Abstract

Bioactive compounds for drug discovery are increasingly extracted and purified from natural sources including marine organisms. Heparin is a therapeutic agent that has been used for several decades as an anticoagulant. However, heparin is known to cause many undesirable complications such as thrombocytopenia and risk of hemorrhage. Hence, there is a need to find alternatives to current widely used anticoagulant drugs. Here, we extract a sulfated polysaccharide from sea hare, that is, Bursatella leachii viscera, by enzymatic digestion. Several analytical approaches including elemental analysis, Fourier-transform infrared spectroscopy, nuclear magnetic resonance, and high-performance liquid chromatography–mass spectrometry analysis show that B. leachii polysaccharides have chemical structures similar to glycosaminoglycans. We explore the anticoagulant activity of the B. leachii extract using the activated partial thromboplastin time and the thrombin time. Our results demonstrate that the extracted sulfated polysaccharide has heparin-like anticoagulant activity, thus showing great promise as an alternative anticoagulant therapy.
1. Introduction
Sulfated polysaccharides are polyanionic macromolecules that exist throughout nature. The structures and bioactivities of these polysaccharides are influenced by their origin and the extraction techniques. Their capacity to interact with cells and physiological proteins provides them a wide variety of bioactivities, such as anticoagulant, antithrombotic, anti-inflammatory, antioxidant, immunostimulatory, and antitumor properties. Thus, they potentially have many useful therapeutic and pharmaceutical applications.1−10
Marine organisms are valuable, yet underexploited sources of sulfated polysaccharides with novel chemical structures and activities. These marine polysaccharides are much safer biomolecules than their terrestrial mammals counterparts,11 which may be linked to their potential to cause infection.12 Many studies have extracted sulfated polysaccharides with high anticoagulant activities from marine organisms. These studies aimed at finding an alternative anticoagulant to heparin, which is a sulfated polysaccharide of the family of glycosaminoglycans (GAGs).13 Alternatives to heparin are desirable for several reasons. For example, certain religious groups are reluctant to use heparin as it is acquired from pig intestines and bovine lungs. Moreover, heparin has been linked to some fatal diseases. Recently, Liu et al. reported that thrombocytopenia-induced heparin represents a high mortality risk in critical COVID-19 patients treated with heparin.14 These disadvantages have encouraged researchers to find safer and more effective alternatives.3,15,16
Marine natural polysaccharides with potent anticoagulant activities have been previously isolated from algae,17,18 fishes,12,19,20 and marine invertebrates including sea cucumbers21,22 and clams.3 However, the extraction of sulfated polysaccharides from sea hares has thus far received little attention from scientists and researchers working in the area of marine natural products.23 The sea hare Bursatella leachii is a marine opisthobranch gastropod mollusk belonging to the Aplysiidae family.24,25 It emerged as an invasive species in the Mediterranean Sea in the mid-1900s.24,26,27
Compounds with an anti-HIV activity and antimalarial properties have been isolated from the purple fluid28 and the internal organs of B. leachii, respectively.29 Braga et al. was interested in the nutritional profile of B. leachii and suggested many potential biotechnological applications in different economic fields.24 To date, limited research has focused on bioactive molecules from B. leachii. In the current study, we extract for the first time a sulfated polysaccharide from B. leachii viscera. We carry out a physicochemical characterization using several techniques including elemental analysis, Fourier-transform infrared spectroscopy (FTIR), solid-state nuclear magnetic resonance (NMR), and high-performance liquid chromatography (HPLC) coupled with mass spectrometry (HLPC–MS). Finally, we study the anticoagulant activity in vitro using classical coagulation assays which show a heparin-like activity and thus could be used for medical application after.
2. Results and Discussion
2.1. Extraction of Polysaccharide from B. leachii
We used papain hydrolysis to extract polysaccharides from marine organisms, which is a safe and cheap technique that neither pollutes the environment nor produces organic residues.30 We extracted the polysaccharide fraction from the viscera of the sea hare B. leachii by enzymatic digestion. We designated the crude polysaccharide that we acquired after dialysis and lyophilization as B. leachii viscera polysaccharides (BLVP). The yield of the polysaccharide fraction extracted from B. leachii viscera was 3%, which was higher than that obtained from fish viscera of Nile tilapia (0.18%) and pacu (0.15%).20 In a previous study, Song et al. yielded crude polysaccharide from scallop viscera by papain digestion of approximately 1%.31 The yield of the current extraction is lower than those obtained from sea cucumber viscera (4.9%)30 and viscera of squid (4.2%).32
2.2. Elemental Analysis
The elemental analysis of the sea hare polysaccharide gave the following composition: 23.45% carbon, 4.59% hydrogen, 46.95% oxygen, 8.21% sulfur, and 2.26% nitrogen (Table 2). The molar ratio of C, H, N, and S (acquired for the polysaccharide extracted from sea hare) was similar to the composition and element ratio of GAGs.33 A comparable molar ratio of C, H, and O was obtained for polysaccharides extracted from seaweed.34 Liu et al. extracted a novel polysaccharide from Angelica sinensis with mass fractions of C, H, O, S, and N of 39.11, 5.65, 54.23, 0.42, and 0.59%, respectively (Liu et al. 2019).96 The presence of nitrogen and sulfur indicates the presence of hexosamine and sulfate groups.35,36 The percentage of sulfur indicates a high amount of sulfate groups and a high degree of substitution (DS) (0.788) in the polysaccharide extracted.
Table 2. Chemical Composition of BLVP.
| yield (%) | uronic acid (%) | sulfate (%) | protein (%) | ||
|---|---|---|---|---|---|
| BLVP | 3.1 | 21.83 ± 6.46 | 28.42 ± 7.93a | 26.45 ± 0.64b | 0.058 ± 0.063 |
Sulfate content determined by turbidimetry.
Sulfate content calculated from sulfur percent according to the formula: sulfate group = 3.22 × S %.39
2.3. Colorimetric Assays
The cellulose-acetate electropherogram revealed a single band migrating near the chondroitin sulfate (CS) standard (Figure 1). The polysaccharides extracted contain a high amount of sulfate (28.42%), which was similar to the amount determined by sulfur analysis (26.45%; Table 1), a high amount of uronic acid (21.83%; Table 2) and a low amount of protein 0.058%.
Figure 1.
Acetate cellulose electrophoresis of the polysaccharide extract (BLVP). Standard GAGs (hyaluronic acid, dermatan sulfate, and CS) (track 1) and BLVP (track 2). The arrow indicates the electrophoretic direction.
Table 1. Elemental Analysis of Polysaccharide Extract.
| N (%) | C (%) | H (%) | S (%) | O (%) | DSa | |
|---|---|---|---|---|---|---|
| BLPV | 2.264035 | 23.45542 | 4.597269 | 8.215622 | 46.9532 | 0.788087 |
2.4. Molecular Weight Determination by Gel Permeation Chromatography–Refractive Index Detector
We estimated the molecular weights of the crude polysaccharide extract BLVP using gel permeation chromatography–refractive index detector (GPC–RID), using Dextran standard for the calibration curve. The results revealed that BLVP consisted of two fractions with high specific average molecular weights of 639,256 and 17,387 g/mol, thus showing that the crude polysaccharide extracted was heterogeneous. These polysaccharides have higher molecular weights than those extracted from other marine invertebrates.
2.5. FT-IR Analysis
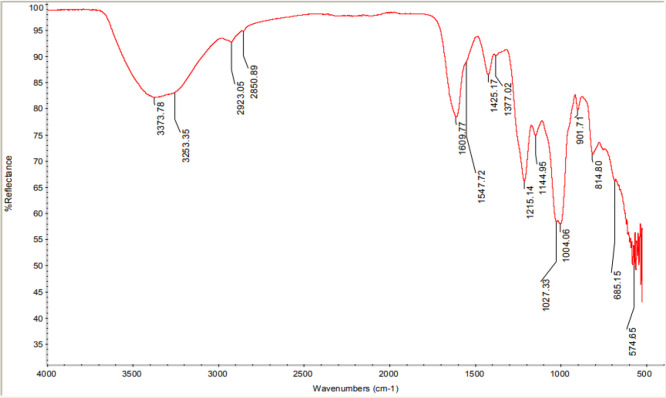
As shown in Figure 2, we assessed BLVP between 4000 and 600 cm–1 to identify the presence of specific functional groups. The absorption bands obtained in the spectrum are typical for extracted sulfated polysaccharides.12,40 The presence of hydroxyl groups and C–H of a sugar ring was evidenced by the absorption bands at 3373 and 2923 cm–1, respectively.34 The signals at 1004, 1027, and 1144 cm–1 confirmed the presence of acidic cycles, which correlated with the vibrations of C–O–C, C–OH, and C–C, respectively.41 The spectrum showed an intense peak at 1609 cm–1, which we attributed to the C=O of uronic acids.30,31 The C–N stretching and the N–H vibration absorptions were evidenced by the signal at 1410 and 1547 cm–1, respectively, indicating the presence of N-acetyl-d-galactosamine.30,42 This absorbance band confirmed the existence of sulfates in the polysaccharide extracted at 1215, 814, and 574 cm–1.30,41−47 The absorption at 1215 cm–1 was assigned to the asymmetric stretching of S=O, and the bands at 814 cm–1 were linked to the C–O–S symmetric stretching of the axial sulfate on C-4, which may represent the occurrence of 4-O-sulfated GalNAc. Absorbance at 574 cm–1 was attributed to the stretching vibration of S–O.30,40 The results obtained confirmed that the polysaccharide extracted was substituted by sulfate esters.30,43 Thus, we determined that BLVP contains a specific characteristic band and has a chemical structure similar to the structural features of GAGs,42 suggesting that BLVP is a GAG-like sulfated polysaccharide.
Figure 2.
FT-IR spectrum of the sulfated polysaccharide extracted from B. leachii viscera.
2.6. Solid-State NMR
NMR is a versatile analytical tool that has been used extensively for molecular identification and for determining the chemical composition of natural products.48−53 NMR is also a quantitative tool that can elucidate the structural features and probe the molecular dynamics54−59 of polysaccharides60,61 and sulfated polysaccharides62−66 because these samples can be analyzed in a solid, dehydrated form.34 This technique uses high-resolution 13C solid-state NMR by magic-angle-spinning (MAS) and the intensity of solid 13C signals using the cross-polarization (CP) technology.30,67,68
As shown in Figure 3 and Table 3, the 13C NMR spectra reveal signals between 90 and 105 ppm that have several peaks characteristic of anomeric carbons.63,69 The signal at 101.87 ppm was assigned to C1 of N acetyl galactosamine and could be assigned to GlcA-GalNAc4S and/or GlcA-GalNAc6S.42 The signals in the region 60–80 ppm were linked to chemical shifts of carbon in the glycosylic ring.70 The signal at 57.29 ppm was attributed to C-2 of N acetyl hexosamine and the presence of the amino group.71 The signal at 175.18 ppm was assigned to the carboxyl group present in glucuronic acids,63 and the signal at 22.97 ppm was characteristic of methyl carbon of the N-acetyl group.30 Signals at 30.53 ppm are because of impurities, persisting proteins, and/or lipids.60 The 13C NMR spectra obtained were similar to those obtained for natural CS, where all signals were found between 50 and 110 ppm, excluding those of carbonyl and acetamido methyl carbons.72−74
Figure 3.

13C-MAS NMR spectra of sulfated polysaccharide-extracted BLVP.
Table 3. 13C Chemical Shifts of Sulfated Polysaccharide-Extracted BLVP.
| 13C chemical shifts (ppm) | assignments | references |
|---|---|---|
| 175.18 | carboxyl group of acid glucuronic | (63,75,76) |
| 101.87 | C1 of N acetyl hexosamine | (42) |
| 98.09 | anomeric carbon | (63,69,77) |
| 69.45–75.93 | osidic CH2–O and CH–O groups | (63,70) |
| 53.23–57.29 | C2 of N acetyl hexosamine | (69,71) |
| 30.53 | impurities | (60) |
| 22.97 | CH3 | (30,78) |
2.7. Direct Analysis by ESI-MS, MALDI-MS, and HPLC–MS
In general, it is necessary to perform hydrolysis of the entire polysaccharide extract to identify the monosaccharide type of molecules. Figures 4 and 5 revealed the presence of identical small-molecular ions such as at m/z 180.5, 243.1, and 349.1. Moreover, m/z 180.5 detected in the matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS) experiment can be assigned to hexosamine isomers without the acetyl group (C6H14O5N). Hence, a repeating unit of 162 Da (C6H10O5) is also observed which reveal the presence of glucose derivatives. Hence, HPLC coupled to a high-resolution mass spectrometer was achieved to identify and extract accurately masses of present monosaccharide ions. Following the hydrolysis of BLVP with TFA, we carried out HPLC–MS analysis. Data treatment was performed using Xcalibur software. HPLC–MS analysis of hydrolyzed BLVP revealed the presence of sodiated O-linked glycan derivatives separated by a unit at 162 amu. m/z 244.07880 (C8H15O6NNa), m/z 203.05250 (C6H12O6Na), m/z 365.10528 (C12H22O11Na), m/z 527.15790 (C18H32O16Na), m/z 851.26595 (C30H52O26Na), and m/z 1013.31942 (C36H62O31Na) (Table 4). Moreover, Figure 6 revealed the presence of sodiated N-acetylhexosamine (C8H15O6NNa) at m/z 244. 07880 (RT: 2.5 min), which could as well indicate the presence of N-acetylgalactosamine. The analytes containing sulfate groups were not seen specifically because its labile O-sulfo groups could fragment during the ionization.79 Interestingly, the presence of the disaccharide of glucuronic acid and N-acetylhexosamine are indicators of GAGs80−82 (Table 4). Compared to the result of electrophoresis that showed that BLVP migrate as far as CS standard, we suggest that the extracted polysaccharide is a GAG-like molecule that contains CS.
Figure 4.

Direct infusion full-scan MS of the hydrolyzed polysaccharide extract using ESI+ and DP 50 V.
Figure 5.
MALDI MS (Ve+) of the hydrolyzed polysaccharide extract.
Table 4. Glucose Derivatives Found during HPLC–MS Analysis.
| m/z | chemical formula | name | error (ppm) |
|---|---|---|---|
| 202.0684 | C6H13O5NNa | hexosamine isomers | 0.93 |
| 203.0525 | C6H12O5Na | glucose | 0.5 |
| 244.0788 | C8H15O6NNa | N-acetylhexosamine | 1.5 |
| 365.1053 | C12H22O11Na | glucose dimer | 0.4 |
| 420.1106 | C14H23O12NNa | acid glucuronic and N-acetylhexosamine | 3.1 |
| 527.1579 | C18H32O16Na | glucose trimer | 0.7 |
| 689.2105 | C24H42O21Na | glucose tetramer | 0.9 |
| 851.266 | C30H52O26Na | glucose pentamer | 2.4 |
| 1013.319 | C36H62O31Na | glucose hexamer | 2.6 |
Figure 6.
(a) Extracted MS chromatogram of sodiated N-acetylhexosamine (RT: 2.56 min) detected (b) at m/z 244.07880 (C8H15O6NNa).
2.8. Anticoagulant Activity
We studied the anticoagulant activity of the extracted BLVP in vitro using the activated partial thromboplastin time (aPTT) and thrombin time (TT) techniques. All measurements were carried out in triplicate, and results were expressed as the mean standard deviation and analyzed using the ANOVA test. As shown in Figure 7, the sulfated polysaccharide-extracted BLVP dose-dependently prolonged the clotting times in various coagulation tests. The prolongation of the aPTT (Figure 7A) and TT (Figure 7B) indicates that BLVP inhibits intrinsic and common coagulation pathways. Interestingly, aPTT and TT were considerably prolonged by the B. leachii polysaccharide at concentrations at or higher than 5 μg/mL, and the maximum prolongation of coagulation times was reached with a concentration of 50 μg/mL for aPTT and 10 μg/mL for TT. Moreover, BLVP prolonged aPTT and TT more effectively than the sodium heparin standard. TT prolonged twice as long in the presence of 5 μg/mL of B. leachii polysaccharide, and aPTT prolonged 4.5-fold with the addition of 25 μg/mL of BLVP. We found that the anticoagulant activity of the B. leachii polysaccharide was higher than other sulfated polysaccharides of marine origin.12,31,83 For instance, Song et al. extracted anticoagulant polysaccharides from Patinopecten yessoensis viscera, and these polysaccharides extended aPTT and TT threefold with the addition of 200 and 1000 μg/mL, respectively. This in vitro anticoagulant may depend on the high sulfate group content15,84−86 and high molecular weights16,87,88 and suggests an antithrombotic activity in vivo.
Figure 7.
Anticoagulant activity analysis of sulfated polysaccharide-extracted BLVP. (A) aPTT and (B) TT. aPTT and TT for control were 33.3 ± 1.8 and 15.4 ± 1.2 s, respectively, and sodium heparin was used as the standard (**p < 0.01).
To the best of our knowledge, this research presents the first report for the isolation of an anticoagulant compound from the sea hare B. leachii. We propose that this extract potentially useful as a drug candidate could be utilized in anticoagulant treatment. However, further investigation should be carried out to study the mechanism of anticoagulant activity and the study of their effects on platelet activation and aggregation.
3. Conclusions
In this study, we extracted an active and potent anticoagulant compound, BLVP, from B. leachii viscera. Several analytical approaches including elemental analysis, FTIR, NMR, and HPLC–MS analysis showed that the extract (BLVP) has a similar chemical structure to that of GAG. The result showed that the BLVP extract possess inhibiting properties for both intrinsic and common coagulation pathways, even more effectively than the sodium heparin standard. This enhanced anticoagulant activity is related to the high degree of sulfation and the high molecular weight of BLVP. These findings will initiate further study of the use of BLVP in medical applications. Further investigations should be carried out to study the mechanism of the anticoagulant activity of the new extracted sulfated polysaccharide.
4. Experimental Section
4.1. Raw Materials and Polysaccharide Extraction
B. leachii was collected from the Mediterranean Sea (Tunisian coast) and rinsed many times with water to eliminate contaminants. The viscera of B. leachii were washed, dried, and ground to powder. The viscera was then defatted with organic solvents and dried to obtain the alcohol-insoluble substances. The polysaccharide was isolated, as described in ref (12). Dried powder (1 g) was dissolved in 25 mL of 0.1 mol/L sodium acetate buffer (pH 6), containing 5.0 mmol/L ethylenediaminetetraacetic acid and 5.0 mmol/L cysteine. The proteolysis was carried out using 100 mg of papain for 24 h at 60 °C. The polyanionic polysaccharides, liberated and recuperated by centrifugation, were precipitated with 0.4% (w/v) of cationic detergent cetyltrimethylammonium bromide, centrifuged, and finally dissolved in NaCl solution prepared in ethanol (100:15, v/v). The polysaccharide was precipitated with ethanol and then recuperated by centrifugation for 15 min at 5000 rpm at 4 °C. The crude polysaccharide, BLVP, was obtained after pellet dissolution, dialysis with deionized water, and lyophilization.
4.2. Cellulose Acetate Electrophoresis
Cellulose acetate electrophoresis was used to evaluate the purity of the BLVP. The BLVP (2 μL) solution or standard GAGs were placed at the origin of the cellulose acetate membrane. The separation was performed at 200 V at room temperature in zinc-acetate buffer 0.1 M, pH 6. The electropherogram was stained by alcian blue after 1 h of migration.89
4.3. Elemental Analysis
The organic elemental analyzer OEA Flash 2000 by Thermo Scientific was used to estimate the molar ratio of carbon, hydrogen, nitrogen, and sulfur in BLVP. The DS and the sulfate content were calculated using the following37−39
4.4. Chemical Composition Analysis
Following the procedure outlined by Dodgson and Price,90 the amount of sulfate was assessed by turbidimetry. The amount of uronic acid was determined by the carbazole reaction.91 The protein content was calculated by the Bradford test using bovine serum albumin as the standard.92
4.5. Molecular Weight Determination by GPC–RID
The polysaccharide-extracted BLVP molecular weight was assessed by GPC with an Agilent 1200 series system and a RID. Two columns connected in series Agilent PL aqua gel-OH 60 and 30 (8 μm) were used for the separation. Milli-Q water was used as the mobile phase at 1 mL/min. The RID was calibrated with Dextran standards solution (MW (g/mol): 1,400,000, 150,000, 250,000, 10,000, and 1000). The polysaccharide extract BLVP (3 mg ) was solubilized in 1 mL water, and then, 100 μL was injected for analysis.93
4.6. FT-IR Analysis
Nicolet FTIR iS10 spectrometer (Thermo Fisher Scientific) was used to measure infrared vibrations of the studied compounds in the solid form. OMNIC Specta software was used for data treatment. The BLVP solid extract was mixed with KBr, pressed, and then analyzed with the FT-IR spectrometer with scanning between 400 and 4000 cm–1.
4.7. NMR Spectroscopy
The solid-state 13C NMR spectrum was recorded using the WB Bruker 400 AVANCE III spectrometer equipped with a 4 mm double-resonance CP MAS Bruker Probe (Bruker BioSpin, Rheinstetten, Germany). The spectrum was recorded at room temperature using a cp pulse program from Bruker pulse library with a recycling delay time of 5 s.67,78,94 Bruker TopSpin 3.5pl7 software was used to collect and process spectra.
4.8. Hydrolysis of Polysaccharide
BLVP (20 mg) was dissolved with 2 mL TFA (4 mol/L). The mixture was sonicated in a bath at 60 °C for 24 h and then centrifuged at 6000 rpm for 10 min after ambient temperature cooling. The dried sample obtained after supernatant evaporation was used for HPLC MS analysis.95
4.9. High-Performance Liquid Chromatography Mass Spectrometry
The separation of the extracted polysaccharide was performed using an Epic Polar Industries column, 5 μm, 4.6 × 150 mm. A gradient composed of water/acetonitrile was used to achieve the separation. The composition of the mobile phase solvents was as following: A: 99.9% water + 0.1% formic acid and B: 100% acetonitrile. The injection volume was 10 μL for each analyte (water blank; caffeine was used as quality control), and the flow rate was adjusted to 300 μL/min. The method development and data treatment was achieved using Xcalibur software (Thermo Scientific).
Thermo LTQ Velos Orbitrap mass spectrometer (Thermo Scientific, Pittsburgh, PA, USA) fitted with a heated electrospray ion source (ESI) was carried out to analyze the BLVP. The mass scan range was set to 100–2000 m/z, with a resolving capacity of 100 000. The m/z calibration of the LTQ-Orbitrap analyzer was carried out in the positive ESI mode using a solution containing caffeine, MRFA (met-arg-phe-ala) peptide, and Ultramark 1621 according to the manufacturer’s guidelines. A heated ion source, fitted with a metal needle and maintained at 4.5 kV, was used to perform the ESI. The source vaporizer temperature was regulated to 120 °C, the declustering potential was set to 50 V, the capillary temperature was set at 275 °C, and the sheath and auxiliary gases (N2) were optimized and set to 8 arbitrary units, respectively.
4.10. Matrix-Assisted Laser Desorption Time-Of-Flight Mass Spectrometry (MALDI-TOF MS)
The matrix was prepared by dissolving 5 mg of 2,5-dihydroxybenzoic acid (Matrix) in 1 mL of 50% (v/v) methanol solution. The hydrolyzed sample and the native polysaccharide BLVP were separately mixed with the Matrix and deposited on the MALDI plate and then dried before analysis.
After air drying, the sample/matrix mixtures were analyzed by the MALDI-TOF-MS Bruker Daltonics Autoflex, equipped with a 337 nm nitrogen laser (Bruker, Germany), in positive and negative ion modes, scanning from 0 to 10,000 m/z.
4.11. Anticoagulant Activity
The anticoagulant activities of extracted polysaccharide BLVP were measured at concentrations ranging from 1 to 100 μg/mL by the classical coagulation assays APTT and TT. Sodium heparin (25,000 UI/5 mL) was used as a reference, and analysis was performed on the STAR analyzer. The platelet-poor plasma PPP was collected by centrifugation at 1200g for 12 min of the platelet-rich plasma, which was recuperated after centrifugation for 15 min at 120 g of blood from ten healthy human volunteers collected on sodium citrate.
aPTT was performed as follows: 50 μL of PPP in the absence or the presence of the sample was mixed with 50 μL of the APTT assay reagent, and after 3 min of incubation at 37 °C, 50 μL of CaCl2 0.025 M was added and the time of coagulation was measured. For TT, 50 μL of PPP with or without the sample was prewarmed for 2 min at 37 °C and the clotting time was recorded after the addition of 100 μL of thrombin.
Acknowledgments
This work was supported by Taibah university and King Abdullah University of Science and Technology (under fund number BAS/1/1085-01-01) to M.J.
The authors declare no competing financial interest.
References
- Sanjeewa K. K. A.; Kang N.; Ahn G.; Jee Y.; Kim Y.-T.; Jeon Y.-J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocolloids 2018, 81, 200–208. 10.1016/j.foodhyd.2018.02.040. [DOI] [Google Scholar]
- Yang W.; Chen D.; He Z.; Zhou L.; Cai Y.; Mao H.; Gao N.; Zuo Z.; Yin R.; Zhao J. NMR characterization and anticoagulant activity of the oligosaccharides from the fucosylated glycosaminoglycan isolated from Holothuria coluber. Carbohydr. Polym. 2020, 233, 115844. 10.1016/j.carbpol.2020.115844. [DOI] [PubMed] [Google Scholar]
- Du Z.; Jia X.; Chen J.; Zhou S.; Chen J.; Liu X.; Cao X.; Zhong S.; Hong P. Isolation and Characterization of a Heparin-Like Compound with Potent Anticoagulant and Fibrinolytic Activity from the Clam Coelomactra antiquata. Mar. Drugs 2019, 18, 6. 10.3390/md18010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M.; Wu J.; Wang S.; Shu H.; Zhang M.; Liu K.; Liu K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. 10.1016/j.ijbiomac.2019.02.043. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Liu Z.; Song S.; Zhu B.; Zhao L.; Jiang J.; Liu N.; Wang J.; Chen X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. 10.1016/j.ijbiomac.2019.09.216. [DOI] [PubMed] [Google Scholar]
- Alencar P. O. C.; Lima G. C.; Barros F. C. N.; Costa L. E. C.; Ribeiro C. V. P. E.; Sousa W. M.; Sombra V. G.; Abreu C. M. W. S.; Abreu E. S.; Pontes E. O. B.; Oliveira A. C.; de Paula R. C. M.; Freitas A. L. P. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocolloids 2019, 90, 28–34. 10.1016/j.foodhyd.2018.12.007. [DOI] [Google Scholar]
- Chen Y.-y.; Xue Y.-t. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. 10.1016/j.carbpol.2018.10.093. [DOI] [PubMed] [Google Scholar]
- Jin W.; Wu W.; Tang H.; Wei B.; Wang H.; Sun J.; Zhang W.; Zhong W. Structure analysis and anti-tumor and anti-angiogenic activities of sulfated galactofucan extracted from Sargassum thunbergii. Mar. Drugs 2019, 17, 52. 10.3390/md17010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Song Q.; Huang L.; Shen M.; Yu Q.; Chen Y.; Xie J. Immunomodulatory activities of sulfated Cyclocarya paliurus polysaccharides with different degrees of substitution on mouse spleen lymphocytes. J. Funct. Foods 2020, 64, 103706. 10.1016/j.jff.2019.103706. [DOI] [Google Scholar]
- Alavi M.; Tabarsa M.; You S.; Gavlighi H. A. Structural characteristics, molecular properties and immunostimulatory effects of sulfated polysaccharide from freshwater Myriophyllum spicatum L. Int. J. Biol. Macromol. 2020, 153, 951–961. 10.1016/j.ijbiomac.2019.11.109. [DOI] [PubMed] [Google Scholar]
- Senni K.; Pereira J.; Gueniche F.; Delbarre-Ladrat C.; Sinquin C.; Ratiskol J.; Godeau G.; Fischer A.-M.; Helley D.; Colliec-Jouault S. Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. 10.3390/md9091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahri M.; Mansour M. B.; Bertholon I.; Ollivier V.; Boughattas N. A.; Hassine M.; Jandrot-Perrus M.; Chaubet F.; Maaroufi R. M. Anticoagulant activity of a dermatan sulfate from the skin of the shark Scyliorhinus canicula. Blood Coagulation Fibrinolysis 2010, 21, 547–557. 10.1097/mbc.0b013e32833b643b. [DOI] [PubMed] [Google Scholar]
- Brito A. S.; Arimatéia D. S.; Souza L. R.; Lima M. A.; Santos V. O.; Medeiros V. P.; Ferreira P. A.; Silva R. A.; Ferreira C. V.; Justo G. Z.; Leite E. L.; Andrade G. P. V.; Oliveira F. W.; Nader H. B.; Chavante S. F. Anti-inflammatory properties of a heparin-like glycosaminoglycan with reduced anti-coagulant activity isolated from a marine shrimp. Bioorg. Med. Chem. 2008, 16, 9588–9595. 10.1016/j.bmc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhang X.; Xiao Y.; Gao T.; Wang G.; Wang Z.; Zhang Z.; Hu Y.; Dong Q.; Zhao S.; Yu L.; Zhang S.; Li H.; Li K.; Chen W.; Bian X.; Mao Q.; Cao C. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. medRxiv 2020, 1–19. 10.1101/2020.04.23.20076851. [DOI] [Google Scholar]
- Tang L.; Chen Y.; Jiang Z.; Zhong S.; Chen W.; Zheng F.; Shi G. Purification, partial characterization and bioactivity of sulfated polysaccharides from Grateloupia livida. Int. J. Biol. Macromol. 2017, 94, 642–652. 10.1016/j.ijbiomac.2016.10.067. [DOI] [PubMed] [Google Scholar]
- Oliveira R. C. R.; Almeida R. R.; Gonçalves T. A. A review of plant sulfated polysaccharides and their relations with anticoagulant activities. J. Dev. Drugs 2016, 5, 166. 10.4172/2329-6631.1000166. [DOI] [Google Scholar]
- Reis S. E.; Andrade R. G. C.; Accardo C. M.; Maia L. F.; Oliveira L. F. C.; Nader H. B.; Aguiar J. A. K.; Medeiros V. P. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Res. 2020, 45, 101750. 10.1016/j.algal.2019.101750. [DOI] [Google Scholar]
- Bittkau K. S.; Neupane S.; Alban S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. 10.1016/j.algal.2019.101759. [DOI] [Google Scholar]
- Bougatef H.; Krichen F.; Capitani F.; Amor I. B.; Gargouri J.; Maccari F.; Mantovani V.; Galeotti F.; Volpi N.; Bougatef A.; Sila A. Purification, compositional analysis, and anticoagulant capacity of chondroitin sulfate/dermatan sulfate from bone of corb (Sciaena umbra). Int. J. Biol. Macromol. 2019, 134, 405–412. 10.1016/j.ijbiomac.2019.05.036. [DOI] [PubMed] [Google Scholar]
- Nogueira A. V.; Rossi G. R.; Iacomini M.; Sassaki G. L.; Trindade E. S.; Cipriani T. R. Viscera of fishes as raw material for extraction of glycosaminoglycans of pharmacological interest. Int. J. Biol. Macromol. 2019, 121, 239–248. 10.1016/j.ijbiomac.2018.09.156. [DOI] [PubMed] [Google Scholar]
- Yan L.; Wang D.; Zhu M.; Yu Y.; Zhang F.; Ye X.; Linhardt R. J.; Chen S. Highly purified fucosylated chondroitin sulfate oligomers with selective intrinsic factor Xase complex inhibition. Carbohydr. Polym. 2019, 222, 115025. 10.1016/j.carbpol.2019.115025. [DOI] [PubMed] [Google Scholar]
- Chahed L.; Balti R.; Elhiss S.; Bouchemal N.; Ajzenberg N.; Ollivier V.; Chaubet F.; Maaroufi R. M.; Mansour M. B. Anticoagulant activity of fucosylated chondroitin sulfate isolated from Cucumaria syracusana. Process Biochem. 2020, 91, 149–157. 10.1016/j.procbio.2019.12.006. [DOI] [Google Scholar]
- Kamiya H.; Sakai R.; Jimbo M.. Bioactive molecules from sea hares. In Molluscs; Cimino G., Gavagnin M., Eds.; Springer: Berlin, 2006; pp 215–239. [DOI] [PubMed] [Google Scholar]
- Braga T.; Rodrigues M. J.; Pereira H.; Varela J.; Barreira L.; González-Wangüemert M.; Custódio L. Bursatella leachii from Mar Menor as a Source of Bioactive Molecules: Preliminary Evaluation of the Nutritional Profile, In Vitro Biological Activities, and Fatty Acids Contents. J. Aquat. Food Prod. Technol. 2017, 26, 1337–1350. 10.1080/10498850.2017.1392670. [DOI] [Google Scholar]
- Sethi S.; Kokane M. R.; Otta S. K.; Sethi G. First record of ragged sea hare Bursatella leachii Blainville, 1817 (Opisthobranchia: Euopisthobranchia: Aplysiidae) in Pulicat Lake, east coast of India. Mar. Biodiversity Rec. 2015, 8, e34 10.1017/s1755267215000147. [DOI] [Google Scholar]
- Travaglini A.; Crocetta F. Natural history collections and alien species: An overlooked sample of Bursatella leachii Blainville, 1817 (Mollusca: Gastropoda: Aplysiida) backdates its confirmed presence in Italy. Thalassas 2019, 35, 137–141. 10.1007/s41208-018-0101-2. [DOI] [Google Scholar]
- Chebaane S.; Sempere-Valverde J.; Dorai S.; Kacem A.; Sghaier Y. R. A Preliminary inventory of alien and cryptogenic species in Monastir Bay, Tunisia: spatial distribution, introduction trends and pathways. Mediterr. Mar. Sci. 2019, 20, 616–626. 10.12681/mms.20229. [DOI] [Google Scholar]
- Rajaganapathi J.; Kathiresan K.; Singh T. P. Purification of anti-HIV protein from purple fluid of the sea hare Bursatella leachii de Blainville. Mar. Biotechnol. 2002, 4, 447–453. 10.1007/s10126-002-0012-2. [DOI] [PubMed] [Google Scholar]
- Suntornchashwej S.; Suwanborirux K.; Koga K.; Isobe M. Malyngamide X: The first (7R)-lyngbic acid that connects to a new tripeptide backbone from the Thai sea hare Bursatella leachii. Chem.—Asian J. 2007, 2, 114–122. 10.1002/asia.200600219. [DOI] [PubMed] [Google Scholar]
- Yang D.; Lin F.; Huang Y.; Ye J.; Xiao M. Separation, purification, structural analysis and immune-enhancing activity of sulfated polysaccharide isolated from sea cucumber viscera. Int. J. Biol. Macromol. 2020, 155, 1003–1018. 10.1016/j.ijbiomac.2019.11.064. [DOI] [PubMed] [Google Scholar]
- Song S.; Wang L.; Wang L.; Yu Q.; Ai C.; Fu Y.; Yan C.; Wen C.; Zhu Z. Structural characterization and anticoagulant activity of two polysaccharides from Patinopecten yessoensis viscera. Int. J. Biol. Macromol. 2019, 136, 579–585. 10.1016/j.ijbiomac.2019.06.116. [DOI] [PubMed] [Google Scholar]
- Ye P.; Li P.; Yang W.; Zhao Y.; Zhao Y.; Sun K.; Wang B.; Chen Y. Structure and Neuroprotective Effect of Polysaccharide from Viscera Autolysates of Squid Ommastrephes bartrami. Mar. Drugs 2019, 17, 188. 10.3390/md17030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. E.; Akhtar N.; Botchway S.; Pennock C. A. Interaction of 1,9-Dimethylmethylene Blue with Glycosaminoglycans. Ann. Clin. Biochem. 1994, 31, 147–152. 10.1177/000456329403100206. [DOI] [PubMed] [Google Scholar]
- Jia R.-B.; Wu J.; Li Z.-R.; Ou Z.-R.; Lin L.; Sun B.; Zhao M. Structural characterization of polysaccharides from three seaweed species and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 1040–1049. 10.1016/j.ijbiomac.2019.11.068. [DOI] [PubMed] [Google Scholar]
- Liu W.; Li W.; Sui Y.; Li X.-Q.; Liu C.; Jing H.; Zhang H.; Cao W. Structure characterization and anti-leukemia activity of a novel polysaccharide from Angelica sinensis (Oliv.) Diels. Int. J. Biol. Macromol. 2019, 121, 161–172. 10.1016/j.ijbiomac.2018.09.213. [DOI] [PubMed] [Google Scholar]
- Na Y. S.; Kim W. J.; Kim S. M.; Park J. K.; Lee S. M.; Kim S. O.; Synytsya A.; Park Y. I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. 10.1016/j.intimp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Seedevi P.; Sudharsan S.; Kumar S. V.; Srinivasan A.; Vairamani S.; Shanmugan A. Isolation and characterization of sulphated polysaccharides from Codium tomentosum (J. Stackhouse, 1797) collected from southeast coast of India. Adv. Appl. Sci. Res. 2013, 4, 72–77. [Google Scholar]
- de Moura Neto É.; Maciel J. d. S.; Cunha P. L. R.; dePaula R. C. M.; Feitosa J. P. A. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J. Braz. Chem. Soc. 2011, 22, 1953–1960. 10.1590/s0103-50532011001000017. [DOI] [Google Scholar]
- Cardozo F. T. G. S.; Camelini C. M.; Cordeiro M. N. S.; Mascarello A.; Malagoli B. G.; Larsen I. v.; Rossi M. J.; Nunes R. J.; Braga F. C.; Brandt C. R.; Simões C. M. O. Characterization and cytotoxic activity of sulfated derivatives of polysaccharides from Agaricus brasiliensis. Int. J. Biol. Macromol. 2013, 57, 265–272. 10.1016/j.ijbiomac.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger O.; Kervarec N.; Ratiskol J.; Colliec-Jouault S.; Chevolot L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus. Carbohydr. Res. 2004, 339, 2371–2380. 10.1016/j.carres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Mansour M. B.; Balti R.; Ollivier V.; Jannet H. B.; Chaubet F.; Maaroufi R. M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym. 2017, 174, 760–771. 10.1016/j.carbpol.2017.06.128. [DOI] [PubMed] [Google Scholar]
- Mansour M. B.; Balti R.; Yacoubi L.; Ollivier V.; Chaubet F.; Maaroufi R. M. Primary structure and anticoagulant activity of fucoidan from the sea cucumber Holothuria polii. Int. J. Biol. Macromol. 2019, 121, 1145–1153. 10.1016/j.ijbiomac.2018.10.129. [DOI] [PubMed] [Google Scholar]
- Bai M.; Han W.; Zhao X.; Wang Q.; Gao Y.; Deng S. Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity. Mar. Drugs 2018, 16, 170. 10.3390/md16050170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.; Cai Y.; Yin R.; Lin L.; Li Z.; Wu M.; Zhao J. Structural analysis and anticoagulant activities of two sulfated polysaccharides from the sea cucumber Holothuria coluber. Int. J. Biol. Macromol. 2018, 115, 1055–1062. 10.1016/j.ijbiomac.2018.04.175. [DOI] [PubMed] [Google Scholar]
- Khan B. M.; Qiu H.-M.; Wang X.-F.; Liu Z.-Y.; Zhang J.-Y.; Guo Y.-J.; Chen W.-Z.; Liu Y.; Cheong K.-L. Physicochemical characterization of Gracilaria chouae sulfated polysaccharides and their antioxidant potential. Int. J. Biol. Macromol. 2019, 134, 255–261. 10.1016/j.ijbiomac.2019.05.055. [DOI] [PubMed] [Google Scholar]
- Myron P.; Siddiquee S.; Al Azad S. Partial structural studies of fucosylated chondroitin sulfate (FuCS) using attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and chemometrics. Vib. Spectrosc. 2017, 89, 26–36. 10.1016/j.vibspec.2016.12.008. [DOI] [Google Scholar]
- Matsuhiro B.; Osorio-Román I. O.; Torres R. Vibrational spectroscopy characterization and anticoagulant activity of a sulfated polysaccharide from sea cucumber Athyonidium chilensis. Carbohydr. Polym. 2012, 88, 959–965. 10.1016/j.carbpol.2012.01.052. [DOI] [Google Scholar]
- Bian C.; Wang Z.; Shi J. Extraction Optimization, Structural Characterization, and Anticoagulant Activity of Acidic Polysaccharides from Auricularia auricula-judae. Molecules 2020, 25, 710. 10.3390/molecules25030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil G.; Thomas L.; Emwas A.-H.; Lens P. N.; Saikaly P. E. NMR and MALDI-TOF MS based characterization of exopolysaccharides in anaerobic microbial aggregates from full-scale reactors. Sci. Rep. 2015, 5, 14316. 10.1038/srep14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Winters H.; Jeong S.; Emwas A.-H.; Vigneswaran S.; Amy G. L. Marine bacterial transparent exopolymer particles (TEP) and TEP precursors: Characterization and RO fouling potential. Desalination 2016, 379, 68–74. 10.1016/j.desal.2015.10.005. [DOI] [Google Scholar]
- Breton R. C.; Reynolds W. F. Using NMR to identify and characterize natural products. Nat. Prod. Rep. 2013, 30, 501–524. 10.1039/c2np20104f. [DOI] [PubMed] [Google Scholar]
- Nageeb A.; Al-Tawashi A.; Emwas A.-H.; Al-Talla Z.; Al-Rifai N. Comparison of artemisia annua bioactivities between traditional medicine and chemical extracts. Curr. Bioact. Compd. 2014, 9, 324–332. 10.2174/157340720904140404151439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D.; Kremb S.; Sioud S.; Emwas A.-H.; Voolstra C. R.; Ravasi T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS One 2017, 12, e0177316 10.1371/journal.pone.0177316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Z. U.; Jeong S.; Tabatabai S. A. A.; Emwas A.-H.; Leiknes T. Advanced characterization of dissolved organic matter released by bloom-forming marine algae. Desalin. Water Treat. 2017, 69, 1–11. 10.5004/dwt.2017.0444. [DOI] [Google Scholar]
- Emwas A.-H.; Roy R.; McKay R. T.; Ryan D.; Brennan L.; Tenori L.; Luchinat C.; Gao X.; Zeri A. C.; Gowda G. A. N.; Raftery D.; Steinbeck C.; Salek R. M.; Wishart D. S. Recommendations and standardization of biomarker quantification using NMR-based metabolomics with particular focus on urinary analysis. J. Proteome Res. 2016, 15, 360–373. 10.1021/acs.jproteome.5b00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Jameel A. G.; Elbaz A. M.; Emwas A.-H.; Roberts W. L.; Sarathy S. M. Calculation of average molecular parameters, functional groups, and a surrogate molecule for heavy fuel oils using 1H and 13C nuclear magnetic resonance spectroscopy. Energy Fuels 2016, 30, 3894–3905. 10.1021/acs.energyfuels.6b00303. [DOI] [Google Scholar]
- Kapla J.; Engström O.; Stevensson B.; Wohlert J.; Widmalm G.; Maliniak A. Molecular dynamics simulations and NMR spectroscopy studies of trehalose–lipid bilayer systems. Phys. Chem. Chem. Phys. 2015, 17, 22438–22447. 10.1039/c5cp02472b. [DOI] [PubMed] [Google Scholar]
- Chu S.; Maltsev S.; Emwas A.-H.; Lorigan G. A. Solid-state NMR paramagnetic relaxation enhancement immersion depth studies in phospholipid bilayers. J. Magn. Reson. 2010, 207, 89–94. 10.1016/j.jmr.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel A. G. A.; Khateeb A.; Elbaz A. M.; Emwas A.-H.; Zhang W.; Roberts W. L.; Sarathy S. M. Characterization of deasphalted heavy fuel oil using APPI (+) FT-ICR mass spectrometry and NMR spectroscopy. Fuel 2019, 253, 950–963. 10.1016/j.fuel.2019.05.061. [DOI] [Google Scholar]
- Naser N.; Abdul Jameel A. G.; Emwas A.-H.; Singh E.; Chung S. H.; Sarathy S. M. The influence of chemical composition on ignition delay times of gasoline fractions. Combust. Flame 2019, 209, 418–429. 10.1016/j.combustflame.2019.07.030. [DOI] [Google Scholar]
- Spěváček J.; Brus J. Solid-State NMR Studies of Polysaccharide Systems. Macromol. Symp. 2008, 265, 69–76. 10.1002/masy.200850508. [DOI] [Google Scholar]
- Schmid F.; Separovic F.; McDougall B. M.; Stone B. A.; Brownlee R. T. C.; Seviour R. J. Characterisation of the extracellular polysaccharides produced by isolates of the fungus Acremonium. Carbohydr. Res. 2007, 342, 2481–2483. 10.1016/j.carres.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Caamal-Fuentes E.; Robledo D.; Freile-Pelegrín Y. Physicochemical characterization and biological activities of sulfated polysaccharides from cultivated Solieria filiformis Rhodophyta. Nat. Prod. Commun. 2017, 12, 803–806. 10.1177/1934578x1701200601. [DOI] [Google Scholar]
- Kolsi R. B. A.; Salah H. B.; Jardak N.; Chaaben R.; Jribi I.; Feki A. E.; Rebai T.; Jamoussi K.; Allouche N.; Blecker C.; Belghith H.; Belghith K. Sulphated polysaccharide isolated from Sargassum vulgare : Characterization and hypolipidemic effects. Carbohydr. Polym. 2017, 170, 148–159. 10.1016/j.carbpol.2017.04.083. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Yang X.; Li P.; Guo C.; Ren X.; Li J. Preparation of chitosan sulfate and vesicle formation with a conventional cationic surfactant. Carbohydr. Polym. 2018, 183, 240–245. 10.1016/j.carbpol.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Ganapathy S.; Lingappa S.; Naidu K.; Selvaraj U.; Ramachandiran S.; Ponnusamy S.; Somasundaram S. T. Isolation and Bioactive Potential of Fucoidan from Marine Macroalgae Turbinaria conoides. ChemistrySelect 2019, 4, 14114–14119. 10.1002/slct.201903548. [DOI] [Google Scholar]
- Jaballi I.; Sallem I.; Feki A.; Cherif B.; Kallel C.; Boudawara O.; Jamoussi K.; Mellouli L.; Nasri M.; Amara I. B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. 10.1016/j.ijbiomac.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Huang T.; Sheng G.; Manchanda P.; Emwas A. H.; Lai Z.; Nunes S. P.; Peinemann K.-V. cyclodextrin polymer networks decorated with subnanometer metal nanoparticles for high-performance low-temperature catalysis. Sci. Adv. 2019, 5, eaax6976 10.1126/sciadv.aax6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Talla Z. A.; Akrawi S. H.; Emwas A.-H. M. Solid state NMR and bioequivalence comparison of the pharmacokinetic parameters of two formulations of clindamycin. Int. J. Clin. Pharmacol. Ther. 2011, 49, 469–476. 10.5414/cp201478. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhou L.; He Z.; Gao N.; Shang F.; Xu J.; Li Z.; Yang Z.; Wu M.; Zhao J. Structural analysis and biological activity of a highly regular glycosaminoglycan from Achatina fulica. Carbohydr. Polym. 2018, 181, 433–441. 10.1016/j.carbpol.2017.10.091. [DOI] [PubMed] [Google Scholar]
- Mou J.; Li Q.; Qi X.; Yang J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018, 185, 41–47. 10.1016/j.carbpol.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Pomin V. H. NMR structural determination of unique invertebrate glycosaminoglycans endowed with medical properties. Carbohydr. Res. 2015, 413, 41–50. 10.1016/j.carres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Mucci A.; Schenetti L.; Volpi N. 1H and 13C nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr. Polym. 2000, 41, 37–45. 10.1016/s0144-8617(99)00075-2. [DOI] [Google Scholar]
- Best S. M.; Duer M. J.; Reid D. G.; Wise E. R.; Zou S. Towards a model of the mineral-organic interface in bone: NMR of the structure of synthetic glycosaminoglycan- and polyaspartate-calcium phosphate composites. Magn. Reson. Chem. 2008, 46, 323–329. 10.1002/mrc.2168. [DOI] [PubMed] [Google Scholar]
- Naji L.; Kaufmann J.; Huster D.; Schiller J.; Arnold K. 13C NMR relaxation studies on cartilage and cartilage components. Carbohydr. Res. 2000, 327, 439–446. 10.1016/s0008-6215(00)00064-1. [DOI] [PubMed] [Google Scholar]
- Alkordi M. H.; Haikal R. R.; Hassan Y. S.; Emwas A.-H.; Belmabkhout Y. Poly-functional porous-organic polymers to access functionality—CO2 sorption energetic relationships. J. Mater. Chem. A 2015, 3, 22584–22590. 10.1039/c5ta05297a. [DOI] [Google Scholar]
- Simoes F. R. F.; Batra N. M.; Emwas A.-H.; Costa P. M. F. J. Validation of alkaline oxidation as a pre-treatment method for elemental quantification in single-walled carbon nanotubes. Anal. Methods 2019, 11, 1884–1890. 10.1039/c8ay02213e. [DOI] [Google Scholar]
- Khan M. T.; Busch M.; Molina V. G.; Emwas A.-H.; Aubry C.; Croue J.-P. How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes?. Water Res. 2014, 59, 271–282. 10.1016/j.watres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- De S.; Gevers L.; Emwas A.-H.; Gascon J. Conversion of Formic Acid into Methanol Using a Bipyridine-Functionalized Molecular Heterogeneous Catalyst. ACS Sustainable Chem. Eng. 2019, 7, 3933–3939. 10.1021/acssuschemeng.8b05070. [DOI] [Google Scholar]
- Volpi N.; Linhardt R. J. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nat. Protoc. 2010, 5, 993. 10.1038/nprot.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Chang Y.; Weyers A. M.; Sterner E.; Linhardt R. J. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2012, 1225, 91–98. 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solakyildirim K. Recent advances in glycosaminoglycan analysis by various mass spectrometry techniques. Anal. Bioanal. Chem. 2019, 411, 3731–3741. 10.1007/s00216-019-01722-4. [DOI] [PubMed] [Google Scholar]
- Palhares L. C. G. F.; Brito A. S.; de Lima M. A.; Nader H. B.; London J. A.; Barsukov I. L.; Andrade G. P. V.; Yates E. A.; Chavante S. F. A further unique chondroitin sulfate from the shrimp Litopenaeus vannamei with antithrombin activity that modulates acute inflammation. Carbohydr. Polym. 2019, 222, 115031. 10.1016/j.carbpol.2019.115031. [DOI] [PubMed] [Google Scholar]
- Faggio C.; Pagano M.; Dottore A.; Genovese G.; Morabito M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. 10.1080/14786419.2015.1086347. [DOI] [PubMed] [Google Scholar]
- Haroun-Bouhedja F.; Ellouali M.; Sinquin C.; Boisson-Vidal C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000, 100, 453–459. 10.1016/s0049-3848(00)00338-8. [DOI] [PubMed] [Google Scholar]
- Nagumo T.; Nishino T.. Fucan sulfates and their anticoagulant activities. In Polysaccharides in Medical Applications; Dumitriu S., Ed.; Marcel Dekker: New York, 1996; pp 545–574. [Google Scholar]
- Franz G.; Alban S. Structure-activity relationship of antithrombotic polysaccharide derivatives. Int. J. Biol. Macromol. 1995, 17, 311–314. 10.1016/0141-8130(96)81837-x. [DOI] [PubMed] [Google Scholar]
- Mestechkina N. M.; Shcherbukhin V. D. Sulfated polysaccharides and their anticoagulant activity: A review. Appl. Biochem. Microbiol. 2010, 46, 267–273. 10.1134/s000368381003004x. [DOI] [PubMed] [Google Scholar]
- Nogueira A. V.; Drehmer D. L.; Iacomini M.; Sassaki G. L.; Cipriani T. R. Biological and structural analyses of bovine heparin fractions of intermediate and high molecular weight. Carbohydr. Polym. 2017, 157, 72–78. 10.1016/j.carbpol.2016.09.061. [DOI] [PubMed] [Google Scholar]
- Wegrowski Y.; Maquart F.-X.. Cellulose acetate electrophoresis of glycosaminoglycans. In Proteoglycan Protocols; Iozzo R. V., Ed.; Humana Press: Totowa, 2001; pp 175–179. [DOI] [PubMed] [Google Scholar]
- Dodgson K.; Price R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106. 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter T.; Muir H. M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gonzaga M. L. C.; Ricardo N. M. P. S.; Heatley F.; Soares S. d. A. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydr. Polym. 2005, 60, 43–49. 10.1016/j.carbpol.2004.11.022. [DOI] [Google Scholar]
- Chisca S.; Duong P. H. H.; Emwas A.-H.; Sougrat R.; Nunes S. P. Crosslinked copolyazoles with a zwitterionic structure for organic solvent resistant membranes. Polym. Chem. 2015, 6, 543–554. 10.1039/c4py01293c. [DOI] [Google Scholar]
- Guo N.; Bai Z.; Jia W.; Sun J.; Wang W.; Chen S.; Wang H. Quantitative Analysis of Polysaccharide Composition in Polyporus umbellatus by HPLC–ESI–TOF–MS. Molecules 2019, 24, 2526. 10.3390/molecules24142526. [DOI] [PMC free article] [PubMed] [Google Scholar]