Abstract

The water generated after the dissociation of gas hydrates is spontaneously imbibed into the matrix pores. It hinders the gas–water flow and decreases the pore pressure, which is not conducive to the continuous depressurization of hydrate sediments. However, there are few research studies on the imbibition capacity and the influencing factors of hydrate sediments. In this paper, spontaneous imbibition experiments are carried out on the samples of hydrate sediments. The imbibition capacity and its influencing factors are analyzed. The results show that as the spontaneous imbibition time increases, the peak of the T2 spectra also shifts to the right, indicating the formation of new larger-sized pores. When the imbibition time exceeds the critical point, the hydrate sediments instantly disperse and reach the maximum imbibition capacity status. The influencing factors of the imbibition capacity include the moisture content, dry–wet cycle, clay minerals content, solution salinity, permeability, and porosity. With the increase of the clay mineral content, the imbibition capacity increases rapidly, and the I/S mixed layer can significantly improve the imbibition capacity. As the number of dry–wet cycles increases, the imbibition capacity gradually increases. In addition, the imbibition capacity is inversely related to the moisture content and the solution salinity. Porosity and permeability have little effect on the imbibition capacity. This study is of great significance for understanding dissociation water retention and optimizing hydrate exploitation systems.
1. Introduction
Hydrate sediments are also called “flammable ice”, and their main component is methane. Hydrates in the deep sea are stable under the pressure of thick water layers, so their environment is low temperature and high pressure.1 The methane gas generated after the dissociation is a clean energy source, and no harmful gas is generated after combustion. During the depressurization exploitation process, 1 cubic meter of flammable ice can release 164 cubic meters of natural gas and 0.8 cubic meters of water at normal temperature and pressure, but only 1% of the dissociation water is produced.2,3 The dissociation water is spontaneously imbibed into the matrix pores. It hinders the gas–water flow and the decrease of pore pressure, which is not conducive to the continuous depressurization of hydrate sediments.4,5 Therefore, studying the imbibition capacity and influencing factors of hydrates will help to further understand the hydrate dissociation process, optimize the hydrate exploitation regime, and improve the hydrate exploitation efficiency.6
The process of gas hydrate extraction and dissociation is related to pressure, temperature, permeability, and saturation. A series of studies have been conducted on the formation and dissociation of natural gas hydrates. The results show that hydrate formation and dissociation processes are accompanied by the change in temperature and pressure of the reservoir.7 The simultaneous decompression and heat injection methods are proved to be effective during hydrate production.8 As for hydrate exploitation, hydrate can be dissociated by depressurization, and gas can be obtained from different methane hydrate sediments.9 In terms of hydrate dissociation, the permeability of sediments seriously affects the process of hydrate dissociation and the efficiency of gas generation. The relationship between permeability and hydrate saturation can be established.10 The increase of hydrate saturation will decrease the gas permeability, but the effect of hydrate morphology on the relative permeability is greater than the hydrate saturation.11 Relative water and gas permeability equations are very important for estimating the gas and water production from hydrate sediments. The pore network model can simulate gas expansion and calculate relative water and gas permeability.12
At present, research studies on spontaneous imbibition mainly focus on sandstone and shale. The anionic surfactant will reduce the imbibition rate of shale matrix pores, and the imbibition rate will be further reduced after the addition of the KCl solution.13,14 The clay mineral content and the water content have significant effects on the imbibition capacity.15 The excess water intake is correlated with the shale mineralogy and petrophysical properties. The water intake of organic shales is controlled by adsorption and capillarity.16 In addition, the fluid pressure could also affect the imbibition rate, and a new analytical model of the forced imbibition is established to study the effects of pressure on the imbibition rate in shale reservoirs.17 Forced imbibition is also affected by boundary conditions and stress sensitivity. The effective stress increases, and microfractures and large pores are compressed, which may decrease the imbibition rate.18,19 The confining pressure would reduce the porosity of the shale formation and thus affect the imbibition process.20,21
Previous researchers carried out a lot of studies on the effects of temperature, pressure, velocity, and saturation on shale imbibition. However, there are few research studies on the imbibition capacity and imbibition capacity and its related influential factors. In this paper, imbibition experiments are carried out on different sediment samples, and the influencing factors of the imbibition capacity are analyzed by comparing with shale samples.
2. Experimental Equipment and Samples
The experiments are divided into three groups. In the first group, seven samples of the A, B, and C formations are used and the experiments are monitored by a NMR instrument. The imbibition characteristics of the hydrate sediment are studied by observing and analyzing the surface morphological changes. Sample physical characteristics are shown in Table 1. The second group of experiments is the imbibition test of the samples with different moisture content. The results are used to study the effects of different moisture content on the imbibition rate and capacity. Three representative formation samples are taken for experimental comparison. The third group of experiments is to study the influences of the dry–wet cycle on the imbibition rate and capacity.
Table 1. Sample Physical Characteristics for the First Groupa.
| sediment | length (cm) | width (cm) | height (cm) | original mass (g) | initial moisture content (%) | porosity (%) | permeability (mD) |
|---|---|---|---|---|---|---|---|
| A1 | 1.266 | 1.148 | 1.508 | 3.752 | 22.25 | 32.5 | 7.8 |
| A2 | 1.235 | 1.213 | 1.725 | 4.791 | 28.58 | 32.4 | 2.0 |
| A3 | 1.394 | 1.156 | 1.845 | 5.615 | 27.38 | 21.5 | 1.0 |
| A4 | 1.377 | 1.450 | 1.842 | 6.373 | 7.75 | 31.4 | 6.6 |
| B1 | 1.083 | 1.113 | 1.595 | 3.072 | 18.81 | 35.4 | 5.1 |
| B2 | 1.120 | 1.183 | 1.600 | 4.078 | 21.76 | 29.5 | 0.3 |
| C1 | 1.268 | 1.148 | 1.849 | 4.146 | 49.02 | 30.6 | 1.9 |
Note: The physical properties of A1, A3, B1, and B2 are from Yang et al.1
2.1. Experimental Sample and Fluids
The samples in this experiment belong to clay hydrate sediments. The original samples and dried samples of three formations are selected for an analysis of the physical properties, the pore structure characteristics, and the mineral composition characteristics. The length, width, and height of the sample are measured before a series of experiments. The water used for the experiments is distilled water. The NaCl solutions with different mass fractions are used to simulate the seawater environment around hydrate sediments.
Table 1 shows the original sample information in the first group of experiments. The average moisture content of the samples in formation A is about 7.75–28.58%, those in formation B is about 18.81–21.76%, and those in formation C is about 49.02%. Among them, the formation C has the highest moisture content and formation A has the lowest water content. The porosity is measured by helium porosimeter from the China University of Petroleum in Beijing. The relative error of porosity measurement is less than 5%. The permeability is determined by nitrogen. The sediment samples are highly compressible and so the permeability is measured under low confining pressure (0.5 MPa). The relative error of permeability measurement is less than 8%. The hydrate sample has a porosity of about 21.5–32.5% and a permeability of about 1.0–7.8 mD, showing the characteristics of high porosity and low permeability. The porosity and permeability of formation A are 29.5% and 4.4 mD on average, those of formation B are 32.5% and 2.7 mD on average, and those of formation C are 30.6% and 1.9 mD, respectively. The porosity of the three formations is relatively close, but the permeability is quite different. The permeability of formation A is much greater than that of formation C. Formation A contains a large number of microfractures, resulting in larger permeability (Figure 1).
Figure 1.
Scanning electron microscopy (SEM) image of the three formations: (a) A, (b) B, and (c) C.
Table 2 shows the mineral composition of the formations. The hydrate sample is mainly composed of quartz and clay minerals. The quartz content is about 36.0–45.7%, and clay mineral content is about 26.1–29.8%. Overall, the samples in formation B have the highest quartz and clay mineral contents. The clay mineral composition is mainly mixed with illite and I/S mixed layer. Because both illite and smectite are more permeable and swellable, the hydrate samples have strong water sensitivity.
Table 2. Results of Mineral Composition Analysis.
| total
mineral composition, wt (%) |
relative
clay abundance, wt (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| label | quartz | feldspar | calcite | dolomite | pyrite | clay | illite | I/Sa | chlorite | kaolinite |
| A1 | 36.7 | 8.7 | 13.7 | 3.5 | 7.6 | 29.8 | 36 | 41 | 14 | 9 |
| A2 | 42.0 | 6.8 | 18.0 | 3.6 | 3.5 | 26.1 | 33 | 42 | 16 | 9 |
| A3 | 36.0 | 5.9 | 23.0 | 3.6 | 2.3 | 29.2 | 32 | 43 | 16 | 9 |
| A4 | 45.7 | 10.4 | 12.6 | 3.0 | 0.5 | 27.8 | 38 | 33 | 19 | 10 |
| B1 | 47.9 | 8.7 | 10.8 | 4.6 | 1.1 | 26.9 | 37 | 34 | 18 | 11 |
| B2 | 44.3 | 7.8 | 12.4 | 4.1 | 1.1 | 30.3 | 38 | 31 | 18 | 13 |
| C1 | 40.7 | 8.5 | 12.5 | 5.2 | 3.1 | 27.9 | 29 | 52 | 13 | 6 |
I/S represents illite/smectite mixed layer. The results of A1, A3, B1, and B2 are from Yang et al.1
Figure 1 shows the results of the SEM analysis. Through SEM scanning, the microstructure of the sample surface, such as the pore structure and composition, can be observed. The pore sizes of the three formations are about 0.1–9 μm. The common feature is that they all contain many pores and fractures. The difference is that formation A is loose and porous. The pore size and microcrack size are much larger, ranging from 0.5 to 9 μm and resulting in higher permeability. The surface of formation B contains large-sized pores and the sediments are cemented into flakes with a high degree of cementation. The pore size is between 0.1 and 1.2 μm, and the pore size and microcracks are not developed. Formation C contains biofossils and debris particles, and its pore size is between 0.5 and 4.5 μm.
2.2. Experimental Equipment and Procedures
The NMR instrument (MiniMR-VTP, Suzhou Niumag Analytical Instrument Corporation) is used for imbibition experiments. The experimental instrument is located at the Chinese Academy of Sciences. The sample diameter is 0–60 mm, and the magnetic field strength is 0.5 T. The control temperature is −20 to 100 °C, and the main frequency is 23 MHz. The imaging analysis rate is 100 μm, and the instrument picture is shown in Figure 2.
Figure 2.
NMR instrument.
The first group of experiments is as follows:
-
(1)
The samples in Table 1 are cut into a rectangular parallelepiped and placed in a beaker. The samples are dried in a drying box at 75 °C for 24 h until the weight does not change. Then, the sample is taken out, wrapped in a plastic wrap, and kept in a cool area to cool to room temperature.
-
(2)
The length, width, and height of the sample are measured, and the initial weight of the sample is recorded.
-
(3)
A long dropper is used to drip distilled water on the surface of the sample. After the water droplet has been completely imbibed into the sample, the dripping process is continued. The sample surface morphology change is observed, the time for the sample to imbibe water is recorded, and NMR tests are performed on the sample after each water drop.
-
(4)
Changes in the T2 spectra over time during water imbibition are plotted.
The second group of experiments is as follows:
-
(1)
Take the dried samples of Table 3, and use a long dropper to drip water on the surfaces of the six samples. All sides can imbibe water uniformly. By this method, different water contents of 5, 10, and 15% are established.
-
(2)
Drip the water on the surface of one sample and record the weight change.
Table 3. Moisture Content Test for the Second Group.
Represents the moisture content of the primitive state.
The third group of experiments is as follows:
-
(1)
The samples of Table 4 are cut, measured, and weighed. Each type of hydrate sample was taken for drying treatment.
-
(2)
Dripping water imbibition experiment is performed on each sample to record the weight change.
-
(3)
Repeat the dripping and drying experiment three times.
Table 4. Dry–Wet Cycle Test for the Third Group.
| label | length (cm) | width (cm) | height (cm) | sectional area (cm2) | drying mass (g) |
|---|---|---|---|---|---|
| A1-2 | 1.691 | 1.055 | 0.724 | 1.224 | 1.776 |
| A3-2 | 1.182 | 0.916 | 1.313 | 1.552 | 1.925 |
| C1-2 | 1.126 | 0.997 | 1.314 | 1.480 | 2.163 |
Figure 3 shows the schematic diagram of the overall experiment procedure.
Figure 3.
Schematic of the overall experiment procedure.
3. Results and Discussions
3.1. Imbibition Characteristics of the Hydrate Sediment
The imbibition capacity of the hydrate can be evaluated by observing the surface changes of the sample, such as fractures initiation and dispersion. The entire imbibition process can be divided into four stages: sediment wetting stage, microfracture initiation stage, fracture network stage, and dispersion stage.1 In the sediment wetting stage, the sample is invaded by water and wetted gradually. With the imbibition development, microfracture initiation appears on the surface of the sample, and the fractures gradually form a fracture network. When the sample reaches the maximum imbibition saturation, it disperses completely. Figure 4 shows the morphological changes of the C1 samples. At 3.3 min, the sample is completely wetted and the color is darkened. At 5.71 min, microcracks begin to grow, indicating that the sample pore size has become larger. When the sample imbibes water at 6.20 min, the fracture network is generated, the volume is expanded, and the pore size is increased further. At 6.58 min, the sample begins to disperse, and it reaches maximum imbibition saturation.
Figure 4.
Sample’s morphological changes during water imbibition.
Figure 5 shows the NMR T2 spectra of the A1 and C1 samples. As the imbibition time increases, the area of the NMR spectra gradually increases. The signal intensity continues to increase, indicating that a large amount of water is imbibed into the pores of the sediment. During this process, the peak gradually slopes to the right, indicating the formation of larger-sized pores and cracks. It should be noted that the right peak does not exist just at the beginning of drip imbibition but starts to appear when the drip imbibition reaches a certain time. Take the C1 sample as an example. The area of the right peak begins to appear and increase rapidly, indicating that the sample has new pores and fractures. In addition, the area of the right peak continues to increase, suggesting that more and more fractures begin to connect together and form a fracture network. It is consistent with the results of the surface morphological changes (Figure 4).
Figure 5.
T2 spectra over soaking time: (a) A1 sample and (b) C1 sample.
According to the Handy model,22 the relationship between the imbibed volume per unit area and time can be given by
where Vimb is the imbibed volume, Ac is the contact area of the sample and water, A is the imbibition rate, and t is the imbibition time.1
Figure 6a,b shows the imbibition curves. The two linear stages of the imbibition curve can represent changes in the pore structure. The first linear phase is related to the imbibition of primary pores. When the imbibition time exceeds the critical imbibition time, a large number of secondary pores begin to appear. The critical imbibition time corresponds to the microfracture initiation time, which is proposed by Yang et al.1 More and more fracture networks are generated, eventually leading to the dispersion of the sediment samples. The second linear phase corresponds to the imbibition of the secondary pores. The primary pore imbibition rate is a1, and the secondary pore imbibition rate is a2. The value of a2 is significantly greater than a1, indicating that the imbibition rate of the secondary pore is significantly accelerated.
Figure 6.
Characteristics of different imbibition curves: (a) curve schematic and (b) imbibition curves of the four samples.
Figure 7 shows the relationship between the primary pore imbibition rate and the secondary pore imbibition rate. The primary pore imbibition rate a1 and the secondary pore imbibition rate a2 have a positive correlation. It is not necessary to use two imbibition rates to evaluate imbibition characteristics. Therefore, the average value A of the imbibition rates a1 and a2 can be taken as the imbibition rate of hydrate sediments. The imbibition capacity can be defined as the imbibition volume per unit sample volume. In the following text, the influencing factors of the imbibition rate and capacity are discussed.
Figure 7.
Relationship between the imbibition rates a1 and a2.
3.2. Influencing Factors
This section discusses the factors that affect the imbibition capacity of the hydrate, including moisture content, dry–wet cycle, clay mineral content, solution salinity, permeability, and porosity.
3.2.1. Effects of Moisture Content
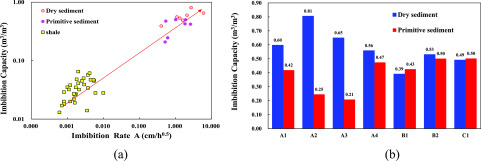
Figure 8a shows the relationship between the imbibition rate and the imbibition capacity of different samples. The primitive sediment samples are A1, A2, A3, A4, B1, B2, and C1 in Table 2. In shale reservoirs, water imbibition can result in a low flowback efficiency of the fracturing fluid. It is similar to the low production of the dissociation water in hydrate sediments. The shale mainly composes of clay minerals and quartz minerals, which resembles the hydrate sediments. Therefore, the imbibition results of the hydrate sediments are compared with the results of shale. From Figure 8b, a positive correlation is observed between the imbibition rate and the imbibition capacity in double logarithmic coordinates. As can be seen from the figure, the imbibition rate of hydrate is about 500 times that of shale, and the imbibition capacity is about 12 times that of shale. Figure 8a,b shows the sample imbibition capacity in different states. It can be seen that the A formation sample (A1, A2, A3, and A4 samples) have a larger imbibition capacity in the dry state than that in the primitive state. In the B and C formations, the imbibition capacity in the dry state is approximately equal to that in the primitive state.
Figure 8.
Effects of moisture content on imbibition characteristics: (a) imbibition rate vs imbibition capacity and (b) imbibition capacity in different states. The experimental results of shale are cited from Ge et al.23 and Yang et al.1
To study the effects of moisture content, the samples A1, A3, and C1 are tested for imbibition under different moisture contents. Figure 9 shows the effects of moisture content on the imbibition rate and capacity. As can be seen from the figure, the imbibition rate and the imbibition capacity of the sample are inversely proportional to the moisture content. With the increase of the moisture content, the imbibition rate and the capacity of samples are decreased.
Figure 9.
Effects of moisture content on imbibition characteristics: (a) imbibition rate and (b) imbibition capacity.
3.2.2. Effects of Dry–Wet Cycle
A certain amount of water is generated during the dissociation process of hydrate sediments. During the flow of the dissociation water, hydrate sediments may be resynthesized under the appropriate temperature and pressure conditions. Therefore, the effect of the dry–wet cycle on the hydrate imbibition capacity needs to be further analyzed and discussed. Figure 10 shows the influence of the dry–wet cycle on samples A1-2, A3-2, and C1-2. The basic information of the samples are presented in Table 4. With the increase in the number of the dry–wet cycles, the imbibed volume of the samples increases significantly, indicating that the imbibition capacity of the samples is improved. On the one hand, this may be due to the fact that the clay minerals imbibe water and expand, resulting in new microcracks. This achieves the additional imbibition capacity beyond the primitive pores. This also explains the generation and existence of primary and secondary pores in the process of sample imbibition. On the other hand, it results from the presence of soluble salt in the inner space of the hydrate. The soluble salt dissolves, leading to sufficient contact between water and clay minerals. It causes the sample to expand and the imbibition capacity to become stronger. In a word, water imbibition into the hydrate pores will cause clay mineral to expand and the formation of microfractures to intensify. The soluble salt inside is easily soluble in water, leading to weakened connections between the particles and the cracks. The two aspects are combined to improve the imbibition capacity.
Figure 10.
Effects of the dry–wet cycle in different samples: (a) A1-2; (b) A3-2; and (c) C1-2.
3.2.3. Effects of Clay Minerals
Figure 11a shows the relationship between the imbibition rate and the total clay mineral content. The results of the clay mineral content are presented in Table 2. It can be seen that the correlation between them is very poor. The small number of samples makes it difficult to reflect the real results. In the future work, it is necessary to conduct more experiments to clarify the effects of clay mineral content on the imbibition rate. Figure 11b shows the relationship between the imbibition capacity and the total clay mineral content. It can be found that the results are well correlated and the imbibition capacity of the samples is proportional to the clay mineral content. More clay minerals correspond to a larger osmotic pressure, which can act as an extra driving force to imbibe water.24
Figure 11.
Effects of the clay mineral content on (a) imbibition rate and (b) imbibition capacity.
Figure 12 shows the effects of I/S concentration on imbibition capacity and rate. It shows a good positive correlation, except for one abnormal point. It suggests that the I/S minerals have significant effects on the imbibition rate and capacity. The I/S minerals have a high specific surface area, which contributes to absorbing large amounts of water. Figure 13 shows the effects of illite content on imbibition capacity and rate. The relationships are very poor. Due to limited data, it may not reflect the true effects of the illite content on the imbibition characteristics.
Figure 12.
Effects of I/S mineral content on (a) imbibition rate and (b) imbibition capacity.
Figure 13.
Effects of illite mineral content on (a) imbibition rate and (b) imbibition capacity.
It is certain that the imbibition capacity of the hydrate sediment samples will increase with the increase in clay mineral content. The I/S mixed layer will lead to the increase of the imbibition capacity and rate. It can be considered that the I/S mixed layer has the most direct effect on the imbibition characteristics of hydrate sediments.
3.2.4. Effects of Solution Salinity
The NaCl solutions have different salinities, such as 5, 10, and 15%. The experiments of the NaCl solution imbibition are carried out and compared with those of distilled water. Figure 14 shows the effects of solution salinity on imbibition characteristics. As can be seen from the figures, the imbibition capacity and the rate of the sample are inversely proportional to the mass fraction of the NaCl solution. With the increase in solution salinity, the imbibition capacity and the rate of the sample decrease. This means that the solution salinity has a significant effect on the imbibition rate and capacity. A high concentration of NaCl solution will inhibit the expansion of the I/S mixed layer and hinder the formation of new pores and fractures. Therefore, it decreases the imbibition rate and capacity of the sediment samples.
Figure 14.
Effects of solution salinity on (a) imbibition rate and (b) imbibition capacity.
3.2.5. Effects of Porosity and Permeability
The shale samples are introduced to compare the imbibition characteristics. The previous results of shale reservoirs can help understand the effects of physical characteristics in hydrate sediments. Figure 15 presents the effects of porosity on imbibition characteristics. The horizontal and vertical coordinates are in logarithmic form. In general, the imbibition rate of shale is between 0.0005 and 0.1 cm/h0.5 and that of hydrate sediment samples is between 0.1 and 10 cm/h0.5. The imbibition capacity of shale is between 0.01 and 0.1 m3/m3, and that of hydrate sediment samples is between 0.3 and 0.9 m3/m3. The general trend is that the imbibition rate and imbibition capacity are approximately proportional to the porosity. However, it presents poor correlations, and the data dispersion degree is very high. Compared with other influencing factors, the porosity may be not the main factor affecting the imbibition rate and the imbibition capacity of the sediment samples.
Figure 15.
Effects of porosity on (a) imbibition rate and (b) imbibition capacity.
Similarly, results from shale samples are compared with those from hydrate sediment samples. Figure 16 shows the relationship between imbibition characteristics and permeability. The horizontal and vertical coordinates are in logarithmic form. The overall trend is positively correlated, but the correlation is poor in the hydrate samples. Due to the limited data, it may not reflect the true effects of permeability on the imbibition characteristics of the sediment samples.24
Figure 16.
Effects of permeability on (a) imbibition rate and (b) imbibition capacity.
4. Conclusions
In this study, the spontaneous imbibition experiments are carried out on the hydrate sediment samples, and the T2 spectra characteristics are analyzed using the NMR. The imbibition characteristics of hydrate sediments and the influencing factors are studied. The following conclusions are obtained.
-
(1)
The imbibition characteristics of hydrate sediments can be characterized by the appearance changes in the sample, NMR T2 spectra, imbibition capacity, and rate. The imbibition curves of the hydrate can be divided into two stages: primary pore imbibition stage and secondary pore imbibition stage. The imbibition rates of the two stages are strongly correlated, so the average value can be used to uniformly characterize the imbibition characteristics.
-
(2)
The imbibition capacity of the hydrate samples will be improved by the dry–wet cycle and the clay minerals. The increase in water content and solution salinity will lead to a decrease in the imbibition capacity in the hydrate sediments.
-
(3)
The imbibition rate and capacity are positively proportional. The imbibition rate of the hydrate sediment is about 500 times that of shale, and the imbibition capacity of the hydrate sediment is about 12 times that of shale.
Acknowledgments
The research was funded by the National Natural Science Foundation of China (No. 11702296), the Natural Science Foundation of China (41941018), the Fundamental Research Funds for the Central Universities (No. 2462019YJRC011), and the China Geological Survey (No. DD20190232).
The authors declare no competing financial interest.
References
- Yang L.; Zhang C.; Cai J.; Lu H. Experimental Investigation of Spontaneous Imbibition of Water into Hydrate Sediments Using Nuclear Magnetic Resonance Method. Energies 2020, 13, 445. 10.3390/en13020445. [DOI] [Google Scholar]
- Cai J.; Perfect E.; Cheng C. L.; Hu X. Y. Generalized modeling of spontaneous imbibition based on Hagen–Poiseuille flow in tortuous capillaries with variably shaped apertures. Langmuir 2014, 30, 5142–5151. 10.1021/la5007204. [DOI] [PubMed] [Google Scholar]
- Meng Q.; Cai J. Recent advances in spontaneous imbibition with different boundary conditions. Capillarity 2018, 1, 19–26. 10.26804/capi.2018.03.01. [DOI] [Google Scholar]
- Li Y.; Liu C.; Liu L.; Sun J.; Liu H.; Meng Q. Experimental study on evolution behaviors of triaxial-shearing parameters for hydrate-bearing intermediate fine sediment. Adv. Geo-Energy Res. 2018, 2, 43–52. 10.26804/ager.2018.01.04. [DOI] [Google Scholar]
- Sun Y.; Lu H.; Lu C.; Li S.; Lv X. Hydrate dissociation induced by gas diffusion from pore water to drilling fluid in a cold wellbore. Adv. Geo-Energy Res. 2018, 2, 410–417. 10.26804/ager.2018.04.06. [DOI] [Google Scholar]
- Cai J.; Xia Y.; Xu S.; Tian H. Advances in multiphase seepage characteristics of natural gas hydrate sediments. Chin. J. Theor. Appl. Mech. 2020, 52, 208–223. [Google Scholar]
- Sun Q.; Du M.; Li X.; Guo X.; Liu A.; Chen G.; Yang L. Study on ethane hydrate formation/dissociation in a sub-millimeter sized capillary. Chem. Eng. Sci. 2019, 206, 1–9. 10.1016/j.ces.2019.05.024. [DOI] [Google Scholar]
- Song B.; Cheng Y.; Yan C.; Lyu Y.; Wei J.; Ding J.; Li Y. Seafloor subsidence response and submarine slope stability evaluation in response to hydrate dissociation. J. Nat. Gas Sci. Eng. 2019, 65, 197–211. 10.1016/j.jngse.2019.02.009. [DOI] [Google Scholar]
- Yang M.; Gao Y.; Zhou H.; Chen B.; Li Y. Gas production from different classes of methane hydrate sediments by the depressurization method. Int. J. Energy Res. 2019, 43, 5493–5505. 10.1002/er.4669. [DOI] [Google Scholar]
- Mahabadi N.; Dai S.; Seol Y.; Jang J. Impact of hydrate saturation on water permeability in hydrate-bearing sediments. J. Pet. Sci. Eng. 2019, 174, 696–703. 10.1016/j.petrol.2018.11.084. [DOI] [Google Scholar]
- Mahabadi N.; Dai S.; Seol Y.; Yun T.; Jang J. The water retention curve and relative permeability for gas production from hydrate-bearing sediments: pore-network model simulation. Geochem., Geophys., Geosyst. 2016, 17, 3099–3110. 10.1002/2016GC006372. [DOI] [Google Scholar]
- Mahabadi N.; Zheng X.; Jang J. The effect of hydrate saturation on water retention curves in hydrate-bearing sediments. Geophys. Res. Lett. 2016, 43, 4279–4287. 10.1002/2016GL068656. [DOI] [Google Scholar]
- Mahabadi N.; Jang J. Relative water and gas permeability for gas production from hydrate-bearing sediments. Geochem., Geophys., Geosyst. 2014, 15, 2346–2353. 10.1002/2014GC005331. [DOI] [Google Scholar]
- Makhanov K.; Habibi A.; Dehghanpour H.; Kuru E. Liquid uptake of gas shales: A workflow to estimate water loss during shut-in periods after fracturing operations. J. Unconv. Oil Gas Resour. 2014, 7, 22–32. 10.1016/j.juogr.2014.04.001. [DOI] [Google Scholar]
- Ghanbari E.; Dehghanpour H. Impact of rock fabric on water imbibition and salt diffusion in gas shales. Int. J. Coal Geol. 2015, 138, 55–67. 10.1016/j.coal.2014.11.003. [DOI] [Google Scholar]
- Zhou Z.; Hoffman T.; Bearinger D.; Li X.; Abass H. Experimental and Numerical Study on Spontaneous Imbibition of Fracturing Fluids in the Horn River Shale Gas Formation. SPE Drill. Completion 2016, 31, 168–177. 10.2118/171600-PA. [DOI] [Google Scholar]
- Zhong Y.; Zhang H.; Kuru E.; Kuang J.; She J. The forced imbibition model for fracturing fluid into gas shales. J. Pet. Sci. Eng. 2019, 179, 684–695. 10.1016/j.petrol.2019.04.076. [DOI] [Google Scholar]
- Dehghanpour H.; Zubair H. A.; Chhabra A.; Ullah A. Liquid intake of organic shales. Energy Fuels 2012, 26, 5750–5758. 10.1021/ef3009794. [DOI] [Google Scholar]
- Lei H.; He L.; Li R.; Hu Z.; He J. Effects of boundary layer and stress sensitivity on the performance of low-velocity and one-phase flow in a shale oil reservoir: Experimental and numerical modeling approaches. J. Pet. Sci. Eng. 2019, 180, 186–196. 10.1016/j.petrol.2019.05.025. [DOI] [Google Scholar]
- Chen M.; Cheng L.; Wang X.; Lyu C.; Cao R. Pore network modeling of fluid flow in tight formations considering boundary layer effect and media deformation. J. Pet. Sci. Eng. 2019, 180, 643–659. 10.1016/j.petrol.2019.05.072. [DOI] [Google Scholar]
- Ehsan M.; Raoof G.; Reza R.; Mohammad R.; Zhong Z.; Mohammad S. A laboratory-based approach to determine Archie’s cementation factor for shale reservoirs. J. Pet. Sci. Eng. 2019, 183, 106399 10.1016/j.petrol.2019.106399. [DOI] [Google Scholar]
- Handy L. L. Determination of effective capillary pressures for porous media from imbibition data. Trans. AIME 1960, 219, 75–80. 10.2118/1361-G. [DOI] [Google Scholar]
- Ge H.; Yang L.; Shen Y.; Ren K.; Meng F.; Ji W.; Wu S. Experimental investigation of shale imbibition capacity and the factors influencing loss of hydraulic fracturing fluids. Pet. Sci. 2015, 12, 636–650. 10.1007/s12182-015-0049-2. [DOI] [Google Scholar]
- Yang L.; Ge H.; Shi X.; Cheng Y.; Zhang K.; Chen H.; Shen Y.; et al. The effect of microstructure and rock mineralogy on water imbibition characteristics in tight reservoirs. J. Nat. Gas Sci. Eng. 2016, 34, 1461–1471. 10.1016/j.jngse.2016.01.002. [DOI] [Google Scholar]