Abstract
Despite its potential to improve metabolic health outcomes, longitudinal physical activity (PA) patterns and their association with cardiometabolic disease among people living with HIV (PLWH) have not been well characterized. We investigated this relationship among PLWH in the Centers for AIDS Research Network of Integrated Clinical Systems with at least one PA self-report between 2008–2015. The 4-item Lipid Research Clinics PA instrument was used to categorize habitual PA levels as: Very Low, Low, Moderate, or High. We analyzed demographic differences in PA patterns. Multivariable generalized estimating equation regression models were fit to assess longitudinal associations of PA with blood pressure, lipid, and glucose levels. Logistic regression modeling was used to assess the odds of being diagnosed with obesity, cardiovascular disease (CVD), cerebrovascular disease, hypertension, diabetes, or multimorbidity. A total of 40,462 unique PA assessments were provided by 11,719 participants. Only 13% of PLWH reported High PA, while 68% reported Very Low/Low PA at baseline and did not increase PA levels during the study period. Compared to those reporting High PA, participants with Very Low PA had almost 2-fold increased risk for CVD. Very Low PA was also associated with several risk factors associated with CVD, most notably elevated triglycerides (odds ratio 25.4), obesity (odds ratio 1.9), hypertension (odds ratio 1.4), and diabetes (odds ratio 2.3; all p<0.01). Low levels of PA over time among PLWH are associated with increased cardiometabolic disease risk.
Keywords: cardiovascular disease, obesity, diabetes, multimorbidity, health outcomes, health disparities
An increasing burden of non-AIDS cardiometabolic diseases is well-documented among people living with HIV (PLWH).1–3 We reported that while most PLWH gain weight during the first six months of antiretroviral therapy use, many continue to gain weight for up to two years after beginning treatment.4 In the current United States epidemic, over 2/3 of PLWH are diagnosed as overweight/obese (25% obese), 40% with hypertension, and 6% cardiovascular disease (CVD).4–6 CVD and other cardiometabolic diseases are associated with increased mortality risk in adults living with and without HIV infection;7, 8 thus these alarming results highlight a need for effective interventions to prevent and treat cardiometabolic disease among PLWH.
One potential intervention is to increase habitual physical activity (PA), which is associated with decreased mortality and improved health outcomes among the general population.9, 10 Unfortunately, PLWH have lower physical fitness levels compared to other vulnerable populations,11 and longitudinal studies conducted in PLWH have primarily focused on specific exercise interventions rather than habitual physical activity.12, 13 Thus it is unknown if the amount of PA needed for meaningful improvement in cardiometabolic disease biomarkers and outcomes is different for PLWH versus those without HIV. Additionally, long-term data regarding PA habits of PLWH are lacking, while cross-sectional studies of PA levels that include smaller sample sizes from single sites reveal a wide disparity in self-reported activity levels.14–16 To develop effective PA strategies, we require a greater understanding of the longitudinal PA patterns reported by PLWH, and the minimal amount of activity needed to reduce cardiometabolic disease risk. Therefore, the objectives of this study were to (1) describe PA patterns among PLWH, and (2) determine which cardiometabolic disease biomarkers/diagnoses are most influenced by PA patterns in this population. To accomplish these objectives, we analyzed PA and cardiometabolic disease data collected over 7.5 years from 11,719 PLWH in the multisite Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort of PLWH in the United States.
METHODS
This retrospective analysis was conducted in the United States from CNICS.17 CNICS is a nationally distributed clinical cohort that includes over 32,000 PLWH receiving routine clinical care. At regular intervals, CNICS sites provide comprehensive data on demographics, laboratory values, pharmaceutical history, HIV/AIDS clinical events, and comorbid conditions collected from electronic medical records and other data sources, including the CNICS clinical assessment of standardized Patient-Reported Outcomes measured at 4–6 month intervals 18, 19 at seven CNICS sites. A rigorous, systematic quality assurance process is in place to maintain this centralized database.
Participants
This study includes all CNICS participants who completed a clinical assessment between January 2008 (first PA measures available) and mid-July 2015. Inclusion criteria were: (1) at least one PA instrument completed, (2) ≥ 19 years of age, and (3) height/weight were available. Written, informed consent for CNICS was obtained from all study participants and documented at each site, and the protocol was approved by the Institutional Review Board (IRB) at each site. Ethical approval for this study was provided by the IRB at the University of Alabama at Birmingham. Procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Study Measures
Physical Activity
Participants completed the 4-question, validated Lipid Research Clinics (LRC) instrument to provide an estimate of self-reported PA.20 The LRC asks about respondents’ perception, compared to peers, of PA amount/intensity at work and outside of work. A 4-point scoring system is used to classify activity levels as (1) high PA (vigorous activity ≥ 3 times weekly), (2) moderate PA (active, but vigorous activity < 3 times weekly), (3) low PA (no vigorous activity but light activity perceived as equivalent to peers), or (4) very low PA (sedentary). The 4-point scoring previously identified physiologically relevant group differences in VO2max: High PA 41.9 ml/kg−1/min−1, Moderate 39.1 ml/kg−1/min−1, Low 33.2 ml/kg−1/min−1, and Very Low 32.9 ml/kg−1/min−1.20
Clinical and Laboratory Parameters
Vital signs and laboratory values closest to the date (± 90 days) of each PA assessment were included. Systolic (SBP) and diastolic (DBP) blood pressure were measured at the beginning of patient care encounters as part of routine clinical care. As participant lab draws occurred throughout the day, both fasting and non-fasting glucose and lipid values (total cholesterol, LDL-c, HDL-c, triglycerides) were included.
Cardiometabolic diseases
We focused on five cardiometabolic outcomes with a prevalence of at least 2% in the CNICS cohort: obesity, CVD, cerebrovascular disease, hypertension, and diabetes – as well as multimorbidity. [1] Obesity. Participants were considered obese with a body mass index (weight [kg] / height [m2]) ≥ 30. [2] CVD. Participants were classified as having CVD if they were diagnosed with a myocardial infarction centrally adjudicated within CNICS. 21 Briefly, two physicians who are experienced myocardial infarction reviewers adjudicate each event based on available EMR data. If there is a discrepancy in the outcome, a third reviewer will also adjudicate the event. Adjudication protocols were not yet developed for other CVD conditions, thus recorded diagnoses (yes/no) in the EMR of chronic ischemic heart disease and congestive heart failure were considered for CVD. [3] Cerebrovascular disease. Similar to other large HIV cohorts, adjudicated stroke data were not available in CNICS. Thus, a participant was considered to have cerebrovascular diseases with a diagnosis in the EMR. [4] Hypertension. Hypertension was based on the CNICS standard definition of a diagnosis of hypertension and the presence of any antihypertensive medication, or the average of at least 2 systolic blood pressure measurements ≥140 mmHg or diastolic blood pressure measurements ≥90 mmHg over 12 months. As we were unable to confirm whether all patients with hypertension or CVD were taking medications as prescribed, we included participants with both pharmaceutically treated and untreated conditions. [5] Diabetes. We defined diabetes based on the following criteria: a) hemoglobin A1c ≥6.5 OR b) use of a diabetes-specific medication such as insulin OR c) use of a diabetes-related medication frequently but not exclusively used to treat diabetes (e.g. biguanides) in the setting of also having a diabetes diagnosis.22
Multimorbidity
Multimorbidity was computed as the presence of two or more of the above 5 cardiometabolic diseases as well as chronic kidney disease and cancer (HIV and non-HIV related). Chronic kidney disease was added to multimorbidity criteria despite <2% population prevalence due to its association with physical activity and risk factors of poorly controlled HTN and obesity.23 Presence of chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 for >3 months (2 values >90 days apart without an intervening normal value), or a charted diagnosis of Stage 2, 3, 4, or 5 kidney disease. The low number of total cancer cases in the CNICS cohort prohibited us from analyzing the association of physical activity with cancer risk. However, cancer diagnoses (classified as dichotomous yes/no) were added to the multimorbidity criteria since physical activity may be associated with cancer risk and metabolic risk factors contributing to development of other cardiometabolic conditions may also increase cancer risk.24, 25
Biomarkers
We further investigated the association of physical activity with available biomarkers of CVD risk, hypertension, and diabetes: Total Cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, and glucose levels.
Independent variables
We examined patient demographic and HIV-related laboratory information including age, sex, self-reported race/ethnicity, CD4+ T-cell count, viral load, HIV transmission risk factor (intravenous drug use, men having sex with men [MSM]), antiretroviral therapy (ART) use (yes/no), insurance status (public, private, uninsured), and mortality (yes/no with confirmed date of death). The “d” drugs didanosine (DDI), stavudine (D4T), and zalcitabine (DDC) may contribute to mitochondrial toxicity and peripheral neuropathy that could impair PA ability;26, 27 thus we included a dichotomous variable (yes/no) representing whether a participant had ever been exposed to d-drugs. Since pharmaceutical therapy may impact the laboratory values evaluated, we documented each prescribed medicine that could affect results, with the list of medicines included for each laboratory value adjudicated by two clinical providers (GB and JW).
Statistical Analyses
Descriptive characteristics were analyzed using chi-square tests, one-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA. Time of first PA assessment was considered the baseline for each participant. We computed prevalence, expressed as unadjusted percentages, for each cardiometabolic condition overall and by PA group. Because race/sex interactions were observed for cardiometabolic disease outcomes, a 6-level race/sex variable was created (black men, black women, white men, white women, other men, other women) for subsequent analyses. The “other” racial category was approximately 40% self-reported Asian/Pacific Islander, 40% unidentified, 11% American Indian, and 9% multiracial.
For each continuous laboratory outcome, generalized estimating equations were fit to estimate the longitudinal effect of PA on laboratory variables over time after controlling for covariates described under “independent variables”. We also controlled for outcome-specific non-ART medications – antihyperlipidemic agents, antihypertensives, oral hypoglycemic agents, insulin - prescribed. For cardiometabolic disease outcomes, multivariable logistic regression was used to compute odds ratio and 95% confidence intervals (CI) of the odds of developing each cardiometabolic condition controlling for the above covariates. Obesity was also a covariate in all models excluding those with multimorbidity and obesity as the dependent variable. For all analyses, site was included as a stratification factor and “High PA” was considered the referent group. With a multilevel modeling approach, adjustment for multiple comparisons was not performed 28. Due to the association of the ART medication zidovudine (azidothymindine) with myopathy we also conducted a sensitivity analysis including “ever used AZT” as a covariate; however, this variable was not associated with PA levels and had no impact on the association between PA and cardiometabolic outcomes. In all models, we further controlled for the following factors described under “independent variables”: age, self-reported ethnicity, CD4+ T-cell count, viral load, HIV transmission risk factor, ART use, insurance status, and mortality. All data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) with a significance level of p<0.05.
RESULTS
Demographics and PA patterns
Over 7 1/2 years, a total of 40,462 unique PA assessments were provided by 11,719 CNICS participants, with 8,157 providing ≥ 2 reports, and 3,029 completing ≥ 5 reports. There were no significant differences in demographic characteristics by those who had completed different numbers of PA assessments; thus descriptive statistics for all 11,719 participants at their initial assessment are presented in Table 1. At time of first PA report, 26% reported being Very Low active, 42% Low active, 19% Moderate active, and 13% High active. Very Low and Low PA participants were younger, less likely to be male, more likely to report black race, and weighed more compared to other PA categories (p < 0.01). Approximately 54% of participants consistently reported Very Low/Low PA and 19% consistently reported High PA at each reporting period; thus the majority (73%) of respondents did not change PA levels during the observation period. There was no increase in Moderate/High PA over time. There was no difference in PA category frequency by year of first PA report, and no difference by PA category in the percentage of participants prescribed ART (87–88%, p=0.50). Of note, individuals identifying as transgender were more likely to report Very Low PA (p = 0.04).
Table 1.
Demographic characteristics and laboratory values (mean ± SD or n (%)) at first physical activity assessment (stratified by physical activity level) for people living with HIV receiving care between January/2008 and July/2015.
| Variable | Total n=11,719 | Very Low n=3058 | Low n=4957 | Moderate n=2177 | High n=1527 | P valuea |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (yrs) | 43.8 ± 10.7 | 44.9 ± 10.2 | 43.7 ± 10.9 | 42.8 ± 10.5 | 43.1 ± 10.8 | < 0.01 |
| Birth Sex | ||||||
| Male | 9772 (83.4) | 2409 (24.7) | 4067 (41.6) | 1956 (20.0) | 1340 (13.7) | < 0.01 |
| Female | 1947 (16.6) | 649 (33.3) | 890 (45.7) | 221 (11.4) | 187 (9.6) | |
| Transgenderb | 0.04 | |||||
| Yes | 96 (1.3) | 37 (38.5) | 32 (33.3) | 18 (18.8) | 9 (9.4) | |
| No | 7104 (98.7) | 1842 (25.9) | 2863 (40.3) | 1445 (20.4) | 954 (13.4) | |
| Race | <0.01 | |||||
| % Black | 3917 (33.4) | 991 (25.3) | 1873 (47.8) | 586 (15.0) | 467 (11.9) | |
| % White | 6735 (57.5) | 1855 (27.5) | 2643 (39.2) | 1338 (19.9) | 899 (13.4) | |
| % Other | 1067 (9.1) | 212 (19.9) | 441 (41.3) | 253 (23.7) | 161 (15.1) | |
| % Hispanic ethnicity | 1597 (13.7) | 358 (11.8) | 666 (13.5) | 350 (16.2) | 223 (14.8) | < 0.01 |
| Body mass index | 26.5 ± 5.5 | 27.0 ± 6.3 | 26.5 ± 5.5 | 26.3 ± 4.8 | 26.0 ± 4.7 | < 0.01 |
| Health insurance | < 0.01 | |||||
| Uninsured | 1167 (9.9) | 279 (23.9) | 477 (40.9) | 240 (20.6) | 171 (14.6) | |
| Public | 6171 (52.7) | 1932 (31.3) | 2552 (41.3) | 1054 (17.1) | 633 (10.3) | |
| Private | 3083 (26.3) | 455 (14.8) | 1416 (45.9) | 643 (20.9) | 569 (18.4) | |
| Unknown | 1298 (11.1) | 392 (30.2) | 512 (39.5) | 240 (18.5) | 154 (11.8) | |
| Transmission Risk Factor | < 0.01 | |||||
| IVDU | 1771 (15.1) | 579 (32.7) | 697 (39.4) | 324 (18.3) | 171 (9.6) | |
| MSM | 6976 (59.5) | 1632 (23.4) | 2956 (42.4) | 1393 (19.9) | 995 (14.3) | |
| Heterosexual | 2615 (22.3) | 725 (27.7) | 1170 (44.7) | 402 (15.4) | 318 (12.2) | |
| Other/Unknown | 357 (3.1) | 122 (34.2) | 134 (37.5) | 58 (16.3) | 43 (12.0) | |
| Study Sitec | <0.01 | |||||
| UCSD | 3345 (28.6) | 908 (27.1) | 1351 (40.4) | 677 (20.3) | 409 (12.2) | |
| UAB | 2827 (24.1) | 728 (25.8) | 1358 (48.0) | 402 (14.2) | 339 (12.0) | |
| UW | 1697 (14.5) | 455 (26.8) | 704 (41.5) | 312 (18.4) | 226 (13.3) | |
| UCSF | 1093 (9.3) | 353 (32.3 0 | 432 (39.5) | 197 (18.0) | 111 (10.2) | |
| Fenway Health | 1028 (8.8) | 194 (18.9) | 359 (34.9) | 285 (27.7) | 190 (18.5) | |
| UNC | 918 (7.8) | 216 (23.5) | 383 (41.7) | 193 (21.0) | 126 (13.8) | |
| John Hopkins | 811 (6.9) | 204 (25.2) | 370 (45.6) | 111 (13.7) | 126 (15.5) | |
| Clinical and Laboratory Parameters | ||||||
| SBP (mmHg) | 125.9 ± 15.4 | 125.2 ± 15.9 | 126.0 ± 15.6 | 126.4 ± 14.9 | 126.3 ± 14.1 | < 0.01 |
| DBP (mmHg) | 78.7 ± 10.3 | 78.9 ± 10.5 | 78.9 ± 10.3 | 78.6 ± 10.2 | 78.2 ± 9.7 | 0.16 |
| Total Cholesterol (mg/dL) | 177.2 ± 42.7 | 176.4 ± 44.3 | 177.8 ± 43.3 | 177.5 ± 41.4 | 176.1 ± 39.2 | 0.60 |
| LDL-c (mg/dL) | 101.9 ± 33.7 | 100.6 ± 34.6 | 102.6 ± 33.4 | 102.4 ± 33.8 | 101.7 ± 32.6 | 0.20 |
| HDL-c (mg/dL) | 43.8 ± 16.4 | 41.7 ± 15.9 | 44.3 ± 16.2 | 44.3 ± 16.2 | 46.1 ± 17.7 | <0.01 |
| Triglycerides (mg/dL) | 176.5 ± 180.5 | 196.2 ± 174.0 | 176.3 ± 211.6 | 164.7 ± 131.2 | 154.0 ± 130.8 | <0.01 |
| Glucose (mg/mL) | 95.1 ± 30.9 | 97.8 ± 37.2 | 94.7 ± 30.2 | 94.2 ± 27.5 | 92.2 ± 22.9 | <0.01 |
| CD4+ T-cell count (cells/μl) | 506.7 ± 294.3 | 488.6 ± 315.7 | 508.7 ± 293.3 | 517.0 ± 275.0 | 521.9 ± 278.2 | < 0.01 |
| Plasma HIV RNA < 200 copies/ml | 7675 (70.5) | 1928 (68.5) | 3242 (70.1) | 1472 (72.6) | 1033 (72.5) | < 0.01 |
| d-drug exposure (ever) | 0.01 | |||||
| Yes | 2533 (21.6) | 724 (28.6) | 1031 (40.7) | 465 (18.4) | 313 (12.3) | |
| No | 9186 (78.4) | 2334 (25.4) | 3926 (42.7) | 1712 (18.7) | 1214 (13.2) | |
| Death during observation period | < 0.01 | |||||
| Yes | 510 (4.4) | 216 (42.4) | 204 (40.0) | 55 (10.8) | 35 (6.8) | |
| No | 11209 (95.6) | 2842 (25.4) | 4753 (42.4) | 2122 (18.9) | 1492 (13.3) | |
One-way ANOVA for continuous measures and chi-square for categorical measures
n(%) only for sites (UCSD, UAB, Fenway Health) collecting data on transgender identify up to 2015 (n=7,200)
Study Sites: UCSD = University of California, San Diego; UAB = University of Alabama at Birmingham; UW = University of Washington, Seattle; UCSF = University of San Francisco;UNC = University of North Carolina at Chapel Hill
Other = American Indian, Asian, Asian/Pacific Islander, Pacific Islander, Multiracial, Missing; IVDU = Intravenous drug use; MSM = Men having sex with men; d-drug = Didanosine, Stavudine, Zalcitabine
SBP = systolic blood pressure; DBP = diastolic blood pressure; LDL-c = low-density lipoprotein cholesterol; HDL-c = high-density lipoprotein cholesterol
A slightly higher percentage of Very Low PA participants (23.7%) had been exposed to d-drugs compared to other groups (range 20.5–21.4%). Baseline CD4+ T-cell counts were significantly increased across PA categories (p < 0.01). Greater mortality was also observed in the Very Low PA group compared to all other groups.
Clinical and Laboratory Parameters
Laboratory values at time of first PA report are shown in Table 1. No significant differences were identified by PA group for DBP, total cholesterol, or LDL-c. Very Low PA participants had slightly lower SBP (125.2 mmHg) compared to other groups (range 126.0–126.4 mmHg). Those in the Very Low PA group presented with significantly lower HDL-c levels, higher triglycerides, and higher glucose compared to all other groups (all at p < 0.01).
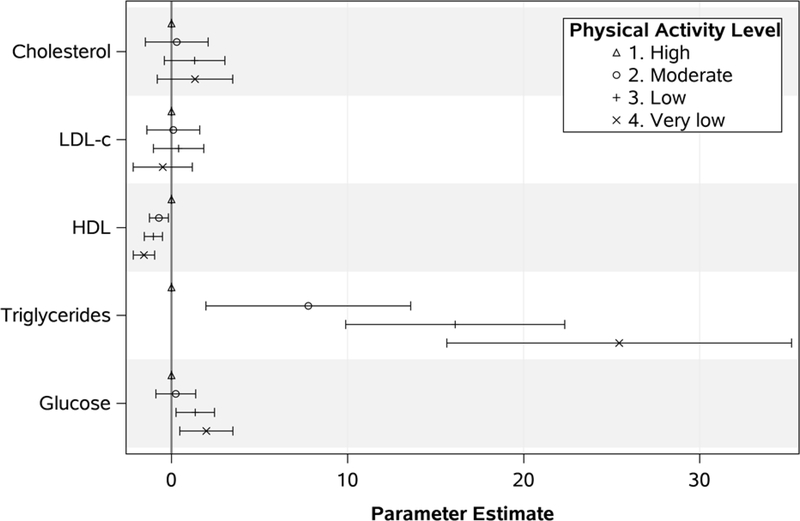
These differences persisted in longitudinal adjusted analyses (Figure 1; Table 2). When compared to the High PA category, a significant stepwise decrease in HDL-c was observed with Moderate (Estimate −0.71; 95% CI −1.25, −0.17; p = 0.009), Low (Estimate −1.03; 95% CI −1.54, −0.51; p < 0.001) and Very Low PA (Estimate −1.56; 95% CI −2.17, −0.96; p < 0.001). An increase in triglyceride levels was also observed for each PA group: Moderate (Estimate 7.78; 95% CI 1.96, 13.59; p = 0.009), Low (Estimate 16.12; 95% CI 9.90, 22.34; p < 0.001), and Very Low (Estimate 25.43; 95% CI 15.64, 35.22; p < 0.001). When glucose was analyzed, the Low PA (Estimate 1.35; 95% CI 0.26, 2.45; p = 0.02) and Very Low PA (Estimate 1.98; 95% CI 0.47, 3.49; p = 0.01) groups had higher levels over time compared to the High PA group. Full models results are shown in Supplementary Table 1.
Figure 1.
Associations between physical activity levels and laboratory values over time among people living with HIV. Values are generalized estimating equationsparameter estimates and 95% confidence intervals. Each model was adjusted for site, first physical activity assessment, race/sex, Hispanic ethnicity, age group, insurance status, HIV risk factor, d-drug use, viral load group, CD4 group, and lab specific non-antiretroviral therapy medications prescribed
Table 2.
Demographic and clinical characteristics associated with cardiometabolic outcomes and longitudinal changes in cardiometabolic disease biomarkers among people living with HIV in adjusted analyses. For diagnosed outcomes, each column shows adjusted odds ratios for separate multivariable logistic regression. For laboratory biomarkers, each column shows parameter estimates and 95% confidence limits for separate generalized estimating equations. All models also adjusted for site as a stratification factor.
| Biomarkers | SBPa(mmHg) | DBP (mmHg) | Total Cholesterol (mg/dl) | LDL-c (mg/dl) | HDL-c (mg/dl) | Triglycerides (mg/dl) | Glucose (mg/dl) |
| PA Levelb | |||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 0.21 (−0.22, 0.64) | 0.41 (0.11, 0.71)‡ | 0.30 (−1.48, 2.08) | 0.10 (−1.40, 1.61) | −0.71 (−1.25, −0.17)‡ | 7.78 (1.96, 13.59)‡ | 0.25 (−0.89, 1.38) |
| Low | −0.20 (−0.62, 0.21) | 0.40 (0.13, 0.68)‡ | 1.31 (−0.41, 3.03) | 0.41 (−1.03, 1.84) | −1.03 (−1.54, −0.51)† | 16.12 (9.90, 22.34)† | 1.35 (0.26, 2.45)* |
| Very Low | −0.64 (−1.11, −0.16)‡ | 0.32 (−0.01, 0.63) | 1.34 (−0.81, 3.48) | −0.49 (−2.17, 1.18) | −1.56 (−2.17, −0.96)† | 25.43 (15.64, 35.22)† | 1.98 (0.47, 3.49)* |
| Diagnosis | Obesity (n=9,930; 3,506 events) | Hypertension (n=9,525; 3,828 events) | Cerebrovascular Disease (n=9,525; 339 events) | Cardiovascular Disease (n=9,525; 560 events) | Diabetes (n=9,525; 976 events) | Multimorbidity (n=9,933; 3,252 events) | |
| PA Levelb | |||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference | |
| Moderate | 1.34 (1.13–1.58) † | 1.16 (0.98–1.38) | 1.42 (0.87–2.31) | 1.09 (0.74–1.60) | 1.19 (0.87–1.62) | 1.32 (1.10–1.58) ‡ | |
| Low | 1.48 (1.28–1.71) † | 1.28 (1.10–1.49) ‡ | 1.38 (0.89–2.13) | 1.48 (1.07–2.06) * | 1.49 (1.14–1.94) ‡ | 1.42 (1.21–1.66) † | |
| Very Low | 1.92 (1.64–2.24) † | 1.43 (1.21–1.68) † | 1.76 (1.13–2.74) * | 1.92 (1.37–2.70) † | 2.32 (1.76–3.05) † | 2.12 (1.80–2.50) † | |
= p < 0.05
= p < 0.01
= p < 0.001
SBP = systolic blood pressure; DBP = diastolic blood pressure; LDL-c = low-density lipoprotein cholesterol; HDL-c = high-density lipoprotein cholesterol; Multimorbidity is defined as two or more diagnoses of obesity, CVD, cerebrovascular disease, hypertension, diabetes, chronic kidney disease, or cancer
PA = physical activity
Cardiometabolic Disease Diagnosis
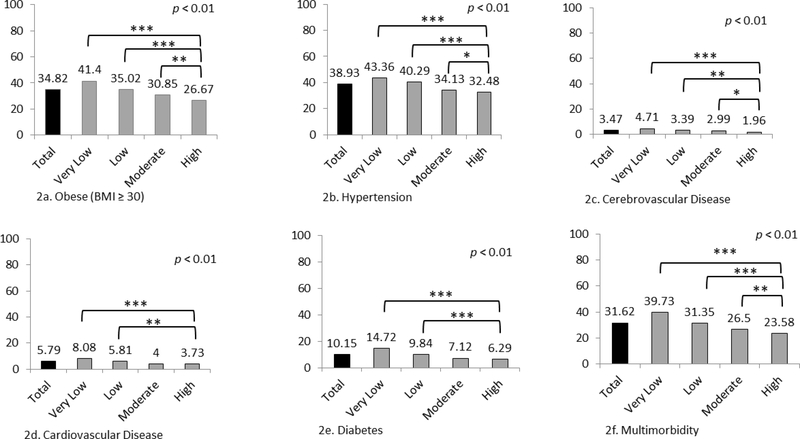
We observed significant differences in the prevalence of five cardiometabolic diseases and multimorbidity by PA group (Figure 2). Over 1/3 (35%) of all participants were obese, but obesity prevalence was significantly different between groups: High PA (27%); Moderate (31%), Low (35%,) and Very Low (41%). Similar differences were observed with hypertension, CVD, and cerebrovascular diagnoses (Figure 2). Finally, while 24% of the High PA group had cardiometabolic multimorbidity, greater prevalence was observed in other PA groups: Moderate (27%, p = 0.003), Low (31%, p < 0.001) and Very Low (40%, p < 0.001).
Figure 2.
Prevalence (%) of cardiometabolic disease and multimorbidity (two or more diagnoses of obesity, CVD, cerebrovascular disease, hypertension, diabetes, chronic kidney disease, or cancer) by physical activity category for 11,719 people living with HIV between 1/2008 and 12/2015
* = p<0.05; ** = p<0.01; *** = p<0.001
In adjusted models using High PA as the reference group (Table 2; Supplementary Table 2), only the Very Low PA group differed in prevalence of cerebrovascular disease (OR 1.76; 95% CI 1.13–2.74). Both Low and Very Low PA were associated with greater risk for hypertension, CVD, and diabetes, while a stepwise increase in risk of obesity and multimorbidity was observed across all PA categories. Participants with Very Low PA experienced 1.5–2 times greater risk of obesity, hypertension, CVD, and multimorbidity. In particular, the Very Low PA group had a 2.3 times greater risk of being diagnosed with diabetes.
DISCUSSION
This investigation revealed that low PA levels are associated with increased risk for CVD and other related chronic diseases (i.e., obesity, hypertenstion, cerebrovascular disease, diabetes, and multimorbidity). Despite nation-wide efforts to promote PA, the majority (68%) of PLWH reported Very Low/Low PA at baseline (compared to 49.8% of the general population), and did not increase PA during the study period.29 Unfortunately, only 13% of PLWH reported High PA, versus 31.2% of the general population.29 Additionally, we found that participants in study sites in the Northeast (Fenway Health and Johns Hopkins) reported slightly higher levels of High PA. Overall, these results highlight the need to develop effective PA promotion strategies for PLWH.
We identified a consistent association of Very Low/Low PA with both cardiometabolic disease biomarkers and outcomes. The only differences in cardiometabolic disease risk for Moderate versus High PA were for obesity and multimorbidity, suggesting that even moderate PA may be sufficient to reduce risk for hypertension, cerebrovascular disease, CVD, and diabetes. In particular, reporting Very Low PA (versus High PA) was associated with higher glucose levels and almost 2.5 times increased diabetes risk.
A recent meta-analysis in HIV-negative adults observed reductions in type 2 diabetes risk of 26% with Moderate PA and 53% with High PA.30 Yarasheski et al. also reported that exercise augments the effects of pharmaceutical treatment in PLWH with diabetes.31 However, due to HIV-related chronic inflammation, pharmaceutical and lifestyle interventions may be less effective in PLWH versus HIV–negative groups.14, 32 Monroe and colleagues14 reported that while low PA was associated with greater insulin resistance (e.g., higher HOMA-IR) regardless of HIV status, men with HIV still had higher HOMA-IR levels at equivalent levels of PA. Collectively, these results highlight uncertainty in exactly how much PA is required to achieve an equivalent benefit in diabetes prevention/treatment for this population.
PA level was associated with both hypertension and cerebrovascular disease. This association may have been attenuated by pharmaceutical interventions for blood pressure control. However, the tendency we observed to see lower blood pressure levels in those reporting Very Low PA could be confounded by a greater prevalence of uncontrolled viremia in this group, which was associated with lower odds of hypertension in our study (Supplementary Table 2) and others.33 The impact of PA frequency or type on HTN risk among PLWH who have not achieved virologic suppression remains to be fully investigated.
Our analysis also associated increased risk of CVD events and atherogenic lipid profile with Low/Very Low PA. An inverse association between PA levels and CVD risk factors/outcomes has been reported in PLWH and HIV-negative populations.34, 35 PA is also associated with higher HDL-c and lower triglycerides in HIV-negative individuals, possibly due to augmentation of lipoprotein lipase activity in skeletal muscle and adipose tissue. However, consistent with our results, two meta-analyses in HIV-negative individuals reported minimal declines in LDL-c and total cholesterol levels of 2,900 men and 1,715 women.36, 37 Future investigations are required to determine to what extent PA can reduce the risk of CVD, and the amount and domains of PA required to meaningfully impact PLWH who have a higher-than-average risk for CVD.
Participants reporting Very Low PA tended to weigh more, have greater mortality, more exposure to d-drugs, and lower CD4+ T-cell counts than other groups. We previously reported that women with HIV have elevated body fat levels regardless of body weight, thus increasing PA may be particularly beneficial for PLWH.38 Regarding the association with CD4+ T-cell count, small transient declines in CD4+ counts are observed with both periods of rest and strenuous activity in PLWH and -negative groups, possibly due to increased autophagy with hypoxia.39, 40 However PA interventions are associated with increased CD4+ count in PLWH in some, but not all, studies.41, 42 This study supports the potential of exercise to complement ART-related immunologic reconstitution as an area that requires additional exploration.
With regard to sex, race, and ethnicity, females had lower PA levels compared to males, and black participants more frequently reported Very Low/Low PA compared to other groups. Previous reports have identified similar patterns of sex or racial health disparities in PA levels in the general population and PLWH,16, 43 confirming the need for programs to reduce sex and race disparities in PA. Interestingly, we also detected differences in PA for PLWH who identify as transgender among the 7,200 participants reporting on transgender status. Within groups, only 9% of transgender participants identified as High PA compared to 13% of other participants, and 38% of transgender participants reported Very Low PA compared to 26% of others. Few investigations have explored unique aspects of health behaviors and risks among transgender individuals, particularly those with HIV. Fredriksen-Goldsen et al.44 reported lower PA levels in transgender adults over age 50 compared to lesbian, gay, and bisexual older adults, while Bryant and colleagues found that compared to other members of the LGBT community, transgender women in particular were at risk for low PA and poor diet.45 The milieu of hormonal and body composition changes, combined with unique barriers to healthy dietary and PA habits, could exacerbate cardiometabolic disease risk in this population. Unfortunately, our small sample limits the general applicability of these findings for transgender PLWH. Additional research is needed to identify barriers, facilitators, and metabolic factors that contribute to disparities in PA for the transgender community.
Our findings are subject to certain limitations. Self-reported PA is less accurate than objectively measured PA and can underestimate Very Low/Low PA while overestimating High PA.46 However, this would possibly mean that Very Low/Low PA is present in more than the 68% of PLWH identified here, and further serves to emphasize the need for PA promotion in this population. Additionally, as our PA data collection only began in 2008, and were unable to determine a reliable date of diagnosis for all cardiometabolic diseases, we are unable to consider the impact of PA habits across the life course and throughout HIV infection, which is an area of significant interest in disease prevention.
Despite these limitations, the present study benefited from several strengths, including a large, diverse sample size inclusive of several geographic regions across the United States, and repeated PA measures assessed over 7 1/2 years of observation. The study included both biomarkers and outcomes of cardiometabolic disease and provides strong evidence that PA can have a meaningful impact on cardiometabolic disease in PLWH. These findings inform clinical practice by highlighting subgroups of PLWH that may benefit from additional PA promotion as well as risk factors that are more likely to be impacted when PA levels change. Additional scientific exploration that determines the appropriate domains and dose-response of PA for specific cardiometabolic disease, as well as strategies to most effectively implement PA programs among PLWH, holds great potential to improve outcomes and reduce health disparities in cardiometabolic disease risk among individuals living and aging with HIV.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Nursing Research #R01 NR018391, the National Institute of Allergy and Infectious Disease #P30-AI27767, the CNICS Research Network #R24-AI067039, and the Mary Fisher CARE Fund. A.L.W. received support from the UAB Center for Outcomes and Effectiveness Research and Education #TL1TR001418, UAB Nutrition Obesity Research Center [National Institute of Diabetes and Digestive and Kidney Diseases #P30DK056336], and the UAB Center for Exercise Medicine. The views expressed are those of the authors, and funding agencies played no role in the design or interpretation of the research.
Abbreviations
- ANOVA
analysis of variance
- BF
black female
- BM
black male
- BMI
body mass index
- CVD
cardiovascular disease
- CFAR
Centers for AIDS Research
- CNICS
CFAR Network of Integrated Clinical Systems
- DBP
diastolic blood pressure
- EMR
electronic medical record
- HDL-c
high-density lipoprotein cholesterol
- HIV
human immunodeficiency virus
- IRB
Institutional Review Board
- IV
intravenous
- LDL-c
low-density lipoprotein cholesterol
- MSM
men who have sex with men
- OF
other female
- OM
other male
- PA
physical activity
- PLWH
people living with HIV
- RNA
ribonucleic acid
- SBP
systolic blood pressure
- WF
white female
- WM
white male
REFERENCES
- 1.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of acquired immune deficiency syndromes. 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 3.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. The Journal of the Association of Nurses in AIDS Care : JANAC. 2011;22:17–25. [DOI] [PubMed] [Google Scholar]
- 4.Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antiviral therapy. 2012;17:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willig AL, Westfall AO, Overton ET, et al. Obesity is associated with race/sex disparities in diabetes and hypertension prevalence, but not cardiovascular disease, among HIV-infected adults. AIDS research and human retroviruses. 2015;31:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ open diabetes research & care. 2017;5:e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, Tang X, Shen P, et al. Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open. 2019;9:e024476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA internal medicine. 2015;175:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654. [DOI] [PubMed] [Google Scholar]
- 11.Vancampfort D, Mugisha J, Rosenbaum S, et al. Cardiorespiratory fitness levels and moderators in people with HIV: A systematic review and meta-analysis. Preventive medicine. 2016;93:106–114. [DOI] [PubMed] [Google Scholar]
- 12.Cade WT, Reeds DN, Overton ET, et al. Pilot study of pioglitazone and exercise training effects on basal myocardial substrate metabolism and left ventricular function in HIV-positive individuals with metabolic complications. HIV clinical trials. 2013;14:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia A, Fraga GA, Vieira RC, Jr., et al. Effects of combined exercise training on immunological, physical and biochemical parameters in individuals with HIV/AIDS. Journal of sports sciences. 2014;32:785–792. [DOI] [PubMed] [Google Scholar]
- 14.Monroe AK, Brown TT, Cox C, et al. Physical Activity and Its Association with Insulin Resistance in Multicenter AIDS Cohort Study Men. AIDS research and human retroviruses. 2015;31:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehm KE, Konkle-Parker D. Physical activity levels and perceived benefits and barriers to physical activity in HIV-infected women living in the deep south of the United States. AIDS care. 2016;28:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webel AR, Barkley J, Longenecker CT, Mittelsteadt A, Gripshover B, Salata RA. A cross-sectional description of age and gender differences in exercise patterns in adults living with HIV. The Journal of the Association of Nurses in AIDS Care : JANAC. 2015;26:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International journal of epidemiology. 2008;37:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Current HIV research. 2007;5:109–118. [DOI] [PubMed] [Google Scholar]
- 19.Fredericksen R, Crane PK, Tufano J, et al. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. Journal of AIDS and HIV research. 2012;4:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Jacobs DR, Jr., Leon AS. Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Medicine and science in sports and exercise. 1993;25:92–98. [DOI] [PubMed] [Google Scholar]
- 21.Crane HM, Heckbert SR, Drozd DR, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. American journal of epidemiology. 2014;179:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane HM, Kadane JB, Crane PK, Kitahata MM . Diabetes case identification methods applied to electronic medical record systems: their use in HIV-infected patients. Current HIV research. 2006;4:97–106. [DOI] [PubMed] [Google Scholar]
- 23.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003;14:479–487. [DOI] [PubMed] [Google Scholar]
- 24.Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Medicine and science in sports and exercise. 2001;33:S530–550; discussion S609–510. [DOI] [PubMed] [Google Scholar]
- 25.Vincent L, Leedy D, Masri SC, Cheng RK. Cardiovascular Disease and Cancer: Is There Increasing Overlap? Curr Oncol Rep. 2019;21:47. [DOI] [PubMed] [Google Scholar]
- 26.Maggiolo F, Roat E, Pinti M, et al. Mitochondrial changes during D-drug-containing once-daily therapy in HIV-positive treatment-naive patients. Antiviral therapy. 2010;15:51–59. [DOI] [PubMed] [Google Scholar]
- 27.Dalakas MC. Peripheral neuropathy and antiretroviral drugs. Journal of the peripheral nervous system : JPNS. 2001;6:14–20. [DOI] [PubMed] [Google Scholar]
- 28.Gelman A, Hill J, Yahiman M. Why we (usually) don’t have to worry about multiple comparisons. J Research on Educational Effectiveness. 2012;5:189–211. [Google Scholar]
- 29.Nutrition Physical Activity, and Data Obesity, Trends and Maps web site. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity and Obesity, ed. Vol 2016 Atlanta, GA: 2015. [Google Scholar]
- 30.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarasheski KE, Cade WT, Overton ET, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. American journal of physiology. Endocrinology and metabolism. 2011;300:E243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han JH, Crane HM, Bellamy SL, et al. HIV infection and glycemic response to newly initiated diabetic medical therapy. Aids. 2012;26:2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11:530–540. [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Yarnell JW, Sweetnam PM, Murray L, Caerphilly s. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hand GA, Lyerly GW, Jaggers JR, Dudgeon WD. Impact of Aerobic and Resistance Exercise on the Health of HIV-Infected Persons. American journal of lifestyle medicine. 2009;3:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: a meta-analysis of randomized controlled trials. The journal of men’s health & gender : the official journal of the International Society for Men’s Health & Gender. 2006;3:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. Journal of women’s health. 2004;13:1148–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willig AL, Kramer PA, Chacko BK, Darley-Usmar VM, Heath SL, Overton ET. Monocyte bioenergetic function is associated with body composition in virologically suppressed HIV-infected women. Redox Biol. 2017;12:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell PJ, Aurelius S, Blowes G, Harvey D. Decrease in CD4 lymphocyte counts with rest; implications for the monitoring of HIV infection. International journal of STD & AIDS. 1997;8:423–426. [DOI] [PubMed] [Google Scholar]
- 40.Weng TP, Huang SC, Chuang YF, Wang JS. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PloS one. 2013;8:e80248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanetti HR, Cruz LG, Lourenco CL, Neves Fde F, Silva-Vergara ML, Mendes EL. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: A randomized controlled trial. European journal of sport science. 2016;16:1232–1239. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC infectious diseases. 2016;16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saffer H, Dave D, Grossman M, Leung LA. Racial, Ethnic, and Gender Differences in Physical Activity. Journal of human capital. 2013;7:378–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredriksen-Goldsen KI, Cook-Daniels L, Kim HJ, et al. Physical and mental health of transgender older adults: an at-risk and underserved population. The Gerontologist. 2014;54:488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smalley KB, Warren JC, Barefoot KN. Differences in health risk behaviors across understudied LGBT subgroups. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2016;35:103–114. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad MH, Salleh R, Mohamad Nor NS, et al. Comparison between self-reported physical activity (IPAQ-SF) and pedometer among overweight and obese women in the MyBFF@home study. BMC Womens Health. 2018;18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.