Abstract
The hypothalamic paraventricular nucleus (PVN) plays a major role in generating increased sympathetic output in hypertension. Although group III metabotropic glutamate receptors (mGluRs) are expressed in the hypothalamus, little is known about their contribution to regulating PVN presympathetic neurons in hypertension. Here we show that activating group III mGluRs with L-2-amino-4-phosphonobutyric acid (L-AP4) consistently inhibited the firing activity of spinally projecting PVN neurons in normotensive rats. However, in spontaneously hypertensive rats (SHRs), L-AP4 inhibited 45% of PVN neurons but excited 37%. L-AP4 significantly reduced glutamatergic and GABAergic input to PVN neurons in both groups. Blocking postsynaptic G protein signaling eliminated the excitatory but not the inhibitory effect of L-AP4 on PVN neurons in SHRs. Remarkably, prior activation of group I mGluRs converted the L-AP4 effect from inhibitory to excitatory in PVN neurons, and L-AP4 consistently inhibited PVN neurons when mGluR5 was blocked in SHRs. Furthermore, the expression level of mGluR4 and mGluR6 in the PVN was significantly higher in SHRs than in normotensive rats. Microinjection of L-AP4 into the PVN decreased blood pressure and lumbar sympathetic nerve discharges in normotensive rats and SHRs. Additionally, blocking group I mGluRs in the PVN potentiated L-AP4’s sympathoinhibitory effect in SHRs. Therefore, activation of presynaptic group III mGluRs inhibits the excitability of PVN presympathetic neurons to attenuate sympathetic vasomotor activity. Through crosstalk with mGluR5, postsynaptic group III mGluR stimulation paradoxically excites PVN presympathetic neurons in SHRs. Concurrently blocking mGluR5 and activating group III mGluRs in the PVN can effectively reduce sympathetic outflow in hypertension.
Keywords: autonomic nervous system, hypothalamus, sympathetic nerve activity, metabotropic glutamate receptor, synaptic plasticity, hypertension
Introduction
The paraventricular nucleus (PVN) in the hypothalamus serves as the interface between the nervous and endocrine systems and is involved in coordinating sympathetic output through its projection to the rostral ventrolateral medulla and spinal intermediolateral cell column. The PVN is the rostral extension of a serial connection of sympathetic-related neurons that extend caudally to the spinal cord (Pyner and Coote, 1999; Strack et al., 1989). Although the critical role of the PVN in the generation of augmented sympathetic outflow in hypertension is well recognized (Allen, 2002; Dampney et al., 2018; Li and Pan, 2007), the mechanisms regulating the excitability of PVN presympathetic neurons are not fully understood.
Glutamate is the predominant excitatory neurotransmitter in the mammalian brain. In spontaneously hypertensive rats (SHRs), the glutamatergic input to PVN presympathetic neurons is augmented, increasing sympathetic vasomotor activity (Li and Pan, 2007; Li et al., 2008b). Glutamate acts on both ionotropic and G protein-coupled metabotropic glutamate receptors (mGluRs). The mGluRs are categorized into 3 major groups on the basis of their amino acid homology, agonist pharmacology, and signal transduction pathway: group I (mGluR1 and mGluR5), group II (mGluR2 and mGluR3), and group III (mGluR4, mGluR6, mGluR7, and mGluR8) (Niswender and Conn, 2010). Group I mGluRs are Gq-coupled, whereas groups II and III mGluRs are linked to Gi/o proteins (De Blasi et al., 2001). Although mGluRs are not typically activated by basally released glutamate, these receptors can be stimulated under conditions in which glutamate release from presynaptic terminals is increased (Cochilla and Alford, 1998; Upreti et al., 2013). The group I mGluR5 is upregulated in the PVN and mediates the hyperactivity of PVN presympathetic neurons and the elevated sympathetic vasomotor activity in SHRs (Li and Pan, 2010; Li et al., 2014). Furthermore, stimulation of group II mGluRs inhibits PVN presympathetic neurons and sympathetic outflow in SHRs but not in normotensive rats (Ye et al., 2013). Group III mGluRs are widely expressed in the brain (Bradley et al., 1998; Ohishi et al., 1995) and are located presynaptically in the hypothalamus (Acuna-Goycolea et al., 2004; Gordon and Bains, 2003; Kuzmiski et al., 2009; Panatier et al., 2004; Yeoh et al., 2019). However, the extent to which group III mGluRs are involved in regulating PVN presympathetic neurons in SHRs remains unknown.
In this study, we determined the role of group III mGluRs in regulating PVN presympathetic neurons and sympathetic outflow in SHRs. Our study provides substantial new information about the contrasting presynaptic and postsynaptic effects of group III mGluR activation on the excitability of PVN presympathetic neurons and sympathetic drive in hypertension.
Materials and Methods
Animal Model
All experimental procedures and protocols were approved (approval #919-RN02) by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. Age-matched male Wistar-Kyoto (WKY, RRID: SCR_002473) rats and SHRs (RRID: SCR_002473) were purchased from Harlan Laboratories (Indianapolis, IN). We used 13-week-old SHRs (weight, 305.3 ± 10.39 g; n = 59) and WKY rats (weight, 295.6 ± 11.12 g; n = 47) for the entire study. All animals were housed 3 rats per cage with free access to food and water in the animal facility. Blood pressure was measured with a tail-cuff system (IITC Life Science Inc., Woodland Hills, CA) for a week before terminal experiments. Fig. 1 is a flowchart showing the timeline for the in vitro and in vivo experiments detailed below.
Figure 1. Flowchart diagrams show the timeline of experimental procedures used in the study.

WKY rats and SHRs were used either for in vivo microinjection experiments (A) or for brain slice recordings (B) at the time point specified. IML, intermediolateral cell column.
Retrograde Labeling of Spinally Projecting PVN Neurons
PVN neurons projecting to the spinal cord were retrogradely labeled, as described previously (Li et al., 2008b; Qiao et al., 2017). In brief, rats were anesthetized with inhalation of 2–3% isoflurane. FluoSpheres (0.04 μm; Molecular Probes, Eugene, OR) were bilaterally injected into the intermediolateral cell column of the spinal cord at the T2 to T4 levels using a glass pipette. Each rat received 4 or 5 separate 50-nL injections. Buprenorphine was administered for 3 days after surgery to minimize postoperative pain (0.2 mg/kg, subcutaneously, every 12 h). The injected rats were allowed to recover for 5 days before being used in brain slice recordings.
Brain Slice Preparation and Whole-Cell Recordings
FluoSphere-injected rats were decapitated while under deep anesthesia with isoflurane. The brains were quickly removed and placed into ice-cold artificial cerebrospinal fluid (aCSF) saturated with 95% O2 and 5% CO2. The coronal hypothalamic slices containing the PVN were sectioned to 300 μm thick using a microtome (Leica Microsystems Inc., Buffalo Grove, IL) and then transferred to aCSF and incubated for 1 hour at 34°C before electrophysiological recordings. The aCSF for brain slice incubation contained (in mM) 126.0 NaCl, 1.2 NaH2PO4, 2.4 CaCl2, 3.0 KCl, 1.5 MgCl2, 10.0 glucose, and 26.0 NaHCO3 (315 mOsm) continuously gassed with 95% O2 and 5% CO2. The aCSF was also used as the external solution in all electrophysiological recordings conducted at 34°C.
Fluorescence-labeled PVN neurons were identified on an upright microscope equipped with epifluorescence and infrared differential interference contrast optics. The resistance of glass electrodes was 4–6 mΩ when filled with internal solution. The action potential was recorded using whole-cell current clamp mode, as described previously (Li et al., 2008a; Li et al., 2014). The recording internal solution contained (in mM) 135.0 K-gluconate, 0.5 CaCl2, 5.0 KCl, 5.0 Mg-ATP, 2.0 MgCl2, 5.0 HEPES. 5.0 EGTA, and 0.5 Na-GTP. Osmolality and pH were adjusted to ~290 mOsm and 7.2, respectively.
Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded using whole-cell voltage-clamp techniques at a holding potential of –60 mV (Li et al., 2008b); the internal solution contained (in mM) 135.0 K-gluconate, 5.0 TEA, 2.0 MgCl2, 5.0 Mg-ATP, 0.5 Na-GTP, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, and 10 lidocaine N-ethyl bromide (adjusted to pH 7.2–7.4 with 1 mol L−1 KOH; 290–300 mOsm L−1). For recording of spontaneous inhibitory postsynaptic currents (sIPSCs), the membrane potential was held at 0 mV (Li et al., 2005; Li et al., 2003), and the internal solution contained (in mM) 110.0 Cs2SO4, 2.0 MgCl2, 0.5 CaCl2, 0.3 Na2-GTP, 10.0 HEPES, 5.0 EGTA, 2.0 Mg-ATP, and 10 lidocaine N-ethyl bromide (pH and osmolality adjusted to 7.25 and 285 mOsm, respectively). The recording signals were processed using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1–2 kHz, and digitized at 20 kHz using DigiData 1440 (Molecular Devices). Access resistance in recorded neurons was assessed by using the membrane test program in Clampex and was kept between 10–15 MΩ throughout the entire recording period.
Quantitative PCR Analysis
RNA was extracted by using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. Tissue samples were taken from the brainstem, frontal cortex, hippocampus, and PVN of WKY rats and SHRs, as we discussed previously (Ma et al., 2018; Qiao et al., 2017). Total RNA (1 μg) was reverse-transcribed into cDNA by using SuperScript III (Invitrogen) in 20-μL reaction volumes. Quantitative PCR was performed using an IQ5 system (Bio-Rad, Hercules, CA). A mixture of 12.5 μL of SYBR Green PCR master mix (Bio-Rad), 1 μL of cDNA, and a 200 nmol/L primer set was used for amplification. All samples were amplified in duplicate in a 96-well plate, and the cycling conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles at 95 °C for 15 s, followed by 1 min at 60 °C. The relative mRNA expression level was determined by calculating the value of Δcycle threshold (ΔCt) by normalizing the average Ct value compared to that of GAPDH (an endogenous control) and then calculating 2−ΔΔCt values. The primer sets used for real-time PCR assays are shown in Table 1.
Table 1.
Primers used for real-time PCR assays
| Gene (Accession No.) | Primer sequence (5’ to 3’) | Tm (°C) | Amplicon size (bp) |
|---|---|---|---|
| mGluR4 (NM_022666) |
F: CAGGTTATGCCACACACAGG R: GCAAAGACGGAAGAGAAACG |
60 | 178 |
| mGluR6 (NM_022920) |
F: GAACGTACAGAAGCGGAAGC R: GGTCGGGGTTTAGCTTTTTC |
60 | 249 |
| mGluR7 (NM_031040) |
F: CCAGGGCGTTATGACATCTT R: TATCCATCACAGGGCTCACA |
60 | 233 |
| mGluR8 (NM_022202) |
F: GGGCACTGGACAAATCAACT R: TGATCCAAAGGGCAAAGTTC |
60 | 215 |
| GAPDH (NM_017008) |
F: TGCCACTCAGAAGACTGTGG R: TTCAGCTCTGGGATGACCTT |
60 | 129 |
F, forward primer; R, reverse primer; Tm, primer melting temperature.
Microinjections and Lumbar Sympathetic Nerve Recording
Rats were anesthetized initially with a mixture of α-chloralose (60–75 mg/kg) and urethane (800 mg/kg) (injected intraperitoneally). The depth of anesthesia was assessed for adequacy by the absence of paw withdrawal responses to a noxious pinch. The trachea was cannulated for mechanical ventilation using a rodent ventilator with 100% O2. Expired CO2 concentration was monitored with a CO2 analyzer (CapStar 100, CWE Inc, Ardmore, PA) and maintained at 4% to 5% by adjusting the ventilation rate and/or tidal volume throughout the experiment. Rectal temperature was monitored through a temperature sensor and maintained at 37–38°C using a heating lamp. The arterial blood pressure (ABP) was monitored via a catheter placed in the left femoral artery, and heart rate was derived from the pulsatile ABP signal. The left lumbar sympathetic nerve was isolated and recorded, as described previously (Li and Pan, 2007; Li et al., 2014). The nerve signal was amplified (gain of 20,000–30,000), band-pass filtered (100–3000 Hz), and processed using the Spike2 system (Cambridge Electronic Design, Cambridge, UK). Background electrical noise was determined after the rats were euthanized with an overdose of sodium phenobarbital. The background noise was subtracted from the integrated values of lumbar sympathetic nerve activity (LSNA), and the percent change in LSNA was calculated.
For PVN microinjections, a glass pipette (tip diameter 20–30 μm) was advanced to the PVN according to the following stereotactic coordinates: 1.8–2.0 mm caudal to the bregma, 0.5 mm lateral to the midline, and 7.0– 7.5 mm ventral to the dura (Li and Pan, 2007; Ma et al., 2018). The microinjection (50 nL) was performed through a calibrated microinjector (Nanoject II; Drumond Scientific, Broomall, PA). The pipette was left in place for about 1 min after each drug injection. The location of the pipette tip and diffusion of the drugs in the PVN were determined by including 5% rhodamine-labeled fluorescent microspheres (0.04 μm; Molecular Probes) in the injection solution. After experiments, the rat brains were removed and fixed in 10% buffered formalin solution for injection site examination.
List of Major Chemicals
(RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG; RRID: SCR_003281) was obtained from Tocris (Minneapolis, MN). L-2-amino-4-phosphonobutyric acid (L-AP4; RRID: SCR_003105), S-3,5-dihydroxyphenylglycine (DHPG, Cat. #D3689), and 2-methyl-6-(phenylethynyl) pyridine (MPEP; Cat. #5046350001) were purchased from Sigma-Aldrich (St. Louis, MO).
Study Design and Data Analysis
All data are presented as means ± SEM. We assigned equal numbers of animals to each group without using randomization. We did not use any statistical methods to predetermine the sample sizes. However, our sample sizes were similar to those utilized in the field (Li and Pan, 2005; Li et al., 2008a; Ye et al., 2013). Animals were excluded from the study if the microinjection occurred outside the PVN. Three WKY rats and two SHRs were excluded from data analysis because of misplaced microinjections of L-AP4. The investigators (JJZ, JP, DPL, and SRC) were blinded during brain slice recordings and in vivo microinjection experiments. The animal in each group was coded before the experiment, and the code was revealed during data analysis. Action potential data were analyzed by using pClamp10 (Molecular Devices, Sunnyvale, CA). A change of >15% in the baseline firing rate with 50 μM L-AP4 was considered to be responsive (Chen et al., 2006; Li and Pan, 2006; Li et al., 2014). Representative traces were selected from the baseline, peak drug effect, and washout period that reflect the summary data in each group. sEPSCs and sIPSCs were analyzed off-line using a peak detection program (MiniAnalysis, Synaptosoft, Leonia, NJ). In all brain slice recording experiments, at least 4 rats were used for recordings in each group. The LSNA was rectified and integrated offline after subtracting the background noise value. Control values were obtained by averaging the signal over a 60-s period immediately before each treatment. Response values after each intervention were averaged over 60 s when the maximal response occurred. D’Agostino-Pearson normality test was used to assess the normality of data, and no test for outliers was conducted. Two-tailed Student t tests were used for comparison between 2 groups, and one-way or two-way ANOVA with Bonferroni’s post hoc test was used to determine differences among 3 or more groups. One-way repeated measures ANOVA with Dunnett’s test was performed to compare different time points within a group. P < 0.05 was considered to be statistically different.
RESULTS
Activation of group III mGluRs inhibits firing activity of PVN presympathetic neurons in WKY rats but produces divergent effects in SHRs
The systolic blood pressure measured with a tail-cuff system in awake animals was significantly higher in 50 SHRs than in age-matched 33 WKY rats (201.34 ± 5.04 vs. 119.90 ± 7.14 mmHg, P < 0.001). To determine the role of group III mGluRs in regulating the excitability of PVN presympathetic neurons, we used a specific agonist for group III mGluRs, L-AP4 (Panatier et al., 2004; Zhang et al., 2009). We tested the effect of L-AP4 on the firing activity of spinally projecting PVN neurons in WKY rats and SHRs. The baseline firing frequency of labeled PVN neurons was significantly higher in SHRs than in WKY rats (P = 0.036, t(35) = 2.173; n = 15 neurons in WKY rats, n = 22 neurons in SHRs; Fig. 2A–C). Similar to what we reported in spinal cord slices previously (Zhang et al., 2009), we observed that the effect of L-AP4 on labeled PVN neurons reached the maximum within 6 min in both WKY rats and SHRs (it took about 3 min to completely exchange the solution in the recording chamber). Thus, L-AP4 was bath applied for 6 min before giving the next concentration, and the peak effect of L-AP4 was averaged over 1 min at each concentration. In WKY rats, bath application of 5–50 μM L-AP4 decreased the firing rate of all 15 labeled PVN neurons in a concentration-dependent manner, and this effect was reversed upon L-AP4 washout (Fig. 2A–C). In SHRs, however, L-AP4 at 50 μM inhibited 10 of 22 (45%; P = 0.0165, F(5,45) = 4.802) labeled PVN neurons but unexpectedly excited 8 of 22 (36%; P = 0.0189, F(5,35) = 6.086) labeled PVN neurons (Fig. 2A–C). L-AP4 had no significant effect on the firing rate of the remaining 4 (18%) labeled PVN neurons in SHRs.
Figure 2. Activation of group III mGluRs decreases the firing activity of spinally projecting PVN neurons in WKY rats but produced divergent effects in SHRs.
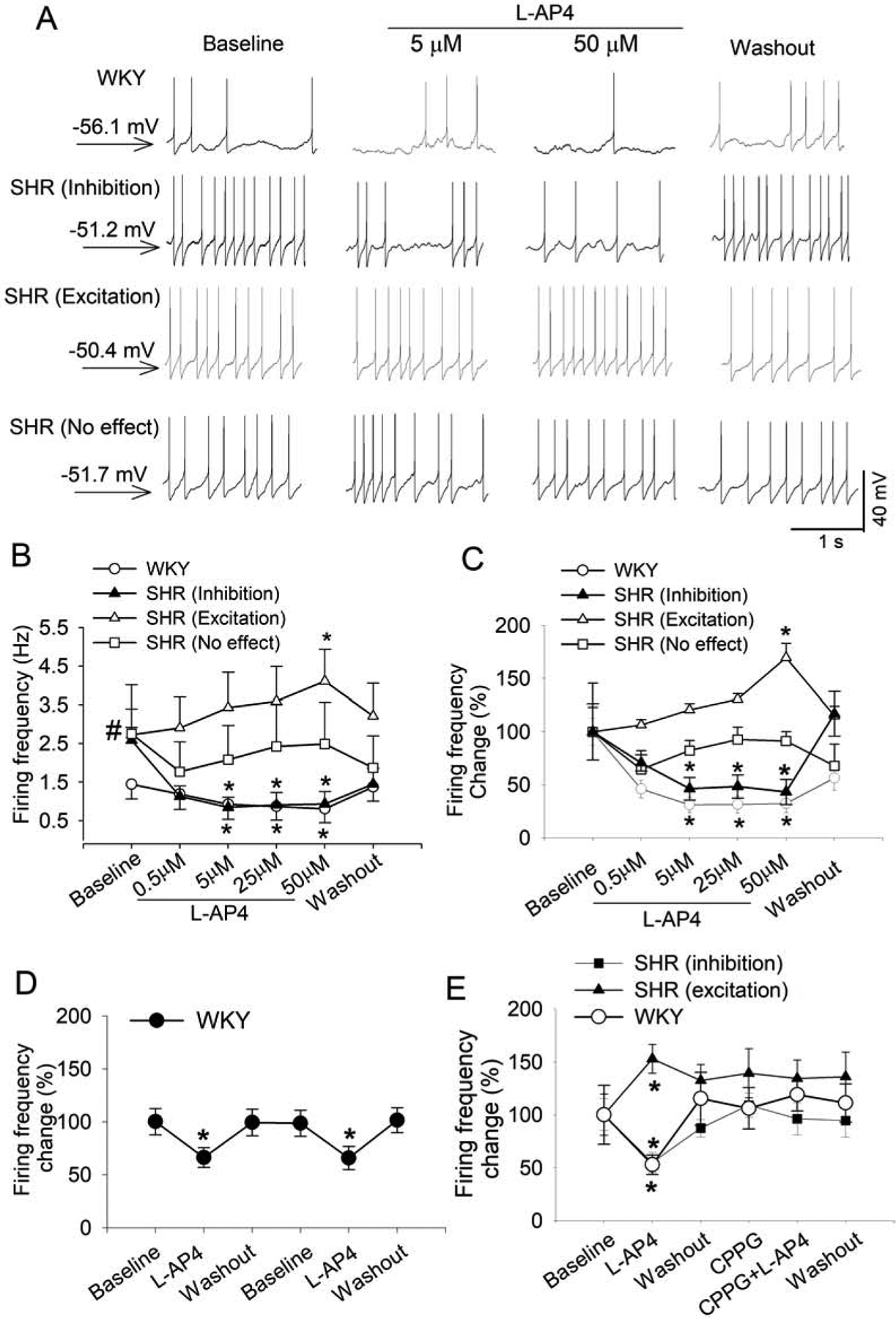
A, Original traces show the effect of L-AP4 on the firing activity of labeled PVN neurons in one WKY rat and one SHR. The membrane potential value was shown on the left. B and C, Mean data (B) and percentage changes (C) show the concentration-dependent effect of L-AP4 on the firing activity of labeled PVN neurons in WKY rats (n = 15 neurons from 4 rats) and SHRs (n = 22 neurons from 6 rats). The firing rate was normalized to the respective baseline in each group. D, Mean data show the effect of repeated bath application of 50 μM L-AP4 on the firing rate of 9 labeled PVN neurons from 4 rats in WKY rats. E, Summary data show that CPPG blocked the effect of L-AP4 on the firing rate of labeled PVN neurons in WKY rats (D, n = 9 neurons from 4 rats) and SHRs (E; inhibition, n = 10 neurons; excitation, n = 7 neurons from 5 rats). Data are presented as mean ± SEM. *P < 0.05, compared with the baseline for the group. #P < 0.05, compared with the baseline in WKY rats.
L-AP4 (50 μM) was first bath applied for 6 min followed by a washout for 6 min and then re-applied for another 6 min. The inhibitory effect produced by the first and second applications of L-AP4 on the firing activity of 9 labeled PVN neurons from WKY rats was similar (Fig. 2D). We next determined whether the effect of L-AP4 on the firing activity of spinally projecting PVN neurons is mediated by group III mGluRs by using CPPG, a specific antagonist for group III mGluRs (Acuna-Goycolea et al., 2004; Zhang et al., 2009). We first tested the effect of 50 μM L-AP4 alone on the firing activity of labeled PVN neurons. After the firing rate returned to the baseline level, we applied 10 μM CPPG for 6 min followed by bath application of L-AP4 (50 μM) in the presence of CPPG for another 6 min. In all 9 PVN neurons from WKY rats, L-AP4 alone significantly decreased the firing rate (P = 0.0047, F(2,16) = 19.12), and this L-AP4-induced inhibition of the firing activity was blocked by CPPG (Fig. 2E). Furthermore, in labeled PVN neurons from SHRs, CPPG abolished both the inhibitory (n = 10 neurons) and excitatory (n = 7 neurons) effects of L-AP4 (Fig. 2E). These results indicate that activation of group III mGluRs consistently inhibits the excitability of PVN presympathetic neurons in normotensive control rats but produces divergent effects on these neurons in SHRs.
Stimulation of group III mGluRs inhibits synaptic glutamatergic and GABAergic input to PVN presympathetic neurons in WKY rats and SHRs
Stimulation of group III mGluRs inhibits synaptic neurotransmitter release to various hypothalamic neurons (Acuna-Goycolea et al., 2004; Muram et al., 2016; Panatier et al., 2004; Schrader and Tasker, 1997). We have shown that blocking glutamatergic input inhibits the firing activity of PVN presympathetic neurons in SHRs (Li et al., 2008b). To determine the role of group III mGluRs in regulating glutamatergic synaptic input to spinally projecting PVN neurons, we recorded sEPSCs in WKY rats and SHRs. The baseline frequency of sEPSCs in labeled PVN neurons was significantly higher in SHRs than in WKY rats (P = 0.0015, t(26) = 1.787; n = 13 neurons from WKY rats, n = 15 neurons from SHRs, Fig. 3A–F), in agreement with previous reports (Li et al., 2008b; Ye et al., 2013). Bath application of 5–50 μM L-AP4 decreased the frequency, but not the amplitude, of sEPSCs in all labeled PVN neurons in a concentration-dependent manner in both WKY rats and SHRs (Fig. 3AG). The frequency of sEPSCs returned to the baseline after L-AP4 washout for 10–15 min in both groups.
Figure 3. Activation of group III mGluRs reduces glutamate release to spinally projecting PVN neurons in WKY rats and SHRs.
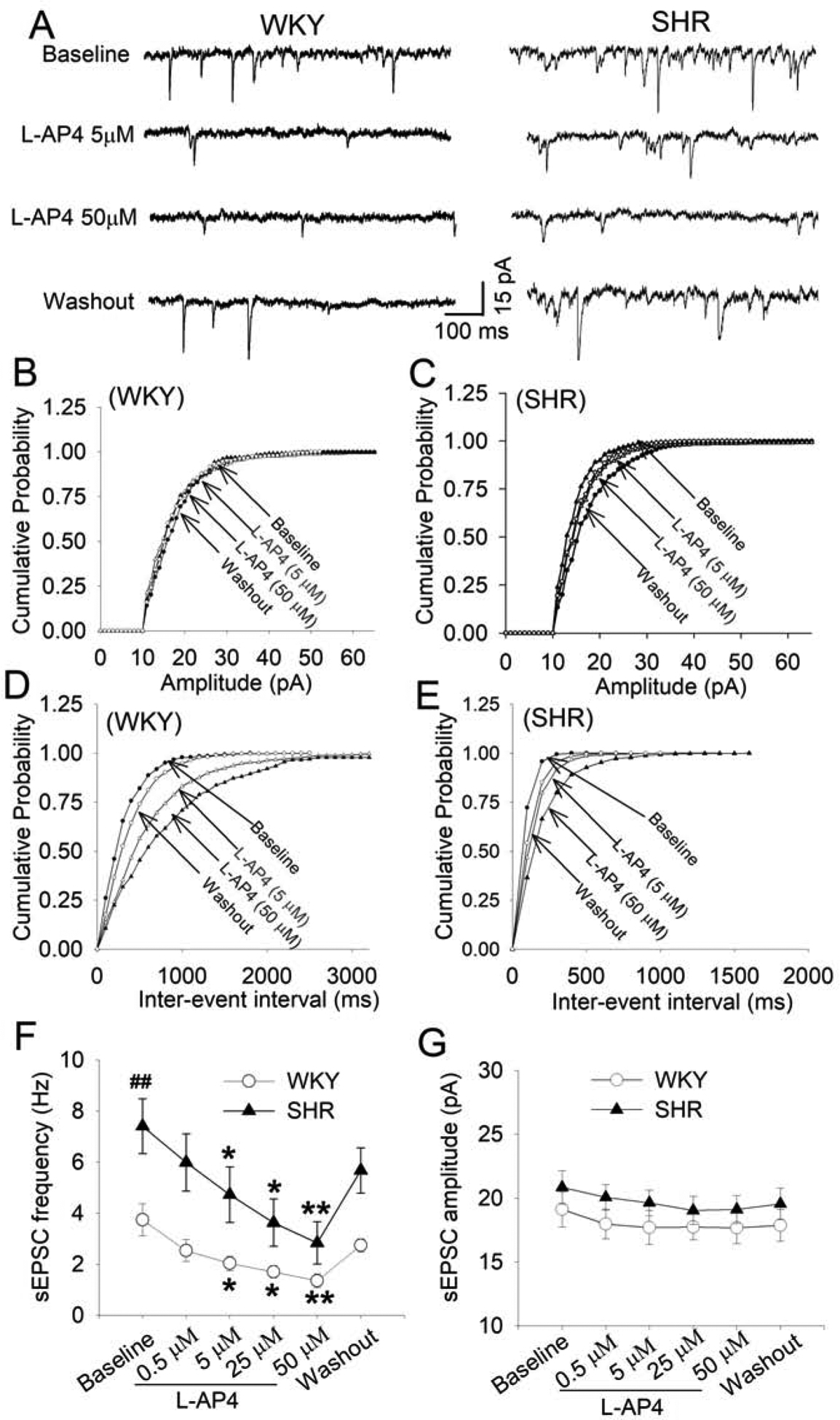
A, Original recordings show that L-AP4 decreased the frequency of sEPSCs in labeled PVN neurons in a concentration-dependent manner in WKY rats and SHRs. B-E, Cumulative probability analyses show that L-AP4 decreased the frequency (D and E), but not the amplitude (B and C), of sEPSCs in labeled PVN neurons in WKY rats and SHRs. F and G: Summary data show the effect of L-AP4 on the frequency (F) and amplitude (G) of sEPSCs in labeled PVN neurons in WKY rats (n = 13 neurons from 4 rats) and SHRs (n = 15 neurons from 4 rats). *P < 0.05 and **P < 0.01, compared with the baseline for the group. ##P < 0.01, compared with the baseline in WKY rats.
We also determined the effect of L-AP4 on GABAergic inhibitory input to spinally projecting PVN neurons by recording sIPSCs. As reported previously (Li et al., 2008a; Ye et al., 2012), the baseline frequency and amplitude of sIPSCs were significantly lower in SHRs than in WKY rats (n = 10 neurons in each group, Fig. 4A–G). Bath application of L-AP4 (50 μM) significantly decreased the frequency, but not the amplitude, of sIPSCs in all labeled PVN neurons in both WKY rats (P = 0.0216, F(2,18) = 5.832) and SHRs (P = 0.035, F(2,18) = 4.890, Fig. 4A–G). The normalized inhibitory effect of L-AP4 on sEPSCs and sIPSCs was not statistically different between WKY rats and SHRs. These data suggest that activation of presynaptic group III mGluRs inhibits glutamatergic and GABAergic input to PVN presympathetic neurons in normotensive control rats and SHRs.
Figure 4. Activation of group III mGluR attenuates GABAergic input to spinally projecting PVN neurons in WKY rats and SHRs.
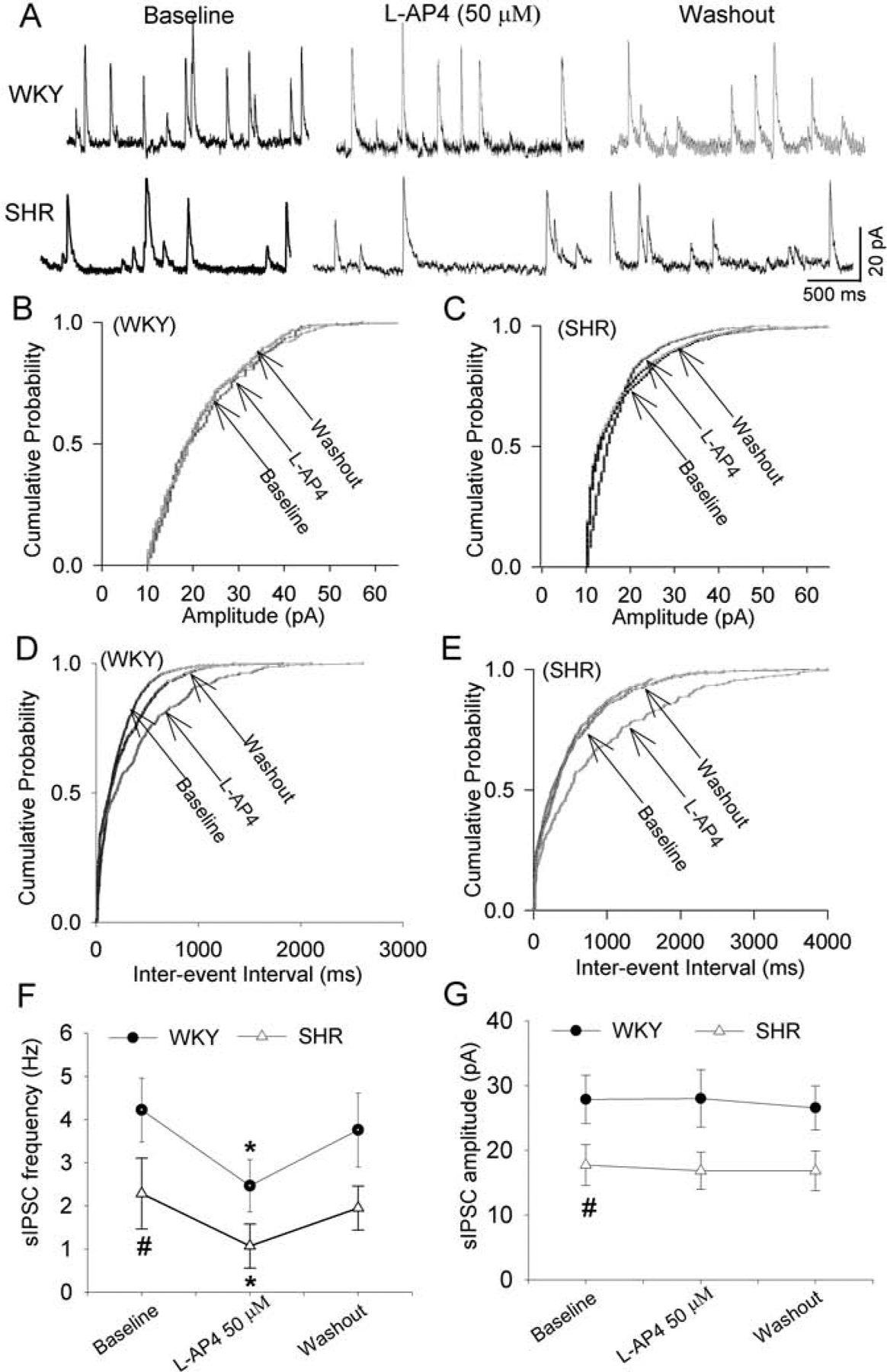
A, Representative traces show the effect of L-AP4 on sIPSCs in labeled PVN neurons in one WKY rats and one SHR. B-E, Cumulative probability analyses show the effect of L-AP4 on the amplitude (B and C) and frequency (D and E) of sIPSCs in the above-labeled PVN neurons. F and G, Summary data show the effect of L-AP4 on the frequency (F) and amplitude (G) of sIPSCs of labeled PVN neurons in WKY rats and SHRs (n = 10 neurons from 4 rats in each group). *P < 0.05, compared with the baseline control. #P < 0.05, compared with the baseline in WKY rats.
Group III mGluR activation excites PVN presympathetic neurons via postsynaptic G protein signaling in SHRs
Because the presynaptic effect of L-AP4 on glutamate and GABA release cannot explain its excitatory effect on some PVN presympathetic neurons in SHRs, we determined whether postsynaptic group III mGluRs are involved in the excitatory effect of L-AP4. Group III mGluRs are coupled to Gi/o proteins (De Blasi et al., 2001), and we performed whole-cell recording by including GDP-β-S (1.0 mM), a non-hydrolyzable GDP analog (Li and Pan, 2005; Li et al., 2008a), in the intracellular recording solution to block postsynaptic G protein signaling. In 10 of 19 (53%) labeled PVN neurons from SHRs, bath application of 50 μM L-AP4 significantly reduced the firing activity (P = 0.0107, F(2,18) = 4.578; Fig. 5A and B). The firing activity of the other 9 (47%) PVN neurons was not significantly changed by L-AP4 (Fig. 5A and B). L-AP4 did not increase the excitability of any of the 19 neurons recorded with the GDP-β-S solution. This finding suggests that postsynaptic group III mGluRs mediate the paradoxical excitatory effect of L-AP4 on PVN presympathetic neurons in SHRs.
Figure 5. Activation of postsynaptic group III mGluRs excites spinally projecting PVN neurons via crosstalk with mGluR5 in SHRs.

A and B, Original traces (A) and summary data (B) show that blocking G protein signaling with intracellular GDP-β-s eliminated the excitatory effect of L-AP4 on the firing activity in labeled PVN neurons in SHRs (n = 10 neurons from 4 rats). C and D, Original traces (C) and summary data (D) show that DHPG treatment converted the effect of L-AP4 to excitatory in labeled PVN neurons in WKY rats (n = 10 neurons from 4 rats) and SHRs (n = 10 neurons from 4 rats). E and F, Original recording traces (E) and summary data (F) show that MPEP treatment blocked the excitatory effect of L-AP4 on labeled PVN neurons in SHRs (n = 13 neurons from 4 rats). The membrane potential value was shown on the left. G and H, Comparison of mRNA levels of mGluR4 (G) and mGluR6 (H) in the brainstem (BS), frontal cortex (FC), hippocampus (HC), and PVN tissues from 7 WKY rats and 9 SHRs. *P < 0.05 and **P < 0.01, compared with the baseline for the group. #P < 0.05 compared with the value of MPEP alone. ###P < 0.001 compared with WKY rats.
mGluR5 is involved in excitation of PVN presympathetic neurons by group III mGluR activation in SHRs
We have previously shown that mGluR5, a major group I mGluR subtype, is upregulated in the PVN and plays a major role in the increased excitability of PVN presympathetic neurons in SHRs (Li et al., 2014). In hippocampal neurons, increased activity of group I mGluRs can sensitize and mediate the excitatory effect of L-AP4 (Charpak et al., 1992). We thus determined whether activation of group I mGluRs with DHPG (Li et al., 2014) alters the effect of L-AP4 on spinally projecting PVN neurons. Bath application of DHPG (100 μM) for 1–2 min significantly increased the firing activity of labeled PVN neurons from both WKY rats and SHRs (WKY rats, P = 0.0255, F(2,16) = 7.929, n = 9 neurons; SHRs, P = 0.022, F(2,18) = 7.832, n = 10 neurons; Fig. 5C and D). After DHPG washout and the return of firing activity to the baseline, subsequent application of L-AP4 (50 μM) increased the firing activity of all 19 labeled PVN neurons tested from WKY rats and SHRs (Fig. 5C and D).
Furthermore, we determined whether blocking mGluR5 activity alters the effect of L-AP4 on the firing activity of spinally projecting PVN neurons in SHRs. MPEP is a highly specific mGluR5 antagonist (Gasparini et al., 1999; Li et al., 2014). We have shown previously that bath application of 10 μM MPEP produced a maximum inhibitory effect within 6 min on the firing activity of labeled PVN neurons in SHRs (Li et al., 2014). In the present study, MPEP (10 μM) was bath applied for 6 min followed by bath application of MPEP plus L-AP4 for another 6 min. Bath application of 10 μM MPEP alone significantly decreased the firing rate of labeled PVN neurons in SHRs (P = 0.0385, F(3,52) = 12.70, n = 13 neurons; Fig. 5E and F). In the presence of MPEP, subsequent application of L-AP4 (50 μM) further significantly decreased the firing rate in these 13 labeled PVN neurons (P = 0.0195, n = 13 neurons; Fig. 5E and F). These data suggest that increased mGluR5 activity is involved in the excitatory effect of group III mGluR activation on PVN presympathetic neurons in SHRs.
The expression level of mGluR4 and mGluR6 in the PVN is increased in SHRs
Because the above electrophysiological data suggested that activation of postsynaptic group III mGluRs can excite a subpopulation of PVN presympathetic neurons in SHRs, we quantified the expression level of all 4 group III mGluR subtypes (mGluR4, mGluR6, mGluR7, and mGluR8) in the PVN in WKY rats and SHRs. We used real-time PCR to measure the mRNA levels of these mGluRs because antibodies against these subtypes are not highly specific. PCR analysis indicated that mRNA level of mGluR4 (P < 0.001, t(14) = 9.286) and mGluR6 (P < 0.001, t(14) = 6.052) in the PVN were significantly higher in SHRs than in WKY rats (SHRs, n = 9 rats; WKY, n = 7 rats; Fig. 5G and H). However, the mRNA levels of mGluR7 and mGluR8 in the PVN tissue from WKY rats and SHRs were below the detection. Additional PCR assays showed that the mRNA levels of mGluR4 and mGluR6 were very low in the brainstem and hippocampus in both WKY rats and SHRs and that the mGluR6 mRNA level in the frontal cortex was significantly lower in SHRs than in WKY rats (P < 0.001, t(14) = 6.382; SHRs, n = 9 rats; WKY, n = 7 rats; Fig. 5G and H).
Group III mGluR activation in the PVN inhibits sympathetic vasomotor activity in WKY rats and SHRs
We next determined whether group III mGluRs in the PVN are involved in controlling sympathetic outflow in vivo. We stimulated group III mGluRs via bilateral microinjection of L-AP4 into the PVN. The baseline mean ABP and heart rate were significantly higher in SHRs than in WKY rats (n = 10 rats per group, Fig. 6A–E). In WKY rats, bilateral microinjection of 10–500 pmol L-AP4 into the PVN significantly decreased mean ABP and LSNA in a dose-dependent manner (n = 10 rats, Fig. 6A–E). By comparison, in SHRs, microinjection of L-AP4 at higher doses (100–500 pmol), but not at a low dose of 10 pmol, significantly decreased mean ABP and LSNA (n = 10 rats, Fig. 6B–E). Microinjection of L-AP4 into the PVN had no significant effect on the heart rate in WKY rats or SHRs (Fig. 6E). Furthermore, the L-AP4-induced sympathoinhibitory effect in both WKY rats and SHRs was completely blocked by prior microinjection of CPPG (500 pmol) into the PVN (Fig. 6A–E). These data suggest that activation of group III mGluRs in the PVN principally reduces sympathetic outflow in normotensive control rats and in SHRs.
Figure 6. Activation of group III mGluRs in the PVN inhibits sympathetic outflow in WKY rats and SHRs.

A and B, Representative traces show the effect of bilateral microinjection of L-AP4 (100 pmol, 50 nL) and CPPG (500 pmol, 50 nL) on mean blood pressure (ABP), lumbar sympathetic nerve activity (LSNA) and heart rate (HR) in one WKY rat (A) and one SHR (B). C–E, Summary data shows the effect of microinjection of L-AP4 (1 to 500 pmol) and CPPG into the PVN on mean ABP, integrated LSNA, and HR in WKY rats and SHRs (n = 10 rats in each group). *P < 0.05, compared with the baseline for the group. #P < 0.05, compared with WKY rats.
Blocking mGluR5 in the PVN potentiates inhibition of sympathetic vasomotor activity by group III mGluR activation in SHRs
Because increased mGluR5 activity participates in the excitatory effect of group III mGluRs on PVN presympathetic neurons in SHRs, we also determined whether blocking mGluR5 in the PVN potentiates the inhibitory effect of group III mGluRs on ABP and sympathetic nerve discharges in SHRs. Bilateral microinjection of MPEP (5 nmol) significantly decreased mean ABP and LSNA in SHRs (P = 0.0316, F(3,25) = 15.87, n = 8 rats, Fig. 7A–C), which is similar to our previous report (Li and Pan, 2010). In contrast, microinjection of MPEP had no significant effect on mean ABP and LSNA in WKY rats (n = 7 rats, Fig. 7B–D). In SHRs, microinjection of 10 pmol L-AP4 alone did not significantly affect mean ABP and LSNA. However, in the presence of MPEP, 10 pmol L-AP4 produced a significant reduction in mean ABP (P = 0.0240, F(3,21) = 15.87), LSNA (P = 0.0152, F(3,21) = 12.22), and heart rate (P = 0.0167, F(3,21) = 9.187) in SHRs (n = 8 rats, Fig. 7B–F). Because of the different baselines between WKY rats and SHRs, we normalized changes of ABP and LSNA to the respective baseline (MPEP alone) value. Microinjection of L-AP4 into the PVN in the presence of MPEP caused a 27.40 ± 3.25% reduction in ABP in SHRs, which was significantly greater than that (10.15 ± 2.19%) in WKY rats. Similarly, microinjection of L-AP4 in the presence of MPEP caused a significant reduction in LSNA in SHRs than in WKY rats (21.11 ± 2.06% vs. 11.70 ± 3.58%). These results suggest that increased mGluR5 activity in the PVN attenuates the inhibitory effect of group III mGluR stimulation on sympathetic outflow in SHRs.
Figure 7. Blocking mGluR5 in the PVN potentiates the inhibitory effect of group III mGluR activation on sympathetic output in SHRs.

A and B, Original traces show the effects of MPEP (5 nmol, 50 nL) and L-AP4 (10 pmol, 50 nL) microinjected into the PVN on mean blood pressure (ABP), lumbar sympathetic nerve activity (LSNA), and heart rate (HR) in one WKY rat (A) and one SHR (B). C-E, Summary data show the effects of MPEP and L-AP4 microinjected to the PVN on mean ABP, integrated LSNA, and HR in WKY rats (n = 7 rats) and SHRs (n = 8 rats). *P < 0.05 and ***P < 0.001, compared with the baseline for the group. #P < 0.05 and ##P < 0.01, compared with the value of MPEP alone in the same group. F, Representative bright-field and fluorescence images and schematic drawing show the microinjection sites in the PVN in WKY rats (gray) and SHRs (black). 3V, third ventricle.
Discussion
Our study provides novel information about the important role of group III mGluRs in regulating the excitability of PVN presympathetic neurons and sympathetic vasomotor tone in hypertension. We found that activation of group III mGluRs with L-AP4 consistently inhibited the firing activity of PVN presympathetic neurons in normotensive control rats. Strikingly, however, in SHRs, L-AP4 inhibited only a subpopulation of PVN neurons and excited other PVN neurons. Notably, divergent responses to receptor stimulation in subgroups of presympathetic PVN neurons under normotensive and hypertensive conditions have been shown previously (Chen et al., 2006; Li and Pan, 2006). Also, activation of group III mGluRs reduced synaptic glutamatergic and GABAergic input to PVN neurons in both SHRs and control rats. Group III mGluRs are predominantly expressed presynaptically, and their activation usually leads to inhibition of synaptic transmission in the brain and spinal cord (Acuna-Goycolea et al., 2004; Zhang et al., 2009). Stimulation of group III mGluRs reduces GABAergic and glutamatergic inputs in the brain, including the hypothalamic supraoptic nucleus (Panatier et al., 2004; Schoepp, 2001). In SHRs, the glutamatergic excitatory input to PVN presympathetic neurons is increased, whereas the GABAergic inhibitory input is diminished (Li and Pan, 2007; Li et al., 2008a; Ye et al., 2012; Ye et al., 2011; Ye et al., 2013). We observed that the inhibitory effect of L-AP4 on firing activity was not blocked by inhibiting postsynaptic G protein signaling in PVN presympathetic neurons in SHRs. Thus, presynaptic inhibition of glutamate release by group III mGluR activation is predominantly responsible for reducing PVN neuronal activity in normotensive rats and in a majority of PVN neurons in SHRs.
Considering the general inhibitory effect of group III mGluR activation on neuronal activity (Acuna-Goycolea et al., 2004; De Blasi et al., 2001), we were surprised that L-AP4 excited a subpopulation of PVN presympathetic neurons in SHRs. This unexpected divergent effect suggests that signaling of group III mGluRs is altered in some PVN neurons in hypertension. The postsynaptic excitatory action of group III mGluRs is supported by the evidence that the stimulatory effect of L-AP4 was abolished by blocking postsynaptic G protein signaling of PVN presympathetic neurons in SHRs. It is possible that the ultimate effect of L-AP4 on PVN neuronal activity in SHRs depends on the dynamic balance between the opposing presynaptic inhibitory and postsynaptic excitatory effects. We found that mGluR4 and mGluR6, but not mGluR7 and mGluR8, were expressed in the PVN. Also, the mRNA levels of mGluR4 and mGluR6 in the PVN were significantly higher in SHRs than in normotensive control rats. In the mammalian brain, mGluR4 is mainly expressed presynaptically (Corti et al., 2002), although mGluR4 is also localized postsynaptically in the hippocampus (Bradley et al., 1996). In contrast, mGluR6 is predominantly expressed postsynaptically in rod bipolar cells (Nomura et al., 1994). It is possible that mGluR4 and mGluR6 are upregulated postsynaptically and interact with mGluR5 to excite PVN presympathetic neurons in SHRs. Because the PVN is a heterogeneous brain region that consists of many types of cells, it is uncertain whether mGluR4 and/or mGluR6 are specifically present in spinally projecting PVN neurons, which is a limitation of quantitative PCR assay used in our study. We did not further examine the specific role of mGluR4 and mGluR6 in regulating PVN neuronal activity owing to the lack of highly selective agonists and antagonists for these mGluRs.
A new finding of our study is that activation of postsynaptic group III mGluRs increases the excitability of a subpopulation of PVN presympathetic neurons via crosstalk with mGluR5 in SHRs (Fig. 8). The increased activity of mGluR5 plays a major role in the hyperactivity of PVN presympathetic neurons in SHRs (Li and Pan, 2010). We showed in this study that when group I mGluRs were activated, the effect of L-AP4 was converted from inhibitory to excitatory in control rats and in SHRs. Furthermore, blocking mGluR5 completely eliminated the excitatory, but not the inhibitory, effect of L-AP4 on PVN neurons in SHRs. These findings indicate that neuronal excitation by postsynaptic group III mGluRs likely occurs through crosstalk with mGluR5 in the PVN in SHRs. It is unclear exactly how activation of postsynaptic group III mGluRs excites PVN presympathetic neurons. One possible mechanism is through crosstalk between the Gq-coupled mGluR5 and Gi/o-coupled mGluR4/mGluR6 in the PVN in SHRs. The functional connection between group III mGluRs and mGluR5 may occur at the level of receptors, G proteins, or second messengers. It has been reported that when group I mGluRs are stimulated, activation of group III mGluRs excites the hippocampal pyramidal neurons (Charpak et al., 1992). Also, Gi-coupled adenosine A1 receptors can enhance the effect of Gq-coupled histamine H1 receptors in cortical neurons (Hill and Kendall, 1987). In addition, the Gβγ exchange seems to mediate the augmenting effects of Gi-coupled adenosine A1 receptors on Gq-coupled bradykinin B2 receptors (Quitterer and Lohse, 1999). Although the crosstalk between group I mGluRs and AMPA/NMDA receptors in the brain has been reported previously (Li et al., 2014; Okubo et al., 2004), our study is the first demonstration of the functional link between mGluR5 and group III mGluRs in the brain. However, the direct interaction between mGluR5 and group III mGluRs in the PVN remains to be substantiated by using other biochemical approaches.
Figure 8. A schematic drawing showing the divergent effect of group III mGluR activation on the firing activity of presympathetic PVN neurons in WKY rats and SHRs.
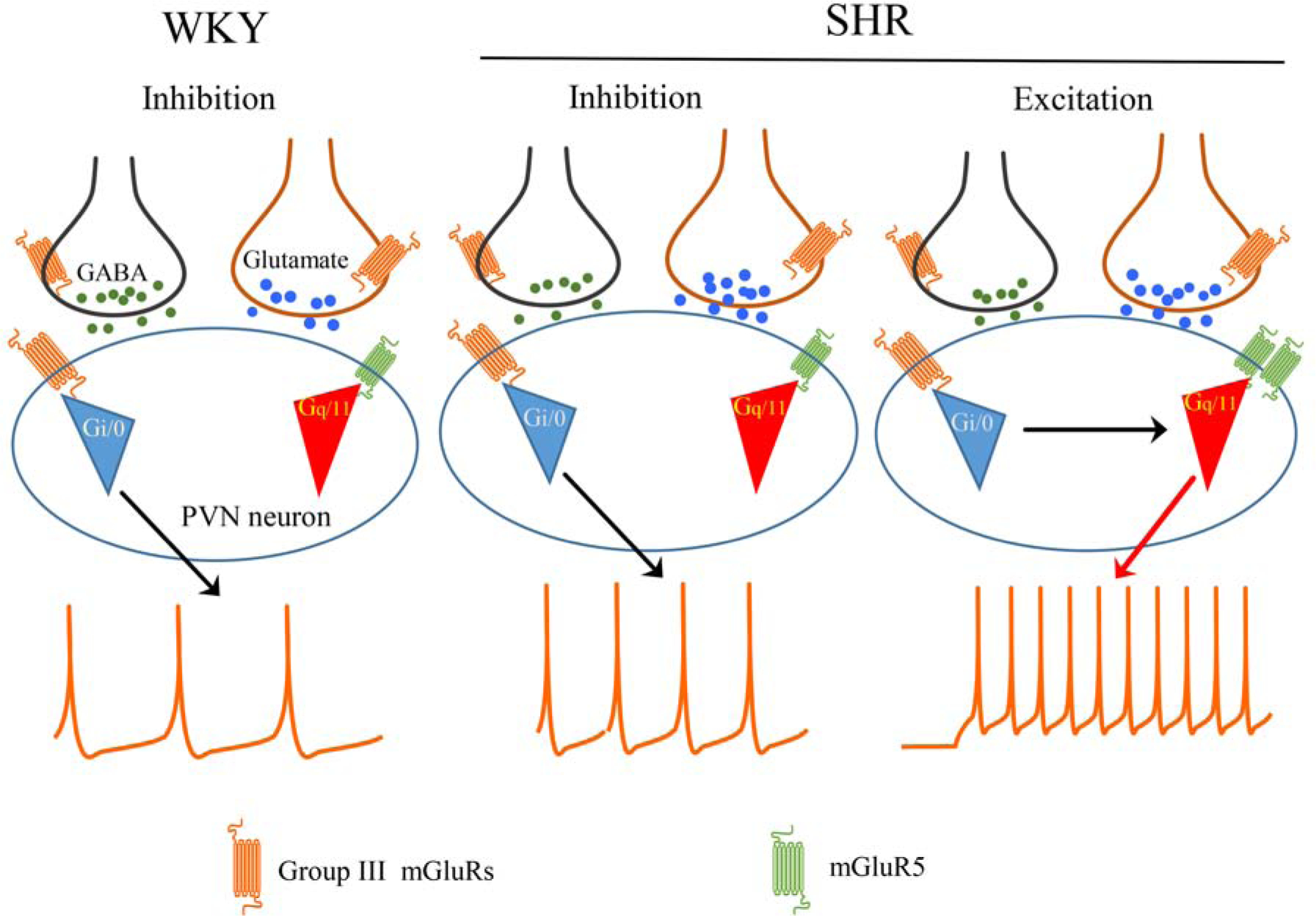
Group III mGluRs, a group of Gi/o-coupled receptors, are expressed at pre- and postsynaptic sites in the PVN. Activation of presynaptic group III mGluRs inhibits GABAergic and glutamatergic synaptic inputs to presympathetic PVN neurons in both WKY rats and SHRs. In WKY rats, stimulation of group III mGluRs consistently inhibits the firing of presympathetic PVN neurons. In SHRs, however, activation of group III mGluRs produces a divergent effect on the excitability of presympathetic PVN neurons. Whereas group III mGluR stimulation reduces the firing of some neurons, it excites other neurons via crosstalk with postsynaptic mGluR5, a Gq/11-coupled receptor.
Another significant finding of our study is that activation of group III mGluRs in the PVN primarily inhibits sympathetic vasomotor activity in normotensive rats and in SHRs. This finding suggests that despite the excitatory effect of group III mGluRs on some PVN presympathetic neurons in SHRs, the overall effect of group III mGluR activation in the PVN is to reduce sympathetic outflow via reducing augmented glutamatergic input (Li and Pan, 2007; Ma et al., 2018; Ye et al., 2011; Ye et al., 2013). Because the baselines were different between WKY rats and SHRs, we normalized changes of ABP and LSNA to the values after MPEP (MPEP alone) so that we can compare the magnitude of the inhibitory effect of L-AP4 microinjection after blocking mGluR5 in the two groups. Remarkably, the sympathoinhibitory effect of L-AP4 in the PVN was augmented by blocking mGluR5 in SHRs, suggesting that increased mGluR5 activity can offset the inhibitory effect of group III mGluR activation on sympathetic vasomotor activity in hypertension. Thus, concurrently blocking mGluR5 and stimulating group III mGluRs (mGluR4/mGluR6) in the PVN could be an effective approach to reducing sympathetic overflow in neurogenic hypertension.
In summary, our study provides new evidence that group III mGluRs regulate glutamatergic and GABAergic synaptic inputs to PVN presympathetic neurons. Stimulation of presynaptic and postsynaptic group III mGluRs in the PVN has a distinct effect on neuronal excitability in hypertension. This information advances our understanding of the cellular and molecular mechanisms of regulating sympathetic outflow in hypertension. Concomitantly activating group III mGluRs and antagonizing mGluR5 may represent an opportune strategy to treat neurogenic hypertension. Further studies are needed to determine whether group III mGluRs have a similar role in regulating sympathetic outflow in other animal models of hypertension and to characterize the interaction between group I and group III mGluR-mediated signaling pathways in the PVN in hypertension.
Highlights:
Group III metabotropic glutamate receptor (mGluR) stimulation consistently inhibits hypothalamic presympathetic neurons in normotension but produces a divergent effect on these neurons in hypertension.
Presynaptic group III mGluR activation reduces excitatory and inhibitory synaptic inputs to hypothalamic presympathetic neurons in both normotension and hypertension.
Stimulation of group III mGluRs paradoxically excites some hypothalamic presympathetic neurons in hypertension through crosstalk with postsynaptic mGluR5.
Group III mGluR activation in the paraventricular nucleus reduces sympathetic vasomotor activity, which is enhanced by blocking mGluR5 in hypertension.
List of Chemical Compounds: γ-aminobutyric acid; (RS)-α-cyclopropyl-4-phosphonophenylglycine; L-2-amino-4-phosphonobutyric acid; S-3,5-dihydroxyphenylglycine; glutamate; guanosine 5′-[β-thio]diphosphate; 2-methyl-6-(phenylethynyl) pyridine.
Acknowledgments
This study was supported by the National Institutes of Health (grants HL131161 and HL142133) and by the N.G. and Helen T. Hawkins Endowment (to H.-L.P.).
Abbreviations:
- ABP
arterial blood pressure
- aCSF
artificial cerebrospinal fluid
- CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
- DHPG
S-3,5-dihroxyphenylglycine
- GDP-β-S
guanosine 5′-[β-thio]diphosphate
- L-AP4
L-2-amino-4-phosphonobutyric acid
- LSNA
lumbar sympathetic nerve activity
- mGluR
metabotropic glutamate receptor
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- PCR
polymerase chain reaction
- sEPSC
spontaneous excitatory synaptic current
- sIPSC
spontaneous inhibitory synaptic current
- PVN
paraventricular nucleus
- RRID
Research Resource Identifier
- SHR
spontaneously hypertensive rat
- WKY
Wistar Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest with the contents of this article.
References
- Acuna-Goycolea C, Li Y, Van Den Pol AN, 2004. Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci 24, 3013–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, 2002. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39, 275–280. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ, 1996. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci 16, 2044–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Rees HD, Yi H, Levey AI, Conn PJ, 1998. Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J Neurochem 71, 636–645. [DOI] [PubMed] [Google Scholar]
- Charpak S, Thompson SM, Gahwiler BH, Gerber U, 1992. Characterization of l-2-Amino-4-Phosphonobutanoate Action Following Sensitization by Quisqualate in Rat Hippocampal Slice Cultures. Eur J Neurosci 4, 491–499. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li DP, Pan HL, 2006. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther 316, 733–742. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Alford S, 1998. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron 20, 1007–1016. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F, 2002. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110, 403–420. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Michelini LC, Li DP, Pan HL, 2018. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 315, H1200–H1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin J, Nicoletti F, 2001. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci 22, 114–120. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R, 1999. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38, 1493–1503. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Bains JS, 2003. Priming of excitatory synapses by alpha1 adrenoceptor-mediated inhibition of group III metabotropic glutamate receptors. J Neurosci 23, 6223–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Kendall DA, 1987. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol 91, 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiski JB, Pittman QJ, Bains JS, 2009. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron 62, 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Atnip LM, Chen SR, Pan HL, 2005. Regulation of synaptic inputs to paraventricular-spinal output neurons by alpha2 adrenergic receptors. J Neurophysiol 93, 393–402. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL, 2003. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23, 5041–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL, 2005. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL, 2006. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290, H1110–1119. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL, 2007. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49, 916–925. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL, 2010. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol 299, R552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL, 2008a. Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295, H807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL, 2008b. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Zhu LH, Pachuau J, Lee HA, Pan HL, 2014. mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J Neurosci 34, 4309–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Chen SR, Chen H, Zhou JJ, Li DP, Pan HL, 2018. alpha2delta-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol 596, 4269–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muram S, Rowe TM, Hirasawa M, 2016. Presynaptic G Protein-Coupled Receptors Differentially Modulate Spontaneous Glutamate Release in the Supraoptic Nucleus. J Neuroendocrinol 28. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ, 2010. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S, 1994. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 77, 361–369. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N, 1995. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol 360, 555–570. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Kakizawa S, Hirose K, Iino M, 2004. Cross talk between metabotropic and ionotropic glutamate receptor-mediated signaling in parallel fiber-induced inositol 1,4,5-trisphosphate production in cerebellar Purkinje cells. J Neurosci 24, 9513–9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Poulain DA, Oliet SH, 2004. Regulation of transmitter release by high-affinity group III mGluRs in the supraoptic nucleus of the rat hypothalamus. Neuropharmacology 47, 333–341. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH, 1999. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience 88, 949–957. [DOI] [PubMed] [Google Scholar]
- Qiao X, Zhou JJ, Li DP, Pan HL, 2017. Src Kinases Regulate Glutamatergic Input to Hypothalamic Presympathetic Neurons and Sympathetic Outflow in Hypertension. Hypertension 69, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitterer U, Lohse MJ, 1999. Crosstalk between Galpha(i)- and Galpha(q)-coupled receptors is mediated by Gbetagamma exchange. Proc Natl Acad Sci U S A 96, 10626–10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, 2001. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299, 12–20. [PubMed] [Google Scholar]
- Schrader LA, Tasker JG, 1997. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol 77, 527–536. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD, 1989. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491, 156–162. [DOI] [PubMed] [Google Scholar]
- Upreti C, Zhang XL, Alford S, Stanton PK, 2013. Role of presynaptic metabotropic glutamate receptors in the induction of long-term synaptic plasticity of vesicular release. Neuropharmacology 66, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Byun HS, Li L, Pan HL, 2012. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci 32, 8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Li L, Pan HL, 2011. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci 31, 8271–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Pan HL, 2013. Regulation of Hypothalamic Presympathetic Neurons and Sympathetic Outflow by Group II Metabotropic Glutamate Receptors in Spontaneously Hypertensive Rats. Hypertension 62, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Adams CD, Bains JS, Sakurai T, Aston-Jones G, Graham BA, Dayas CV, 2019. Activation of lateral hypothalamic group III metabotropic glutamate receptors suppresses cocaine-seeking following abstinence and normalizes drug-associated increases in excitatory drive to orexin/hypocretin cells. Neuropharmacology 154, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL, 2009. Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience 158, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]


