Abstract
Levulinic acid (LA) is considered to be one of the promising organic bio-platform chemicals and intermediates for the synthesis of fuels, chemicals, and polymers. In the present study, heterogeneous catalytic dehydration of hexose sugars, fructose and glucose, using a strong cation exchange resin (hydrogen form) as an acid catalyst, was performed to produce LA in an aqueous medium. The effect of salts such as NaCl, KCl, CaCl2, Na2CO3, and Na2SO4 in the medium on the rate of sugar conversion and LA yield was evaluated. Under optimum reaction conditions, 10% (w/w) fructose was dehydrated to LA (with 74.6% yield) in 10% (w/w) NaCl aqueous solution in 24 h at 110 °C using the catalyst at 30% (w/w sugar). Even 10% (w/w) glucose monohydrate was directly dehydrated to LA (with 70.7% yield) under similar conditions but at 145 °C. This study shows that the salts enhance the rate of catalytic dehydration in the order of Cl– > CO32– > SO42–. Thus, the combination of high sugar concentration and heterogeneous catalysis in an aqueous system under relatively mild conditions could provide a high-yielding and sustainable process for bio-based LA production.
Introduction
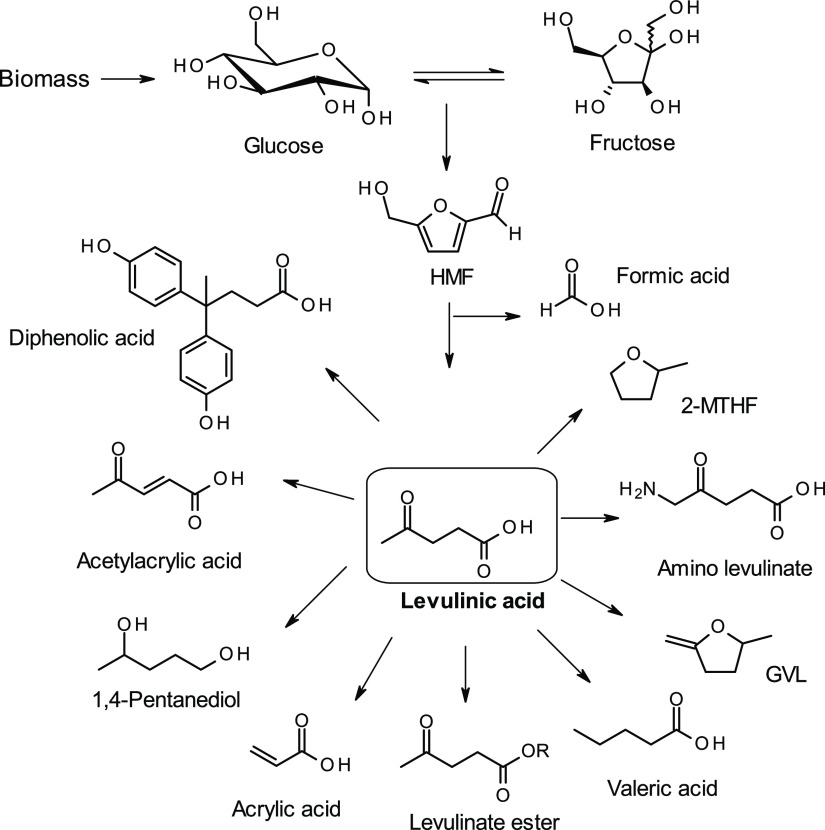
As the main source of functional carbon-building blocks for the fuel, chemical, and polymer industries is still based on fossil resources, one of the greatest challenges of the 21st century is to enable transition to an economy based on renewable resources. Therefore, production of these materials from bio-based renewable resources using environment-friendly routes for reducing the carbon footprint is drawing increasing attention.1−5 Levulinic acid (LA; also known as 4-oxopentanoic acid or γ-ketovaleric acid) is classified by the United States Department of Energy as one of the top 12 promising bio-based building blocks for the synthesis of fuels and chemicals.2,6,7 It is also a specialty chemical that finds applications as a component in polymer resins, animal feed, food as well as flavor and fragrance industry products, solvents, textile dyes, additives, extenders for fuels, antimicrobial agents, herbicides, and plasticizers.2,7 LA is a bifunctional chemical containing ketone and carboxylic acid groups, which are important for the production of a wide range of chemicals such as levulinate esters, γ-valerolactone (GVL), acrylic acid, 1,4-pentanediol, angelica lactone, 2-methyltetrahydrofuran (MTHF), δ-aminolevulinic acid (DALA), etc (Scheme 1).2,8
Scheme 1. Heterogeneous Catalytic Dehydration of Hexose Sugars (Glucose and Fructose) to Levulinic Acid and Formic Acid, and the Conversion of Levulinic Acid.
Production of LA from monosaccharides (e.g. glucose and fructose), polysaccharides, and lignocellulosic biomass has been extensively investigated using homogeneous or heterogeneous catalysts (Scheme 1). Although fructose is the preferred substrate for LA production with respect to yield and selectivity, several reports on its production from glucose via isomerization to fructose, as well as directly from cellulose are also available, however, with relatively low yields.9−11 Mineral acids such as HCl and H2SO4 are the most common homogeneous catalysts used. Recently, high experimental yield (74 mol %) of LA was obtained from 0.1 M d-fructose using 1 M sulfuric acid at 140 °C.12 In another study, 83 and 51% yields of LA were obtained from fructose and glucose using 0.25 M HCl at 130 °C, respectively.13 In general, lower yields of LA have been reported when using glucose as the starting material with HCl/H2SO4 as a catalyst as compared to that obtained with fructose.13 Although production of LA directly from polysaccharides results in comparable yields as that from monosaccharides, longer reaction times or higher amounts of acid catalysts are needed.13
However, the drawbacks associated with the use of mineral acids, such as the corrosion of equipment and human tissue, difficult recovery, and recyclability,14−16 have prompted the development and use of heterogeneous acid catalysts (e.g. zeolites, Amberlyst resins, or Dowex) that are easily recovered from reaction media.17−19 Several studies have been carried out on the production of LA from fructose, glucose and sucrose, and polysaccharides using different heterogeneous catalysts for example Fe/HY zeolite, Dowex 50 × 8–100, and cellulase-mimetic solid acid catalysts, in media based on water or a mixture of water and a polar solvent.13,17−19 The product yield was found to be higher in the presence of a solvent than in pure water, for example LA yield from fructose in a reaction catalyzed by Dowex 50 × 8–100 was increased from 58% in water to 72% in a 50:50 mixture of water/gamma-valerolactone.18 However, the processes with good LA yields from sugars and cellulose under milder reaction conditions still require further development. Water is preferable as a reaction medium for attaining minimal environmental impact in (bio)chemical reactions and for minimizing energy-intensive downstream operations for solvent recovery but is generally inefficient for dehydration of sugars into LA and formic acid, and other products. Meanwhile, inclusion of metal halides (e.g. FeCl3) has been shown to enhance the reaction rate and product yield during production of furfural from xylose.20−22
This paper reports a study performed on the effect of salts on the conversion of fructose and glucose to LA in an aqueous medium using an ion exchange resin, Dowex DR-2030, as the catalyst. Furthermore, reusability of the catalyst was evaluated in repeated batch reactions.
Results and Discussion
Catalytic Conversion of Fructose and Glucose Using Acidic Ion Exchanger DR-2030
Recently, we demonstrated catalytic dehydration of fructose used at high concentration (300 g/L) to 5-HMF using the acidic ion exchanger, DR-2030 in DMSO at 110 °C in batch and continuous modes.23 Our preliminary studies on using the catalyst in an aqueous solution for dehydration of fructose and glucose showed the formation of LA and formic acid. It is likely that DR-2030 catalyzes the isomerization of glucose to fructose through the 1,2-enediolate, an enediol–IEx complex,24 as has also been reported earlier for other heterogeneous catalysts like Fe/HY zeolite,17 cation-exchange zeolites and hydrotalcites,24 Yb(OTf)3/[Bmim]Cl,25 and CuCl2/[EMIM]Cl26 Subsequently, fructose undergoes dehydration with loss of three water molecules yielding 5-HMF, which in the presence of water is rehydrated 4 times and dehydrated two times to give the final products LA with FA.7,27,28 This has been confirmed in a study using Amberlyst 70 as a catalyst in DMSO and 13C-labeled fructose on the C1 and C6 positions.28,29 Reaction using the DR-2030 catalyst in the present study showed a transient 5-HMF peak by HPLC analysis, which was also confirmed by GC–MS (calculated 126.11, analyzed 126.8). However, the peak of fructose in the reaction with glucose was not detected during HPLC analysis, which suggests that isomerization of glucose to fructose is perhaps a reaction-limiting step that requires a higher temperature than that required for the conversion of fructose to LA.
Further experiments were then performed to evaluate the effect of salt and other reaction parameters on DR-2030-catalyzed conversion of both glucose and fructose to LA and FA, and also reusability of the catalyst. Initial studies were performed with fructose and based on the results obtained, the reaction with glucose was investigated under similar conditions but at higher temperature.
Effect of Inorganic Salt on Dehydration of Fructose
Reaction of the fructose solution (1.1 mmol, 2 mL) in the presence of DR-2030 (60 mg, 0.3 w/w fructose) at 110 °C resulted in the maximum sugar conversion of 75% and LA yield of 32.7% in 24 h. The reaction was then run in the presence of different types of inorganic salts including NaCl, KCl, CaCl2, Na2CO3, and Na2SO4, respectively (Figure 1).
Figure 1.
(A) Effect of salts (NaCl □, 10% (w/v)), KCl (△, 12.7% (w/v)), CaCl2 (*, 25% (w/v)), Na2CO3 (◇, 18% (w/v)), and Na2SO4 (○, 21.4% (w/v)) on the dehydration of 1.1 mmol fructose catalyzed by DOWEX DR-2030 (60 mg) in 2 mL at 110 °C. (A1) Conversion of fructose and (A2) yield of LA. (B) Effect of NaCl amounts (w/w to fructose), 0 (◇), 0.5 (○), 1 (□), and 2 (△) under similar reaction conditions on (B1) conversion of fructose and (B2) yield of LA.
The effect of the salts on fructose conversion and LA yields could be grouped into two profiles. In the first group, Cl– is a common ion with different metal cations, which provided similar results, while in the second group Na+ is the common ion with CO32– and SO42– counter ions. High conversion (over 98%) and yields (over 65% of LA) were obtained with NaCl, KCl, and CaCl2 (1.0 mol/mol fructose) in contrast to 41.5% fructose conversion and 21.1% yield in the absence of any salt. Addition of Na2CO3 resulted in 78.1% conversion but with less than 20% yield of LA. On the other hand, Na2SO4 suppressed the reaction, resulting in only 35.3% conversion and 14.1% yield of LA (Figure 1A).
Further reactions with increasing NaCl ratio in the order of 0, 0.5, 1, and 2 (w/w fructose) showed a linear increase in the fructose conversion and LA yield within initial 6 h (Figure 1B). The maximum yield of LA obtained in the presence of NaCl was in the range of 75–78% at 0.5–2 (w/w fructose) of NaCl (Table 1). Substrate conversions of 100% and 97.1% were achieved within 6 h at the salt ratios of 1 and 2 to fructose, respectively (Figure 1B). After reaching the highest LA yield, the reaction continued but with a decrease in LA yield to below 70% within 24 h for all salt concentrations. The effect of NaCl (0.5–1 w/w fructose) was also studied in the absence of the catalyst DR-2030 at 110 °C, which gave only 1.5–2.5% LA yield in 24 h.
Table 1. Summary for the Dehydration of Hexose Sugars under Aqueous Conditions by Using the Acidic Ion Exchange Catalystc.
| run | asugar, mmol | bsalt, (w/w) | temp. (°C) | cat. (w/w) | time (h) | conversion (%) | yield (%) LA |
|---|---|---|---|---|---|---|---|
| 1 | fructose, 1.1 | NaCl, 1 | 110 | 0.3 | 12 | 100 | 74.6 |
| 2 | fructose, 1.1 | KCl, 1.27 | 110 | 0.3 | 12 | 100 | 76.7 |
| 3 | fructose, 1.1 | CaCl2, 2.5 | 110 | 0.3 | 12 | 100 | 64.8 |
| 4 | fructose, 1.1 | Na2CO3, 1.8 | 110 | 0.3 | 12 | 78.1 | 16.8 |
| 5 | fructose, 1.1 | Na2SO4, 2.14 | 110 | 0.3 | 12 | 35.3 | 14.1 |
| 6 | fructose, 1.1 | None | 110 | 0.3 | 12 | 41.5 | 21.1 |
| 7 | fructose, 1.1 | NaCl, 0.5 | 110 | 0.3 | 12 | 99.2 | 74.9 |
| 8 | fructose, 1.1 | NaCl, 2 | 110 | 0.3 | 12 | 100 | 78.9 |
| 9 | fructose, 1.1 | NaCl, 1 | 95 | 0.3 | 24 | 84.1 | 52.8 |
| 10 | fructose, 1.1 | NaCl, 1 | 130 | 0.3 | 6 | 100 | 77.1 |
| 11 | fructose, 0.55 | NaCl, 1 | 110 | 0.3 | 24 | 100 | 79.8 |
| 12 | fructose, 0.88 | NaCl, 1 | 110 | 0.3 | 24 | 100 | 76.5 |
| 13 | fructose, 0.2 | NaCl, 1 | 110 | 0.3 | 24 | 100 | 53.8 |
| 14 | fructose, 1.1 | NaCl, 1 | 110 | 0.1 | 24 | 99.4 | 53.4 |
| 15 | fructose, 1.1 | NaCl, 1 | 110 | 0.2 | 24 | 99.7 | 65.5 |
| 16 | fructose, 1.1 | NaCl, 1 | 110 | 0.4 | 12 | 100 | 75.1 |
| 17 | glucose, 1.1 | NaCl, 1 | 130 | 0.3 | 24 | 65.6 | 34.4 |
| 18 | glucose, 1.1 | NaCl, 1 | 145 | 0.3 | 24 | 100 | 70.7 |
| 19 | glucose, 1.1 | none | 145 | 0.3 | 24 | 62.3 | 27.6 |
Sugar amounts in 2 mL aqueous solution.
Salt ratio to sugar (w/w).
Catalyst ratio to sugar (w/w).
The above results indicate the predominant influence of anions on the dehydration of fructose to be in the order of Cl– ≫ CO32– > SO42– with Na+ as the common counter ion. The chloride anions possibly stabilize the cationic (monovalent C+) intermediates formed during the dehydration of fructose to LA and FA.20 Enhanced production of furfural from xylose (C5 sugar) in the aqueous acidic (H2SO4) solution was earlier shown in the presence of chloride salts,21,22 and with dissolved metal halides.20 Furthermore, we noted that pH of the aqueous solution containing DR-2030 (30 mg/mL) was decreased from 2.98 to 0.78, 1.02, 0.67, and 1.76 by adding 100 mg (10% w/v) of NaCl, KCl, CaCl2, and Na2SO4, respectively. Increasing the amount of NaCl to 20% (w/v) decreased the pH further down to 0.55. This indicates that addition of chloride salt promoted the exchange of cations and release of H+ from the ion exchanger, thus promoting a decrease in pH and consequent fructose conversion to LA. The sulfate, on the other hand, had an inhibitory effect despite the decrease in pH. Our observations are comparable to the recent reports showing LA yield of 74 mol % from 0.1 M fructose using 1 M sulfuric acid at 140 °C12 and 83% using 0.25 M HCl at 130 °C,13 and may imply that the catalysis occurs in the homogeneous mode. Addition of Na2CO3 increased the solution pH to 9.98, which affected the conversion of fructose to LA.
Effect of Temperature on Fructose Dehydration
While the reaction temperature influences the substrate conversion rate, product yield, and selectivity, the choice of temperature is influenced by many reaction factors including kind of reaction, properties of substrates and solvents used, and also the catalyst. Besides enhancing the reaction rate, the increase in temperature also increases the side reactions leading to the formation of byproducts, for example, humins in this study, and consequently reducing the product yield and selectivity.
Performing the reaction using 1.1 mmol fructose and 60 mg (w/w) of DR-2030 with a 1 ratio (w/w fructose) of NaCl in 2 mL aqueous solution at 95, 110, and 130 °C, respectively, revealed a marginal increase in the reaction rate at temperature above 110 °C (Figure 2A), and similar yield of LA at 110 and 130 °C (Figure 2B), resulting in over 98% fructose conversion and over 75% LA yield within 12 h of reaction (Table 1). The lowest conversion rate (84.1%) and yield (52.8%) were obtained at 95 °C (Figure 2). These observations are in agreement with the previous study involving dehydration of fructose at high concentrations in DMSO using DR-2030 to HMF with high yield and catalyst stability at 110 °C but without the formation of LA.23
Figure 2.
Effect of catalytic dehydration of 1.1 mmol fructose with 200 mg NaCl in 2 mL aqueous solution using DOWEX DR-2030 (60 mg) at 95 °C (◇), 110 °C (○), and 135 °C (□) on (A) conversion of fructose, and (B) molar yield of LA.
Effect of the Catalyst Amount and the Initial Fructose Concentration on the Dehydration
Ion exchange resins have been employed as heterogeneous catalysts in various reactions for production of chemicals in aqueous and organic solvent systems.18,23 Varying the concentration of DR-2030 (ratios of 0.1–0.4 (w/w) of fructose) for dehydration of 550 mM fructose in the NaCl aqueous solution at 110 °C showed complete fructose conversion and about 75% yield of LA with 0.3–0.4 catalyst ratios to fructose at 12 h (Figure 3).
Figure 3.

Effect of catalytic dehydration of 1.1 mmol fructose with 200 mg NaCl in 2 mL aqueous solution at 110 °C using DOWEX DR-2030 at ratios of 0.1, 0.2, 0.3, and 0.4 (w/w) to fructose on conversion of fructose (gray ■) and molar yield of LA (orange ■) at a reaction time of 12 h.
At lower catalyst concentrations, a longer time was needed for the substrate conversion but the LA yield remained at around 54 and 65% for 0.1 and 0.2 catalyst ratios, respectively (Table 1).
Using the 0.3 catalyst ratio to fructose, the concentration of fructose was varied between 280 and 1100 mM in 10% (w/v) NaCl solution. Fructose at a concentration of 550–1100 mM was completely converted in about 6 h, and 280 mM fructose in 12 h. Maximum LA yield of 75–78% was obtained for 550–825 mM fructose at 12 h and for 280 mM fructose at 24 h. With 1100 mM fructose, maximum LA yield of 54% was reached at 6 h (Figure 4).
Figure 4.

Effect of the initial fructose concentration treated with DOWEX DR-2030 (60 mg, 0.3 ratio (w/w) to fructose) in 200 mg NaCl in 2 mL aqueous solution at 110 °C on: (A) fructose conversion and (B) molar yield of LA. The initial fructose concentrations used were 280 mM (◇), 550 mM (○), 825 mM (□), and 1100 mM (△), respectively.
Production of LA from Glucose
Dehydration of glucose to HMF and/or LA may be more challenging, requiring harsher conditions with low yield than that with fructose because the equilibrium shift is required for the additional step of isomerization of glucose to fructose.30 For instance, a recent report on the aqueous phase conversion of fructose and glucose to HMF and LA in the presence of hydrochloric acid13 showed complete conversion of fructose and 83% LA yield at 130 °C, while only 21.1% yield of LA (46% conversion and 43% selectivity) was obtained from glucose. Evaluation of various homogeneous Brønsted acids (sulfuric acid, hydrochloric acid, and phosphoric acid) and Lewis acids (aluminum chloride, copper chloride, iron chloride, and chromium chloride) for glucose dehydration to LA showed that coupling of CrCl3 and H3PO4 as a mixed catalyst had a synergistic catalytic effect on the reaction compared to the individual CrCl3 or H3PO4 catalysts. The highest LA yield of 54.2% from 100% glucose conversion after 4 h at 170 °C was reported.31 Meanwhile, 5-(chloromethyl)furfural (CMF) was produced as a main product (with over 70% yield) from saccharides such as glucose, sucrose, and corn stover in a two-phase system of aqueous HCl with LiCl and 1,2-dichloroethane at 100 °C.32
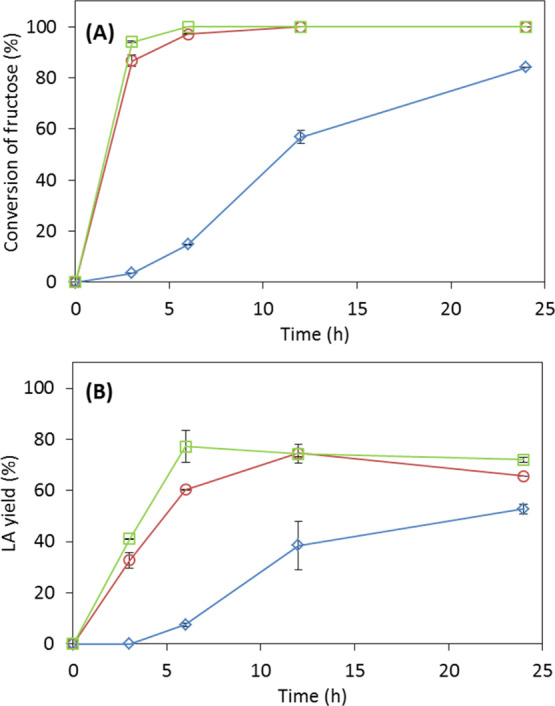
In this study, the optimal conditions determined from the experiments on fructose dehydration (1.1 mmol sugar, 200 mg (1 ratio, w/w sugar) NaCl, and 60 mg (0.3 ratio, w/w sugar) catalyst in 2 mL aqueous solution) shown above were employed for glucose dehydration to produce LA and FA, but at a higher temperature. The reaction efficiency and the product profile were investigated for the reaction of 1.1 mmol glucose using 60 mg of the catalyst with and without NaCl at 110, 130, and 145 °C, respectively (Figure 5). Only 15% LA was obtained from the reaction at 110 °C during the reaction time of 24 h, while 71% LA yield resulting from 100% glucose conversion was obtained at 145 °C. Increasing LA yield (34.4 and 71%) and glucose conversion (65.6 and 100%) were achieved upon increasing temperature to 130 and 145 °C, respectively. Meanwhile, in the absence of NaCl, 62.3% glucose conversion and 27.6% LA yield were observed at 145 °C.
Figure 5.

Catalytic dehydration of 1.1 mmol glucose (monohydrate) to LA using DOWEX DR-2030 (60 mg) at: (◇) 110 °C in 2 mL reaction medium with 200 mg NaCl, (○) 130 °C with 200 mg NaCl, (□) 145 °C with 200 mg NaCl, and (△) 145 °C without NaCl. (A) Glucose conversion and (B) molar yield of LA.
Hence, the results clearly indicate that elevated temperature and use of salt were critical factors for glucose isomerization and subsequent dehydration to LA. The salt could significantly improve the yield of LA from glucose compared to the dehydration without salts by mineral and heterogeneous acid catalysts, for example, 51% yield of LA from glucose was reported using 0.25 M HCl as the catalyst at 130 °C.13
Stability and Reusability of the Ion Exchange Catalyst
Stability and reusability of the catalyst, especially ion exchange resin, are important factors because of the risk of thermal inactivation and decomposition of the polymer matrix at elevated temperatures. In process stability of the ion exchanger, DR-2030 was investigated by recycling the catalyst for 3 consecutive batch reactions for dehydration of fructose and glucose at 560 mM initial concentration using a 0.3 (w/w) ratio of catalyst to sugars at 110 and 145 °C, respectively. In between the consecutive reactions, the catalyst was collected from the reaction medium, washed with 0.5 mL deionized water, 1 N HCl, followed again by deionized water prior to reuse in the next reaction with a fresh sugar solution.
In the first batch, the conversion of fructose reached up to 100% within 12 h with the highest LA yield of 74.9% (Figure 6). The reactions slowed down with consecutive batches compared to the previous run (Figure 6A), hence decreasing conversion rates and yields were obtained at the same reaction times in consequent batches. A similar trend was observed during dehydration of glucose, where 94.7% glucose conversion and 65.1% LA yield were obtained in the first batch (Figure 6B). Even though heterogeneous catalysts are interesting catalysts for LA synthesis, the main challenge will be the development of methodology to prevent catalyst deactivation, which is most likely caused by excessive deposition of humins formed as the by-products and leading to blocking of the catalyst surface or active sites. Elemental analysis of a similar strong acidic cation exchange catalyst (DOWEX 50WX8-100) used for dehydration of fructose to 5-HMF showed a distinct increase in the C content along with a minor increase and decrease in H and S contents, respectively.33 The increase in C and H contents was suggested to be because of adsorbed humins or other residues that are difficult to wash off from the resin, while the decrease in S was ascribed to some loss of sulfonate groups on the resin. Accumulation of humins is promoted because of the reaction being performed for a long time in a batch mode, which can be significantly reduced by a continuous mode of operation as shown in our recent studies on 5-HMF production from fructose.23 Under such conditions, the exposure of sugar to high temperature can be controlled by the residence time of the solution in the reactor. Even inclusion of a solvent that would minimize the aldol condensation reactions of HMF could improve the selectivity of the reaction.34 Moreover, screening of more ion exchange catalysts and appropriate regeneration conditions are necessary for catalyst recycling.
Figure 6.

Reusability of the DR-2030 catalyst (60 mg, ratio of 0.3 w/w to sugars) in 200 mg NaCl in 2 mL aqueous solution for dehydration of (A) fructose (1.1 mmol) at 110 °C, and (B) glucose (1.1 mmol) at 145 °C in three consequent batches. Sugar conversion (gray ■), LA yield (green ■), and FA yield (orange ■) at 12 h.
Conclusions
In this work, heterogeneous catalysis and a salt water system were employed to develop a facile and clean process for the production of LA from fructose and glucose. Furthermore, we show that salt anions play an important role in dehydration of the sugars to LA. The reaction was enhanced with salt anions in the order of Cl– > CO32– > SO42–, which seems to be because of the release of H+ ions from the ion exchange catalyst and further decrease in acidity of the reaction medium. Especially, glucose was directly dehydrated to LA with high conversion and yield (70.7% yield) in an aqueous medium, which can be highlighted as a significantly improved observation as compared to the reports so far. The results presented here are promising and the process can be further improved especially concerning catalyst performance at high temperature and recycling.
Materials and Methods
Materials
LA (97% purity), (d)-fructose (99% purity), 5-hydroxymethyl-2-furfural (HMF, 99% purity), glucose monohydrate, formic acid, and Dowex DR-2030 hydrogen form (strong cation ion exchange resin) were procured from Sigma-Aldrich. DOWEX DR-2030 is a macroporous styrene-divinylbenzene resin functionalized with sulfonic acid groups, >4.7 meq/g dry weight capacity, 16–40 mesh, 30 m2/g surface area, and <3% moisture content, with an upper limit for safe operation at 130 °C. All chemicals were used without further treatment.
Dehydration of Fructose and Glucose to LA
The stock solutions of fructose/glucose and salt were prepared at the given concentrations in 20 mL volume (final volume adjusted by water). Typically, 2 mL of the solution was placed in a 4 mL glass vial, followed by the addition of the ion exchange resin, DR-2030. The vials were placed in a ThermoMixer (HTMR 131, HLC BioTech, Germany), heated, and shaken for the reaction to take place. The effect of the initial fructose concentration, 280–1100 mM (5–20%, w/v) in water using a 0.3 ratio of the resin (w/w to fructose) was evaluated at 110 °C and shaking at 500 rpm. The effect of reaction temperature (95, 110, and 125 °C, respectively) was evaluated using 1.1 mmol (200 mg) fructose and 60 mg (0.3 w/w to fructose) resin in 2 mL volume (550 mM fructose), and shaking at 500 rpm. The amount of catalyst was varied between 0.1 and 0.4 (w/w) at 1.1 mmol fructose in a 2 mL reaction volume. The effect of salt (NaCl, KCl, Na2CO3, and Na2SO4) on dehydration of 1.1 mmol fructose to LA at 110 °C by 60 mg of the catalyst was also investigated. To verify the reaction conditions in a larger volume, 100 mL aqueous solution containing 0.55 mol fructose in a 250 mL flask was placed in an oil bath, followed by the addition of 3 g ion exchange resin, and magnetic stirring at 110 °C. Dehydration of glucose was performed at 110, 130, and 145 °C, respectively, under otherwise similar conditions to those used for fructose dehydration. Samples (20 μL each) were collected from the reaction solution at different time intervals after cooling (to below 80 °C) briefly, and analyzed for the concentrations of residual fructose and products.
Regeneration and Recycling of the Ion Exchange Resin in the Batch Reaction
Two milliliter solution containing 1.1 mmol (200 mg) fructose and 200 mg NaCl was mixed with 60 mg of the resin in a 4 mL glass vial and incubated at 110 °C with shaking at 500 rpm in a ThermoMixer. After 12 h of the reaction, the resin was separated, washed with deionized water, and then regenerated by suspending in 0.5 mL of 1 N HCl for 1 h, and washing with deionized water until reaching neutral pH. The resin was recycled for 5 consecutive runs using a fresh reaction solution under similar conditions of temperature and shaking. Samples (20 μL each) were collected at 12 h of the reaction, and analyzed for concentrations of fructose and products.
Analytical Procedures
The concentrations of fructose, glucose, and products were determined using HPLC (JASCO, Tokyo, Japan) equipped with Aminex Bio-Rad Fast Acid Analysis chromatographic column connected to a Micro-Guard column cation H (Biorad, Richmond, CA, USA), a RI detector (ERC, Kawaguchi, Japan), and a JASCO intelligent autosampler.23 The column temperature was maintained at 65 °C in a chromatographic oven (Shimadzu, Tokyo, Japan). Samples were diluted with deionized water and mixed with 20% v/v sulfuric acid (20 μL/mL sample) and then filtered. A 40 μL aliquot was injected in the 0.5 mM H2SO4 mobile phase flowing at a rate of 0.6 mL/min. The peaks for the substrate, fructose and glucose, and products, LA, HMF, and formic acid, were confirmed and quantified from the standard curves obtained using external standards.
All the data were obtained from two independent experiments and are provided as the average of the replicates ± standard deviation. The reaction parameters calculated were percentage of sugar conversion, percentage of LA yield (with respect to the substrate), and selectivity using the following equations:
Acknowledgments
This work was performed within the research program, Sustainable Plastics and Transition Pathways (STEPS) at Lund University supported by the Swedish Foundation for Strategic Environmental Research (Mistra) (grant no. 2016/1489). R.H.K. and S.J.G. also acknowledge the financial support of Formas for the project Surplus Agricultural Residues to Furanics (Farm2Furan) (grant no. 942-2016-33).
The authors declare no competing financial interest.
References
- Hatti-Kaul R.; Törnvall U.; Gustafsson L.; Börjesson P. Industrial biotechnology for the production of bio-based chemicals–a cradle-to-grave perspective. Trends Biotechnol. 2007, 25, 119–124. 10.1016/j.tibtech.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Pileidis F. D.; Titirici M.-M. Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. 10.1002/cssc.201501405. [DOI] [PubMed] [Google Scholar]
- van Putten R.-J.; van der Waal J. C.; De Jong E.; Rasrendra C. B.; Heeres H. J.; de Vries J. G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- Saha B.; Abu-Omar M. M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. 10.1039/C3GC41324A. [DOI] [Google Scholar]
- Shrotri A.; Kobayashi H.; Fukuoka A. Catalytic conversion of structural carbohydrates and lignin to chemicals. Adv. Catal. 2017, 60, 59–123. 10.1016/bs.acat.2017.09.002. [DOI] [Google Scholar]
- Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; United States Department of Energy (DOE), 2004.
- Yan K.; Jarvis C.; Gu J.; Yan Y. Production and catalytic transformation of levulinic acid: A platform for specialty chemicals and fuels. Renewable Sustainable Energy Rev. 2015, 51, 986–997. 10.1016/j.rser.2015.07.021. [DOI] [Google Scholar]
- Isikgor F. H.; Becer C. R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. 10.1039/C5PY00263J. [DOI] [Google Scholar]
- Kang S.; Fu J.; Zhang G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renewable Sustainable Energy Rev. 2018, 94, 340–362. 10.1016/j.rser.2018.06.016. [DOI] [Google Scholar]
- Wang K.; Jiang J.; Liang X.; Wu H.; Xu J. Direct conversion of cellulose to levulinic acid over multifunctional sulfonated humins in sulfolane–water solution. ACS Sustainable Chem. Eng. 2018, 6, 15092–15099. 10.1021/acssuschemeng.8b03558. [DOI] [Google Scholar]
- Girisuta B.; Heeres H. J.. Levulinic acid from biomass: synthesis and applications. In Production of Platform Chemicals from Sustainable Resources; Fang Z., Smith R. Jr., Qi X., Eds.; Springer: Singapore, 2017; pp 143–169. [Google Scholar]
- Fachri B. A.; Abdilla R. M.; Bovenkamp H. H. v. d.; Rasrendra C. B.; Heeres H. J. Experimental and kinetic modeling studies on the sulfuric acid catalyzed conversion of D-fructose to 5-hydroxymethylfurfural and levulinic acid in water. ACS Sustainable Chem. Eng. 2015, 3, 3024–3034. 10.1021/acssuschemeng.5b00023. [DOI] [Google Scholar]
- Garcés D.; Díaz E.; Ordóñez S. Aqueous phase conversion of hexoses into 5-Hydroxymethylfurfural and levulinic Acid in the presence of hydrochloric acid: mechanism and kinetics. Ind. Eng. Chem. Res. 2017, 56, 5221–5230. 10.1021/acs.iecr.7b00952. [DOI] [Google Scholar]
- Zuo Y.; Zhang Y.; Fu Y. Catalytic conversion of cellulose into levulinic acid by a sulfonated chloromethyl polystyrene solid acid catalyst. ChemCatChem 2014, 6, 753–757. 10.1002/cctc.201300956. [DOI] [Google Scholar]
- Cirujano F. G.; Corma A.; Llabrés i Xamena F. X. Conversion of levulinic acid into chemicals: synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. 10.1016/j.ces.2014.09.047. [DOI] [Google Scholar]
- Khiratkar A. G.; Balinge K. R.; Krishnamurthy M.; Cheralathan K. K.; Patle D. S.; Singh V.; Arora S.; Bhagat P. R. Sulphonic acid-functionalized benzimidazolium based poly ionic liquid catalyzed esterification of levulinic acid. Catal. Lett. 2018, 148, 680–690. 10.1007/s10562-017-2284-1. [DOI] [Google Scholar]
- Ramli N. A. S.; Amin N. A. S. Optimization of renewable levulinic acid production from glucose conversion catalyzed by Fe/HY zeolite catalyst in aqueous medium. Energy Convers. Manage. 2015, 95, 10–19. 10.1016/j.enconman.2015.02.013. [DOI] [Google Scholar]
- Thapa I.; Mullen B.; Saleem A.; Leibig C.; Baker R. T.; Giorgi J. B. Efficient green catalysis for the conversion of fructose to levulinic acid. Appl. Catal., A 2017, 539, 70–79. 10.1016/j.apcata.2017.03.016. [DOI] [Google Scholar]
- Shen F.; Smith R. L.; Li L.; Yan L.; Qi X. Eco-friendly method for efficient conversion of cellulose into levulinic acid in pure water with cellulase-mimetic solid acid catalyst. ACS Sustainable Chem. Eng. 2017, 5, 2421–2427. 10.1021/acssuschemeng.6b02765. [DOI] [Google Scholar]
- Enslow K. R.; Bell A. T. The role of metal halides in enhancing the dehydration of xylose to furfural. ChemCatChem 2015, 7, 479–489. 10.1002/cctc.201402842. [DOI] [Google Scholar]
- Marcotullio G.; De Jong W. Chloride ions enhance furfural formation from D-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. 10.1039/B927424C. [DOI] [Google Scholar]
- Mao L.; Zhang L.; Gao N.; Li A. FeCl3 and acetic acid co-catalyzed hydrolysis of corncob for improving furfural production and lignin removal from residue. Bioresour. Technol. 2012, 123, 324–331. 10.1016/j.biortech.2012.07.058. [DOI] [PubMed] [Google Scholar]
- Pyo S.-H.; Sayed M.; Hatti-Kaul R. Batch and continuous flow production of 5-hydroxymethylfurfural from high concentration of fructose using acidic ion exchange catalyst. Org. Process Res. Dev. 2019, 23, 952–960. 10.1021/acs.oprd.9b00044. [DOI] [Google Scholar]
- Moreau C.; Durand R.; Roux A.; Tichit D. Isomerization of glucose into fructose in the presence of cation-exchanged zeolites and hydrotalcites. Appl. Catal., A 2000, 193, 257–264. 10.1016/S0926-860X(99)00435-4. [DOI] [Google Scholar]
- Utami S. P.; Amin N. S. Optimization of glucose conversion to 5-hydroxymethylfulfural using [BMIM] Cl with ytterbium triflate. Ind. Crops Prod. 2013, 41, 64–70. 10.1016/j.indcrop.2012.03.036. [DOI] [Google Scholar]
- Zhao H.; Holladay J. E.; Brown H.; Zhang Z. C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. 10.1126/science.1141199. [DOI] [PubMed] [Google Scholar]
- Antonetti C.; Melloni M.; Licursi D.; Fulignati S.; Ribechini E.; Rivas S.; Parajó J. C.; Cavani F.; Raspolli Galletti A. M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal., B 2017, 206, 364–377. 10.1016/j.apcatb.2017.01.056. [DOI] [Google Scholar]
- Girisuta B.; Heeres H. J.. Levulinic acid from biomass: synthesis and applications, 143- 169. In Production of Platform Chemicals from Sustainable Resources; Fang Z., Qi X., Eds.; Springer: New York, 2017. [Google Scholar]
- Zhang J.; Weitz E. An in situ NMR study of the mechanism for the catalytic conversion of fructose to 5-hydroxymethylfurfural and then to levulinic acid using C-13 labeled D-fructose. ACS Catal. 2012, 2, 1211–1218. 10.1021/cs300045r. [DOI] [Google Scholar]
- Hansen T. S.; Mielby J.; Riisager A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. 10.1039/C0GC00355G. [DOI] [Google Scholar]
- Weiqi W.; Shubin W. Experimental and kinetic study of glucose conversion to levulinic acid catalyzed by synergy of Lewis and Brønsted acids. Chem. Eng. J. 2017, 307, 389–398. 10.1016/j.cej.2016.08.099. [DOI] [Google Scholar]
- Mascal M.; Nikitin E. B. Dramatic advancements in the saccharide to 5-(chloromethyl) furfural conversion reaction. ChemSusChem 2009, 2, 859–861. 10.1002/cssc.200900136. [DOI] [PubMed] [Google Scholar]
- Qi X.; Watanabe M.; Aida T. M.; Smith R. L. Jr. Selective conversion of D-fructose to 5-hydroxymethylfurfural by ion-exchange resin in acetone/dimethyl sulfoxide solvent mixtures. Ind. Eng. Chem. Res. 2008, 47, 9234–9239. 10.1021/ie801016s. [DOI] [Google Scholar]
- Tsilomelekis G.; Josephson T. R.; Nikolakis V.; Caratzoulas S. Origin of 5-hydroxymethylfurfural stability in water/dimethyl sulfoxide mixtures. ChemSusChem 2014, 7, 117–126. 10.1002/cssc.201300786. [DOI] [PubMed] [Google Scholar]