Abstract
SPARC is a matricellular protein, able to modulate cell/ECM interactions and influence cell responses to growth factors, and therefore is particularly attuned to contribute to physiological processes involving changes in ECM and cell mobilization. Indeed, the list of biological processes affected by SPARC includes wound healing, tumor progression, bone formation, fibrosis, and angiogenesis. The process of angiogenesis is complex and involves a number of cellular processes such as endothelial cell proliferation, migration, ECM degradation, and synthesis, as well as pericyte recruitment to stabilize nascent vessels. In this review, we will summarize current results that explore the function of SPARC in the regulation of angiogenic events with a particular emphasis on the modulation of growth factor activity by SPARC in the context of blood vessel formation. The primary function of SPARC in angiogenesis remains unclear, as SPARC activity in some circumstances promotes angiogenesis and in others is more consistent with an anti-angiogenic activity. Undoubtedly, the mercurial nature of SPARC belies a redundancy of functional proteins in angiogenesis as well as cell-type-specific activities that alter signal transduction events in response to unique cellular milieus. Nonetheless, the investigation of cellular mechanisms that define functional activities of SPARC continue to contribute novel and exciting paradigms to vascular biology.
Keywords: SPARC/osteonectin, TGF-β, Angiogenesis, Extracellular matrix
Introduction
Secreted protein acidic and rich in cysteine (SPARC), also referred to as osteonectin or BM-40, is a 32-kDa Ca2+-binding glycoprotein secreted by a variety of cell types. SPARC was originally reported to be a major constituent of non-collagenous fetal calf bone protein extracts [1, 2]. Subsequently, Sage et al. [3] described SPARC as an unidentified albumin-binding protein expressed and secreted by cultured fibroblasts, endothelial cells, vascular smooth muscle cells, and tumor cells. Shortly thereafter, the gene encoding SPARC was cloned from mouse parietal endoderm cells [4]. These studies laid the foundation for the study of SPARC and its function. Thirty years since its initial discovery, we now know SPARC as a matricellular protein able to regulate a myriad of processes including cell migration, cell proliferation, tissue morphogenesis, and tissue repair, and we now have a more detailed understanding of its molecular and biochemical properties [5, 6].
In early studies, in vitro activity assays implicated SPARC as an important regulator of angiogenesis. SPARC was isolated initially from proliferating cultured endothelial cells and, paradoxically, the addition of purified SPARC was shown to decrease proliferation of endothelial cells [3, 7, 8]. Addition of purified SPARC to bovine aortic endothelial cells inhibited 3H-thymidine incorporation and the onset of S-phase in a dose-dependent manner [9]. Exogenous SPARC was subsequently shown to have differential effects on fibroblasts distinct from its effects on endothelial cells, suggesting that effects of SPARC on proliferation were not direct and were cell-type-dependent [10].
Results from animal studies have also supported the concept of SPARC as a regulator of angiogenesis. These include immunohistochemical detection and in situ hybridization studies in which SPARC expression was detected in embryonic brain capillaries, dermal wounds capillaries, and in newly formed vessels of the allantoic membrane [11, 12]. Studies in SPARC −/− mice have demonstrated that SPARC influences angiogenic responses, though whether it serves as a promoter or inhibitor of the angiogenic response is dependent on the model being used. Somewhat surprisingly, given the high levels of SPARC expression in endothelial cells, SPARC −/− mice were not found to exhibit abnormalities in developmental angiogenesis. However, adult SPARC −/− mice have been shown to demonstrate differential response to angiogenic stimuli, most well characterized in angiogenic events associated with tumor growth.
Brekken et al. [13] first showed that LLC cells (Lewis lung carcinoma) grown subcutaneously in SPARC −/− mice exhibited reduced vascular area compared to those grown in SPARC +/+ mice. Puolakkainen et al. [14] then demonstrated that Pan02 cells (mouse pancreatic carcinoma) grown subcutaneously in SPARC −/− mice exhibited decreased pericyte-associated vessels compared to those grown in SPARC +/+. These results suggested that SPARC positively regulates tumor angiogenesis. In support of this, Pan02 tumors grown in the pancreata of SPARC −/− mice exhibited a decrease in both vessel number and vessel maturity compared to those grown in SPARC +/+ mice [15]. In other models of angiogenesis using implanted materials, results have been, on first glance, contradictory. Whereas studies using dermally implanted sponges showed increased fibrovascular invasion in SPARC −/− versus SPARC +/+ mice, the vascular capsule formed in response to implanted silicone discs was decreased in SPARC −/− versus SPARC +/+ animals. Altogether, one conclusion that can be drawn from these studies is that SPARC activity is a critical regulator of the angiogenic process; however, these studies also highlight the complexity of SPARC function in vivo.
SPARC was originally described as an extracellular matrix (ECM)-associated protein with implications in bone mineralization, and subsequent studies have found SPARC to localize to the ECM of developing tissues; therefore much work has been done to characterize the nature of SPARC–ECM interactions and was recently reviewed by Bradshaw [16]. In this review, we will highlight the activity of SPARC in the regulation of growth factor signaling with particular emphasis on events that regulate angiogenesis and those that have been shown to be relevant in vivo. The contribution of SPARC to the effect of three different growth factors that regulate the angiogenic process, VEGF, FGF2, and TGF-β, will be discussed with inclusion of a discussion of SPARC and integrin-signaling pathways (summarized in Table 1; Fig. 1).
Table 1.
Angiogenic molecules regulated by SPARC
| Molecule | Role in angiogenesis | Effect of SPARC | References |
|---|---|---|---|
| VEGF-A | Stimulates endothelial cell activation and initiates angiogenesis through VEGFR2 | SPARC directly interacts with VEGF-A and prevents it from inducing VEGFR1 activity in cultured endothelial cells and in vivo. VEGF-A is predicted to interact with SPARC at its VEGFR1-binding site | [18–20] |
| FGF2 | Stimulates endothelial cell activation and initiates angiogenesis through FGFR1 | SPARC inhibits the FGF2-induced activation of endothelial cells in vitro | [22] |
| PDGFB | Stimulates pericyte proliferation and migration through PDGFRβ | SPARC directly interacts with PDGF-BB and -AB and prevents PDGF-induced responses in fibroblasts and mural cells in vitro | [70, 71] |
| TGF-β1 | Can stimulate endothelial cell activation through ALK1 and inhibit activation through ALK5. Inhibits pericyte migration and induces their differentiation through ALK5 | TGF-β1 induces expression of SPARC in a variety of cell types including fibroblasts. SPARC also regulates expression of TGF-β1. Activation of TGF-β1 can be induced or inhibited by SPARC | [31–33, 35–37] |
| α6 Integrin | Heterodimerizes with β1 and β4 integrins to form laminin receptors. Effects of these integrins on angiogenesis vary. Their function is inconclusive | SPARC decreases expression and activity of α6β1 in lens epithelial cells and α6 integrins in preadipocytes in vitro | [48, 72] |
| β1 Integrin | Forms heterodimers with 11 distinct α subunits. Mediates pericyte recruitment and endothelial survival | SPARC interacts with β1 integrin and induces ILK activity in lens epithelial cells | [49] |
| αvβ3 and αvβ5 Integrins | Vitronectin receptors (also bind fibrinogen and fibronectin). Induce endothelial cell survival in response to ligand interaction, and induce cell death in the absence of ligand | SPARC can increase αvβ3 and αvβ5-mediated migration of prostate tumor cells and dental pulp cells in vitro. SPARC blocks αvβ3 and αvβ5-mediated attachment and surface expression in several ovarian cancer cell lines | [63, 66, 73] |
Fig. 1.
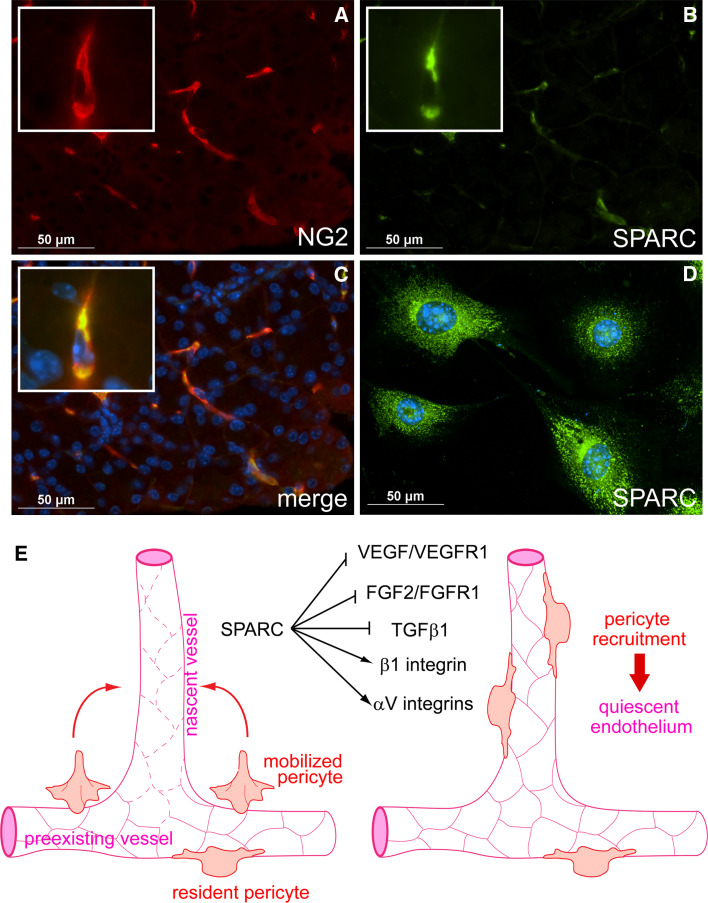
Regulation of angiogenesis by SPARC. a–c Pericyte expression of SPARC in murine pancreas. Pancreas was harvested from a 3-week-old mouse, sectioned, and subjected to immunofluorescent staining for the pericyte marker NG2 (a) and SPARC (b). The merged image is presented with nuclear DAPI staining in c. Pericytes express SPARC in vitro (d). Whole murine pancreata were subjected to collagenase digest; NG2+ cells were then isolated using anti-NG2 IgG immunomagnetic bead separation. Early passage cells were subjected to immunofluorescent staining for SPARC. SPARC staining is presented with nuclear DAPI staining in d. Note cytoplasmic staining of SPARC in b and d. e Model illustrating potential regulation by SPARC of integrin activity and multiple growth factor signaling pathways critical to angiogenesis. During angiogenesis, VEGF, FGF2, and TGF-β1 are secreted by cells or released from the ECM into an angiogenic milieu. These growth factors induce formation of new blood vessels that are marked by excessive leakiness and endothelial cell proliferation (left diagram; leaky vessels are indicated by dashed endothelial cell–cell junctions). Pericytes are mobilized and secrete SPARC into the angiogenic microenvironment, where it negatively regulates VEGF/VEGFR1 and FGF2/FGFR1 signaling, diminishing endothelial cell proliferation. SPARC also influences the activities of β1, αVβ3, and αVβ5 integrins. SPARC binds β1 integrin and stimulates downstream signaling in an interaction that may adversely affect ECM interactions. In contrast, SPARC seems to promote αV integrin–ECM interactions. SPARC also regulates activation of TGF-β1 signaling, however, the mechanism and outcome of this regulation is unclear and is perhaps cell-type-dependent. Evidence in support of an inhibitory activity of SPARC that blocks the activation of TGF-β1 in pericytes has been reported [14, 37, 40]. SPARC inhibition of TGF-β1 gives pericytes opportunity to migrate to the nascent vessel and engage the activated endothelium (right diagram). Subsequently, TGF-β1 is activated and pericytes induce vessel quiescence. The resulting mature vessel thus consists of both pericytes and endothelial cells. In total, the diversity of pathways that are influenced by SPARC places it as an important regulator of microenvironment-perception
Vascular endothelial growth factor (VEGF)
VEGF-A is a member of a subfamily of the PDGF family of growth factors. Three known receptor tyrosine kinases bind to members of this subfamily, but only VEGF-receptor 1 (VEGFR1) and VEGF-receptor 2 (VEGFR2) interact with VEGF-A [17]. Kupprion et al. [18] first demonstrated that 125I-labeled SPARC can to bind to immobilized VEGF-A and that this interaction was inhibited by unlabeled SPARC. Furthermore, a peptide corresponding to SPARC residues 254–273 (peptide 4.2) blocked binding of 125I-labeled VEGF-A to human microvascular endothelial cells, and SPARC was shown to specifically block VEGF-A-induced VEGFR1 phosphorylation while having no effect on VEGFR2 phosphorylation. Molecular docking simulations have predicted that the extracellular Ca2+-binding domain of SPARC interacts with the VEGFR1-binding site of VEGF-A, in support of a scenario in which SPARC specifically prevents VEGF-A from interacting with VEGFR1 [19].
VEGF-A drives angiogenesis by binding to and activating VEGFR2, which results in receptor autophosphorylation. SPARC bound to VEGF-A is predicted to decrease phosphorylation of VEGFR1 while having no effect on the phosphorylation of VEGFR2 [18]. Nozaki et al. [20] demonstrated that blockade of the VEGF-A/VEGFR1 interaction by SPARC enhances VEGF-A/VEGFR2 signaling to drive angiogenesis in a model of choroidal neovascularization (CNV) in which a laser is used to induce an angiogenic response in the eye. VEGF-A inhibited angiogenesis when it was injected into the animal after laser injury, but induced angiogenesis when added prior to laser injury. The anti-angiogenic effect of VEGF-A post injury was reversed if the animals were treated with a VEGFR1-blocking antibody, implicating VEGFR1 activity as a primary inhibitor of VEGF-A/VEGFR2-induced angiogenesis. SPARC was found to be expressed constitutively in the region of the injured eye. However, expression decreased over time after CNV was induced, consistent with SPARC blocking the antagonistic effect of VEGFR1 at the time of injury. In support of this, VEGF-A was unable to stimulate CNV when administered before injury in SPARC −/− mice and injection of recombinant SPARC (rSPARC) blocked the inhibitory effect of VEGF-A on CNV post injury. Conclusions from these studies included that SPARC can function to block VEGFR1-mediated recruitment of the phosphatase SHP-1 to VEGFR2, thereby allowing phosphorylated VEGFR2 to induce an angiogenic response.
Fibroblast growth factor 2 (FGF2)
SPARC has been shown to influence FGF2/fibroblast growth factor receptor 1 (FGFR1) signaling, though not through direct binding with soluble growth factor [21, 22]. FGF2 binds and signals through the FGFR family of receptor tyrosine kinases, and formation of a ternary complex of FGF-bound to FGFR with heparan sulfate proteoglycans is required for many FGF-induced responses [23]. Hasselaar et al. [21] demonstrated that SPARC inhibited FGF2-induced migration of endothelial cells without inhibiting interaction of 125I-labeled FGF2 with cells. Motamed et al. [22] showed that biotinylated SPARC, even at a threefold molar excess, was unable to interfere with the binding of FGF2 to recombinant human FGFR1-Fc chimeric protein. However, SPARC inhibited FGF2-induced phosphorylation of FGFR1, MAPK activation, and DNA synthesis and did not require heparan sulfate proteoglycans. Furthermore, addition of SPARC blocked the effect of FGF2 on MM14 terminal differentiation, a murine myoblast cell line that requires FGF2/FGFR1 signaling to maintain an undifferentiated state [24].
Activated FGFR1 receptor induces many of the same intracellular signaling cascades as those activated by VEGFR2 and therefore promotes endothelial cell survival and induces proliferation and migration [25]. Experiments with human microvascular cells have shown that SPARC can inhibit the effect of FGF2-induced proliferation of endothelial cells through inhibition of DNA synthesis, MAPK phosphorylation, and FGFR1 phosphorylation (Motamed et al. 2003). Interestingly, the FGF2 inhibitory activity of SPARC was mapped to a region within SPARC residues 254–273, the same region that binds to VEGF-A and inhibits interaction of VEGF-A with VEGFR1, indicating that the effect of SPARC on FGF2 signaling might be linked to effects on VEGF-A receptor signaling. Endothelial cells likely express endogenous VEGF-A as autocrine VEGF-A signaling is required for endothelial cell survival [26]. A recent report by Lichtenberger et al. [27] demonstrated that an autocrine VEGF-A/VEGFR1 signaling axis was required for EGF/EGFR-induced proliferation of squamous cell carcinoma cells. As a direct interaction between SPARC and FGF2 has not been shown, perhaps autocrine VEGF-A/VEGFR1 signaling is required for the proliferative effects of FGF2 and SPARC inhibition of activity. Thus diminished interaction of VEGF-A with VEGFR1 mediated by SPARC might decrease FGF2-induced signal transduction through FGFR1. Further examination of the function of SPARC in the regulation of each of these pathways is needed to characterize the molecular basis of SPARC activity.
Transforming growth factor-β1 (TGF-β1)
Upon association of pericytes with endothelial cells, TGF-β1 activation induces pericyte maturation and blood vessel stabilization. Genetic ablation of TGF-β1 results in abnormal vessel formation and embryonic lethality in mice [28, 29]. A connection between SPARC and TGF-β1 was first shown at the level of expression. Wrana et al. [30] demonstrated that TGF-β1 induced SPARC expression twofold in a population of fibroblast-like fetal calvarial cells. Later studies revealed that SPARC expression was induced by TGF-β1 in a variety of cell types [31–33]. Conversely, SPARC can also regulate expression of TGF-β1. Francki et al. [34] found that lack of SPARC expression in mesangial cells resulted in decreased TGF-β1 expression and that addition of rSPARC restored TGF-β1 expression to wild-type levels. The bidirectional regulation of expression between SPARC and TGF-β1 indicates that perhaps these two proteins function in cooperation with one another.
In addition to expression, SPARC also has the capacity to regulate TGF-β1 activity, though the precise mechanism behind this regulation is unclear. Mesangial cells isolated from SPARC −/− mice exhibited over 50% reduction in basal SMAD2 phosphorylation compared to cells isolated from SPARC +/+ mice [35]. Interestingly, addition of rSPARC to SPARC −/− cells for 30 min was sufficient to increase basal SMAD2 phosphorylation, a result consistent with a direct effect of SPARC and not an increase in TGF-β1 expression. In support of this, rSPARC had a synergistic effect on TGF-β1-induced SMAD2 phosphorylation in SPARC +/+ cells [35]. Furthermore, Schiemann et al. [36] found that SPARC-induced SMAD2 phosphorylation in endothelial and epithelial cells, an effect that was blocked with a neutralizing TGF-β antibody.
In contrast to these results, Chlenski et al. [37] found that SPARC had a negative effect on TGF-β1 activity in vivo and in vitro. Transformed human embryonic kidney cells (HEK293) transfected with SPARC or an empty vector were used in a mouse xenograft model. Tumors expressing SPARC exhibited a significant decrease in the level of activated fibroblasts as detected by SMAD2-induced α-SMA expression. Forced expression of SPARC by HEK293 cells suppressed the capacity of the cells to induce 3T3 fibroblast expression of α-SMA in co-culture experiments. Likewise, conditioned media from HEK293 cells expressing SPARC was less able to stimulate α-SMA expression compared to conditioned media from control cells. Whereas incubation with purified TGF-β1 induced α-SMA expression in 3T3 cells, rSPARC blocked this effect. Lastly, rSPARC blocked TGF-β1-induced phosphorylation of SMAD2 in both 3T3 cells and primary human fibroblasts. Interestingly, SPARC also controlled FGF2-induced effects on 3T3 cells as conditioned media from HEK293 cells expressing SPARC was able to enhance the effect of FGF2-induced migration.
TGF-β1/SMAD2 signaling induces pericyte expression of α-SMA as well as components of the ECM, and inhibits their migration; therefore TGF-β1 activation must be spatially regulated to prevent premature differentiation of pericytes [38–41]. The results of Chlenski et al. [37] would predict that SPARC promotes pericyte migration toward nascent endothelial tubes by diminishing activation of TGF-β1 signaling. In support of this, SPARC activity was shown to promote pericyte recruitment in an orthotopic tumor model [14, 15]. In addition, we have recently uncovered that SPARC decreases TGF-β1 activity in pericytes in an endoglin- and integrin-dependent manner [42].
The apparently contradictory activities exhibited by SPARC in terms of TGF-β activity might be due to differences in cell-type-specific TGF-β1 signaling pathways, tissue microenvironment, and/or the source of SPARC protein used in different laboratories. Furthermore, SPARC is differentially glycosylated in various cell types, which is known to influence fibrillar collagen binding and might dictate alternate functions of SPARC in a number of in vitro and in vivo assays [43]. As the functional relationship of SPARC with TGF-β1 is currently unclear, future studies examining differences in cell lines exhibiting opposing TGF-β1-dependent responses to SPARC would likely reveal important cellular mechanisms of SPARC and TGF-β1.
Integrins
Control of integrin activity by SPARC provides another mechanism through which this matricellular protein might impact angiogenesis and perhaps influence growth factor activity. SPARC, a counter-adhesive protein, was first reported to participate in substrate/cell interactions in studies using bovine aortic endothelial cells in which addition of SPARC resulted in a loss of focal adhesions as assayed using interference reflective microscopy [44]. SPARC also induced diffusion of vinculin out of focal adhesions and redistribution of actin to the cell periphery. Since these early experiments, SPARC has been shown to regulate the expression, surface level, and activity of many integrins and integrin subunits involved in the angiogenic cascade.
Regulation of β1 integrins
The β1 integrin subunit forms heterodimers with 11 different α subunits to generate substrate-specific ECM receptors [45–47]. In lens epithelial cells, the absence of SPARC was associated with increased levels of the laminin receptor heterodimer, α6β1 integrin. In addition, SPARC decreased expression of the α6 integrin subunit in preadipocytes when added exogenously to cells in vitro [48]. Thus, in at least two cell types, expression of SPARC regulated levels of α6 integrin and adhesive events mediated by α6 integrin. A function of SPARC in the regulation of α6 integrin levels in endothelial cells has not been assessed.
Recently, SPARC has been shown to interact directly with β1 integrin [49]. Weaver et al. [49] found that SPARC expression in cultured mouse lens epithelial cells increased in response to cell stress. Furthermore, SPARC −/− cells demonstrated an increased susceptibility to stress-induced apoptosis compared to their SPARC +/+ counterparts. Integrin linked kinase (ILK) is a critical regulator of cell death via its direct phosphorylation of a variety of proteins including Akt [50]. As SPARC regulates ILK activity in fibroblasts and its downstream effectors such as Akt in glioma cell lines, Weaver et al. investigated the function of ILK in the pro-survival effect of SPARC and found that ILK activity was induced in response to stress and was required for the pro-survival effect of SPARC [49, 51, 52]. Immunoprecipitation experiments revealed that β1 integrins associated in a complex with SPARC and ILK. The β1 binding site on SPARC was mapped to residues 113–130, a region that contains the previously characterized angiogenic, Cu2+-binding sequence GHK [10, 11, 53]. Whether SPARC preferentially interacts with specific β1 heterodimers (i.e., β1 in association with specific α subunits) was not addressed in these studies.
In the vasculature, β1 integrins are expressed both by endothelial cells and pericytes in resting vasculature as well as during angiogenesis [47, 54]. Various studies have pointed to a proangiogenic function for β1 integrins expressed by both mural cells and endothelial cells. As SPARC is expressed and secreted by vascular cells during angiogenesis, it seems highly plausible that a mechanism of SPARC-mediated cell survival that acts through the extracellular interaction of the GHK domain of SPARC with β1 integrins might positively regulate vascular cell behavior during blood vessel formation. Derivative peptides of SPARC promoted chick chorioallantoic membrane angiogenesis, bovine aortic endothelial cell tube formation, and endothelial cell proliferation [10, 11]. Though a mechanism in which SPARC, through its GHK domain, induces angiogenesis through interactions with β1 integrin heterodimers seems likely, experiments specifically addressing the dependency of SPARC-induced angiogenesis on β1 integrin expression by pericytes and endothelial cells are currently lacking.
In a separate study, Weaver et al. demonstrated that, in lens epithelial cells, an antibody against β1 integrin that inhibited ECM binding by integrin heterodimers, blocked SPARC from associating with β1 integrin receptors. SPARC might therefore compete with ECM substrates for engagement of integrin receptors [49]. As ECM interactions with β1 integrins are required for normal angiogenesis, the activity of SPARC to modulate β1 integrin engagement is likely to be temporally regulated. Indeed, Iruela-Arispe et al. [12] found that SPARC protein but not message was highest in newly formed chick chorioallantoic membrane vasculature between days 9 and 15 but dropped afterwards. The highest levels of SPARC protein spatially overlapped regions of plasmin protease activity, suggesting that plasmin-induced angiogenic SPARC cleavage products are beneficial only in the early phases of new blood vessel formation.
Regulation of αV integrins
αV integrin receptors represent the most studied group of integrins involved in angiogenesis to date [55]. Genetic ablation of αV integrin expression results in perinatal lethality due to abnormal association of cerebral blood vessels with the surrounding parenchyma [56]. A plethora of studies has revealed an increase in αV integrin expression in vascular cells in response to various angiogenic stimuli [55, 57]. αV integrins can dimerize with β3, β5, or β8 integrin subunits to form vitronectin receptors, and with β6 subunits to form fibronectin receptors. The majority of reports have demonstrated that αVβ3 and αVβ5 are the only αV integrins expressed by vascular cells, and these were found expressed in endothelial cells only. However, we and others have found that stellate cells, which function as pericytes in various organs including the pancreas and the liver, express αV, β3, β5, β6, and β8 [58–61]. Specific antagonism of αVβ3 and αVβ5 has proven to be an effective strategy for blocking tumor angiogenesis in clinical trials with glioblastoma patients [62]. Studies using human dental pulp cells and various tumor lines have shown that SPARC is able to modulate expression and activity of both αVβ3 and αVβ5 integrins.
For example, in a study of bone metastasis in prostate cancer, SPARC was shown to enhance migration of a human prostate cell line [63]. The stimulatory effect of SPARC on cell migration required αVβ3 and αVβ5 integrins, as specific blockade of either of these receptors significantly decreased SPARC-induced migration. Interestingly, blocking VEGFR2 had a similar effect on SPARC-induced migration. Ligation of SPARC by αVβ5 integrin was found to stimulate VEGF expression, an effect that was blocked with the αVβ5 integrin-specific inhibitor cRGDfV. Similarly, SPARC induced the migration of human dental pulp cells in a dose-dependent manner and was blocked by αVβ3 integrin-inhibiting antibodies [64].
In contrast, SPARC appeared to have a negative effect on αV integrins in human ovarian cancer cells. rSPARC was able to block Mn2+-induced, αVβ3-mediated adhesion of several human ovarian cancer cell lines to vitronectin [65]. Incubation of SKOV3 cells with SPARC decreased surface expression of αVβ3 by 74%, and αVβ5 by 32%. SPARC also blocked αV integrin-induced adhesion of ID8 mouse ovarian cancer cells to vitronectin and to peritoneal explants [66]. The results from ovarian cancer cell lines implied that SPARC blocks αVβ3 and αVβ5 activity, whereas studies using other cell types suggested SPARC enhanced activity of these receptors [63, 64]. The discrepancy perhaps indicates that SPARC influences αV integrin activity through an indirect mechanism that is dependent upon cell-type-specific protein expression. A detailed analysis of SPARC and αV integrins in endothelial cells would contribute to our understanding of how SPARC affects angiogenesis.
Conclusions
That SPARC is involved in regulating angiogenesis is supported by: (1) the induction of SPARC expression in the vasculature during both physiological and pathological angiogenesis, (2) the capacity of SPARC to modulate growth factor activity required for angiogenesis, and (3) functional modulation by SPARC of integrin activity utilized by endothelial cells and pericytes during angiogenesis. For these reasons, manipulation of SPARC activity may prove to be a beneficial strategy against diseases characterized by blood vessel formation. Perhaps the most immediately relevant of such diseases is cancer [67]. Tumor-associated blood vessels are typically quite different from the vasculature of normal tissues. They are tortuous, leaky, and are often discontinuous, characteristic features of an unstable vasculature, and are comprised of abnormal endothelial cells and pericytes [67]. That pericytes represent a beneficial target for anti-angiogenic therapy is established. Targeting pericytes by blocking PDGFRβ enhanced the activity of anti-VEGF-A therapy to decrease tumor blood vessel number and control tumor growth in a mouse model of islet carcinoma [68]. As SPARC was shown to promote pericyte recruitment in orthotopic pancreatic tumors grown in mice, a SPARC-mediated block of PDGF-B binding to PDGFRβ is not favored as a primary effector of SPARC on pericyte behavior [69]. SPARC has been shown to bind and reduce PDGF binding to receptor in vitro using relatively high amounts of SPARC protein. However, SPARC −/− mice exhibited reduced pericyte recruitment in models of pancreatic cancer. Therefore, the effects of SPARC on TGF-β-mediated pericyte response is likely more relevant in vivo than that of PDGF [30–37]. Regulation of TGF-β activity in pericytes by SPARC may represent a novel mechanism for modulating pericyte behavior in vivo with clinical implications in tumor therapy.
Based on current knowledge, a unifying hypothesis of the function of SPARC in angiogenesis is not readily apparent. Perhaps the complexity of cellular events required for the generation of new blood vessels from existing vasculature hinder labeling of some angiogenic factors as either pro or anti-angiogenic. Endothelial cell proliferation, migration, and ECM deposition are all required for vascular remodeling, it is plausible factors that promote one phase of angiogenesis might inhibit a subsequent process.
Acknowledgments
This work was supported in part by the NIH, NCI through R01CA118240 (RAB), NIGMS through T32 GM008203 (LBR), NIDCR through P20RR017696 (ADB), NIHLB through 094517 (ADB), and a Merit Award from the Veteran’s Administration to ADB.
Abbreviations
- SPARC
Secreted protein acidic and rich in cysteine
- ECM
Extracellular matrix
- PDGF
Platelet-derived growth factor
- VEGF
Vascular endothelial growth factor
- VEGFR1
VEGF receptor 1
- VEGFR2
VEGF receptor 2
- FGF
Fibroblast growth factor
- TGF-β
Transforming growth factor-beta
- BMP
Bone morphogenic protein
- αSMA
Alpha smooth muscle actin
- ER
Endoplasmic reticulum
- ILK
Integrin-linked kinase
- SHP-1
Src homology region 2 domain-containing phosphatase-1
- CNV
Choroidal neovascularization
Contributor Information
Amy D. Bradshaw, Phone: +1-843-7924959, FAX: +1-843-8765068, Email: bradshad@musc.edu
Rolf A. Brekken, Phone: +1-214-6485151, FAX: +1-214-6484940, Email: rolf.brekken@utsouthwestern.edu
References
- 1.Termine JD, Belcourt AB, Conn KM, Kleinman HK. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981;256:10403–10408. [PubMed] [Google Scholar]
- 2.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 3.Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984;259:3993–4007. [PubMed] [Google Scholar]
- 4.Mason IJ, Taylor A, Williams JG, Sage H, Hogan BL. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell ‘culture shock’ glycoprotein of Mr 43,000. EMBO J. 1986;5:1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/S0945-053X(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 6.Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an evolutionarily conserved collagen chaperone? J Dent Res. 2007;86:296–305. doi: 10.1177/154405910708600402. [DOI] [PubMed] [Google Scholar]
- 7.Sage H. Culture shock. Selective uptake and rapid release of a novel serum protein by endothelial cells in vitro. J Biol Chem. 1986;261:7082–7092. [PubMed] [Google Scholar]
- 8.Sage H, Tupper J, Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cell Physiol. 1986;127:373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- 9.Funk SE, Sage EH. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk SE, Sage EH. Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol. 1993;154:53–63. doi: 10.1002/jcp.1041540108. [DOI] [PubMed] [Google Scholar]
- 11.Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iruela-Arispe ML, Lane TF, Redmond D, Reilly M, Bolender RP, Kavanagh TJ, et al. Expression of SPARC during development of the chicken chorioallantoic membrane: evidence for regulated proteolysis in vivo. Mol Biol Cell. 1995;6:327–343. doi: 10.1091/mbc.6.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]
- 15.Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, et al. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech. 2010;3:57–72. doi: 10.1242/dmm.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan LA, Carbon JG, Roland CL, Toombs JE, Nyquist-Andersen M, Kavlie A, et al. r84, a novel therapeutic antibody against mouse and human VEGF with potent anti-tumor activity and limited toxicity induction. PLoS One. 2010;5:e12031. doi: 10.1371/journal.pone.0012031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekaran V, Ambati J, Ambati BK, Taylor EW. Molecular docking and analysis of interactions between vascular endothelial growth factor (VEGF) and SPARC protein. J Mol Graph Model. 2007;26:775–782. doi: 10.1016/j.jmgm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, et al. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006;116:422–429. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasselaar P, Sage EH. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J Cell Biochem. 1992;49:272–283. doi: 10.1002/jcb.240490310. [DOI] [PubMed] [Google Scholar]
- 22.Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, et al. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem. 2003;90:408–423. doi: 10.1002/jcb.10645. [DOI] [PubMed] [Google Scholar]
- 23.Quarto N, Amalric F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J Cell Sci. 1994;107(Pt 11):3201–3212. doi: 10.1242/jcs.107.11.3201. [DOI] [PubMed] [Google Scholar]
- 24.Templeton TJ, Hauschka SD. FGF-mediated aspects of skeletal muscle growth and differentiation are controlled by a high affinity receptor, FGFR1. Dev Biol. 1992;154:169–181. doi: 10.1016/0012-1606(92)90057-N. [DOI] [PubMed] [Google Scholar]
- 25.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/S0165-6147(00)01676-X. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 29.Dickson K, Philip A, Warshawsky H, O’Connor-McCourt M, Bergeron JJ. Specific binding of endocrine transforming growth factor-beta 1 to vascular endothelium. J Clin Invest. 1995;95:2539–2554. doi: 10.1172/JCI117955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrana JL, Maeno M, Hawrylyshyn B, Yao KL, Domenicucci C, Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988;106:915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrana JL, Overall CM, Sodek J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur J Biochem. 1991;197:519–528. doi: 10.1111/j.1432-1033.1991.tb15940.x. [DOI] [PubMed] [Google Scholar]
- 32.Ford R, Wang G, Jannati P, Adler D, Racanelli P, Higgins PJ, et al. Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes: regulation via a TGF-beta-dependent pathway. Exp Cell Res. 1993;206:261–275. doi: 10.1006/excr.1993.1146. [DOI] [PubMed] [Google Scholar]
- 33.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 34.Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 35.Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, et al. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91:915–925. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- 36.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlenski A, Guerrero LJ, Yang Q, Tian Y, Peddinti R, Salwen HR, et al. SPARC enhances tumor stroma formation and prevents fibroblast activation. Oncogene. 2007;26:4513–4522. doi: 10.1038/sj.onc.1210247. [DOI] [PubMed] [Google Scholar]
- 38.Antonelli-Orlidge A, Smith SR, D’Amore PA. Influence of pericytes on capillary endothelial cell growth. Am Rev Respir Dis. 1989;140:1129–1131. doi: 10.1164/ajrccm/140.4.1129. [DOI] [PubMed] [Google Scholar]
- 39.Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera LB, Brekken RA. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-β1 activity. J Cell Biol. 2011;193:1305–1319. doi: 10.1083/jcb.201011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann B, Muller S, Hanisch FG, Hartmann U, Paulsson M, Maurer P, et al. Structural variability of BM-40/SPARC/osteonectin glycosylation: implications for collagen affinity. Glycobiology. 2004;14:609–619. doi: 10.1093/glycob/cwh063. [DOI] [PubMed] [Google Scholar]
- 44.Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J Cell Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- 45.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham S, Kogata N, Fassler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 47.Carnevale E, Fogel E, Aplin AC, Gelati M, Howson KM, Zhu WH, et al. Regulation of postangiogenic neovessel survival by beta1 and beta3 integrins in collagen and fibrin matrices. J Vasc Res. 2007;44:40–50. doi: 10.1159/000097976. [DOI] [PubMed] [Google Scholar]
- 48.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29:281–291. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker TH, Baneyx G, Cardo-Vila M, Workman GA, Weaver M, Menon PM, et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem. 2005;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- 52.Shi Q, Bao S, Maxwell JA, Reese ED, Friedman HS, Bigner DD, et al. Secreted protein acidic, rich in cysteine (SPARC), mediates cellular survival of gliomas through AKT activation. J Biol Chem. 2004;279:52200–52209. doi: 10.1074/jbc.M409630200. [DOI] [PubMed] [Google Scholar]
- 53.Thompson WD, Stirk CM, Melvin WT, Smith EB. Plasmin, fibrin degradation and angiogenesis. Nat Med. 1996;2:493. doi: 10.1038/nm0596-493. [DOI] [PubMed] [Google Scholar]
- 54.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 55.Hynes RO. Cell–matrix adhesion in vascular development. J Thromb Haemost. 2007;5 Suppl 1:32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 56.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 58.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somanath PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. 2009;53:53–64. doi: 10.1007/s12013-008-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beer AJ, Schwaiger M. Imaging of integrin alphavbeta3 expression. Cancer Metastasis Rev. 2008;27:631–644. doi: 10.1007/s10555-008-9158-3. [DOI] [PubMed] [Google Scholar]
- 61.Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- 62.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De S, Chen J, Narizhneva NV, Heston W, Brainard J, Sage EH, et al. Molecular pathway for cancer metastasis to bone. J Biol Chem. 2003;278:39044–39050. doi: 10.1074/jbc.M304494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavasant P, Yongchaitrakul T, Pattamapun K, Arksornnukit M. The synergistic effect of TGF-beta and 1, 25-dihydroxyvitamin D3 on SPARC synthesis and alkaline phosphatase activity in human pulp fibroblasts. Arch Oral Biol. 2003;48:717–722. doi: 10.1016/S0003-9969(03)00134-1. [DOI] [PubMed] [Google Scholar]
- 65.Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol. 2005;167:1739–1752. doi: 10.1016/S0002-9440(10)61255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Said N, Najwer I, Motamed K. Secreted protein acidic and rich in cysteine (SPARC) inhibits integrin-mediated adhesion and growth factor-dependent survival signaling in ovarian cancer. Am J Pathol. 2007;170:1054–1063. doi: 10.2353/ajpath.2007.060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 68.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puolakkainen P, Bradshaw AD, Kyriakides TR, Reed M, Brekken R, Wight T, et al. Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials. Am J Pathol. 2003;162:627–635. doi: 10.1016/S0002-9440(10)63856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem. 2002;84:759–771. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 71.Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weaver MS, Sage EH, Yan Q. Absence of SPARC in lens epithelial cells results in altered adhesion and extracellular matrix production in vitro. J Cell Biochem. 2006;97:423–432. doi: 10.1002/jcb.20654. [DOI] [PubMed] [Google Scholar]
- 73.Pavasant P, Yongchaitrakul T. Secreted protein acidic, rich in cysteine induces pulp cell migration via alphavbeta3 integrin and extracellular signal-regulated kinase. Oral Dis. 2008;14:335–340. doi: 10.1111/j.1601-0825.2007.01383.x. [DOI] [PubMed] [Google Scholar]