Abstract
The spleen is a key participant in the pathophysiology of sepsis and inflammatory disease. Many splenocytes exhibit a cholinergic phenotype, but our knowledge regarding their cholinergic biology and how they are affected by sepsis is incomplete. We evaluated effects of acute sepsis on the spleen using the cecal ligation and puncture (CLP) model in C57BL/6 and ChATBAC-eGFP mice. Quantification of cholinergic gene expression showed that choline acetyltransferase and vesicular acetylcholine transporter (VAChT) are present and that VAChT is upregulated in sepsis, suggesting increased capacity for release of acetylcholine (ACh). High affinity choline transporter is not expressed but organic acid transporters are, providing additional mechanisms for release. Flow cytometry studies identified subpopulations of cholinergic T and B cells as well as monocytes/macrophages. Neither abundance nor GFP intensity of cholinergic T cells changed in sepsis, suggesting that ACh synthetic capacity was not altered. Spleens have low acetylcholinesterase activity, and the enzyme is localized primarily in red pulp, characteristics expected to favor cholinergic signaling. For cellular studies, ACh was quantified by mass spectroscopy using d4-ACh internal standard. Isolated splenocytes from male mice contain more ACh than females, suggesting the potential for gender-dependent differences in cholinergic immune function. Isolated splenocytes exhibit basal ACh release, which can be increased by isoproterenol (4 and 24 h) or by T cell activation with antibodies to CD3 and CD28 (24 h). Collectively, these data support the concept that sepsis enhances cholinergic function in the spleen and that release of ACh can be triggered by stimuli via different mechanisms.
Keywords: Sepsis, Cholinergic biology, Acetylcholine release, Splenocytes, Isoproterenol, T cell activation
1. Introduction
Sepsis is a serious, life-threatening condition that develops as an excessive and abnormal immune response to infections [1,2]. It is a major cause of mortality and morbidity worldwide, and specific therapies targeting the underlying pathophysiology are lacking. Acute sepsis is characterized by an excessive pro-inflammatory response that contributes to organ damage, but anti-inflammatory features and immunosuppression develop as disease progresses and constitute a major problem in sepsis survivors [1–3]. The spleen, which is the largest secondary lymphoid organ, plays a major role in the pathophysiology of sepsis and is thought to be the main source of damaging proinflammatory cytokines during the acute stage of disease [2,4]. For this reason, the cholinergic anti-inflammatory pathway could offer novel approaches for treating sepsis and other inflammatory conditions [5,6].
The cholinergic anti-inflammatory pathway includes sympathetic neurons in the celiac/superior mesenteric ganglion complex, which send projections to the splenic white pulp where cholinergic T cells are located [6–8]. Norepinephrine (NE), released from sympathetic nerves, stimulates β2-adrenoceptors on cholinergic T cells to cause release of acetylcholine (ACh). Proposed anti-inflammatory effects of this pathways require diffusion of ACh to the marginal zone where it stimulates α7 nicotinic ACh receptors (α7nAChRs) on macrophages and possibly monocytes. While there is some controversy about the preganglionic component of this pathway, it can be activated by stimulation of the efferent and afferent vagus and by splanchnic nerve stimulation [7,9–11]. Further evidence has shown that this pathway can be activated as a reflex triggered by inflammatory signals [12,13]. The therapeutic potential of this system in sepsis is supported by studies showing that activation of the pathway by central mechanisms or transcutaneous vagal nerve stimulation can increase survival in a murine model of sepsis [12,13]. However, there is concern that excessive activation of cholinergic anti-inflammatory mechanisms might tip the balance toward immunosuppression [14].
Comprehensive understanding of cholinergic leukocytes and their role in disease will be essential for careful targeting of these cells in sepsis and other inflammatory disease. Since reflex activation of the cholinergic anti-inflammatory pathways is expected to occur during acute sepsis, the first goal for this study was to determine how cholinergic biology of the spleen is affected by experimental sepsis in mice. Our second goal was to characterize the cholinergic biology of isolated splenocytes, focusing on stimuli and dynamics of ACh release.
2. Materials and methods
2.1. Animals
Adult C57BL/6J mice and B6-Cg-Tg(RP23–268L19 EGFP)2Mik/J mice, also known as ChATBAC-eGFP mice, were used in this study. ChATBAC-eGFP mice were bred in house. For sepsis studies, we used only male mice (4–6 months of age), since female mice are less susceptible to sepsis [15,16]. We also used primarily male mice for cell culture studies, since early experiments showed that splenocytes from female mice contain less ACh. Animal protocols (P130304, P160204 and P190202) were approved by the East Tennessee State University Animal Care and Use Committee and conformed to guidelines of the National Institutes of Health published in the Guide for the Care and Use of Laboratory Animals (Eighth Edition, National Academy of Sciences, 2011).
2.2. Experimental sepsis model
Polymicrobial sepsis was induced, as described previously, by cecal ligation and puncture [17]. While under isoflurane anesthesia the full cecum was ligated with a 0 suture, punctured once with a 20-gauge needle, and a small amount of feces was extruded. The abdomen was closed in two layers, and mice received a subcutaneous injection of 1 ml warm, sterile saline as resuscitation fluid. Mice received no post-operative antibiotic. Sham mice received the same procedure without ligation or puncture.
2.3. RNA isolation and quantitative polymerase chain reaction (PCR) analysis
Mice were euthanized with CO2 at 8 and 16 h after surgery, and spleens were removed and immediately immersed in RNALater solution (Ambion). Tissue was homogenized using a Polytron PT2500 benchtop homogenizer (Kinematica Inc., Bohemia NY) in 1 ml cold Trizol reagent (Invitrogen). Trizol was phase separated and ethanol-precipitated as per the manufacturer’s instructions, and then loaded onto RNeasy Mini nucleic acid-binding columns (Qiagen, Valencia CA) for DNase treatment and additional clean-up steps. DNase digestion was performed on-column using Qiagen RNase-free DNase, followed by multiple washes, and then elution of RNA in RNase-free water. RNA concentration and integrity (RIN) were examined using an Agilent Microfluidics Bioanalyzer (Agilent Technologies, Santa Clara, CA); all purified RNA samples displayed RIN values > 7.5. Superscript III (LifeTechnologies) was used to reverse transcribe cDNA from 2 μg total RNA. All sample cDNAs were amplified for GAPDH in end-point PCR, with positive & negative reverse-transcriptase controls, and electrophoresed on 1% TAE agarose gels to assess success of cDNA synthesis reaction and potential genomic DNA contamination in RNA preparations. Standard amplicons were prepared in a 100 μl PCR reaction using 2× GoTaq Hot-start PCR Mastermix (Promega, Madison, WI), 10× gene-specific QuantiTect Primer Assay (Qiagen), and 50 ng control mouse brain cDNA. An aliquot of amplicon PCR was examined by 2% agarose gel electrophoresis to evaluate the amplicon specificity and expected size, then the remaining PCR reaction (90 μl) was purified using a nucleic acid-binding bead-based PCR Purification Kit (Qiagen). Quantitative PCR analysis was performed using a BioRad Cfx-96 Real-time PCRdetection system (Bio-Rad, Hercules, CA) and SsoFast Evagreen qPCR mastermix (Bio-Rad), as per the manufacturer’s instructions, in a 20 μl volume containing 1× QuantiTect primers (Qiagen; Supplement Table 1) and 1 μl cDNA per well. All samples were analyzed a minimum of twice in triplicate; transcript starting quantities were determined, using Cfx Manager software (Bio-Rad), by comparison with a standard 8-point amplicon dilution series run on the same plate in triplicate. Data are presented as the starting quantify of mRNA for the gene of interest (number of transcripts/50 μg RNA) and as relative expression normalized to reference genes as recommended by Bustin et al [18]. Relative expression was calculated as starting quantity of gene of interest/geometric mean of GAPDH and HPRT starting quantities.
2.4. Immunohistochemistry and image analysis
Paraffin-embedded spleen sections were cut at 5 μm and processed for immunohistochemistry as described previously [19–21]. Sections were stained for VAChT (rabbit anti-VAChT, 1:1000, Synaptic Systems #P40101–1) using a rabbit ABC kit (Vector Laboratories) and developed using Vector ImmPact VIP chromogen (Vector Laboratories), which generated a purple reaction product. White pulp regions in labeled sections were photographed, at a 20× magnification, using an Olympus BX41 microscope equipped with a Q-Color 3 digital camera. Digital images were imported into ImageJ software (NIH), converted to 8-bit and threshold adjusted. Staining density was then calculated as the percent of image area occupied by purple stain.
2.5. Flow cytometry
Flow cytometry to measure GFP expression and the phenotype of GFP+ cells was performed on splenic leukocytes at 16 h post-operatively. Splenocytes were isolated by teasing the tissue apart to liberate the cells and the stroma was discarded. Erythrocytes were lysed with PharmLyse buffer (BD Pharmingen). Leukocytes were blocked with 5% rabbit serum, 0.5% bovine serum albumin (BSA), and 5 mM EDTA with anti-murine CD16/32 (clone 2.4G2, BD Pharmingen) prior to staining. Leukocytes were stained with anti-CD19 PE (B cells, clone 1D3, BD Biosciences), anti-CD3 PerCP (T cells, clone 145–2c11, BD Biosciences), and anti-F4/80 APC (monocytes/macrophages, clone Cl:A3–1, AbD Serotec), or their isotype control antibodies. Staining was performed according to the protocol described by BD Pharmingen. Cells were suspended in Pharmingen Stain Buffer and analyzed using a FACScalibur flow cytometer with CellQuest software (BD Biosciences, Mountain View, CA). Phenotype of cells was determined on an antibody vs. SSC plot. Histograms for GFP were generated using the cell populations as a gate, and mean GFP fluorescence was determined.
2.6. Acetylcholinesterase (AChE) assay and histochemistry
Spleens were removed from sham and CLP mice 16 h post-operative, flash frozen on dry ice, and stored at −80 °C. Samples were thawed on ice with the addition of 2 ml ice-cold HEPES saline buffer (15 mM HEPES, 1 M NaCl) containing 1× protease inhibitor cocktail (Sigma). Samples were homogenized for 30 s on power level 5 using a Polytron PT2500 benchtop homogenizer (Kinematica Inc), then centrifuged at 16,000g for 30 min at 4 °C in an Eppendorf refrigerated microfuge. First extraction supernatants, containing loosely bound AChEs and membrane fragments in suspension, were transferred to clean microfuge tubes and protein concentrations were determined using the Bradford Reagent Assay. The tissue pellet was resuspended in HEPES saline containing 1× protease inhibitor and 1% Triton X-100 to release membrane bound AChE, homogenized and centrifuged as described for the first extraction. First and second extractions were assayed separately and in combination for AChE activity using the DetectX® Acetylcholinesterase Fluorescent Activity Kit (Arbor Assays) in the presence and absence of tetraisopropyl pyrophosphoramide (Sigma), a potent inhibitor of butyrylcholinesterase. Readings were obtained using a Promega Glo-Max fluorescent plate reader with fluorescent emission at 510 nm and excitation at 370–410 nm.
The histochemical method of Koelle [22] was used to identify AChE in 30 μm sections of spleen and brain. Briefly, slide-mounted sections were first preincubated in a 24% Na2SO4 solution containing 1 μM tetraisopropyl pyrophosphoramide for 30 min at 37 °C to inhibit butyrylcholinesterase irreversibly. Slides were then transferred to a solution containing 4 mM acetylthiocholine iodide (ATCh), and incubated for an additional 2 h at 37 °C. The latter solution was prepared as described by Koelle [22–24] and contained the following components at pH 6.0: 6 mM CuSO4, 40 mM MgCl2, 54 mM sodium maleate, 20 mM glycine and 24% Na2SO4. During the incubation, ATCh is converted to thiocholine (TCh), which reacts rapidly with Cu2+ forming a white precipitate of CuTCh. After brief washing, the slides are placed in 4% ammonium sulfide in water for 1 min, which converts CuTCh to a brown reaction product of CuS. Slides are washed again, fixed in 10% formalin, and dehydrated before applying a cover glass with Cytoseal XYL (Thermo Scientific).
2.7. Isolation of splenocytes
Mice were euthanized by overdose with isoflurane, and the spleen was transferred to a 75 mm culture dish containing 3 ml of sterile phosphate-buffered saline (PBS) on ice. The splenic tissue is teased apart using 21ga needles and then macerated using the tip of a 5 cc syringe. Dissociated cells were collected in the syringe, leaving the stroma behind, and transferred to a vacutainer tube. Cells were collected by centrifugation at 200g for 5 min at 10 °C, red blood cells in the pellet were lysed, and samples were spun again to obtain a pellet containing splenocytes. These cells were washed once in cold PBS, and resuspended in 10 ml cold PBS. Live and dead cell counts in the suspension were determined using a Countess II Automated Cell Counter (Invitrogen). Live cell yields ranged from 79 to 89% of total cells. The cell suspension was washed again and the cells were either suspended in cell culture medium for experiments or treated with distilled water containing 0.5 μM neostigmine (Sigma) to extract ACh.
2.8. Cell culture experiments
Splenocytes were resuspended in cell culture medium, comprising DMEM/F12 + GlutaMax (Gibco), 1% heat inactivated fetal bovine serum, 1× penicillin/streptomycin (Gibco), and 0.5 μM neostigmine. They were then transferred to 12-well plates with 2 ml/well and 106 live splenocytes/ml. Splenocytes were maintained in an incubator (5% CO2 and 37 °C) for 4 or 24 h before collecting 1.9 ml aliquots of medium, which were spun at 500 g for 5n min at 4 °C. Supernatant (1.8 ml) was transferred to a cryotube, spiked with 10 μl d4-ACh internal standard (0.5 μg/ml; CDN Isotopes, Pointe-Claire, Quebec, Canada) and mixed gently before freezing on dry ice. Calibration standards were prepared by adding 10 μl ACh chloride standards (0.1–50 μg/ml) and d4-ACh internal standard to 1.9 ml medium in cryotubes. Frozen samples were lyophilized using a freeze dryer (Labconco 6L FreeZone Console).
Cells were incubated with 1 μM (−) isoproterenol hydrochloride (Sigma) to evaluate effects of β-adrenoceptor stimulation on ACh release. Effects of T cell activation were studied in two ways as specified in figure legends. Antibodies to CD3 (Biolegend, #100340) and CD28 (Biolegend, #102116) were added directly to the wells (1 μg/ml of each) containing splenocytes, or plates were coated with anti-CD3 (1 ml of 10 μg/ml) overnight, washed before adding splenocytes, and then anti-CD28 (5 μg/ml) was added.
2.9. Analysis of ACh by liquid chromatography with tandem mass spectrometry
A Shimadzu LCMS-IT-TOF (liquid chromatography mass spectrometry – ion trap- time of flight) system was used for all mass spectrometric measurements (Shimadzu Scientific, Kyoto, Japan). The LC system was equipped with column oven, in-line degasser, and autosampler, and the mass spectrometer was operated in positive electrospray (+ESI) mode with direct MS/MS channels. ACh was quantified in cell culture samples using LC-MS/MS, with a matrix matched calibration curve run on each sample day. The chromatographic separation was achieved using an Atlantis HILIC column (2.1 × 100 mm, 3 μm), maintained at 30 °C under isocratic conditions. The aqueous component of the mobile phase was a 10 mM ammonium formate buffer, pH 3 (14%) and the organic component was acetonitrile (86%), maintained at a flow rate of 0.5 ml/min. Injection volume of 10 μl was used for all calibrants and study samples. All solvents were of LC-MS grade (Thermo Fisher, Waltham, MA, USA). Liquid nitrogen (1.5 L/min) was used as the nebulizing gas, and the +ESI source was maintained at 200 °C, with a detector voltage of 1.85 kV. The MS/MS detection channels were set at 146.1191, 150.1390, 104.1127, and 108.1312 for ACh, deuterium (d4) labeled ACh, choline, and deuterium (d4) labeled choline. Experimental samples and standards were reconstituted in 500 μl LC-MS grade acetonitrile and vortexed for 30 s, filtered through 0.22-μm membranes using non-sterile Costar centrifuge filter tubes, and transferred to autosampler vials for LC-MS analysis. Area under the curve (AUC) for LC-MS peaks was calculated using LCMSsolutions, v 3.0 (Shimadzu Scientific, Kyoto, Japan).
2.10. RNA isolation from splenocytes and real time PCR screening
Total RNA was extracted from mouse splenocytes (~2 × 106 cells) using the RNeasy Mini kit (Qiagen, cat. no. 74104). Genomic DNA was removed by DNase digestion using the RNase-free DNase Set (Qiagen, cat. no. 79256). RNA was evaluated on an Agilent 2100 Bioanalyzer using the Agilent RNA 6000 NANO chip kit (Agilent Technologies, cat. no. 5067–1511). RIN values ranged from 8.5 to 9.6. Splenocyte RNA (500 ng) was reverse transcribed using the SuperScript IV First-Strand cDNA Synthesis kit (Invitrogen, cat. no. 18091–050). cDNA positives (with SSIV enzyme) and negative (dH2O) reactions for each sample as well as a Hela total RNA in-kit control were processed using the cycling parameters outlined in the cDNA synthesis protocol on a Bio-Rad T100 Thermocycler. PCR was performed using GoTaq HotStart colorless master mix (Promega, #PRM5132). Each reaction was run using 1 μl template. Primer sets are listed in Supplement Table 2. Cycling parameters were 95 °C, 3 min; 94 °C, 30 s; 53 or 57 °C, 30 s; 72 °C, 1 min, 25 or 35 cycles; 72 °C, 5 min. PCR products were separated on a 1% agarose gel and visualized using a G-Box imaging system (Syngen).
2.11. Statistical analysis
Statistical comparisons were conducted using Graph Pad Prism, v. 8.3.0 (La Jolla, CA, USA). Primary analysis was done using unpaired t test or one-way analysis of variance (ANOVA) for paired or unpaired samples, as appropriate. Post hoc comparisons were made by the Tukey test when results of ANOVA were significant. Values of P < 0.05 were considered significant.
3. Results
3.1. ChAT and VAChT are expressed in spleen, and VAChT is upregulated in acute sepsis
Although it is well known that the spleen can make ACh, it was unclear how similar the cholinergic phenotype was in spleen compared to neurons. We evaluated this point by performing qPCR for neuronal cholinergic markers using RNA extracted from mouse spleen. Using this approach, we quantified mRNA for ChAT and VAChT in control mouse spleens (Fig. 1). However, mRNA for the high affinity choline transporter (CHT1) was not detectable by qPCR. Since previous studies of isolated immune cells showed that inflammatory stimuli can upregulate ChAT, we evaluated the effect of acute sepsis (CLP) on expression of ChAT and VAChT. These experiments showed that ChAT expression was not altered at 8 h after CLP (Fig. 1A, D). However, absolute quantity of VAChT mRNA in spleen was increased significantly at 8 and 16 h after CLP compared to values from control spleens or spleens from mice that received sham surgery (Fig. 1B, C). The same trend occurred when VAChT mRNA was normalized to housekeeping genes (Supplement Fig. 1), but the differences were not statistically significant (Fig. 1E, F).
Fig. 1.
Sepsis increases the expression of VAChT but not ChAT in mouse spleen. Spleens from Sham and CLP mice were collected at 8 or 16 h post-surgery. RNA from naïve control, sham, and CLP mouse spleens were evaluated by qPCR. A-C: Absolute amount of RNA. E-F: Relative expression – starting quantity of ChAT or VAChT mRNA was normalized to the geometric mean of the starting quantity for GAPDH and HPRT reference genes. These experiments were done using male C57BL/6J mice. Data in each panel were evaluated by one-way ANOVA, and post hoc comparisons were made using the Tukey test when overall group differences were detected. Group data are graphed as means ± SEM for the indicated n values. *P < 0.05 vs other groups.
Immunohistochemical labeling of ChAT was not possible in sections of mouse spleen, likely due to limitations of available antibodies and/or low expression levels. Staining for VAChT with a well-validated antibody [25], gave negligible staining in the white pulp of spleens from sham mice (Fig. 2A, C), but scattered, punctate staining occurred in white pulp of CLP mice (Fig. 2B). This staining appeared to be associated with splenocytes in high magnification images (Fig. 2D, E). Quantification of stained sections showed that VAChT immunoreactivity was more abundant in the white pulp at 16 h after CLP compared to sham surgery (Fig. 2F). Scatter cells in the red pulp of both groups also stained for VAChT (Fig. 2C).
Fig. 2.
Acute sepsis increases VAChT immunoreactivity in the spleen. Sections of spleen were immunostained for VAChT at 16 h after sham and CPL surgery. A and B: Photomicrograph showing immunohistochemical staining for VAChT (purple reaction product) in splenic white pulp of a Sham (A) and CLP (B) mouse. C: VAChT staining of spleen from another mouse showing VAChT + cells (arrows) in the red pulp. Such cells were also seen in red pulp of CLP mice. D and E: High magnification images (100× oil objective) showing the localization of VAChT to leukocytes in white pulp from a CLP mouse. Arrows indicated specific cells. VAChT in some cells appeared to be localized to the membrane and/or underlying cytosol. F: Quantification of staining for VAChT in the white pulp by analysis of digital images using ImageJ. These experiments were done using male C57BL/6J mice. Values are means ± SEM for the indicated n values. *P < 0.05 vs Sham by unpaired t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Different classes of splenocytes exhibit a cholinergic phenotype but the relative abundance of cholinergic T and B cells is not altered by acute sepsis
To further evaluate effects of acute sepsis on cholinergic leukocytes, we used ChATBAC-eGFP transgenic mice [5,8,26,27]. Splenocytes were isolated at 16 h after sham or CLP surgery and evaluated by flow cytometry. The relative abundance of B cells, T cells and monocytes/macrophages was not affected by CLP (Fig. 3B). As demonstrated by others, we found that a subpopulation of cells in each of these major groups of splenic leukocytes showed eGFP fluorescence, indicative of the cholinergic phenotype (Fig. 3C). A significant increase in number of GFP + monocytes/macrophages was detected at 16 h post-CLP. The fluorescence intensity of cholinergic leukocytes was not altered significant by CLP, but showed an upward trend in the monocyte/macrophage group (Fig. 3D).
Fig. 3.
Effects of sepsis on the phenotype of non-cholinergic and cholinergic splenocytes determined by flow cytometry at 16 h post-surgery. These experiments were done using male ChATBAC-eGFP mice. A: Flow cytometry gating strategy and representative raw flow cytometry data. Phenotyping antibody vs. side scatter graphs were constructed, and the specific cell population gates were identified. Histogram plots of GFP fluorescence were then constructed using these gates. B: CLP did not affect the gross phenotype distribution of splenocytes. C: CLP increased the percentage of monocytes/macrophages that were cholinergic (i.e., GFP+). D: CLP did not affect the intensity of GFP fluorescence in cholinergic splenocytes, significantly, but there was an upward trend for the monocyte/macrophage population (P = 0.1324). For each cell type and parameter, Sham and CLP values were compared using an unpaired t-test. Values are means ± SEM, with n = 5 for Sham and n = 6 for CLP. *P < 0.05 vs Sham.
3.3. AChE activity in spleen is low, occurs primarily in red pulp and is unaffected by acute sepsis
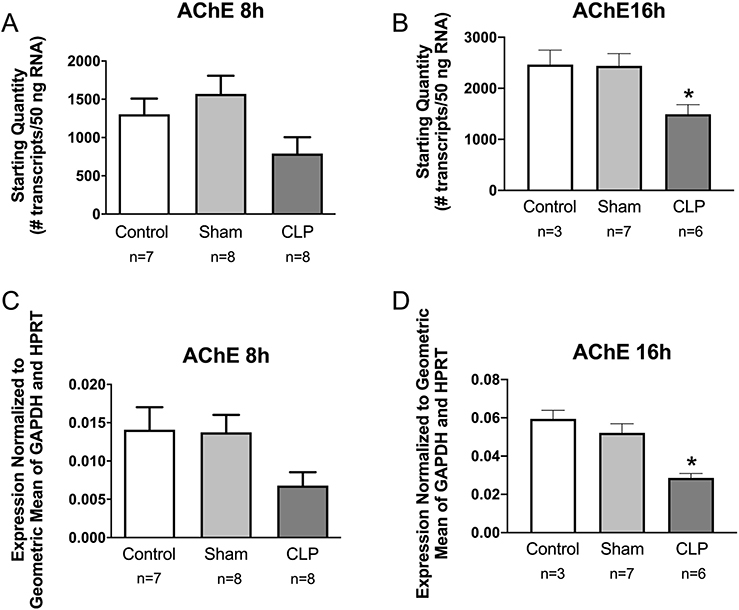
Although AChE is not a specific marker for cholinergic neurons, it is expressed by all cholinergic neurons and by many cholinoceptive cells [22]. We found a trend toward decreased abundance of AChE mRNA in the spleen at 8 h post-CLP and a significant decrease at 16 h (Fig. 4). Since this enzyme plays an essential role in regulating the magnitude and duration of responses evoked by released ACh, we also evaluated the localization and activity of AChE in mouse spleen. Histochemical staining for AChE showed that the enzyme occurred primarily in the red pulp but was very sparse in white pulp (Fig. 5A, B). Within the red pulp, staining was particularly intense in large cells presumed to be megakaryocytes. AChE staining in spleen was low compared to that in brain (Fig. 5C). Analysis of AChE activity in homogenates of spleen and brain confirmed the relatively low enzymatic activity in spleen and showed that this was not affected by acute sepsis (Fig. 5D).
Fig. 4.
Sepsis decreases the expression of AChE in mouse spleen. Spleens from Sham and CLP mice were collected at 8 or 16 h post-surgery. RNA from naïve control, sham, and CLP mouse spleens were evaluated by qPCR. A and B: Absolute amount of RNA. C and D: Relative expression – starting quantity of AChE mRNA was normalized to the geometric mean of the starting quantity for GAPDH and HPRT reference genes. These experiments were done using male C57BL/6J mice. Data in each panel were evaluated by one-way ANOVA, and post hoc comparisons were made using the Tukey test when overall group differences were detected. Group data are graphed as means ± SEM for the indicated n values. *P < 0.05 vs other groups.
Fig. 5.
Localization and activity of AChE in mouse spleen compared to brain. A-C: Photomicrographs of spleen (A and B) and brain (C) sections stained for AChE by enzyme histochemistry using the Koelle method. Note that AChE activity in the spleen is much more abundant in the red pulp (rp) compared to the white pulp (wp). Arrow (A and B) indicate presumed megakaryocytes that stain intensely for AChE. D: Quantitative analysis of AChE activity shows that brain contains much higher activity than spleen and that activity in the spleen is not changed 16 h post-CLP. Group data are graphed as means ± SEM for the indicated n values. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Acute sepsis alters the expression of α7 but not α4 nicotinic ACh receptors in spleen
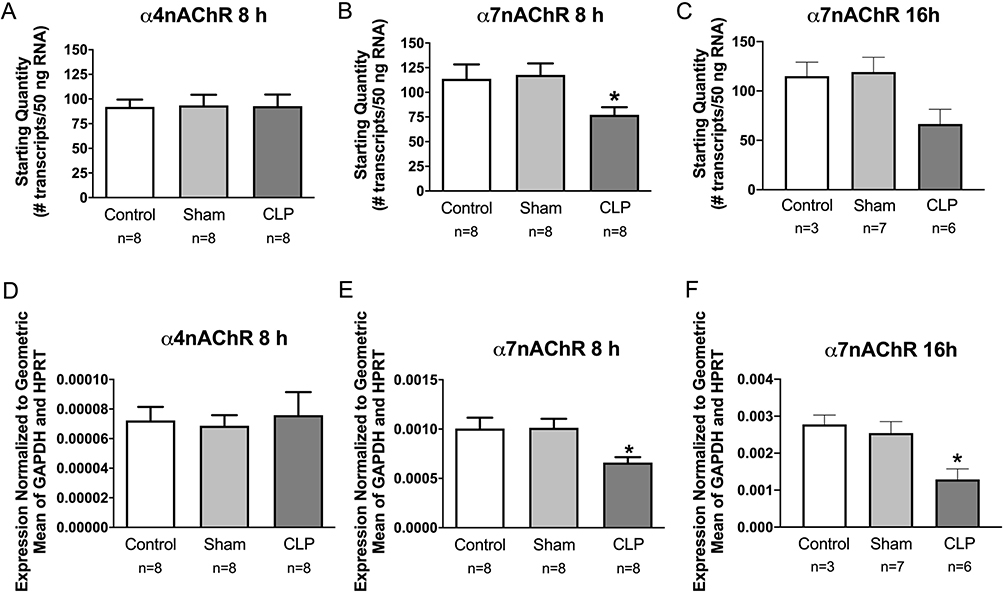
Previous studies showed that most types of leukocytes express muscarinic and nicotinic receptors [28,29], and there is strong evidence that α7 receptors on monocytes/macrophages mediate anti-inflammatory effects on these cells [6,8,30,31]. We used qPCR to evaluate the effect of acute sepsis on α4 and α7 receptor subunits in mouse spleen. Expression of α7 was decreased at 8 and 16 h post CLP (Fig. 6B, C, E, F). Expression of α4 was not affected at 8 h (Fig. 6A, D).
Fig. 6.
Sepsis decreases expression of α7nAChRs in mouse spleen but does not affect α4nAChRs. Spleens from Sham and CLP mice were collected at 8 or 16 h post-surgery. RNA from naïve control, sham, and CLP mouse spleens were evaluated by qPCR. A-C: Absolute amount of RNA. E-F: Relative expression – starting quantity of α7nAChR or α4nAChR mRNA was normalized to the geometric mean of the starting quantity for GAPDH and HPRT reference genes. These experiments were done using male C57BL/6J mice. Data in each panel were evaluated by one-way ANOVA, and post hoc comparisons were made using the Tukey test when overall group differences were detected. Group data are graphed as means ± SEM for the indicated n values. *P < 0.05 vs other groups.
3.5. ACh is present in isolated splenocytes and is more abundant in males
Our first step toward measuring ACh content and release from splenocytes was to develop a quantitative analysis method. For this purpose, we selected LC-MS/MS since this approach clearly separates ACh from choline and enables addition of a d4-ACh internal standard to each sample. A typical ion chromatogram and standard curve are shown is Supplement Fig. 2. Note that ACh concentrations are based on the ratio of ACh:d4-ACh.
Using this method, we measured the ACh content of splenocytes isolated from male and female ChATBAC-eGFP mice. This strain was used in all subsequent experiments because GFP fluorescence of cholinergic cells will facilitate analysis of pure subpopulations of cholinergic leukocytes in future studies. We found that ACh is more abundant in splenocytes from male mice compared to females (Fig. 7). For this reason, we used male mice for remaining experiments to enhance detection of ACh release.
Fig. 7.
Splenocytes collected from male C57BL/6 mice contain more ACh than splenocytes collected from female mice. Values are means ± SEM, n = 3 per group. *P < 0.05 vs male.
3.6. Splenocytes exhibit basal and stimulated release of ACh
As a prelude to our ACh release experiments, we evaluated the stability of d4-ACh in cell culture medium with and without added splenocytes. Neostigmine was used in all of these experiments as an added precaution. This experiment showed that the presence of splenocytes did not affect hydrolysis of ACh, but ~5% of d4-ACh was converted to d4-Ch over 24 h incubation at 37° C in cell culture medium. Since cell culture medium is pH 7.4, we attribute this small change to chemical hydrolysis.
It is thought that release of ACh in the spleen during activation of the cholinergic anti-inflammatory pathway occurs through stimulation of β2-adrenergic receptors on T cells [6–8,32]. To test this hypothesis in vitro, we treated isolated splenocytes with (−)-isoproterenol, a selective β-adrenergic agonist, for 4 and 24 h in culture. Basal release of ACh into the medium was detected but this did not increase over 24 h. Treatment with isoproterenol evoked significant release of ACh compared to unstimulated controls after 4 and 24 h incubation, and the concentration of ACh released was significantly higher after 24 h (Fig. 8A). Treatment of isolated splenocytes with T cell activating antibodies also increased ACh release into the medium after 24 h but not after 4 h (Fig. 8B). Combined treatment with isoproterenol and T cell activating antibodies caused more ACh release than either treatment alone (Fig. 8C).
Fig. 8.
Isoproterenol and T cell activation stimulated the release of ACh by isolated splenocytes from male ChATBAC-eGFP mice. Isolated splenocytes were incubated in 2 ml of medium for indicated times with no treatment, 1 μM (−) isoproterenol, or T cell activating antibodies (anti-CD3 and anti-CD28). For each experiment, data were evaluated by repeated measures ANOVA followed by post hoc comparison of group data. A: Isoproterenol increased ACh release after 4 and 24 h incubation, with 24 h causing significantly higher ACh concentration in the medium. B: Addition of T cell antibodies to the medium caused a significant increase in ACh release only after 24 h. C: Treatment with isoproterenol + T cell activation caused greater ACh release than either treatment alone. For T cell activation in this experiment, plates were coated with anti-CD3 overnight, washed before adding cells, and treated with anti-CD28 added to the medium. Data in each panel were evaluated by repeated measures one-way ANOVA, and post hoc comparisons were made using the Tukey test when overall group differences were detected. Values are means ± SEM with n = 7 in A, n = 3 in B and n = 5 in C. *P < 0.05 vs indicated groups.
3.7. Splenocytes express several organic cation transporters implicated in ACh release
Several organic cation ion transporters have been implicated in the release of ACh from non-neuronal cholinergic cells [33,34], so preliminary PCR experiments were done to screen splenocytes for the expression of these. We found that cells from C57BL/6 and ChATBAC-eGFP mice expressed OCT2, OCT3, and OCTN2, but neither OCT1 nor OCTN1 was detected (Fig. 9).
Fig. 9.
Expression of OCT1, OCT2 and OCTN organic acid transporter by splenocytes isolated from C57BL/6 (C57) and ChATBAC-eGFP (TG) mice. Expression determined by RT-PCR. (−), no RT added; NTC, no template control.
4. Discussion
This study has expanded our knowledge regarding cholinergic biology of the spleen and how this system is affected by acute sepsis. Our qPCR experiments established that ChAT is expressed in the spleen at baseline, and our flow cytometry studies with ChATBAC-eGFP mice confirmed previous reports that this expression occurs in splenic T cells, B cells and monocytes/macrophages [26,28]. Acute sepsis did not affect either the expression of ChAT or the relative abundance of eGFP+ cells in T or B cell populations, but it did increase the proportion of GFP+ monocytes/macrophages. Evaluation of two other markers, which are required by cholinergic neurons, showed that VAChT was expressed by spleen at baseline, but CHT1 was not. While VAChT could have a role in storage and release of ACh by leukocytes, the lack of CHT1 suggests that choline uptake by these cells occurs by another mechanism. VAChT immunoreactivity was negligible in white pulp of normal spleens, however, acute sepsis increased VAChT expression in the spleen and caused punctate VAChT staining of leukocytes in the white pulp. These findings suggest that acute sepsis might increase ACh release by means of VAChT upregulation. Organic cation transporters, identified by our RT-PCR analysis of isolated splenocytes, might also contribute to ACh release from leukocytes. The stability of released ACh would depend of the presence and localization of AChE. We found low levels of AChE activity in spleen homogenates, and this activity did not change in acute sepsis. Importantly, histochemistry for AChE showed that most of the enzyme activity occurs in the red pulp and not the white pulp where cholinergic T cells reside. This distribution would favor diffusion of ACh to target monocytes and macrophages in the red pulp and marginal zone. Lastly, we found that isolated splenocytes from naive mice exhibit basal release of ACh and that release can be evoked by stimulation of β-adrenergic receptors on naive splenocytes or by activation of T cells with antibodies to CD3 and CD28.
Since the cholinergic anti-inflammatory pathway that targets the spleen presumably operates as a homeostatic mechanism [6,12,13], we assumed that it would be stimulated reflexively during the acute phase of sepsis. This scenario would involve activation of afferent input to the brain by early cytokines like IL1β and toll receptor agonists, central processing of this information, and subsequent stimulation of the sympathetic nervous system and hypothalamic pituitary adrenocortical axis. Indeed, previous studies of CLP sepsis have shown that plasma catecholamine concentrations and turnover of catecholamines in the spleen are increased during the time period we studied [35–37]. Our observation that ChAT expression is not increased for at least 8 h after CLP suggests that the ACh synthetic capacity of the spleen is not increased by catecholamines or other mediators generated during early sepsis. However, upregulation of VAChT does suggest that acute sepsis enhances the capacity for ACh release. Upregulation of VAChT alone was unexpected, since ChAT and VAChT have a common gene locus and are coordinately regulated [38,39]. However, there is evidence that the efficacy for transcription of these genes can differ and that they can be regulated differentially [40]. For example, relative expression of ChAT and VAChT vary inversely during development of chick dorsal root ganglia [41], and VAChT mRNA levels in developing rat brain peak much earlier than ChAT mRNA [42]. Also, specific chemical treatments can differentially affect ChAT and VAChT expression in NG 108–15 cells [43]. Further, some non-neuronal cells express ChAT but not VAChT, for example bladder urothelial cells [44].
The presence of ChAT in cholinergic leukocytes and the dominant role it plays in ACh synthesis by these cells are well established [28,45], but other elements of cholinergic biology of leukocytes are still unclear and could vary between cell types and with the activation state of these cells. Several candidate molecules have been identified for the uptake of choline, which is required for synthesis of ACh, and for the released of ACh into the extracellular compartment. Evidence from our study indicates that CHT1, which is essential for ACh synthesis by cholinergic neurons, does not play a role in ACh synthesis by leukocytes. Previous work identified CHT1 expression in one of three leukemic T cell lines [46], which could differ from normal leukocytes. The most likely candidates for this role are choline transporter-like proteins (CTL1–5), which have an intermediate affinity for choline and are expressed widely. While these transporters have not been studied extensively in leukocytes, CTL1 is expressed by THP-1 monocyte-derived macrophages and bone marrow-derived macrophages, where it mediates choline uptake [47,48]. Furthermore, CTL4 has been linked to ACh synthesis and release in H82 lung cancer cell lines [49].
Several mechanisms for release of ACh from non-neuronal cells have been identified, including VAChT, which is essential for quantal release from cholinergic nerves. We identified VAChT expression in mouse spleen and showed that expression of RNA and protein are upregulated during acute sepsis. The functional significance of these observations is unknown, but they do suggest the possibility for increased release of ACh by some splenocytes during sepsis. In contrast, Fujii et al did not detect VAChT expression by Southern blot analysis of human peripheral blood mononuclear cells [50]. It remains possible that VAChT might be upregulated by these leukocytes under inflammatory conditions. The same group proposed that ACh is released directly from the cytosol of lymphocytes without storage, and they provided convincing evidence that such release can be mediated by a protein called mediatophore, which is structurally similar to a vacuolar H+-ATPase with known ability to translocate ACh [51]. These experiments were performed using human acute lymphoblastic leukemia cell lines, so further study of this mechanism is needed using primary lymphocyte cultures. Studies of other non-neuronal cholinergic cells have demonstrated that various organic cation transporters (OCTs) also have the ability to release ACh directly from the cytosol [33,34]. These include OCT(1–3), OCTN1 and OCTN2. Such transporters function in a sodium-independent manner and move cations like ACh down their concentration gradient [52]. Thus, they would enable movement of ACh from the cytosol of leukocytes into the extracellular compartment. While it is not known if T cell activation or adrenergic stimulation of T cells alter the function of OCTs, there is evidence that OCTs in other cells can be stimulated or inhibited through activation of specific intracellular signaling mechanisms [52]. There is clear evidence that some of these transporters mediate ACh release from non-neuronal cholinergic tissues such as airway epithelium, peritoneal mesothelial cells, and placenta [33,34], but their role in cholinergic leukocytes has received little attention. While Fujii et al reported that OCT(1–3) are not expressed by human acute lymphoblastic leukemia cells [51], we identified expression of OCT2, OCT3, and OCTN2 in splenocytes isolated from C57BL/6 and ChATBAC-eGFP mice. Therefore, OCTs remain viable candidates for ACh release from cholinergic leukocytes, especially since VAChT+ cells have a much lower abundance than GFP+ cells in ChATBAC-eGFP mice. Collectively, these data suggest that cholinergic leukocytes have multiple mechanisms for release of ACh, but further studies are needed to understand the relative importance of specific transporters according to cell phenotype, how these release mechanisms are engaged, and the dynamic properties of release from leukocytes.
ChAT is the rate-limiting enzyme for ACh synthesis, so upregulation of ChAT is a potential mechanism for regulating ACh release from cholinergic splenocytes. In fact, previous studies of the MOLT-3T cell lines have shown that treatment with phytohemagglutinin, a T cell activator, caused a rapid increase in ChAT mRNA, which reached over 10-fold after 12 h [53]. Likewise, increased ChAT mRNA was reported after treatment of murine mononuclear leukocytes with concanavalin A for 24 h and after treating dendritic cells for 24 h with endotoxin [54]. However, we did not observe rapid upregulation of ChAT in the spleen during experimental sepsis, suggesting that in vitro treatments do not adequately model the conditions of acute sepsis pathophysiology. Nevertheless, sepsis did upregulate VAChT suggesting an alternative mechanism for increased ACh release. Additionally, it is possible that ACh synthesis and release might be increased secondary to upregulation of a CLT, mediatophore, and/or organic cation transporters.
Previous studies have established the presence of ACh in various immune cell lines and primary leukocytes isolated from various species including humans and rats by using a specific radioimmunoassay [28,29,45,55]. Using a GC–MS method, we detected a higher content of ACh in murine splenocytes than reported for rat splenocytes [56]. This higher content might be attributed to our analysis of freshly isolated cells rather than cells maintained in culture. Further, we found that splenocytes from males had higher ACh content than those from females. While this finding requires deeper investigation, it does imply that immunoregulatory effects of ACh may differ between sexes.
We also demonstrated basal and evoked release of ACh from isolated murine splenocytes. Stimulation with isoproterenol, to mimic sympathetic activation of β2-adrenoceptors on T cells, caused significant release of ACh into the medium after 4 and 24 h incubation. This finding builds on a previous report that non-adherent splenocytes released ACh in response to a very high concentration of the nonselective adrenergic agonist NE [8]. However, the latter study detected low nanomolar amounts of ACh, whereas we detected low micromolar concentrations capable of activating multiple cholinergic receptors including α7nAChRs linked to cholinergic anti-inflammatory responses. More recently other investigators, using more sensitive mass spectroscopy methods combined with microdialysis, showed that acute vagal stimulation evokes rapid, β2-adrenoceptor-mediated release of ACh in high nanomolar quantities into dialysate [7]. Clearly, such a rapid, evoked response must depend on existing release mechanisms. While isoproterenol does not cause T cell activation, we found that T cell activation alone by antibody stimulation also evokes ACh release, although not as quickly as β2-adrenoceptor stimulation. Collectively, these data suggest that ACh release can be stimulated and accomplished by multiple mechanisms.
Our experiments also showed that acute sepsis down-regulated α7nAChRs and AChE expression in the spleen. While alpha7 receptors are expressed by several types of leukocytes [28], they are most noted for mediating anti-inflammatory effects on monocytes and macrophages [6,13,31]. Reduction of alpha7 RNA in the spleen during sepsis might be attributed to trafficking of these cells to sites of infection, but additional explanations are possible. Downregulation of AChE mRNA might lead to reduction of already low levels of AChE activity, further supporting cholinergic modulation of immune function in the spleen.
In summary, our findings demonstrate that acute sepsis affects the cholinergic biology of the spleen in ways that would promote cholinergic modulation of immune function. This work also adds to our understanding of the complex mechanisms capable of triggering and mediating ACh release from splenocytes. Further analysis of cholinergic function and signaling by different classes of leukocytes in the spleen and throughout the immune systems offers the potential for discrete therapeutic modulation of immune function.
Supplementary Material
Acknowledgements
This work was supported, in part, by NIH grants R15GM107949 (DBH) GM083016 (DLW), GM119197 (DLW), GM122934 (TRO) and C06RR0306551 (ETSU). Thanks to Dr. Michelle Duffourc for designing primers for RT-PCR and to Rheas Dykes for doing RT-PCR analysis.
Footnotes
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106359.
References
- [1].Delano MJ, Ward PA, Sepsis-induced immune dysfunction: can immune therapies reduce mortality, J. Clin. Invest. 126 (2016) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL, Sepsis and septic shock, Nat. Rev. Dis. Primers 2 (2016) 16045, 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Delano MJ, Ward PA, The immune system’s role in sepsis progression, resolution, and long-term outcome, Immunol. Rev. 274 (2016) 330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huston JM, Wang H, Ochani M, Ochani K, Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ, Yang H, Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels, J. Immunol. 181 (2008) 3535–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hoover DB, Cholinergic modulation of the immune system presents new approaches for treating inflammation, Pharmacol. Ther. 179 (2017) 1–16, 10.1016/j.pharmthera.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pavlov VA, Tracey KJ, Neural regulation of immunity: molecular mechanisms and clinical translation, Nat. Neurosci. 20 (2017) 156–166. [DOI] [PubMed] [Google Scholar]
- [7].Murray K, Barboza M, Rude KM, Brust-Mascher I, Reardon C, Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen, Brain. Behav. Immun. 82 (2019) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ, Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit, Science 334 (2011) 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM, Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons, Exp. Physiol. 97 (2012) 1180–1185. [DOI] [PubMed] [Google Scholar]
- [10].Martelli D, McKinley MJ, McAllen RM, The cholinergic anti-inflammatory pathway: a critical review, Auton. Neurosci. 182 (2014) 65–69. [DOI] [PubMed] [Google Scholar]
- [11].Murray K, Reardon C, The cholinergic anti-inflammatory pathway revisited, Neurogastroenterol. Motil. 30 (2018) 1–12, 10.1111/nmo.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andersson U, Tracey KJ, Reflex principles of immunological homeostasis, Annu. Rev. Immunol. 30 (2012) 313–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tracey KJ, Reflex control of immunity, Nat. Rev. Immunol. 9 (2009) 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kanashiro A, Sônego F, Ferreira RG, Castanheira FV, Leite CA, Borges VF, Nascimento DC, Cólon DF, Alves-Filho JC, Ulloa L, Cunha FQ, Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis, Pharmacol. Res. 117 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choudhry MA, Bland KI, Chaudry IH, Gender and susceptibility to sepsis following trauma, Endocr. Metab. Immune. Disord. Drug. Targets 6 (2006) 127–135. [DOI] [PubMed] [Google Scholar]
- [16].Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH, Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males, Crit. Care. Med. 25 (1997) 106–110. [DOI] [PubMed] [Google Scholar]
- [17].Hoover DB, Ozment TR, Wondergem R, Li C, Williams DL, Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis, Shock 43 (2015) 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin. Chem. 55 (2009) 611–622. [DOI] [PubMed] [Google Scholar]
- [19].Downs AM, Bond CE, Hoover DB, Localization of α7 nicotinic acetylcholine receptor mRNA and protein within the cholinergic anti-inflammatory pathway, Neuroscience 266 (2014) 178–185. [DOI] [PubMed] [Google Scholar]
- [20].Fregoso SP, Hoover DB, Development of cardiac parasympathetic neurons, glial cells, and regional cholinergic innervation of the mouse heart, Neuroscience 221 (2012) 28–36. [DOI] [PubMed] [Google Scholar]
- [21].Hoover DB, Brown TC, Miller MK, Schweitzer JB, Williams DL, Loss of sympathetic nerves in spleens from patients with end stage sepsis, Front. Immunol 8 (2017) 1712, 10.3389/fimmu.2017.01712.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koelle GB, Cytological distributions and physiological functions of cholinesterases, Handb. Exp. Pharmacol. 15 (1963) 187–298. [Google Scholar]
- [23].Koelle GB, The elimination of enzymatic diffusion artifacts in the histochemical localization of cholinesterases and a survey of their cellular distributions, J. Pharmacol. Exp. Ther. 103 (1951) 153–171. [PubMed] [Google Scholar]
- [24].Koelle GB, The histochemical identification of acetylcholinesterase in cholinergic, adrenergic and sensory neurons, J. Pharmacol. Exp. Ther. 114 (1955) 167–184. [PubMed] [Google Scholar]
- [25].Brown TC, Bond CE, Hoover DB, Variable expression of GFP in different populations of peripheral cholinergic neurons of ChATBAC-eGFP transgenic mice, Auton. Neurosci. 210 (2018) 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reardon C, Duncan GS, Brüstle A, Brenner D, Tusche MW, Olofsson PS, Olofsson P, Rosas-Ballina M, Tracey KJ, Mak TW, Lymphocyte-derived ACh regulates local innate but not adaptive immunity, Proc. Natl. Acad. Sci. USA 110 (2013) 1410–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI, BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons, Physiol. Genomics 27 (2006) 391–397. [DOI] [PubMed] [Google Scholar]
- [28].Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K, Expression and function of the cholinergic system in immune cells, Front. Immunol 8 (2017) 1085, 10.3389/fimmu.2017.01085.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawashima K, Fujii T, Moriwaki Y, Misawa H, Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function, Life. Sci 91 (2012) 1027–1032. [DOI] [PubMed] [Google Scholar]
- [30].Kawashima K, Fujii T, Moriwaki Y, Misawa H, Horiguchi K, Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs, Int. Immunopharmacol. 29 (2015) 127–134. [DOI] [PubMed] [Google Scholar]
- [31].Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation, Nature 421 (2003) 384–388. [DOI] [PubMed] [Google Scholar]
- [32].Vida G, Peña G, Kanashiro A, Thompson-Bonilla MR, Palange D, Deitch EA, Ulloa L, β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system, FASEB. J. 25 (2011) 4476–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kummer W, Krasteva-Christ G, Non-neuronal cholinergic airway epithelium biology, Curr. Opin. Pharmacol. 16 (2014) 43–49. [DOI] [PubMed] [Google Scholar]
- [34].Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ, The non-neuronal cholinergic system in humans: expression, function and pathophysiology, Life. Sci. 72 (2003) 2055–2061. [DOI] [PubMed] [Google Scholar]
- [35].Hahn PY, Wang P, Tait SM, Ba ZF, Reich SS, Chaudry IH, Sustained elevation in circulating catecholamine levels during polymicrobial sepsis, Shock 4 (1995) 269–273. [DOI] [PubMed] [Google Scholar]
- [36].Jones SB, Kovarik MF, Romano FD, Cardiac and splenic norepinephrine turnover during septic peritonitis, Am. J. Physiol. 250 (1986) R892–R897. [DOI] [PubMed] [Google Scholar]
- [37].Kovarik MF, Jones SB, Romano FD, Plasma catecholamines following cecal ligation and puncture in the rat, Circ. Shock 22 (1987) 281–290. [PubMed] [Google Scholar]
- [38].Eiden LE, The cholinergic gene locus, J. Neurochem. 70 (1998) 2227–2240. [DOI] [PubMed] [Google Scholar]
- [39].Erickson JD, Varoqui H, Schäfer MK, Modi W, Diebler MF, Weihe E, Rand J, Eiden LE, Bonner TI, Usdin TB, Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus, J. Biol. Chem. 269 (1994) 21929–21932. [PubMed] [Google Scholar]
- [40].Cervini R, Houhou L, Pradat PF, Béjanin S, Mallet J, Berrard S, Specific vesicular acetylcholine transporter promoters lie within the first intron of the rat choline acetyltransferase gene, J. Biol. Chem. 270 (1995) 24654–24657. [DOI] [PubMed] [Google Scholar]
- [41].Corsetti V, Mozzetta C, Biagioni S, Augusti Tocco G, Tata AM, The mechanisms and possible sites of acetylcholine release during chick primary sensory neuron differentiation, Life. Sci. 91 (2012) 783–788. [DOI] [PubMed] [Google Scholar]
- [42].Holler T, Berse B, Cermak JM, Diebler MF, Blusztajn JK, Differences in the developmental expression of the vesicular acetylcholine transporter and choline acetyltransferase in the rat brain, Neurosci. Lett. 212 (1996) 107–110. [DOI] [PubMed] [Google Scholar]
- [43].Castell X, Diebler MF, Tomasi M, Bigari C, De Gois S, Berrard S, Mallet J, Israël M, Dolezal V, More than one way to toy with ChAT and VAChT, J. Physiol. Paris 96 (2002) 61–72. [DOI] [PubMed] [Google Scholar]
- [44].Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA, Non-neuronal acetylcholine and urinary bladder urothelium, Life. Sci. 80 (2007) 2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K, Physiological functions of the cholinergic system in immune cells, J. Pharmacol. Sci. 134 (2017) 1–21. [DOI] [PubMed] [Google Scholar]
- [46].Fujii T, Okuda T, Haga T, Kawashima K, Detection of the high-affinity choline transporter in the MOLT-3 human leukemic T-cell line, Life Sci. 72 (2003) 2131–2134. [DOI] [PubMed] [Google Scholar]
- [47].Fullerton MD, Wagner L, Yuan Z, Bakovic M, Impaired trafficking of choline transporter-like protein-1 at plasma membrane and inhibition of choline transport in THP-1 monocyte-derived macrophages, Am. J. Physiol. Cell. Physiol. 290 (2006) C1230–C1238. [DOI] [PubMed] [Google Scholar]
- [48].Snider SA, Margison KD, Ghorbani P, LeBlond ND, O’Dwyer C, Nunes JRC, Nguyen T, Xu H, Bennett SAL, Fullerton MD, Choline transport links macrophage phospholipid metabolism and inflammation, J. Biol. Chem. 293 (2018) 11600–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Song P, Rekow SS, Singleton CA, Sekhon HS, Dissen GA, Zhou M, Campling B, Lindstrom J, Spindel ER, Choline transporter-like protein 4 (CTL4) links to non-neuronal acetylcholine synthesis, J. Neurochem. 126 (2013) 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fujii T, Yamada S, Watanabe Y, Misawa H, Tajima S, Fujimoto K, Kasahara T, Kawashima K, Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator, J. Neuroimmunol. 82 (1998) 101–107. [DOI] [PubMed] [Google Scholar]
- [51].Fujii T, Takada-Takatori Y, Horiguchi K, Kawashima K, Mediatophore regulates acetylcholine release from T cells, J. Neuroimmunol. 244 (2012) 16–22. [DOI] [PubMed] [Google Scholar]
- [52].Koepsell H, Organic Cation Transporters in Health and Disease, Pharmacol. Rev. 72 (2020) 253–319. [DOI] [PubMed] [Google Scholar]
- [53].Mashimo M, Iwasaki Y, Inoue S, Saito S, Kawashima K, Fujii T, Acetylcholine released from T cells regulates intracellular Ca2+, IL-2 secretion and T cell proliferation through nicotinic acetylcholine receptor, Life Sci. 172 (2017) 13–18. [DOI] [PubMed] [Google Scholar]
- [54].Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H, Expression and function of genes encoding cholinergic components in murine immune cells, Life. Sci. 80 (2007) 2314–2319. [DOI] [PubMed] [Google Scholar]
- [55].Kawashima K, Fujii T, Watanabe Y, Misawa H, Acetylcholine synthesis and muscarinic receptor subtype mRNA expression in T-lymphocytes, Life. Sci. 62 (1998) 1701–1705. [DOI] [PubMed] [Google Scholar]
- [56].Rinner I, Kawashima K, Schauenstein K, Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation, J. Neuroimmunol 81 (1998) 31–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.