure 29-4.

Peripheral blood smear showing atypical lymphocytes (arrows) in a patient with Epstein–Barr virus mononucleosis. Note the abundant cytoplasm with vacuoles, and deformation of cell by surrounding cells.
(Courtesy of J.H. Brien.©)
The acute pharyngitis caused by Arcanobacterium haemolyticum can resemble GAS pharyngitis, including the presence of a scarlitiniform rash. In rare cases, A. haemolyticum can produce a membranous pharyngitis that can be confused with diphtheria.
Pharyngeal diphtheria is characterized by a grayish brown pseudomembrane that can be limited to one or both tonsils or can extend widely to involve the nares, uvula, soft palate, pharynx, larynx, and tracheobronchial tree. Involvement of the tracheobronchial tree may lead to life-threatening respiratory obstruction. Soft-tissue edema and prominent cervical and submental lymphadenopathy may create a bull-neck appearance.
DIAGNOSIS
When seeing a child or adolescent with acute pharyngitis, physicians in the United States must primarily distinguish between GAS and viral pharyngitis. Efforts have been made to incorporate clinical and epidemiological features of acute pharyngitis into scoring systems that attempt to predict the probability that a particular illness is caused by GAS.7–9 These clinical scoring systems are helpful in identifying patients at such low risk of GAS infection that a throat culture or RADT is usually unnecessary. However, the signs and symptoms of GAS and non-GAS pharyngitis overlap broadly, and the clinical diagnosis of GAS pharyngitis cannot be made with accuracy, even by the most experienced physicians. Therefore, recent guidelines from the Infectious Diseases Society of America (IDSA),10 as well as guidelines from the American Academy of Pediatrics (AAP)11 and the American Heart Association (AHA),12 indicate that microbiologic confirmation (either with a throat culture or RADT) is required for the diagnosis of GAS pharyngitis.
The decision to perform a microbiologic test on a child or adolescent with acute pharyngitis should be based on the clinical and epidemiologic characteristics of the illness (see Box 29-1). A history of close contact with a documented case of GAS pharyngitis or a high prevalence of GAS infections in the community can also be helpful. Testing usually need not be performed on patients with acute pharyngitis whose clinical and epidemiologic features do not suggest GAS as the etiology. Selective use of diagnostic studies for GAS will not only increase the proportion of positive test results, but also the percentage of patients with positive tests who are truly infected rather than merely GAS carriers.
Recently, new practice guidelines from the Centers for Disease Control and Prevention (CDC), the American Academy of Family Physicians (AAFP), and the American College of Physicians–American Society of Internal Medicine (ACP–ASIM) recommended the use of a clinical algorithm without microbiologic confirmation as an acceptable approach to the diagnosis of GAS pharyngitis in adults only.13 Although the goal of this algorithm-based strategy was to reduce the inappropriate use of antibiotics in adults with pharyngitis, there is concern that use would result in the administration of antimicrobial treatment to an unacceptably large number of adults with non-GAS pharyngitis.14
A study was recently performed to assess the impact of six different guidelines (including the IDSA and CDC/AAFP/ACP–ASIM guidelines) on the identification and treatment of GAS pharyngitis in children and adults.15 Guidelines that recommended selective use of RADTs and/or throat culture and treatment based only on positive test results significantly reduced the inappropriate use of antibiotics in adults. In contrast, the empiric strategy proposed in the CDC/AAFP/ACP–ASIM guidelines resulted in the administration of unnecessary antibiotics to an unacceptably large number of adults. Before abandoning the concept of treatment only after laboratory confirmation of GAS in adults with pharyngitis (IDSA guideline), additional studies need to be performed to compare empiric and laboratory-based strategies in terms of relevant patient outcomes and cost.
THROAT CULTURES
Culture on sheep blood agar of a specimen obtained by throat swab is the standard laboratory procedure for the microbiologic confirmation of GAS pharyngitis.16 If performed correctly, a throat culture has a sensitivity of 90% to 95% in detecting the presence of GAS in the pharynx.17
Several variables impact on the accuracy of throat culture results. One of the most important is the manner in which the swab is obtained.18, 19 Throat swab specimens should be obtained from the surface of both tonsils (or tonsillar fossae) and the posterior pharyngeal wall. Other areas of the pharynx and mouth are not acceptable sampling sites and should not be touched during the culturing procedure. Even with an appropriately collected specimen, false-negative results can occur if the patient has received antimicrobials before the throat swab is taken.
Anaerobic incubation and the use of selective culture media have been reported to increase the sensitivity of throat cultures.20, 21 However, data regarding the impact of the atmosphere of incubation and the culture media are conflicting, and, in the absence of definite benefit, the increased cost and effort associated with anaerobic incubation and selective culture media are difficult to justify.21–24
Duration of incubation can impact the yield of throat cultures. Once plated, cultures should be incubated at 35 to 37°C for 18 to 24 hours prior to reading. An additional overnight incubation at room temperature, however, identifies a considerable number of positive cultures that would not otherwise have been identified. Recently, Armengol and coworkers found that more than 40% of the positive confirmatory throat cultures performed on patients with pharyngitis and negative RADT results were negative after 24 hours of incubation but positive after 48 hours.25 Therefore, although initial therapeutic decisions can be guided by negative result at 24 hours, it is advisable to wait 48 hours for definitive results.
The clinical significance of the number of colonies of GAS present on the throat culture plate is controversial. Density of bacteria is likely to be greater in patients with bona fide acute GAS pharyngitis than in GAS carriers. However, there is too much overlap in the colony counts between those acutely infected with GAS and GAS carriers to permit differentiation on the basis of degree of positivity alone.24
The bacitracin disk test is the most widely used method in physicians' offices for the differentiation of GAS from other beta-hemolytic streptococci on a sheep blood agar plate. This test provides a presumptive identification based on the observation that >95% of GAS demonstrate a zone of inhibition around a disk containing 0.04 units of bacitracin, whereas 83% to 97% of non-GAS are not inhibited by bacitracin.24 An alternative and highly specific method for the differentiation of beta-hemolytic streptococci is the performance of group-specific cell wall carbohydrate antigen detection test directly on isolated bacterial colonies. Commercial kits employing group-specific antisera are available for this purpose. Additional expense for the minimal improvement in accuracy may not be justified.24
RAPID ANTIGEN DETECTION TESTS
RADTs have been developed for the identification of GAS directly from throat swabs. Although these RADTs are more expensive than blood agar cultures, the advantage they offer over the traditional procedure is the speed with which they can provide results. Rapid identification and treatment of patients with GAS pharyngitis can reduce the risk of the spread of GAS, allow the patient to return to school or work sooner, and speed clinical improvement.17, 26 In addition, in certain environments (e.g., emergency departments) the use of RADTs compared with throat cultures has significantly increased the number of patients appropriately treated for GAS pharyngitis.27
The majority of currently available RADTs have specificities of 95% or greater compared with blood agar cultures.28 Therapeutic decisions, therefore, can be made with confidence on the basis of a positive RADT result. However, the sensitivity of RADTs is between 70% and 90%.28 Although it has been suggested that many of the falsely negative RADT results occur in patients who are merely GAS carriers, it has been demonstrated that a large proportion of patients with falsely negative RADT results truly are infected with GAS.29
The first RADTs utilized latex agglutination methodology, were relatively insensitive, and had unclear endpoints.28 Subsequent tests based on enzyme immunoassay techniques had a more sharply defined endpoint and increased sensitivity. More recently, RADTs using optical immunoassay (OIA) and chemiluminescent DNA probes have been developed.28 These tests may be more sensitive than other RADTs and perhaps even as sensitive as blood agar plate cultures.28 However, because of conflicting and limited data about the OIA and other commercially available RADTs, advisory groups recommend that physicians continue to perform a confirmatory blood agar culture on children and adolescents suspected on clinical grounds of having GAS pharyngitis who have a negative RADT result. Physicians electing to use any RADT in children and adolescents without culture backup of negative results should do so only after demonstrating with adequate sample size calculation that the RADT is as sensitive as throat culture in their own practice.10, 11
Because of considerable variability in study designs and culture techniques, it is difficult to compare the sensitivity of an RADT as determined in one study to the sensitivity of another RADT as determined in a different study.28 The relative sensitivities of different RADTs can only be determined by direct comparisons. There have been only four reports of direct comparisons of different RADTs reported in the English literature.30–33 Few studies have investigated the performance of RADTs in office practice.25, 31–35 In one study,25 performed over three winter periods and using the on-site physician office laboratory at the pediatric group practice, RADT had a sensitivity of approximately 85% compared with a single blood agar plate culture. The investigators concluded that the sensitivity of this particular RADT was too low to consider abandoning the confirmatory throat culture in their practice. Investigators in a different pediatric group practice reviewed their experience with 11427 RADTs performed between 1996 and 1999.36 Only 2.4% of specimens negative by RADT were positive by culture. Investigators concluded that culture confirmation of specimens negative for RADT was costly and may not be necessary in all circumstances.36
Neither blood agar culture nor RADT accurately differentiates individuals with bona fide GAS pharyngitis from GAS carriers. However, they facilitate withholding antimicrobial therapy from the great majority of patients with sore throats whose cultures or RADTs are negative. There are an estimated 6.7 million visits to primary care providers by adults who complain of sore throat each year in the United States, and antimicrobial therapy is prescribed at 73% of these visits.37 Recent trends show a modest decline in the use of antibiotics in children and adolescents diagnosed with pharyngitis to 68.6% in one study in 1999 to 2000,38 and to 54% in another study in 2003.39
Antistreptococcal antibody titers have no value in the diagnosis of acute GAS pharyngitis. They are useful in prospective epidemiologic studies to differentiate true GAS infections from GAS carriage. Antistreptococcal antibodies are valuable for confirmation of prior GAS infections in patients suspected of having acute rheumatic fever or poststreptococcal acute glomerulonephritis.
REPEAT DIAGNOSTIC TESTING
The majority of asymptomatic persons who have a positive throat culture or RADTs after completing a course of appropriate antimicrobial therapy for GAS pharyngitis are GAS carriers.40 Follow-up throat cultures (or RADTs) are not indicated for such patients. Follow-up throat culture (or RADTs) for an asymptomatic individual should be performed in those with a history of rheumatic fever, and should be considered in patients who develop acute pharyngitis during outbreaks of acute rheumatic fever or poststreptococcal acute glomerulonephritis, and in individuals in closed or semiclosed communities during outbreaks of GAS pharyngitis.40
TREATMENT
Antimicrobial therapy is indicated for individuals with symptomatic pharyngitis after the presence of GAS in the throat has been confirmed by either throat culture or RADT. In situations in which the clinical and epidemiologic findings are highly suggestive of GAS, antimicrobial therapy can be initiated while awaiting microbiologic confirmation, provided that such therapy is discontinued if culture or RADT is negative. Antimicrobial therapy for GAS pharyngitis shortens the clinical course of the illness.26 However, GAS pharyngitis is usually a self-limited disease, and most signs and symptoms resolve spontaneously within 3 or 4 days of onset without antimicrobial therapy.41 In addition, the initiation of antimicrobial therapy can be delayed for up to 9 days after the onset of GAS pharyngitis and still prevent the occurrence of acute rheumatic fever.42 Therefore, there can be flexibility in initiating antimicrobial therapy during the evaluation of an individual patient with presumed GAS pharyngitis.
Children and adolescents with viral pharyngitis should be treated symptomatically; antimicrobial therapy is not indicated and is ineffective.
Penicillin and its congeners (such as ampicillin and amoxicillin), as well as numerous cephalosporins, macrolides, and clindamycin, are effective treatment for GAS pharyngitis. Several advisory groups have recommend penicillin as the treatment of choice for this infection.10–12 Group A streptococcus has remained exquisitely susceptible to beta-lactam agents over five decades.43 Amoxicillin is often used because of acceptable taste of suspension; efficacy appears to equal penicillin. Orally administered erythromycin is indicated for patients allergic to penicillin. Other macrolides, such as clarithromycin or azithromycin, are also effective. First-generation cephalosporins are acceptable in penicillin-allergic patients who do not manifest immediate-type hypersensitivity to beta-lactam antibiotics. Sulfa drugs, including trimethoprim-sulfamethoxazole, and tetracyclines are not effective and should not be used for GAS pharyngitis.
Some investigators concluded that cephalosporins should be treatments of choice for GAS tonsillopharyngitis44 following a meta-analysis performed of 35 clinical trials completed between 1970 and 1999 in which a cephalosporin was compared with penicillin for the treatment. However, others concluded that several major flaws make it impossible to accept the validity of this conclusion.45 Although the use of cephalosporins for GAS pharyngitis could reduce the number of persons (most merely chronic carriers) who harbor GAS in their throats after completing therapy, their use would be associated with substantial economic and ecologic costs. Penicillin has stood the test of time satisfactorily for five decades, and there are compelling reasons (e.g., its narrow antimicrobial spectrum, inexpensive cost, and impressive safety profile) to continue to recommend it as the drug of choice for GAS pharyngitis.
Oral penicillin must be administered multiple times a day for 10 days in order to achieve maximal rates of eradication of GAS. Reduced frequency of dosing and shorter treatment courses (<10 days) may result in better patient adherence to therapy than is seen with 10 days of oral penicillin. It has been reported that several antimicrobial agents, including clarithromycin, cefuroxime, cefixime, ceftibuten, cefdinir, and cefpodoxime, are effective in eradication of GAS from the pharynx when administered for ≤ 5 days.46–49 However, many of the studies of short-course therapy have serious methodologic flaws that cloud validity of conclusions. In addition, the spectra of these antibiotics are much broader than that of penicillin, and, even if administered for short courses, they are more expensive.46 Therefore, additional studies are needed before these short-course regimens can be recommended.10
Attempts to treat GAS pharyngitis with a single daily dose of penicillin have been unsuccessful.52 In recent years, investigators have demonstrated that several antimicrobial agents, including azithromycin, cefadroxil, cefixime, ceftibuten, cefpodoxime, cefprozil, and cefdinir, are effective in eradicating pharyngeal streptococci when given as a single daily dose.10, 46, 49, 53 However, these agents are expensive and have broad spectra of activity compared with penicillin. Preliminary investigations have demonstrated that once-daily amoxicillin therapy is effective in the treatment of GAS pharyngitis.54, 55 If confirmed by additional investigations, once-daily amoxicillin therapy, because of its low cost and relatively narrow spectrum, could become an alternative regimen for the treatment of GAS pharyngitis. Table 29-2 gives recommendations for several agents proven to be effective for the treatment of GAS pharyngitis.10 Intramuscular benzathine penicillin G is preferred in those patients unlikely to complete a full 10-day course of oral therapy.
TABLE 29-2.

Antimicrobial Therapy for Group A Beta-Hemolytic Streptococci (GAS) Pharyngitis
Rights were not granted to include this table in electronic media. Please refer to the printed book.
Modified from Bisno AL, Gerber MA, Gwaltney JM, et al. Practice guideline for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113–125, with permission.
© 2008
Although penicillin resistance has not occurred in GAS anywhere in the world,56 there have been geographic areas with relatively high levels of resistance to macrolide antibiotics.50, 51 The rate of macrolide resistance among isolates of GAS in the United States has generally remained <5%. In an investigation of 245 pharyngeal isolates and 56 invasive isolates of GAS obtained between 1994 and 1997 from 24 states and the District of Columbia, only 8 (2.6%) of the 301 isolates were determined to be macrolide-resistant.43 However, higher resistance rates have occasionally been reported. For example, 9% of pharyngeal and 32% of invasive GAS strains collected in San Francisco during 1994 to 1995 were reported to be macrolide-resistant.57 During a longitudinal investigation of GAS disease in a single elementary school in Pittsburgh, investigators found that 48% of isolates of GAS collected between 2000 and 2001 were resistant to erythromycin; none was resistant to clindamycin.58 Molecular typing indicated that this outbreak was due to a single strain of GAS. In addition, of 100 randomly selected isolates of GAS obtained from the community in 2001, 38 (38%) were resistant to erythromycin.58
The results of a prospective, multicenter, United States community- based surveillance of pharyngeal isolates of GAS recovered from children 3 to 18 years of age during three successive respiratory seasons between 2000 and 2003 have been reported.59 Macrolide resistance was <5%, clindamycin resistance was 1.04%, and rates were stable. In addition, there was no evidence of wide dissemination of specific macrolide-resistance clones, increasing clindamycin resistance, or increasing erythromycin minimum inhibitory concentrations over the 3-year study period. There was, however, considerable geographic variability in macrolide resistance rates in each study year, as well as year-to-year variability at individual study sites.59 Clinicians should be aware of local resistance rates.
Acute rheumatic fever has not been described as a complication of either group C or group G streptococcal pharyngitis. Although acute glomerulonephritis has been reported as a complication of group C streptococcal pharyngitis, it is extremely unusual and a causal relationship has not been established. Therefore, the primary reason to identify either group C or group G streptococcus as the cause of acute pharyngitis is to initiate antimicrobial therapy that may mitigate the clinical course of the infection. However, there is currently no convincing evidence from controlled studies of clinical response to antimicrobial therapy in patients with acute pharyngitis and either group C or group G streptococcus isolated from their pharynx. If one elects to treat either group C or group G streptococcal pharyngitis, the treatment should be similar to that for GAS pharyngitis with penicillin as the antimicrobial agent of choice.6
COMPLICATIONS
GAS pharyngitis can be associated with suppurative and nonsuppurative complications. Suppurative complications result from the spread of GAS to adjacent structures and include peritonsillar abscess, retropharyngeal abscess, cervical lymphadenitis, sinusitis, otitis media, and mastoiditis. Before antimicrobial agents were available, suppurative complications of GAS pharyngitis were common; however, antimicrobial therapy has greatly reduced the frequency of such complications.
Acute rheumatic fever, acute poststreptococcal glomerulonephritis, and poststreptococcal reactive arthritis are recognized nonsuppurative sequelae of GAS pharyngitis (see Chapter 118, Streptococcus pyogenes). Acute rheumatic fever occurs after an episode of GAS pharyngitis (usually after a 2- to 4-week latent period) and not after GAS infections of the skin. Appropriate antimicrobial therapy begun within 9 days of the onset of pharyngitis can prevent this complication. In contrast to acute rheumatic fever, acute poststreptococcal glomerulonephritis can occur after a GAS infection of either the pharynx or skin and does not appear to be prevented by antimicrobial therapy of the antecedent GAS infection. The latent period for glomerulonephritis is about 3 weeks following skin infection and 10 days following upper respiratory tract infection. Poststreptococcal reactive arthritis is similar to other postinfectious arthritides. The relationship of this entity to acute rheumatic fever is still unclear.
TREATMENT FAILURES, CHRONIC CARRIAGE, AND RECURRENCES
Antimicrobial treatment failures with GAS pharyngitis have traditionally been classified as either clinical or bacteriologic failures. However, the significance of clinical treatment failures (usually defined as persistent or recurrent signs or symptoms suggestive of GAS pharyngitis) is difficult to determine because GAS pharyngitis is a self-limited illness even without antimicrobial therapy.41 In addition, without the repeated isolation of the infecting strain of GAS (i.e., true bacteriologic treatment failure), it is particularly difficult to determine the clinical significance of persistent or recurrent signs or symptoms suggestive of GAS pharyngitis.
Bacteriologic treatment failures can be classified as either true or apparent failures.
True bacteriologic failure refers to the inability to eradicate the specific strain of GAS causing an acute episode of pharyngitis with a complete course of appropriate antimicrobial therapy. Apparent bacteriologic treatment failure can reflect a variety of circumstances.
Apparent bacteriologic failures can occur when newly acquired GAS isolates are mistaken for the original infecting strain of GAS, when the infecting strain of GAS is eradicated but then rapidly reacquired, or when adherence to antimicrobial therapy is poor – the majority of apparent bacteriologic treatment failures are manifestations of the GAS carrier state. These chronic carriers have GAS in their pharynx but no immunologic response to the organism. They may be colonized for 6 to 12 months or more, during which time they may experience episodes of intercurrent viral pharyngitis and be identified to have a positive test for GAS. Carriers are unlikely to spread GAS to their close contacts and are at very low, if any risk, for developing suppurative or nonsuppurative complications.60, 61 During the winter and spring in temperate climates, as many as 20% of asymptomatic school-aged children carry GAS.60 GAS carriers should not be given antimicrobial therapy; the primary approach to the suspected or confirmed carrier is reassurance. A throat culture or RADT should be performed whenever the patient has symptoms and signs suggestive of GAS pharyngitis, but should be avoided when symptoms are more typical of viral illnesses (see Box 29-1). Each clinical episode confirmed with a positive throat culture or RADT should be treated. Identification and eradication of the streptococcal carrier state are desirable in certain specific situations (Box 29-2 ). When antimicrobial therapy is employed, oral clindamycin (20 mg/kg per day up to 450 mg, divided into three equal doses) for 10 days is preferred,43 but intramuscular benzathine penicillin (alone or in combination with procaine penicillin) plus oral rifampin (20 mg/kg per day divided into two equal doses (maximum dose 300 mg) for 4 days beginning on the day of the penicillin injection)32 is also effective. Chronic carriage can recur upon re-exposure to GAS.
BOX 29-2. Indications for Identification and Eradication of the Streptococcal Carrier State.
-
•
A personal or family history of rheumatic fever
-
•
During outbreaks of acute rheumatic fever or acute poststreptococcal glomerulonephritis
-
•
Outbreaks of GAS in closed communities, such as military barracks or prisons, or semiclosed communities, such as college campuses or boarding schools
-
•
Family with repeated episodes of spread of GAS among members
-
•
Case in which tonsillectomy is only being considered because of chronic carriage of GAS
-
•
An inordinate amount of anxiety about GAS among family members despite education and reassurance
GAS, group A beta-hemolytic streptococci.
Several explanations have been proposed for true bacteriologic treatment failures. No penicillin-resistant strains of GAS have been identified. The role of penicillin tolerance (i.e., a discordance between the concentration of penicillin required to inhibit and to kill the organisms) in true bacteriologic treatment failures has never been established.62, 63 The precise role that bacteria present in the normal pharyngeal flora contribute to true bacteriologic failures either by enhancing the colonization and growth of GAS in the upper respiratory tract or by producing beta-lactamases that inactive penicillin has not been determined.56
Routine throat culture (or RADT) is not indicated for asymptomatic individuals following completion of antimicrobial therapy for GAS pharyngitis. In a symptomatic patient, a throat culture (or RADT) is usually performed and, if positive, many clinicians would elect to administer a second course of antimicrobial therapy.
When repeated episodes of spread of GAS occurs among family members, some physicians would perform simultaneous cultures of all family contacts and treat those whose cultures are positive. There is no credible evidence that family pets are reservoirs for GAS, nor do they contribute to spread.
The patient with repeated episodes of acute pharyngitis associated with a positive throat culture (or RADT) is a common and difficult problem for the practicing physician. The fundamental question that must be addressed is whether this patient is experiencing repeated episodes of bona fide GAS pharyngitis or is a GAS carrier experiencing repeated episodes of viral pharyngitis. The latter situation is considerably more common than the former. Such a patient is likely to be a GAS carrier if: (1) the clinical and epidemiologic findings suggest a viral etiology; (2) there is little clinical response to appropriate antimicrobial therapy; (3) throat culture (or RADT) is positive between episodes of pharyngitis; and (4) there is no serologic response to GAS extracellular antigen (e.g., antistreptolysin O, antideoxyribonucleases B). In contrast, the patient with repeated episodes of acute pharyngitis associated with positive throat cultures (or RADTs) for GAS is likely to be experiencing repeated episodes of bona fide GAS pharyngitis if: (1) the clinical and epidemiologic findings suggest GAS pharyngitis; (2) there is a demonstrable clinical response to appropriate antimicrobial therapy; (3) throat culture (or RADT) is negative between episodes of pharyngitis; and (4) there is a serologic response to GAS extracellar antigens. If determined that the patient is experiencing repeated episodes of bona fide GAS pharyngitis, some physicians have suggested use of prophylactic oral penicillin V. However, the efficacy of this regimen has not been proven, and antimicrobial prophylaxis is not recommended except to prevent recurrences of rheumatic fever in patients who have experienced a previous episode of rheumatic fever. Tonsillectomy may be considered in the rare patient whose symptomatic episodes do not diminish in frequency over time and in whom no alternative explanation for the recurrent GAS pharyngitis is evident. However, tonsillectomy has been demonstrated to be beneficial for a relatively small group of these patients, and any benefit can be expected to be relatively short-lived.64–66
References
- 1.Woodwell D. Office Visits to Pediatric Specialists. 1989. (Advance Data from Vital and Health Statistics, no. 208) National Center for Health Statistic; Hyatttsville MD: 1992. [PubMed] [Google Scholar]
- 2.Meier FA, Centor RM, Graham I., Jr Clinical and microbiological evidence for endemic pharyngitis among adults due to group C streptococci. Arch Intern Med. 1990;150:825–829. [PubMed] [Google Scholar]
- 3.Turner JC, Hayden FG, Lobo MC. Epidemiologic evidence for Lancefield group C beta-hemolytic streptococci as a cause of exudative pharyngitis in college students. J Clin Microbiol. 1997;35:1–4. doi: 10.1128/jcm.35.1.1-4.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arditi M, Shulman ST, Davis AT. Group C beta-hemolytic streptococcal infections in children: nine pediatric cases and review. Rev Infect Dis. 1989;11:34–45. doi: 10.1093/clinids/11.1.34. [DOI] [PubMed] [Google Scholar]
- 5.Gerber MA, Randolp MF, Martin NJ. Community wide outbreak of group G streptococcal pharyngitis. Pediatrics. 1991;87:598–603. [PubMed] [Google Scholar]
- 6.Gerber MA. Non-group A or B streptococci. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 17th ed. Saunders; Philadelphia: 2004. pp. 883–884. [Google Scholar]
- 7.Centor RM, Witherspoon JM, Dalton HP. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239–1246. doi: 10.1177/0272989X8100100304. [DOI] [PubMed] [Google Scholar]
- 8.Wald ER, Green MD, Schwartz B. A streptococcal score card revisited. Pediatr Emerg Care. 1998;14:109–111. doi: 10.1097/00006565-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Attia MW, Zaoutis T, Klein JD. Performance of a predictive model for streptococcal pharyngitis in children. Arch Pediatr Adolesc Med. 2001;155:687–691. doi: 10.1001/archpedi.155.6.687. [DOI] [PubMed] [Google Scholar]
- 10.Bisno AL, Gerber MA, Gwaltney JM. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis. 2002;35:113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics . Group A streptococcal infections. In: Pickering LK, editor. Red Book: Report of the Committee on Infectious Diseases. 27th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2006. pp. 610–620. [Google Scholar]
- 12.Dajani A, Taubert K, Ferrieri P. Treatment of acute streptococcal pharyngitis and prevention of acute rheumatic fever: a statement for health professionals. Pediatrics. 1995;96:758–764. [PubMed] [Google Scholar]
- 13.Cooper JR, Hoffman JR, Bartlett JG. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med. 2001;134:509–517. doi: 10.7326/0003-4819-134-6-200103200-00019. [DOI] [PubMed] [Google Scholar]
- 14.Bisno AL, Peter GS, Kaplan EL. Diagnosis of strep throat in adults: are clinical criteria really good enough? Clin Infect Dis. 2002;35:126–129. doi: 10.1086/342056. [DOI] [PubMed] [Google Scholar]
- 15.McIsaac WJ, Kellner JD, Aufricht P. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291:1587–1595. doi: 10.1001/jama.291.13.1587. [DOI] [PubMed] [Google Scholar]
- 16.Breese BB, Disney FA. The accuracy of diagnosis of beta-streptococcal infections on clinical grounds. J Pediatr. 1954;44:670–673. doi: 10.1016/s0022-3476(54)80008-4. [DOI] [PubMed] [Google Scholar]
- 17.Gerber MA. Comparison of throat cultures and rapid strep tests for diagnosis of streptococcal pharyngitis. Pediatr Infect Dis J. 1989;8:820–824. doi: 10.1097/00006454-198911000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Brien JH, Bass JW. Streptococcal pharyngitis: optimal site for throat culture. J Pediatr. 1985;106:781–783. doi: 10.1016/s0022-3476(85)80354-1. [DOI] [PubMed] [Google Scholar]
- 19.Gunn BA, Mesrobian R, Keiser JF. Cultures of Streptococcus pyogenes from the oropharynx. Lab Med. 1985;16:369–371. [Google Scholar]
- 20.Lauer BA, Reller LB, Mirrett S. Effect of atmosphere and duration of incubation on primary isolation of group A streptococci from throat cultures. J Clin Microbiol. 1983;17:338–340. doi: 10.1128/jcm.17.2.338-340.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz RH, Gerber MA, McCoy P. Effect of atmosphere of incubation on the isolation of group A streptococci from throat cultures. J Lab Clin Med. 1985;106:88–92. [PubMed] [Google Scholar]
- 22.Kellogg JA, Manzella JP. Detection of group A streptococci in the laboratory or physician's office: culture vs antibody methods. JAMA. 1986;255:2638–2642. [PubMed] [Google Scholar]
- 23.Roddey OF, Jr, Clegg HW, Martin ES. Comparison of throat culture methods for the recovery of group A streptococci in a pediatric office setting. JAMA. 1995;274:1863–1865. [PubMed] [Google Scholar]
- 24.Gerber MA. Diagnosis of pharyngitis: methodology of throat cultures. In: Shulman ST, editor. Pharyngitis: Management in an Era of Declining Rheumatic Fever. Praeger; New York: 1984. pp. 61–72. [Google Scholar]
- 25.Armengol CE, Schlager TA, Hendley JO. Sensitivity of a rapid antigen detection test for group A streptococci in a private pediatric office setting: answering the Red Book's request for validation. Pediatrics. 2004;113:924–926. doi: 10.1542/peds.113.4.924. [DOI] [PubMed] [Google Scholar]
- 26.Randolph MF, Gerber MA, DeMeo KK. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J Pediatr. 1985;106:870–875. doi: 10.1016/s0022-3476(85)80228-6. [DOI] [PubMed] [Google Scholar]
- 27.Lieu TA, Fleisher GR, Schwartz JS. Clinical evaluation of a latex agglutination test for streptococcal pharyngitis: performance and impact on treatment rates. Pediatr Infect Dis J. 1988;7:847–854. [PubMed] [Google Scholar]
- 28.Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17:571–580. doi: 10.1128/CMR.17.3.571-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber MA, Randolph MF, Chanatry J. Antigen detection test for streptococcal pharyngitis: evaluation of sensitivity with respect to true infections. J Pediatr. 1986;108:654–658. doi: 10.1016/s0022-3476(86)81036-8. [DOI] [PubMed] [Google Scholar]
- 30.Roe M, Kishiyama C, Davidson K. Comparison of BioStar Strep A OIA optical immunoassay, Abbott Test Pack Plus Strep A, and culture with selective media for diagnosis of group A streptococcal pharyngitis. J. Clin Microbiol. 1995;33:1551–1553. doi: 10.1128/jcm.33.6.1551-1553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roosevelt GE, Kaulkarni MS, Shulman ST. Critical evaluation of a CLIA-waived streptococcal antigen detection test in the emergency department. Ann Emerg Med. 2001;37:377–381. doi: 10.1067/mem.2001.114090. [DOI] [PubMed] [Google Scholar]
- 32.Gieseker KE, MacKenzie T, Roe MH. Comparison of two rapid Streptococcus pyogenes diagnostic tests with a rigorous culture standard. Pediatr Infect Dis J. 2002;21:922–926. doi: 10.1097/00006454-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz RH. Evaluation of rapid streptococcal detection tests. Pediatr Infect Dis J. 1997;16:1099–2000. doi: 10.1097/00006454-199711000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Nerbrand C, Jasir A, Schalen C. Are current rapid detection tests for group A streptococci sensitive enough? Scand J Infect Dis. 2002;34:797–799. doi: 10.1080/0036554021000026953. [DOI] [PubMed] [Google Scholar]
- 35.Gieseker KE, Roe MH, MacKenzie T. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111:e666–e670. doi: 10.1542/peds.111.6.e666. [DOI] [PubMed] [Google Scholar]
- 36.Mayes T, Pichichero ME. Are follow-up throat cultures necessary when rapid antigen detection tests are negative for group A streptococci? Clin Pediatr. 2001;40:191–195. doi: 10.1177/000992280104000402. [DOI] [PubMed] [Google Scholar]
- 37.Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989–1999. JAMA. 2001;286:1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]
- 38.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 39.Linder JA, Bates DW, Lee GM. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315–2322. doi: 10.1001/jama.294.18.2315. [DOI] [PubMed] [Google Scholar]
- 40.Gerber MA. Treatment failures and carriers: perception or problems? Pediatr Infect Dis J. 1994;13:576–579. doi: 10.1097/00006454-199406000-00036. [DOI] [PubMed] [Google Scholar]
- 41.Brink WR, Rammelkamp CH, Jr, Denny FW. Effect of penicillin and aureomycin on the natural course of streptococcal tonsillitis and pharyngitis. Am J Med. 1951;10:300–308. doi: 10.1016/0002-9343(51)90274-4. [DOI] [PubMed] [Google Scholar]
- 42.Catanzaro FJ, Stetson CA, Morris AJ. The role of streptococcus in the pathogenesis of rheumatic fever. Am J Med. 1954;17:749–756. doi: 10.1016/0002-9343(54)90219-3. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan EL. Johnson DR, Del Rosario MC, et al. Susceptibility of group A beta- hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr Infect Dis J. 1999;18:1069–1072. doi: 10.1097/00006454-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Casey JR, Pichichero ME. Meta-analysis of cephalosporin versus penicillin treatment of group A streptococcal tonsillopharyngitis in children. Pediatrics. 2004;113:866–882. doi: 10.1542/peds.113.4.866. [DOI] [PubMed] [Google Scholar]
- 45.Shulman ST, Gerber MA. So what's wrong with penicillin for strep throat? Pediatrics. 2004;113:1816–1818. doi: 10.1542/peds.113.6.1816. [DOI] [PubMed] [Google Scholar]
- 46.Gerber MA, Tanz RR. New approaches to the treatment of group A streptococcal pharyngitis. Curr Opin Pediatr. 2001;13:51–55. doi: 10.1097/00008480-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Scholz H. Streptococcal A tonsillopharyngitis: a 5-day course of cefuroxime axetil versus a 10-day course of penicillin V, results depending on children's age. Chemotherapy. 2004;50:51–54. doi: 10.1159/000077286. [DOI] [PubMed] [Google Scholar]
- 48.Syrogiannopoulos GA, Bozdogan B, Grivea IN. Two dosages of clarithromycin for five days, amoxicillin/clavulinate for five days or penicillin V for ten days in acute group A streptococcal tonsillopharyngitis. Pediatr Infect Dis J. 2004;23:857–865. doi: 10.1097/01.inf.0000138080.74674.a2. [DOI] [PubMed] [Google Scholar]
- 49.Cohen R. Defining the optimum treatment regimen for azithromycin in acute tonsilliopharyngitis. Pediatr Infect Dis J. 2004;23:S129–S134. doi: 10.1097/01.inf.0000112527.33870.0d. [DOI] [PubMed] [Google Scholar]
- 50.Cornaglia G, Ligozzi M, Mazzariol A. Resistance of Streptococcus pyogenes to erythromycin and related antibiotics in Italy. Clin Infect Dis. 1998;27(suppl 1):S87–S92. doi: 10.1086/514908. [DOI] [PubMed] [Google Scholar]
- 51.Seppala H, Klaukka T, Vuopio-Varkila J. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 52.Gerber MA, Randolph MF, DeMeo K. Failure of once-daily penicillin V therapy for streptococcal pharyngitis. Am J Dis Child. 1989;143:153–155. doi: 10.1001/archpedi.1989.02150140039016. [DOI] [PubMed] [Google Scholar]
- 53.Curtin CD, Casey JR, Murray PC. Efficacy of cephalexin two vs. three times daily vs. cefadroxil once daily in streptococcal pharyngitis. Clin Pediatr. 2003;42:519–526. doi: 10.1177/000992280304200606. [DOI] [PubMed] [Google Scholar]
- 54.Shvartzman P, Tabenkin H, Rosentzwaig A. Treatment of streptococcal pharyngitis with amoxycillin once a day. Br Med J. 1993;306:1170–1172. doi: 10.1136/bmj.306.6886.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feder HMJ, Gerber MA, Randolph MF. Once-daily therapy for streptococcal pharyngitis with amoxicillin. Pediatrics. 1999;103:47–51. doi: 10.1542/peds.103.1.47. [DOI] [PubMed] [Google Scholar]
- 56.Gerber MA. Antibiotic resistance: relationship to persistence of group A streptococci in the upper respiratory tract. Pediatrics. 1996;97:971–975. [PubMed] [Google Scholar]
- 57.York MK, Gibbs L, Perdreau-Remington R. Characterization of antimicrobial resistance in Streptococcus pyogenes isolates from the San Francisco Bay area of Northern California. J Clin Microbiol. 1999;37:1727–1731. doi: 10.1128/jcm.37.6.1727-1731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin JM, Green M, Barbadora KA. Erthromycin-resistant group A streptococci in school children in Pittsburgh. N Engl J Med. 2002;346:1200–1206. doi: 10.1056/NEJMoa013169. [DOI] [PubMed] [Google Scholar]
- 59.Tanz RR, Shulman ST, Shortridge VD. Community-based surveillance in the United States of macrolide-resistant pediatric pharyngeal group A streptococci during 3 respiratory disease seasons. Clin Infect Dis. 2004;39:1794–1801. doi: 10.1086/426025. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan EL. The group A streptococcal carrier state: an enigma. J Pediatr. 1980;97:337–345. doi: 10.1016/s0022-3476(80)80178-8. [DOI] [PubMed] [Google Scholar]
- 61.Martin JM, Green M, Barbadora KA. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–1219. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- 62.Smith TD, Huskins C, Kim KS. Efficacy of beta-lactamase-resistant penicillin and influence of penicillin tolerance in eradicating streptococci from the pharynx after failure of penicillin therapy for group A streptococcal pharyngitis. J Pediatr. 1987;110:777–782. doi: 10.1016/s0022-3476(87)80023-9. [DOI] [PubMed] [Google Scholar]
- 63.Kim KS, Kaplan EL. Association of penicillin tolerance with failure to eradicate group A streptococci from patients with pharyngitis. J Pediatr. 1985;107:681–684. doi: 10.1016/s0022-3476(85)80392-9. [DOI] [PubMed] [Google Scholar]
- 64.Paradise JL, Blueston CD, Bachman RZ. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. N Engl J Med. 1984;310:674–683. doi: 10.1056/NEJM198403153101102. [DOI] [PubMed] [Google Scholar]
- 65.Paradise JL, Bluestone CD, Colborn DK. Tonsillectomy and adenoidectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110:7–15. doi: 10.1542/peds.110.1.7. [DOI] [PubMed] [Google Scholar]
- 66.Discolo CM, Darrow DH, Koltai PJ. Infection indications for tonsillectomy. Pediatr Clin North Am. 2003;50:455–458. doi: 10.1016/s0031-3955(03)00030-0. [DOI] [PubMed] [Google Scholar]


