Abstract
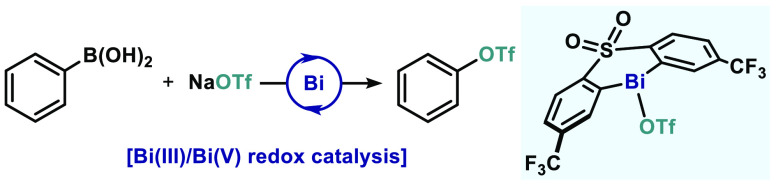
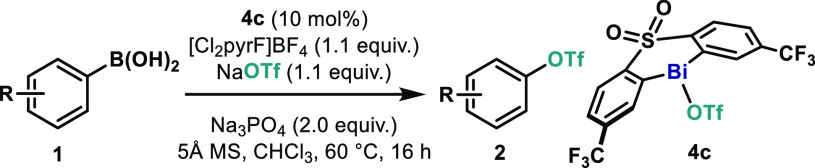
Herein we present a Bi-catalyzed cross-coupling of arylboronic acids with perfluoroalkyl sulfonate salts based on a Bi(III)/Bi(V) redox cycle. An electron-deficient sulfone ligand proved to be key for the successful implementation of this protocol, which allows the unusual construction of C(sp2)–O bonds using commercially available NaOTf and KONf as coupling partners. Preliminary mechanistic studies as well as theoretical investigations reveal the intermediacy of a highly electrophilic Bi(V) species, which rapidly eliminates phenyl triflate.
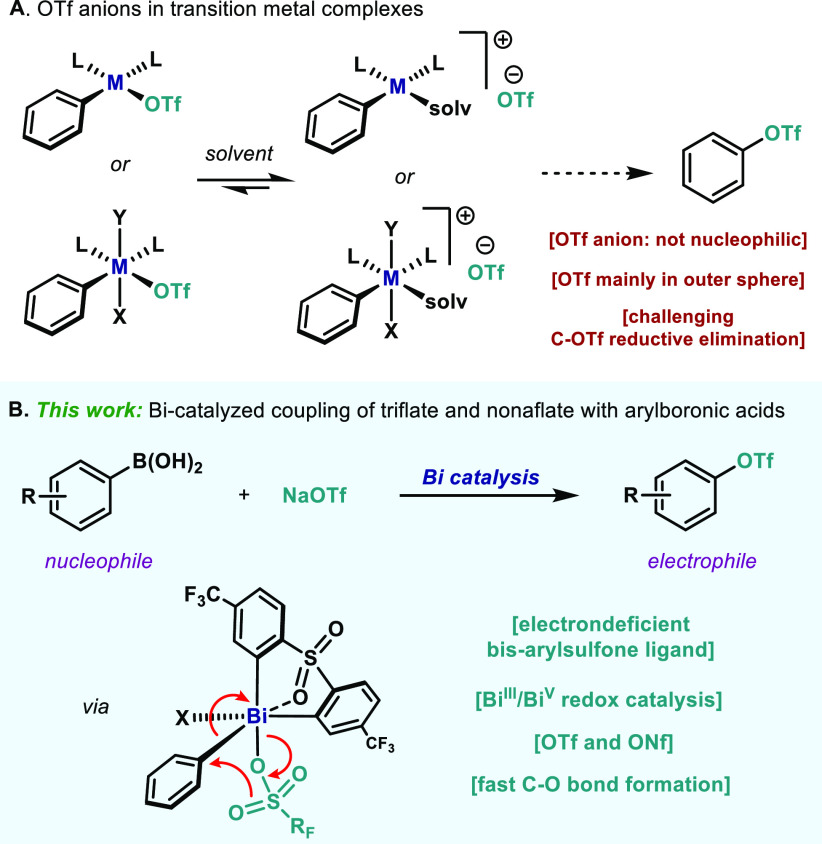
Functional groups such as the trifluoromethanesulfonate (triflate, OTf) or the nonafluorobutanesulfonate (nonaflate, ONf) are highly useful moieties when present in organic compounds, especially when attached to a carbon atom (C–OTf or C–ONf).1 Indeed, C(sp2)–OTf and C(sp2)–ONf have been utilized as surrogates of aryl halides (aka pseudohalides) due to their ability to heavily polarize the C–O bond, facilitating oxidative addition by d-block metals. This strategy has been largely exploited with a myriad of combinations of both transition metals and coupling partners,2 thus placing aryl triflates and nonaflates as routine electrophiles in this large arena.3 From the organometallic standpoint, OTf anions have also many attractive features. The coordinating properties of triflate anions have been a matter of intense debate in the recent literature.4 However, it is evident that differently than Ar–Cl, oxidative addition complexes of Ar–OTf would result in a remarkably weaker interaction of the OTf anion and the metal center in solution. Furthermore, in polar and coordinating solvents the OTf anion is generally relegated to the outer sphere, leaving a vacant coordination site (Figure 1A), which has been exploited for a variety of organometallic and coordination purposes.5 Yet, the great attributes of OTf anions—highly electronegative, poor nucleophiles and labile ligands—inherently situates them as one of the foulest anions to undergo C–O reductive elimination.6
Figure 1.
(A) OTf anions as ligands in transition metal chemistry. (B) Catalytic Ar–OTf formation through a Bi(III)/Bi(V) redox system.
Many examples with high-valent transition metals have been reported to accommodate OTf anions in the primary coordination sphere.7 However, reductive elimination primarily occurred at other anionic ligand sites and the M–OTf bond remained unaltered.8 During the synthesis of trisubstituted olefins, Gaunt and co-workers suggested that C(sp2)–OTf bonds could be formed through an unusual reductive elimination from a Cu(III) center,9 although further evidence was not provided. Indeed, examples of well-defined transition metal complexes that forge C(sp2)–OTf bonds still remain elusive. Notwithstanding, the development of a catalytic protocol which enables the formation of Ar–OTf from the corresponding organometallic reagent (Ar–M) and a commercially available triflate salt (MOTf) would be highly desirable from both the synthetic and fundamental point of view.
Our group has recently started a program to study the catalytic redox properties of bismuth (Bi) complexes,10 to facilitate transformations beyond the reactivity of transition metals.11 Hence, based on the known oxophilicity of Bi complexes12 and their ability to bind triflate,13 we envisaged that an oxidative protocol based on the redox couple Bi(III)/Bi(V) could fulfill this synthetic challenge. Indeed, a decade ago Mukaiyama and co-workers demonstrated that C–OTf bonds could be forged from Bi(V) compounds and HOTf, albeit in low yields.14 Inspired by this early precedent, herein we report on a catalytic oxidative coupling between arylboronic acids and triflate salts to furnish Ar–OTf species (Figure 1B). A rationally designed Bi complex bearing an electron-deficient diarylsulfone ligand unlocks a catalytic redox process and enables the use of triflate (OTf) and nonaflate (ONf) salts as coupling partners. Preliminary mechanistic investigations and theoretical analysis revealed that the C(sp2)–O bond formation is extremely fast from Bi(V) and is suggested to proceed through a five-membered transition state.
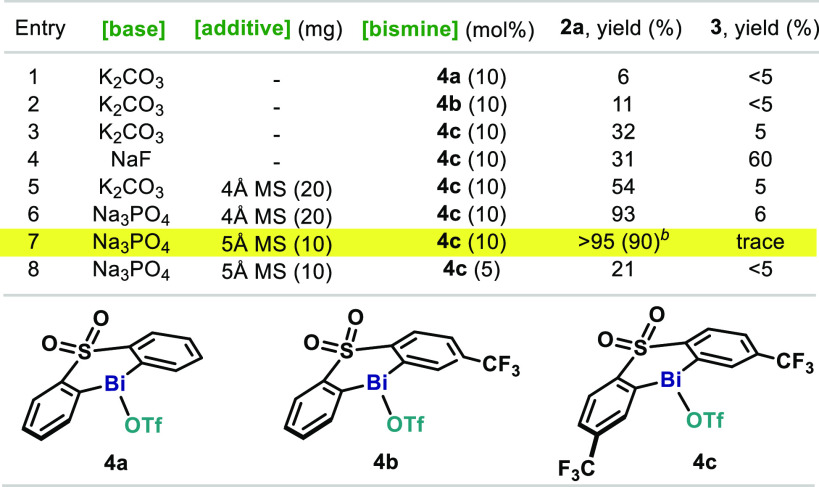
We started our investigations by optimizing the coupling of phenylboronic acid (1a) with NaOTf to generate phenyltriflate (2a) (Table 1). Based on our previous studies on Bi-catalyzed fluorination, bismines featuring a diarylsulfone backbone (4a–c) were selected as catalysts,15 together with N-fluoro-2,6-dichloropyiridinium tetrafluoroborate ([Cl2pyrF]BF4) as oxidant. To promote transmetalation, we selected K2CO3, as it has been recently demonstrated to be an excellent base for this purpose.16 In our initial attempts, the unsubstituted bismine catalyst (4a) provided no reactivity toward 2a (entry 1). However, when a CF3 group was introduced in meta-position to the Bi (4b), an encouraging 11% of 2a was obtained; interestingly, the formation of protodeboronation byproduct 3 was largely suppressed (entry 2). In line with these results, when two CF3 are introduced in the backbone of the sulfone (4c) the reactivity toward 2a increased to 32%, while the formation of 3 was still largely reduced (entry 3). When K2CO3 is replaced by NaF, a reversed trend in the product distribution is observed, substantially favoring undesired 3 (entry 4). Surprisingly, addition of 4 Å molecular sieves (MS) boosted the formation of 2a to 54% yield, while formation of 3 was still minimized (entry 5). Remarkably, when K2CO3 was replaced by the weaker Na3PO4, nearly quantitative formation of 2a was achieved (entry 6). The use of 5 Å MS proved crucial to completely suppress the formation of 3, thus obtaining the desired 2a in >95% yield (90% isolated) (entry 7). Unfortunately, lower catalyst loadings resulted in poor yields (entry 8).
Table 1. Optimization of the Reaction Conditionsa.
Reactions performed at 0.025 mmol of 1a. Yields determined by 19F NMR using 1-fluoro-4-nitrobenzene as internal standard.
Isolated yield of pure material of a reaction performed at 0.3 mmol of 1a.
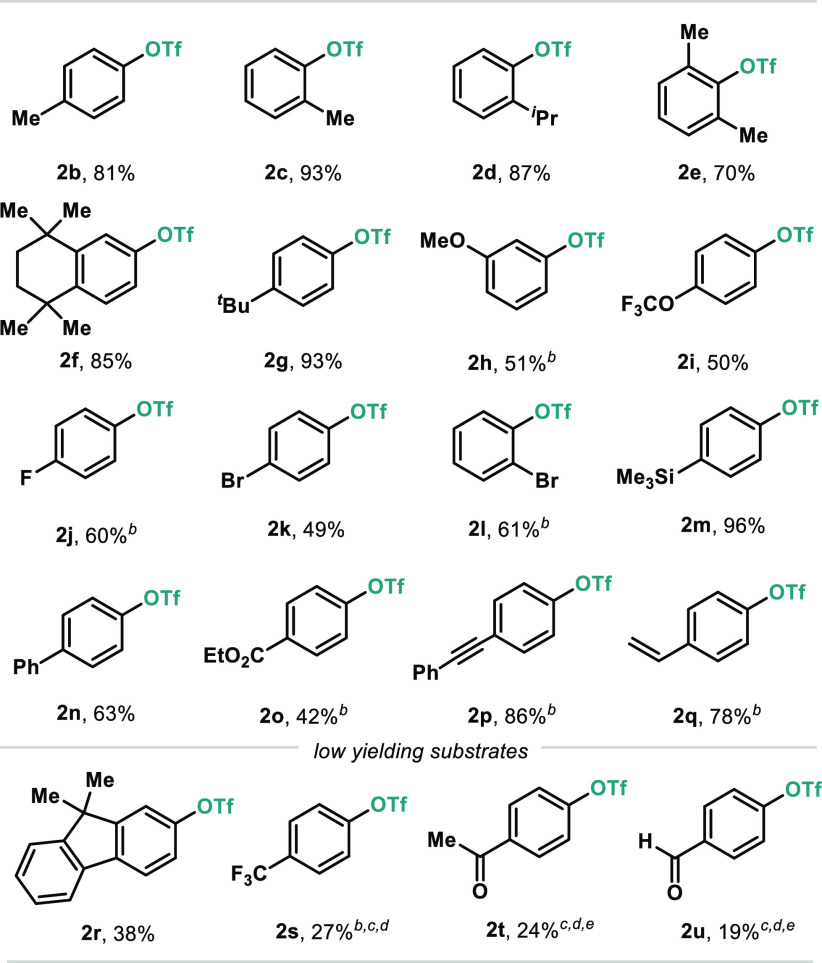
With the optimal conditions in hand, the scope of the Bi-catalyzed C(sp2)–OTf bond reaction was investigated using a variety of arylboronic acid derivatives (Table 2). The methodology boded well with Me groups in both para- (2b) and ortho-positions (2c). Remarkably, when the steric encumbrance at the ortho-position was further increased, excellent yields of the corresponding triflate were obtained (2d and 2e). Furthermore, the presence of alkyl moieties in other positions of the aryl ring did not affect the reactivity (2f and 2g). The protocol accommodates various functional groups, including ethers (2h and 2i) and halogens (2j, 2k, and 2l), albeit in moderate yields. Arylboronic acids substituted with a trimethylsilyl group (TMS), Ph, or an ester at the para-position afforded good to excellent yields of the corresponding aryl triflates (2m–2o). Arylboronic acids bearing unsaturated moieties boded well in this methodology, as exemplified by the presence of alkynyl (2p) and vinyl (2q) groups. In spite of the large variety of arylboronic acids amenable for this transformation, moderate yields were obtained in the presence of certain functionalities. Due to the high reactivity toward oxidation with [Cl2pyrF]BF4, fluorene derivative 2r was obtained in 38% yield.15 Substrates bearing strong electron-withdrawing groups such as CF3 (2s) and reactive carbonyl functionalities at the para-position (2t and 2u) also struggled to undergo C–O bond formation, demonstrating some limitations to the scope of this reaction.
Table 2. Scope of the Bi-Catalyzed Oxidative Coupling of Arylboronic Acids and Sodium Triflatea.
Reaction conditions: 1 (0.3 mmol), NaOTf (0.33 mmol), 4c (0.03 mmol), [Cl2pyrF]BF4 (0.33 mmol), Na3PO4 (0.6 mmol), and 5 Å MS (120 mg) in CHCl3 at 60 °C for 16 h. Yields of isolated pure material.
Reaction performed at 90 °C with 2.0 equiv of NaF as base.
Yields determined by 19F NMR using 1-fluoro-4-nitrobenzene as internal standard.
Reactions performed at 0.025 mmol of the corresponding arylboronic acids.
Reaction performed at 90 °C with 4.0 equiv of Na3PO4 as base.
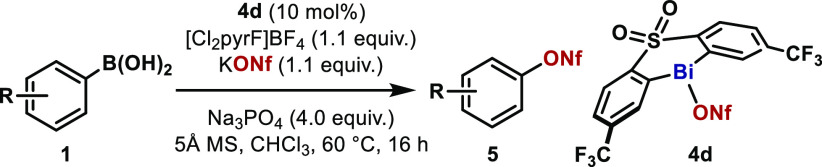
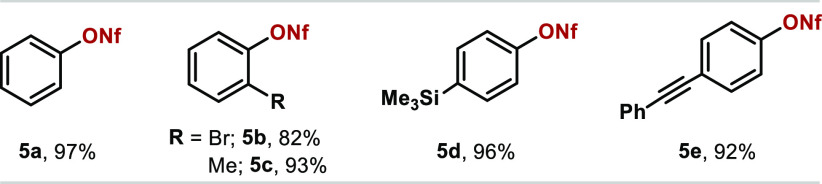
Having established a protocol for the successful coupling of NaOTf, we turned our attention to the use of less nucleophilic nonaflate salts as coupling partners. A brief re-examination of the reaction parameters revealed bismine nonaflate 4d as the catalyst of choice to couple arylboronic acids with commercially available KONf.15 With the optimized conditions shown in Table 3, Ph–ONf (5a) was isolated in a satisfactory 97% yield. The various arylboronic acids scrutinized in the nonaflate synthesis revealed comparable reactivity to NaOTf. Aryl nonaflates containing ortho-substituents such as Br (5b) and Me (5c) were obtained in excellent yields. Furthermore, a TMS moiety can also be accommodated to the protocol (5d) as well as unsaturated alkynyl functionalities (5e).
Table 3. Scope of the Bi-Catalyzed Oxidative Coupling of Arylboronic Acids and Potassium Nonaflatea.
Reaction conditions: 1 (0.3 mmol), KONf (0.33 mmol), 4d (0.03 mmol), [Cl2pyrF]BF4 (0.33 mmol), Na3PO4 (1.2 mmol) and 5 Å MS (120 mg) in CHCl3 at 60 °C for 16 h. Yields of isolated pure material.
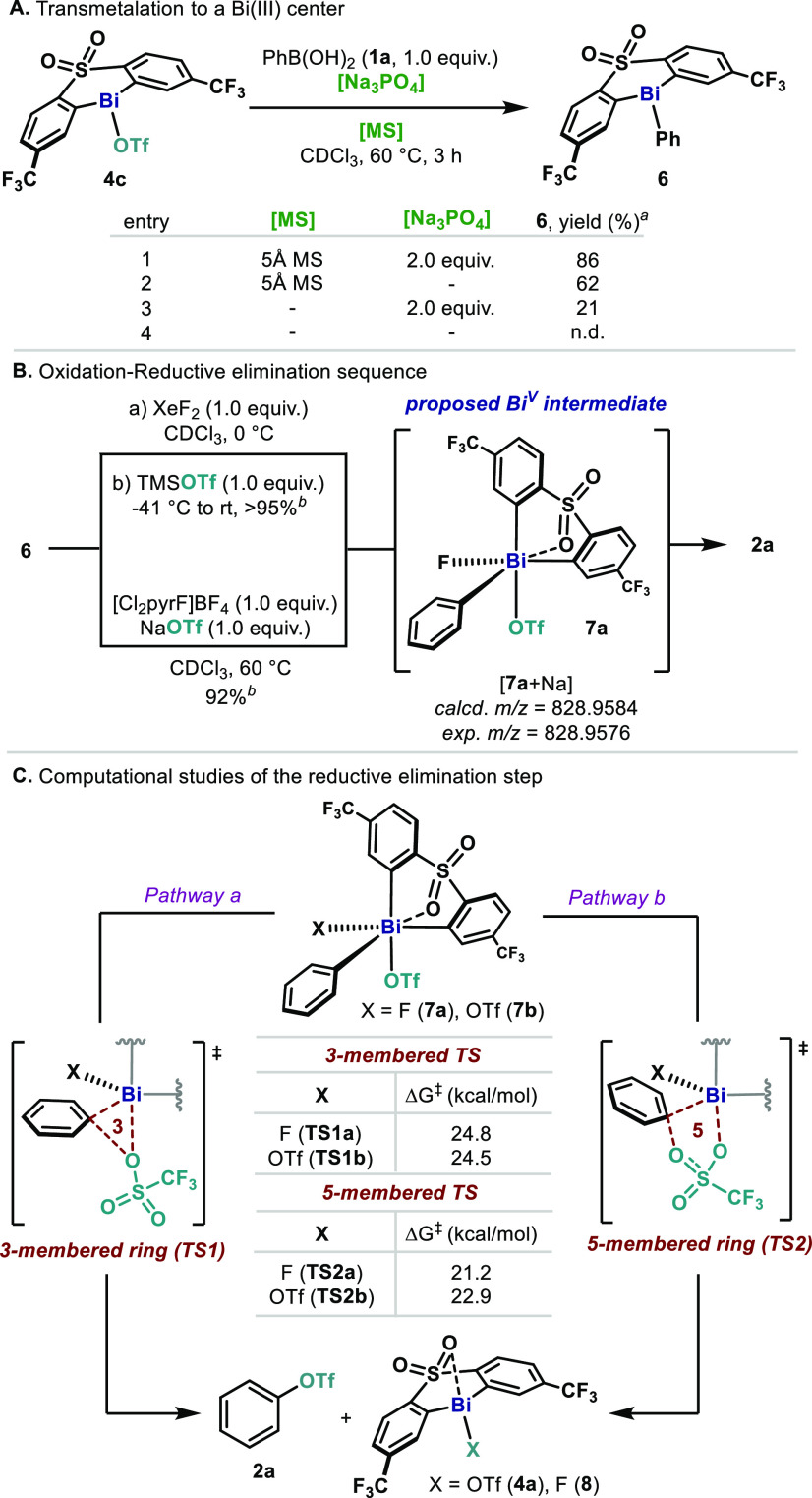
The unprecedented catalytic C–OTf and C–ONf bond forming reaction using 4c and 4d led us to explore the operative mechanism governing this transformation. First, we interrogated the transmetalation step between 1a and 4c (Figure 2A). When the reaction was performed in the presence of Na3PO4 and 5 Å MS, transmetalation occurred efficiently and 6 was obtained in 86% yield (entry 1). In the absence of base, 6 was also obtained in slightly lower yields (62%, entry 2). In sharp contrast, when the reaction was performed without MS (entry 3), formation of 6 was dramatically reduced (21%). In the absence of both MS and Na3PO4, 6 was not detected. These results demonstrate the importance of molecular sieves in this transformation, not only as a dehydrating agent17 but also as a potential heterogeneous Brønsted base,18 promoting transmetalation to the Bi(III) center. At this point, the oxidation–reductive elimination sequence from phenylbismine 6 was studied utilizing different oxidants and triflate sources (Figure 2B, top). After oxidizing 6 with XeF2 to the high-valent Bi(V) difluoride species,10b,15 TMSOTf was added, resulting in a rapid color change from pale to dark yellow. Analysis of the reaction crude revealed quantitative formation of 2a. This result points to the formation of a highly electrophilic Bi(V) intermediate (7a) bearing an OTf moiety, as a consequence of fluoride abstraction by TMSOTf. Indeed, when TMSOTf was added at −41 °C, intermediate 7a could be detected by HRMS (Figure 2B). Furthermore, using [Cl2pyrF]BF4 as an oxidant together with NaOTf similar yields for 2a were obtained (92%, Figure 2B, bottom). It is important to mention that only trace amounts of fluorobenzene were detected, which shows the preferential formation of C–OTf over C–F bonds (vide infra).19 Related intermediates have been previously postulated by Mukaiyama, in the C(sp2)–OTs coupling from Bi(V) intermediates.14 Based on these experimental results, preliminary theoretical studies were performed to investigate a putative reductive elimination from 7, bearing both a F (7a) or an OTf (7b) as counterions.15 As shown in Figure 2C, two possible scenarios were postulated. On one hand, reductive elimination can occur through a three-membered transition state (Figure 2C, pathway a), reminiscent of concerted reductive eliminations performed by d-block elements. Alternatively, reductive elimination could also occur via a five-membered transition state (Figure 2C, pathway b), where two oxygens of the OTf are involved. This latter hypothesis has been previously invoked to explain the selectivity of Bi-mediated couplings such as α-arylation of phenols20 and N-arylation of pyridones,21 among other transformations.22 In accordance with these previous reports, our theoretical analysis predicts that the five-membered TS2 is slightly favored over TS1, pointing toward TS2 as the preferable pathway for the C–O bond forming event. NBO analysis on the Bi center also provided additional information about this process.23 In the case of 7a, the NBO charge on the Bi decreases from 2.17 to 1.84 in TS1 and 1.88 in TS2 and is further reduced to 1.50 in 8. The same trend is observed from 7b.15 This progressive change in charge at the metal center has been previously observed in high-valent Cu cross-couplings,24 suggesting a concerted reductive elimination through the metal.
Figure 2.
(A) Study of the transmetalation step: influence of the molecular sieves and the base. (B) Stoichiometric sequence of oxidative addition–reductive elimination. (C) Theoretical analysis of the C–O bond forming step. aYields determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard. bYields determined by 19F NMR using 1-fluoro-4-nitrobenzen as internal standard.
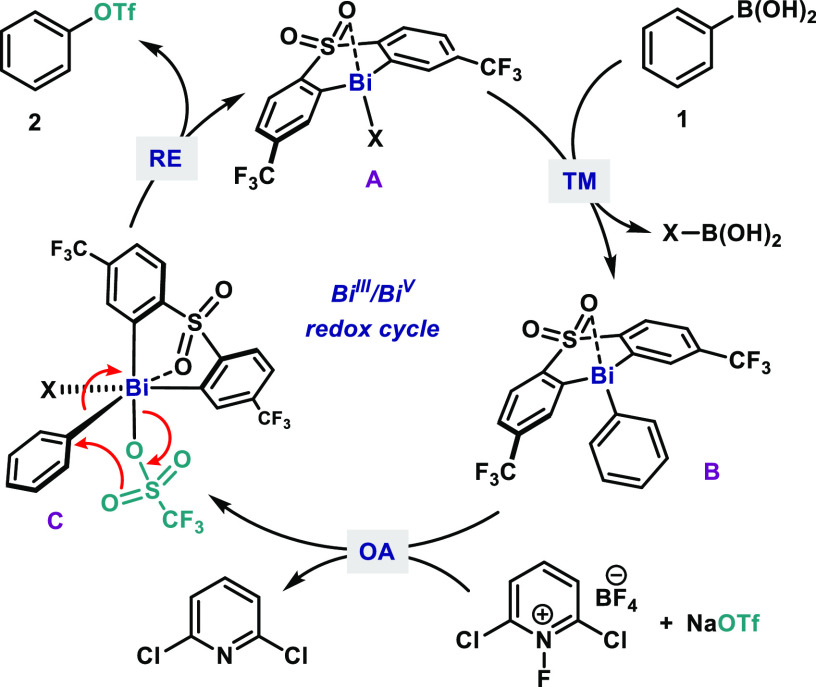
Taken these results together, the reaction is proposed to follow the catalytic cycle depicted in Figure 3. Initially, bismine A undergoes transmetalation (TM) with the corresponding arylboronic acid, thus forming aryl bismine B. Subsequently, B undergoes formal oxidative addition (OA) with [Cl2pyrF]BF4, furnishing the proposed high-valent Bi(V) intermediate C. Reductive elimination (RE) from C delivers the desired aryl triflate with concomitant regeneration of A. Due to the structural similarities between OTf and ONf, we believe that a similar mechanism is operating for the coupling of the latter.
Figure 3.
Postulated mechanism for the Bi-catalyzed oxidative coupling of arylboronic acids and triflate salts.
In summary, an unprecedented oxidative coupling of arylboronic acids with triflate and nonaflate salts has been developed exploiting the reactivity of the Bi(III)/Bi(V) redox couple. A highly electron-withdrawing diarylsulfone ligand unlocked a catalytic process which proceeds under mild conditions and accommodates various functional groups. The results presented in this study unveil bismuth redox catalysis as a promising tool to perform transformations beyond the scope of transition metals, while mimicking their fundamental organometallic steps.
Acknowledgments
Financial support for this work was provided by Max-Planck-Gesellschaft, Max-Planck-Institut für Kohlenforschung, Fonds der Chemischen Industrie (FCI-VCI). This project has received funding from European Union’s Horizon 2020 research and innovation programme under Agreements Nos. 850496 (ERC Starting Grant, J.C.) and 833361 (Marie Skłodowska Curie Fellowship, O.P.). We thank Prof. Dr. A. Fürstner for insightful discussions and generous support. We also thank Dr. Kalishankar Bhattacharyya and Dr. Dimitrios Pantazis for insightful suggestions and support in the computational studies, as well as the analytical department at the MPI-Kohlenforschung for support in the characterization of compounds.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c05343.
Experimental procedures, analytical data (1H, 19F, 11B, and 13C NMR, HRMS) for all new compounds, computational results, including Tables (S1–S9) and Figures (S1–S20) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Hansen R. L. Perfluoroalkanesulfonate Esters as Alkylating Agents. J. Org. Chem. 1965, 30, 4322–4324. 10.1021/jo01023a511. [DOI] [Google Scholar]; b Stang P. J.; Hanack M.; Subramanian L. R. Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry. Synthesis 1982, 1982, 85–126. 10.1055/s-1982-29711. [DOI] [Google Scholar]; c Ritter K. Synthetic Transformations of Vinyl and Aryl Triflates. Synthesis 1993, 1993, 735–762. 10.1055/s-1993-25931. [DOI] [Google Scholar]; d Högermeier J.; Reissig H.-U. Nine Times Fluoride can be Good for your Syntheses. Not just Cheaper: Nonafluorobutanesulfonates as Intermediates for Transition Metal-Catalyzed Reactions. Adv. Synth. Catal. 2009, 351 (17), 2747–2763. 10.1002/adsc.200900566. [DOI] [Google Scholar]
- a Zeni G.; Larock R. C. Synthesis of Heterocycles via Palladium-Catalyzed Oxidative Addition. Chem. Rev. 2006, 106, 4644–4680. 10.1021/cr0683966. [DOI] [PubMed] [Google Scholar]; b Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bisz E.; Szostak M. Iron-Catalyzed C–O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. 10.1002/cssc.201701287. [DOI] [PubMed] [Google Scholar]
- For representative transition-metal catalyzed C–OTf and C–ONf functionalization reactions, see:; a Echavarren A. M.; Stille J. K. Palladium-catalyzed coupling of aryl triflates with organostannanes. J. Am. Chem. Soc. 1987, 109, 5478–5486. 10.1021/ja00252a029. [DOI] [Google Scholar]; b Ohe T.; Miyaura N.; Suzuki A. Palladium-catalyzed cross-coupling reaction of organoboron compounds with organic triflates. J. Org. Chem. 1993, 58, 2201–2208. 10.1021/jo00060a041. [DOI] [Google Scholar]; c Wolfe J. P.; Buchwald S. L. Palladium-Catalyzed Amination of Aryl Triflates. J. Org. Chem. 1997, 62, 1264–1267. 10.1021/jo961915s. [DOI] [Google Scholar]; d Rottländer M.; Knochel P. Palladium-Catalyzed Cross-Coupling Reactions with Aryl Nonaflates: A Practical Alternative to Aryl Triflates. J. Org. Chem. 1998, 63, 203–208. 10.1021/jo971636k. [DOI] [PubMed] [Google Scholar]; e Anderson K. W.; Mendez-Perez M.; Priego J.; Buchwald S. L. Palladium-Catalyzed Amination of Aryl Nonaflates. J. Org. Chem. 2003, 68, 9563–9573. 10.1021/jo034962a. [DOI] [PubMed] [Google Scholar]; f Lee D.-Y.; Hartwig J. F. Zinc Trimethylsilylamide as a Mild Ammonia Equivalent and Base for the Amination of Aryl Halides and Triflates. Org. Lett. 2005, 7, 1169–1172. 10.1021/ol050141b. [DOI] [PubMed] [Google Scholar]; g Gooßen L. J.; Rodríguez N.; Linder C. Decarboxylative Biaryl Synthesis from Aromatic Carboxylates and Aryl Triflates. J. Am. Chem. Soc. 2008, 130, 15248–15249. 10.1021/ja8050926. [DOI] [PubMed] [Google Scholar]; h Uemura M.; Yorimitsu H.; Oshima K. Cp*Li as a base: application to palladium-catalyzed cross-coupling reaction of aryl-X or alkenyl-X (X = I, Br, OTf, ONf) with terminal acetylenes. Tetrahedron 2008, 64, 1829–1833. 10.1016/j.tet.2007.11.095. [DOI] [Google Scholar]; i Watson D. A.; Su M.; Teverovskiy G.; Zhang Y.; García-Fortanet J.; Kinzel T.; Buchwald S. L. Formation of ArF from LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides. Science 2009, 325, 1661–1664. 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Gooßen L. J.; Linder C.; Rodríguez N.; Lange P. P. Biaryl and Aryl Ketone Synthesis via Pd-Catalyzed Decarboxylative Coupling of Carboxylate Salts with Aryl Triflates. Chem. - Eur. J. 2009, 15, 9336–9349. 10.1002/chem.200900892. [DOI] [PubMed] [Google Scholar]; k Shekhar S.; Dunn T. B.; Kotecki B. J.; Montavon D. K.; Cullen S. C. A General Method for Palladium-Catalyzed Reactions of Primary Sulfonamides with Aryl Nonaflates. J. Org. Chem. 2011, 76, 4552–4563. 10.1021/jo200443u. [DOI] [PubMed] [Google Scholar]; l Si T.; Li B.; Xiong W.; Xu B.; Tang W. Efficient cross-coupling of aryl/alkenyl triflates with acyclic secondary alkylboronic acids. Org. Biomol. Chem. 2017, 15, 9903–9909. 10.1039/C7OB02531A. [DOI] [PubMed] [Google Scholar]
- Krossing I.; Raabe I. Noncoordinating Anions—Fact or Fiction? A Survey of Likely Candidates. Angew. Chem., Int. Ed. 2004, 43, 2066–2090. 10.1002/anie.200300620. [DOI] [PubMed] [Google Scholar]
- a Hartwig J. F.Organotransition metal chemistry: From bonding to catalysis; University Science Books: Mill Valley, CA, 2010. [Google Scholar]; b Maleckis A.; Sanford M. S. Facial Tridentate Ligands for Stabilizing Palladium(IV) Complexes. Organometallics 2011, 30, 6617–6627. 10.1021/om200779j. [DOI] [Google Scholar]; c Racowski J. M.; Gary J. B.; Sanford M. S. Carbon(sp3)–Fluorine Bond-Forming Reductive Elimination from Palladium(IV) Complexes. Angew. Chem., Int. Ed. 2012, 51, 3414–3417. 10.1002/anie.201107816. [DOI] [PubMed] [Google Scholar]
- Dhakal B.; Bohé L.; Crich D. Trifluoromethanesulfonate Anion as Nucleophile in Organic Chemistry. J. Org. Chem. 2017, 82, 9263–9269. 10.1021/acs.joc.7b01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lawrance G. A. Coordinated trifluoromethanesulfonate and fluorosulfate. Chem. Rev. 1986, 86, 17–33. 10.1021/cr00071a002. [DOI] [Google Scholar]; b Beck W.; Suenkel K. Metal complexes of weakly coordinating anions. Precursors of strong cationic organometallic Lewis acids. Chem. Rev. 1988, 88, 1405–1421. 10.1021/cr00089a017. [DOI] [Google Scholar]; c Ball N. D.; Kampf J. W.; Sanford M. S. Aryl–CF3 Bond-Forming Reductive Elimination from Palladium(IV). J. Am. Chem. Soc. 2010, 132, 2878–2879. 10.1021/ja100955x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lyons T. W.; Sanford M. S. Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Canty A. J.Higher Oxidation State Organopalladium and Platinum Chemistry; Springer-Verlag: Berlin, Heidelberg, Germany, 2011. [Google Scholar]
- a Bour J. R.; Camasso N. M.; Sanford M. S. Oxidation of Ni(II) to Ni(IV) with Aryl Electrophiles Enables Ni-Mediated Aryl–CF3 Coupling. J. Am. Chem. Soc. 2015, 137, 8034–8037. 10.1021/jacs.5b04892. [DOI] [PubMed] [Google Scholar]; b Camasso N. M.; Sanford M. S. Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes. Science 2015, 347, 1218–1220. 10.1126/science.aaa4526. [DOI] [PubMed] [Google Scholar]; c Canty A. J.; Ariafard A.; Camasso N. M.; Higgs A. T.; Yates B. F.; Sanford M. S. Computational study of C(sp3)–O bond formation at a PdIV centre. Dalton Trans 2017, 46, 3742–3748. 10.1039/C7DT00096K. [DOI] [PubMed] [Google Scholar]; d Nebra N. High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art. Molecules 2020, 25, 1141. 10.3390/molecules25051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suero M. G.; Bayle E. D.; Collins B. S. L.; Gaunt M. J. Copper-Catalyzed Electrophilic Carbofunctionalization of Alkynes to Highly Functionalized Tetrasubstituted Alkenes. J. Am. Chem. Soc. 2013, 135, 5332–5335. 10.1021/ja401840j. [DOI] [PubMed] [Google Scholar]
- a Wang F.; Planas O.; Cornella J. Bi(I)-Catalyzed Transfer-Hydrogenation with Ammonia-Borane. J. Am. Chem. Soc. 2019, 141, 4235–4240. 10.1021/jacs.9b00594. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Planas O.; Wang F.; Leutzsch M.; Cornella J. Fluorination of arylboronic esters enabled by bismuth redox catalysis. Science 2020, 367, 313–317. 10.1126/science.aaz2258. [DOI] [PubMed] [Google Scholar]
- a Power P. P. Main-group elements as transition metals. Nature 2010, 463, 171–177. 10.1038/nature08634. [DOI] [PubMed] [Google Scholar]; b Weetman C.; Inoue S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem 2018, 10, 4213–4228. 10.1002/cctc.201800963. [DOI] [Google Scholar]; c Melen R. L. Frontiers in molecular p-block chemistry: From structure to reactivity. Science 2019, 363, 479–484. 10.1126/science.aau5105. [DOI] [PubMed] [Google Scholar]; d Janssen-Müller D.; Oestreich M. Transition-Metal-Like Catalysis with a Main-Group Element: Bismuth-Catalyzed C–F Coupling of Aryl Boronic Esters. Angew. Chem., Int. Ed. 2020, 59, 8328–8330. 10.1002/anie.201914729. [DOI] [PubMed] [Google Scholar]
- a Stewart C. A.; Calabrese J. C.; Arduengo A. J. Synthesis and structure of the first 20-Bi-9 system: a discrete nine-coordinate 20-electron bismuth. J. Am. Chem. Soc. 1985, 107, 3397–3398. 10.1021/ja00297a084. [DOI] [Google Scholar]; b Boyer B.; Keramane E. M.; Montero J.-L.; Roque J.-P. BiCl3: An Efficient Agent for Selective Chlorination of Alcohols or for Halogen Exchange Reaction. Synth. Commun. 1998, 28, 1737–1741. 10.1080/00397919808007004. [DOI] [Google Scholar]; c Matano Y.; Nomura H. Dimeric Triarylbismuthane Oxide: A Novel Efficient Oxidant for the Conversion of Alcohols to Carbonyl Compounds. J. Am. Chem. Soc. 2001, 123, 6443–6444. 10.1021/ja010584k. [DOI] [PubMed] [Google Scholar]; d Yin S.-F.; Maruyama J.; Yamashita T.; Shimada S. Efficient Fixation of Carbon Dioxide by Hypervalent Organobismuth Oxide, Hydroxide, and Alkoxide. Angew. Chem., Int. Ed. 2008, 47, 6590–6593. 10.1002/anie.200802277. [DOI] [PubMed] [Google Scholar]; e Raţ C. I.; Silvestru C.; Breunig H. J. Hypervalent organoantimony and -bismuth compounds with pendant arm ligands. Coord. Chem. Rev. 2013, 257, 818–879. 10.1016/j.ccr.2012.07.026. [DOI] [Google Scholar]
- For selected recent examples of Bi–OTf species, see:; a Tschersich C.; Hoof S.; Frank N.; Herwig C.; Limberg C. The Effect of Substituents at Lewis Acidic Bismuth(III) Centers on Its Propensity to Bind a Noble Metal Donor. Inorg. Chem. 2016, 55, 1837–1842. 10.1021/acs.inorgchem.5b02740. [DOI] [PubMed] [Google Scholar]; b Kannan R.; Kumar S.; Andrews A. P.; Jemmis E. D.; Venugopal A. Consequence of Ligand Bite Angle on Bismuth Lewis Acidity. Inorg. Chem. 2017, 56, 9391–9395. 10.1021/acs.inorgchem.7b01243. [DOI] [PubMed] [Google Scholar]; c Ritschel B.; Poater J.; Dengel H.; Bickelhaupt F. M.; Lichtenberg C. Double CH Activation of a Masked Cationic Bismuth Amide. Angew. Chem., Int. Ed. 2018, 57, 3825–3829. 10.1002/anie.201712725. [DOI] [PubMed] [Google Scholar]; d Ramler J.; Poater J.; Hirsch F.; Ritschel B.; Fischer I.; Bickelhaupt F. M.; Lichtenberg C. Carbon monoxide insertion at a heavy p-block element: unprecedented formation of a cationic bismuth carbamoyl. Chem, Sci. 2019, 10, 4169–4176. 10.1039/C9SC00278B. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ramler J.; Hofmann K.; Lichtenberg C. Neutral and Cationic Bismuth Compounds: Structure, Heteroaromaticity, and Lewis Acidity of Bismepines. Inorg. Chem. 2020, 59, 3367–3376. 10.1021/acs.inorgchem.9b03189. [DOI] [PubMed] [Google Scholar]
- a Imachi S.; Mukaiyama T. Oxidative Coupling of Carbonyl Compounds by Using Pentavalent Biphenyl-2,2′-ylenebismuth Reagents. Chem. Lett. 2007, 36, 718–719. 10.1246/cl.2007.718. [DOI] [Google Scholar]; b Sakurai N.; Mukaiyama T. A New Preparative Method of Aryl Sulfonate Esters by Using Cyclic Organobismuth Reagents. Heterocycles 2007, 74, 771–790. 10.3987/COM-07-S(W)63. [DOI] [Google Scholar]
- See Supporting Information for further details.
- Jurrat M.; Maggi L.; Lewis W.; Ball L. T. Modular bismacycles for the selective C–H arylation of phenols and naphthols. Nat. Chem. 2020, 12, 260–269. 10.1038/s41557-020-0425-4. [DOI] [PubMed] [Google Scholar]
- West M. J.; Fyfe J. W. B.; Vantourout J. C.; Watson A. J. B. Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev. 2019, 119, 12491–12523. 10.1021/acs.chemrev.9b00491. [DOI] [PubMed] [Google Scholar]
- Steinhoff B. A.; King A. E.; Stahl S. S. Unexpected Roles of Molecular Sieves in Palladium-Catalyzed Aerobic Alcohol Oxidation. J. Org. Chem. 2006, 71, 1861–1868. 10.1021/jo052192s. [DOI] [PubMed] [Google Scholar]
- Furuya T.; Kamlet A.; Ritter T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. H. R.; Bhatnagar N. Y.; Finet J.-P.; Motherwell W. B. Pentavalent organobismuth reagents. Part VI. Comparative migratory aptitudes of aryl groups in the arylation of phenols and enols by pentavalent bismuth reagents. Tetrahedron 1986, 42, 3111–3122. 10.1016/S0040-4020(01)87378-6. [DOI] [Google Scholar]
- Ikegai K.; Mukaiyama T. Synthesis of N-Aryl Pyridin-2-ones via Ligand Coupling Reactions Using Pentavalent Organobismuth Reagents. Chem. Lett. 2005, 34, 1496–1497. 10.1246/cl.2005.1496. [DOI] [Google Scholar]
- a Finet J.-P.Ligand Coupling Reactions with Heteroatomic Compounds; Elsevier: Oxford, 1998. [Google Scholar]; b Finet J.-P. Arylation reactions with organobismuth reagents. Chem. Rev. 1989, 89, 1487–1501. 10.1021/cr00097a005. [DOI] [Google Scholar]
- a Reed A. E.; Curtiss L. A.; Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. 10.1021/cr00088a005. [DOI] [Google Scholar]; b Weinhold F.; Landis C. R.. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge University Press: Cambridge, United Kingdom, 2005. [Google Scholar]
- Paeth M.; Tyndall S. B.; Chen L.-Y.; Hong J.-C.; Carson W. P.; Liu X.; Sun X.; Liu J.; Yang K.; Hale E. M.; Tierney D. L.; Liu B.; Cao Z.; Cheng M.-J.; Goddard W. A.; Liu W. Csp3–Csp3 Bond-Forming Reductive Elimination from Well-Defined Copper(III) Complexes. J. Am. Chem. Soc. 2019, 141, 3153–3159. 10.1021/jacs.8b12632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.