Abstract
Axial spondyloarthritis (axSpA) is a group of debilitating, chronic, rheumatic conditions characterized by inflammation and new bone formation, mainly involving the spine and the sacroiliac joints. The lack of biomarkers in axSpA is well known. Despite significant treatment advances in recent years thanks to the introduction of drugs with a new mode of action, such as new biologic and targeted synthetic disease-modifying antirheumatic drugs, no relevant improvement in the identification of disease biomarkers has been achieved. Common parameters, such as erythrocyte sedimentation rate and C-reactive protein, which are routinely used to measure systemic inflammation, are the sole markers available to date and are not adequate to assess disease activity in all patients.
The aim of this study is to review the most promising serum biomarkers that may help treatment decision in axSpA via a proper assessment of disease activity and identification of negative prognostic factors.
Keywords: ankylosing spondylitis, anti-drug antibodies, axial spondyloarthritis, biomarkers, disease activity, radiographic progression
Introduction
Spondyloarthritis (SpA) is a group of chronic, inflammatory, rheumatic diseases characterized by overlapping clinical signs and symptoms and a common genetic background.1 Depending on the predominant pattern of clinical symptoms, a primary peripheral or axial involvement can be distinguished.1,2 Axial spondyloarthritis (axSpA), which mainly affects the spine and the sacroiliac joints (SIJs) and with a symptom onset usually before the age of 45 years, can be further divided into nonradiographic axSpA (nr-axSpA) and radiographic axSpA, the latter also known as ankylosing spondylitis (AS).2 If undiagnosed and untreated, long-term outcomes of axSpA are physical and functional permanent damage as well as lifelong disability.2,3 Importantly, patients with early disease also experience fatigue and functional impairment with subsequent depression.4 Reduced social life and working ability result in a poor quality of life with consequent increase in the economic burden of the disease.5
Significant steps forward have been taken in the management of axSpA in clinical practice, following the introduction of tumor necrosis factor (TNF)-α inhibitors (TNFi). Patients with axSpA respond quickly to TNFi, which provide an Assessment of Spondyloarthritis International Society response criteria (ASAS40) improvement in about 40% of patients in the first 3–6 months of treatment. Similar response rates have also been observed following treatment with biologic disease-modifying antirheumatic drugs (bDMARDs) targeting interleukin (IL)-17.6,7 The role of other bDMARDs targeting IL-12/IL-238,9 needs further evaluation in axSpA. Furthermore, the targeted synthetic DMARDs (tsDMARDs), which inhibit intracellular signaling molecules such as the Janus kinases (JAK) showed some preliminary promising results and may be approved in a short time in axSpA treatment.10,11 The introduction of these new targeted treatments allowed a better understanding of the pathogenesis of axSpA, but such improvements were not paralleled by the discovery of new biomarkers. Because of the absence of rheumatoid factor, axSpA has been long considered to have a seronegative property. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), routinely used inflammatory indices, in addition to human leucocyte antigen (HLA) B27, had been considered until now to be the main diagnostic and therapeutic biomarkers in axSpA. ESR and CRP levels are often within the normal range even in the case of very active disease, and are not adequate to monitor all axSpA patients.12–15 As a result of the growing awareness of the impact of chronic back pain in axSpA patients and of new breakthroughs on etiopathogenetic mechanisms and in the field of genetics thanks to genome-wide association studies, recent studies have investigated novel potential biomarkers of disease activity to identify patients at higher risk for a worse outcome.16–20 The National Institute of Health Biomarkers and Surrogate Endpoint Working Group have elaborated the definition of biomarker as a “characteristic that can be objectively measured and evaluated as an indicator of normal biological or pathogenic processes or pharmacological responses to a therapeutic intervention”.21 However, clinical characteristics and comorbidities are also important biomarkers toward the definition and diagnosis of axSpA. The Italian board for the TAilored BIOlogic therapy (ITABIO) group attempted to develop an algorithm to guide first- and second-line biological treatments that mainly relies on clinical features of the disease.22,23 Likewise, the American College of Rheumatology recommendations for spondyloarthritides also take into account comorbidities to select the preferable treatment options.24 In this regard, rheumatologists have to take into account concomitant cardiovascular disease and metabolic syndrome, the desire of a pregnancy, the risk of infections, and importantly the patients’ preference. Furthermore, concomitant skin psoriasis, uveitis and inflammatory bowel disease (IBD) may be used to characterize the disease in SpA. Along with these factors, other characteristics may help to profile the patient with axSpA and to personalize the treatment. Male sex,25 smoking,26,27 presence of syndesmophytes28 and active inflammatory or post-inflammatory modifications on MRI29 are known predictors of a more severe disease with higher Ankylosing Spondylitis Disease Activity Score (ASDAS) or Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and with a radiographic progression.
A large number of biomarkers have been investigated and suggested in axSpA and variable evidence is available on their role in the diagnosis, clinical and prognostic assessment and evaluation of the response to treatment.
The purpose of our review was to summarize the available biomarkers in axSpA towards a more tailored therapeutic approach. Specifically, we focused on markers associated with disease activity and treatment response (clinical and radiological). We performed a PubMed search of articles published between 1984 and 2019. The search terms included: axial spondyloarthritis, ankylosing spondylitis, biomarkers, serological markers, antibodies, immunogenicity. Biomarkers are presented according to five main categories: (1) systemic markers of inflammation; (2) molecules implicated in the bone homeostasis; (3) miscellaneous; (4) anti-drug antibodies (ADAbs) and drug through levels; and (5) combination of biomarkers. A schematic summary of their potential use in axSpA is reported in Table 1.
Table 1.
A schematic summary of the potential use in the clinical practice of selected promising biomarkers in axial spondyloarthritis.
| Biomarker | Association with disease activity | Association with treatment response | Predictor of structural damage |
|---|---|---|---|
| Systemic markers of inflammation | |||
| CRP | Positive association with disease activity indices12,14,18,19,30 | Decreases following treatment response13,14,19 | High levels predict further damage13,14,19 |
| Erythrocyte sedimentation rate | High levels predict further damage13 | ||
| Calprotectin | Positive association with disease activity indices and structural damage31–33 | Decreases following treatment response34–36 | High levels predict damage33 |
| Serum amyloid A proteins | Positive association with systemic inflammation12,37,38 | Inconclusive9,12 | |
| Tumor necrosis factor-α | Inconclusive39,40 | Inconclusive39,40 | |
| IL-6 | Inconclusive association with disease activity indices;18,39,41 positive association with structural damage18,42–45 | High levels predict damage18,45 | |
| IL-17 | Inconclusive43,46,47 | Decreases following treatment response43 | |
| IL-23 | Inconclusive43,47 | ||
| Molecules implicated in the bone homeostasis | |||
| Sclerostin | Negative association with disease activity indices48,49 | Low levels predict damage50–52 | |
| Wnt proteins and Dickkop-1 | Inconclusive53 | Inconclusive54 | |
| Osteoprotegerin | Positive association with disease activity indices51,55,56 | Inconclusive51,55,56 | |
| Matrix metalloproteinase 3 | Positive association with disease activity indices57–60 | Inconclusive9,61–63 | Inconclusive45,64,65 |
| Citrullinated fragments of vimentin | Positive association with systemic inflammation66,67 | Inconclusive66–68 | |
| CTX-I and type II collagen | Positive association with systemic inflammation63,69,70 and structural damage19 (CTX-II only) | Decreases following treatment response (CTX-II only)18,19,71 | High levels predict damage63,70,72,73 |
| Osteocalcin | High levels predict damage14,19,74,75 | ||
| Miscellaneous | |||
| Vascular endothelial growth factor | Positive association with systemic inflammation76–78 | High levels predict damage76–78 | |
| Leptin | Negative association with disease activity indices79 | Inconclusive results45,80,81 | |
| High-molecular-weight adiponectin | Low levels predict damage78 | ||
| p40 IL-12/23 | Serum IL-12p40 levels were raised in AS and correlated with CRP and ASDAS82 | ||
| Hepatocyte growth factor | Pleiotropic effects in the suppression of inflammation60,83 | Molecule involved in the osteoproliferation and osteoporosis in AS84 | |
| Anti-drug antibodies and drug through levels | |||
| Anti-drug antibodies | In patients with unsatisfactory clinical response, detectable levels may predict treatment failure85–92 and subsequent good response to another drug with the same mechanism of action93 | ||
| Drug through levels | In patients with unsatisfactory clinical response, low levels are associated with good response to dose increase88 | ||
| Combination of biomarkers | |||
| ENRADAS trial combination biomarker | Positive association with disease activity indices78 | Inconclusive78 | |
AS, ankylosing spondylitis; ASDAS, Ankylosing Spondylitis Disease Activity Score; CRP, C-reactive protein; CTX-I, C-terminal cross-linking telopeptide of type I; CTX-II, C-terminal cross-linking telopeptide of type II; IL, interleukin.
Systemic markers of inflammation
CRP and ESR, are acute-phase proteins used in clinical practice as markers of systemic inflammation.94 Unfortunately, they may not fully represent the inflammatory process in axSpA due to their low sensitivity and specificity.95 An elevated CRP is one of the items included in the classification criteria of the ASAS for axSpA.2 CRP levels are also taken into consideration in the ASDAS and they were shown to correlate with the BASDAI.96 However, elevated CRP or ESR levels are detected in only 40–50% of patients with AS.97 In fact, the degree of inflammation fluctuates during the course of axSpA. In general, CRP levels are higher in patients with radiographic axSpA compared with nr-axSpA.30 Although CRP is within the normal range in a large proportion of patients with active axSpA, it is still widely considered a reliable marker of disease activity. In fact, CRP levels are moderately correlated with magnetic resonance imaging (MRI) inflammation.14,18,19,30 In addition, CRP has been found to be a reliable biomarker for monitoring treatment response and predicting further radiographic progression.30,98 Several studies have shown that CRP levels drop significantly during TNFi treatment.13–15 Modifications in CRP levels correlate with changes in BASDAI and MRI scores. Elevated baseline CRP levels are associated with a good treatment response12,14 and represent a strong positive predictor of radiographic sacroiliitis progression, especially for nr-axSpA into AS.14 Several prospective studies have also demonstrated that elevated CRP levels are independently associated with radiographic spinal progression in axSpA patients.98 The ESR seems to be a nonspecific measure of inflammation that may be influenced by a variety of other nonrheumatic conditions and comorbidities. However, some studies reported that high ESR levels and increased CRP levels are independently associated with structural disease progression in patients with nr-axSpA.13
Besides the two most common inflammation markers, calprotectin (CPT) is a promising marker of inflammation that has recently gained interest in several inflammatory rheumatic diseases. CPT is a heterodimeric calcium- and zinc-binding protein complex, composed of S100A8 and S100A9 subunits, expressed in the cytosol of keratinocytes, neutrophils and monocytes. This protein exerts diverse intra- and extracellular functions, acting on different target tissues/organs, such as muscle, cartilage, bone, synovial tissue, vessels and epithelium, among others. Modulation of the inflammatory response by binding to different cell-surface proteins such as Toll-like receptor, as well as oxidant-scavenging, antimicrobial and apoptosis-inducing activities are among the extracellular functions attributed to CPT.99 Interestingly, both pro- and anti-inflammatory roles have been reported for CPT.50,100 Additionally, previous studies showed that CPT is a sensitive and specific biomarker of local inflammation, mirroring intestinal or synovial inflammation. Fecal CPT levels have been associated with disease activity in AS and are associated with inflammatory activity in the bowel.101–103 Serum CPT levels can be used as a marker of inflammation in nr-axSpA, AS and reactive arthritis without significant difference in the levels between these diseases. Some authors reported that elevated CPT levels positively correlated with ESR, CRP, BASDAI, and ASDAS as well as Spondyloarthritis Research Consortium of Canada (SPARCC) scoring.31,32 CPT might also be used to monitor treatment response. Serum levels of CPT were reported to decrease following treatment with TNFi.34,35 S100A8 and S100A9 subunits were reduced after 6 weeks of IL-17 inhibition with secukinumab and correlated with ASAS response.36 A significant decrease in serum CPT was noted also after intensive physiotherapy in patients with nr-axSpA and AS.104 Serum CPT has a potential role as a prognostic factor, being associated with radiographic spinal progression.33 Baseline CPT serum levels have been found significantly increased in patients with a higher modified Stoke Ankylosing Spondylitis Spinal Scores (mSASSS) versus those without.33 A few studies reported an association of CPT with adverse lipid profiles, in addition to the known association with classical inflammatory biomarkers, which strengthens the association between inflammation and development of atherosclerosis and may help to identify patients with axSpA at high risk of cardiovascular events.55,105–107
Serum amyloid A (SAA) protein was also suggested to help in disease monitoring although it is less reliable compared with CPT. SAA is a family of apolipoproteins associated with high-density lipoproteins that are produced in response to an inflammatory trigger. Patients with AS have higher serum SAA levels with respect to healthy controls; SAA levels also correlated with inflammatory markers and disease activity variables.12,37,38 Similarly to CRP, baseline high SAA levels were shown to be associated with higher odds of treatment response to TNFi, especially in patients with elevated CRP.12 SAA levels were decreased following treatment with ustekinumab, and anti-IL-12/23, in patients with AS; however, no correlation with clinical response was observed.9
Cytokines are key mediators in the pathogenesis of rheumatic diseases. However, their role as biomarkers are widely debated. TNF-α has long been the only cytokine known to be involved in the pathogenesis of axSpA, leading to the development of therapeutic antibodies targeting this cytokine. Notably, TNF-α provides a potential link between inflammatory response and disturbed bone homeostasis,39 exerting several effector and biological functions via proinflammatory cytokines and chemokine release, the activation of endothelial cells with upregulation of adhesive molecules, leucocyte accumulation, angiogenesis, lymphocyte activation, fibroblast proliferation and chondrocyte and osteoclast activation. TNF-α expression is strongly upregulated in SIJ biopsies of patients with AS40 and serum levels are significantly higher in patients with AS compared with subjects with noninflammatory back pain or healthy controls.39,108 In addition to the interesting findings that support TNF-α involvement in the disease, the serum levels did not prove to be reliable biomarkers of disease activity.
IL-6, similarly to TNF-α, is one of the most extensively studied cytokine in rheumatic diseases, especially in rheumatoid arthritis (RA), but it has also been evaluated in axSpA. IL-6 is produced by a variety of immune cells that further induce the production of several acute-phase proteins.39 IL-6 is involved in the very early stage of the inflammation process by inducing the gathering of neutrophils at the inflammation site and modulating T-cell activation and differentiation. Elevated IL-6 levels were also found to be expressed in cartilage, synovial fluid and connective tissue in SIJ biopsies of patients with AS, particularly in early stages.109 Higher IL-6 serum levels are usually found in patients with AS compared with healthy controls.39,110,111 An association between IL-6 serum levels and activity indices or inflammatory markers was demonstrated by some authors,39,41–43 but was not confirmed by others.18,44 Particularly, IL-6 does not seem to be associated with other emerging cytokines in the pathogenesis of axSpA, that is, IL-17 and IL-23.112 Notably, IL-6 is associated with MRI changes. Lower baseline levels were found to be associated with lower MRI inflammation scores following TNFi treatment.18,45 Interestingly, the change of IL-6 levels from baseline to week 4 and week 14 was associated with spinal inflammation in MRI. IL-6 appears to have a potential prognostic role, as high baseline levels were predictive of a high probability of structural response to TNFi as assessed with mSASSS.45
Following the introduction of biologics with new modes of action, the interest moved to other cytokines, namely IL-17 and IL-23, which are the targets of these drugs. Both of them are involved in the helper T cell type 17 (TH17) pathway and are often tested together due to their tight connection. In fact, IL-17 is mainly produced by TH17 following IL-23 production. The IL-23–IL-17 immune axis is implicated in the pathogenesis of axSpA,9,80,113–115 particularly at the entheseal level.116–118 Most studies reported elevated IL-17 and IL-23 levels in the plasma and serum of patients with AS.9,46,47,69,110,116,119 The association with clinical variables is not consistently reported in all studies. IL-17 and peripheral TH17 cells are reported to be reduced following successful TNFi treatment43 and IL-17 correlates with CRP and disease activity indices, such as BASDAI.43,46 Other authors failed to find correlation between IL-17 levels and inflammatory indices, disease activity or MRI changes.46,47 Very similar findings have been found for IL-23 in the same studies.43,46 Chen et al., showed that IL-17 and IL-23 performed even better compared to ESR and CRP in discriminating patients with disease activity, assessed by BASDAI.46
In addition to the above discussed cytokines, IL-21 and IL-22, both linked to IL-17 and TH17 cells, are elevated in patients with AS.46,120–122 IL-22 was strongly associated with IL-23 in one study, but no associations with clinical, serological and imaging variables were found.119 IL-31 emerged as the only cytokine to be higher in patients with SpA compared with controls in a French cohort, and it was associated with less structural damage.112 IL-33, a member of the IL-1 family, was found to be elevated in the serum of patients with AS in China and to correlate with disease activity or other inflammatory markers.123–125
Molecules involved in bone homeostasis
Molecules mirroring bone metabolism that can be detected in the serum represent an opportunity to better understand and monitor axSpA. Molecules driving bone resorption and formation and enzymes degrading extracellular matrix are among the best studied. Components of the extracellular matrix and its degradation products have been extensively studied in the field of osteoporosis and associations between their serum levels with axSpA disease activity have also been investigated.
The human receptor activator of nuclear factor-κB ligand (RANKL) and monocyte colony-stimulating factor (M-CSF) are the two key molecules involved in osteoclast formation from peripheral blood monocytes and in osteoclast activation126 and are the best studied. These cytokines play a critical role in physiological bone turnover and their dysregulation leads to impairment of osteoclast generation and excessive bone formation, as observed in several rheumatic diseases, including SpA. However, there is substantial discrepancy regarding serum RANKL levels in patients with SpA (especially axSpA). Some studies reported either higher or lower serum RANKL levels in patients with axSpA in comparison to healthy subjects.48,51,127,128
Sclerostin (SOST) is a bone morphogenic protein antagonist. SOST, being an antagonist of the Wnt/β-catenin pathway, exerts anti-anabolic effects on bone formation and a reduced biological effect of SOST seems responsible for the bone neo-ossification that is typical of AS.129 Nonetheless, studies focusing on SOST levels in patients with AS gave diverging results. Most of the studies found lower levels in individuals with AS compared with controls,50,56,64 and an association with disease activity was found.49,51 Some studies also suggested a prognostic value, as low baseline SOST levels in AS and axSpA were associated with new syndesmophyte formation.50–52 Interestingly, anti-SOST antibodies were found together with low SOST levels, thereby implying a possible pathogenic role of the antibodies neutralizing SOST.130,131
The Dickkopf (Dkk) proteins are one of the two main families of soluble inhibitors of the Wnt proteins, and include the most important protein, Dkk-1. The activation of Wnt signaling induces osteoblastogenesis and is implicated in new bone formation. As Dkk-1 is an inhibitor of the Wnt pathway, one might expect Dkk-1 concentrations to be low in the diseases characterized by an increase of new bone formation. Nevertheless, only a few studies found lower Dkk-1 levels in patients with AS.74,132 Furthermore, consistently with most other studies, Dkk-1 concentrations were higher in patients with AS compared with healthy subjects,54,55,133,134 similarly in patients with peripheral SpA compared with healthy controls; and higher in patients with AS compared with psoriatic arthritis (PsA).133 Thus, it has been suggested that Dkk-1 serum levels may be higher in axial disease because of a pathologically dysfunctional Dkk-1 molecule, which does not inhibit Wnt-mediated osteoproliferation in axSpA. In fact, possible explanations are provided by the observation that Dkk-1at high levels binds to its receptor LRP6 with low avidity in AS,54,133 and that Dkk-1 even has a stimulatory effect on the Wnt pathway in the presence of anti-Dkk-1 monoclonal antibodies.133 Dkk-1 is not correlated with CRP level,53 and TNFi treatment does not affect Dkk-1 levels.54 The latter observation may be unexpected because recent data showed a retardation of radiographic progression under TNFi in different patient groups (e.g. with treatment initiation within the first 10 years after symptoms,135 after long-term treatment136 or in the case of a good treatment response137).
Osteoprotegerin (OPG), a member of the TNF receptor superfamily, inhibits the RANK–RANKL interaction, impeding osteoclastogenesis with anti-resorptive effects. Several studies found OPG levels higher in patients with AS compared with controls,8,55,127,138 whereas others found the opposite.51,56 OPG seems to correlate with BASDAI score, being higher in patients with more active disease, but not with TNFi treatment response.51,55,56
Matrix metalloproteinases (MMPs) are relevant enzymes involved in the degradation of extracellular matrix proteins. These zinc-dependent endopeptidases also play a role in cell proliferation, migration, differentiation, angiogenesis, apoptosis and host defence.139–141 MMP-3 is the most studied among these molecules in axSpA. MMP-3 serum levels are increased in patients with AS with respect to healthy controls.139–141 Furthermore, some investigators have reported that MMP-3 correlated with ESR and CRP levels, disease activity and functional status, as assessed by the BASDAI.142 Similar findings have been also found in PsA, further confirming the potential role of this molecule as a biomarker.57–59 TNFi induce a significant and rapid decrease in serum MMP-3 levels together with a reduction in conventional variables (ESR and BASDAI).61–63 A similar effect was observed after treatment with ustekinumab, although MMP-3 decrease was not associated with clinical variables.9 MMP-3 association with structural damage has been reported.64,143 Maksymowych et al. showed that MMP-3 was a significant independent predictor of radiographic progression over a 2-year observation period in axSpA,143 suggesting that MMP-3 could be considered a parameter of bone metabolism. Other studies did not confirm the association of MMP-3 with inflammation in the SIJs detected by MRI,144 with radiographic progression.45,65 Although fewer studies have been carried out on other MMPs, MMP-8 and MMP-9 (but not MMP-3) were found to be associated with disease activity, as assessed by the BASDAI score.60
Cathepsin K is a potent collagenase predominantly expressed in osteoclasts and is involved in bone remodeling and resorption. Cathepsin K expression follows the signal of inflammatory cytokines released after tissue injury. Reports of cathepsin K levels in AS are controversial; this molecule seems to be expressed at a higher level by mononuclear cells, fibroblast-like cells, and other cells attached to bone but cathepsin K serum levels were not different compared with healthy controls and no significant modification following TNFi was observed.145,146
The cartilage oligomeric matrix (COMP) protein catalyzes the assembly of collagen in the extracellular matrix. Elevated COMP levels were observed in a variety of inflammatory joint diseases;18 however, its association with other clinical and disease activity markers in AS have been inconsistently reported. Nevertheless, one study reported an inverse relationship between COMP levels and MRI inflammation in patients with axSpA.19 Bone-specific alkaline phosphatase (BAP) is a marker of active bone formation. High levels of BAP have been found in patients with axSpA and are associated with disease activity.53,64
Recently, extracellular matrix turnover products have gained interest as possible biomarkers in SpA. Citrullinated fragments of vimentin (VICM) are MMP-degraded fragments modified by citrullination, a process occurring unspecifically during the inflammatory phase. VICM was found to be higher in patients with AS compared with controls and to be associated with high CRP serum levels.66,67 VICM levels, together with high baseline mSASSS values, were found to be predictors of radiographic progression.66 However, other researchers did not confirm this finding.67,68 Furthermore, indirect biomarkers of VICM, anti-citrullinated vimentin antibodies, are higher in SpA, including individuals with AS versus controls and appeared to show promising molecules associated with radiographic damage and correlated with CRP.65
Other products of collagen degradation are fragments of type I (C1M), II (C2M), IV (C4M), V (C5M), and VI (C6M) collagens generated by MMP. Their high levels in AS, compared with controls, supports their association with tissue remodeling during active disease.66,147,148 C1M and C6M are also correlated with CRP.65,66 C1M was associated with mSASSS progression in one study; however, the association was not confirmed after adjustment for confounders.67 A combination of C2M, C3M and C6M was found to be a marker of structural progression as it correlated with mSASSS.66 More recently C1M, C5M and C6M showed no relationship with mSASSS progression.68
An MMP-mediated metabolite of CRP (CRPM) was found to have higher levels in AS compared with nr-axSpA and was associated with some disease activity indices, such as CRP and ASDAS-CRP.65
Type I and type II collagen are proteins of cartilage and components of connective tissue. Studies have evaluated both serum and urinary type I and II C-terminal telopeptides (CTX-I and CTX-II). CTX-I is a marker of osteoclast activity reflecting bone degradation.149 Both urinary and serum CTX-I levels are higher in patients with AS compared with healthy controls.72,150 CTX-II levels were shown to correlate with MRI inflammation scores in SIJs and/or lumbar spine in patients with axSpA .19 CTX-II serum levels were also correlated with serological markers of disease activity,63,70,72 and they decreased after treatment with TNFi.18,19,71 CTX-II seems to be a biomarker of radiographic progression in axSpA. In multivariate analyses with a 2-year change in mSASSS,70 CTX-II seems to significantly and independently contribute to explaining variation in radiological damage and progression and the association remained after the adjustment for markers of inflammation (which also correlated with CTX-II). This observation suggests that cartilage degradation might be related to the process of syndesmophyte formation. Notably, CTX-II and CTX-I may serve as prognostic markers to identify patients prone to radiographic spinal progression with a more robust evidence for CTX-II.63,70,72,73
Osteocalcin is a small molecule of the mineralized bone matrix. Previous studies have shown that osteocalcin serum levels in AS are lower than in controls;151 however, recent studies have reported high serum levels in AS, associated with radiographic progression, especially for the development of new syndesmophytes.14,19,74,75 Osteocalcin was also found to increase early following TNFi treatment,19 which is intriguing because recent data showed a retardation of radiographic progression under TNFi.
Serum human cartilage glycoprotein-39 (YKL-40), a secretory protein of human articular chondrocytes and synoviocytes, is a marker of cartilage remodeling and is associated with disease activity in AS.18 YKL-40 serum levels are significantly higher in patients with SpA with respect to healthy controls but YKL-40 does not seem to perform as well as other inflammatory biomarkers, such as CRP and MMP-3, in the disease assessment.111 Aggrecan is the central component of cartilage extracellular matrix. The replacement of the glycosaminoglycan side chain with aggrecan with charge density modification determines osmotic processes, which are fundamental for the biomechanical properties of cartilage. Patients with AxSpA have been found to have decreased levels of total aggrecan compared with healthy subjects and aggrecan seems to increase following TNFi treatment.18
Miscellaneous
Vascular endothelial growth factor (VEGF) is a driver of angiogenesis and seems to be implicated in the initial phase of bone remodeling, which is characterized by inflammation and leads to bone formation. VEGF serum levels were reported to be higher in patients with AS and axSpA than in controls.18,70,76,77,123 High VEGF serum levels are associated with active disease and worse radiographic progression according to mSASSS.59,76–78
The fetuin family encompasses a series of proteins that have been implicated in numerous biological functions. Particularly, fetuin proteins seem to play a role in inflammatory processes and are implicated in bone remodeling, osteogenesis and bone resorption.152 Fetuin-A was found to be higher in patients with AS with syndesmophytes compared with patients with AS without syndesmophytes or controls.153
Adipokines are cytokines secreted by adipose tissue, which also have a role in mediating immune responses and regulating bone mass. Leptin, visfatin and high-molecular-weight adiponectin (HMW-ADN) are those mostly investigated in axSpA; however, inconsistent results have been reported. Leptin serum levels in AS have been reported to be either higher154,155 or lower compared with controls.78,81 A negative association of leptin with BASMI79 has been found and with radiographic progression independently of CRP and baseline structural damage,80,81 but other authors did not confirm this association.45 HMW-ADN serum levels were also found to be reduced in AS compared with controls and they were associated with syndesmophyte formation.78 Visfatin is reported to be increased compared with controls and seems to be associated with radiographic spinal progression, like resistin.156
Cytotoxic T lymphocyte-associated molecule (CTLA-4) is an inhibitor of immune response. CTLA-4 serum levels were lower in healthy controls compared with patients with axSpA, where they were correlated with CRP and BASDAI.157
Recently, anti-CD74 antibodies (or anti-CLIP antibodies) directed against CD74, a class II-associated invariant chain peptide, were found in patients with axSpA.158 Binding of CLIP antibodies to CD74 may lead to activation of cells and production of proinflammatory cytokines such as TNF-α. Baraliakos et al.159 found anti-CLIP antibodies are mostly present in patients with early axSpA compared with controls, but the frequency of such antibodies decreases overtime158 and further studies did not confirm a significant diagnostic value in patients suffering with inflammatory low back pain.160
Some researchers considered the possible role of p40 subunit, common to IL-12 and IL-23, as a biomarker of disease activity, as higher levels of synovial p40 IL12/23 were found higher in 27 patients with SpA versus patients with osteoarthritis and versus paired serum SpA levels.161 Ivanova et al. reported that serum IL-12 and IL-23p40 were raised among patients with AS and correlated with CRP and ASDAS;82 however, the findings warrant further confirmation in larger cohort studies.
Two reports have also recently described the hepatocyte growth factor (HGF) as a promising biomarker.60,83 HGF is secreted by mesenchymal cells and has pleiotropic effects, including suppression of inflammation and enhanced osteoblastic differentiation of mesenchymal cells. HGF appears to be involved in the osteoproliferation and osteoporosis in AS.84 Consistent associations between HGF and parameters of bone remodeling and lesions on imaging, especially on MRI, have yet to emerge.
Anti-drug antibodies and drug through levels
Immunogenicity has been implicated as a cause of treatment failure of bDMARDs, as all biological drugs have immunogenic potential85,162,163 and may induce formation of ADAbs. ADAb development is well known in RA and IBD, and it is described in all patients treated with monoclonal antibodies.164 ADAbs are associated with reduced effectiveness of treatments and adverse events and correlated negatively with drug serum levels.165 A considerable proportion of patients with axSpA treated with TNFi fail to respond ab initio (primary failure), or lose response over time despite an initial good response (secondary failure).164,166 Some patients may also discontinue TNFi treatment due to significant adverse events.86,167 ADAbs reduce drug blood levels by neutralizing the binding sites of the target molecules, or by forming immune complexes with the biological drugs and enhancing their clearance.168–171 ADAbs are described during treatment with all five TNFi to varying degrees, depending on the specific TNFi,171 and also following secukinumab and ustekinumab treatments.172,173 A comprehensive literature search using three databases (PubMed, Web of Science, and the Cochrane Library) on immunogenicity of TNFi in autoimmune diseases between 1966 and 2017, showed that ADAbs are present in 10% of patients with SpA treated with a TNFi.86 In these patients, lower drug through levels are also found, which are usually associated with poorer clinical response.85–89
ADAbs are often found in patients presenting secondary failure to the treatment and their detection predicted treatment failure over time.85,87,88,174 Overall, these observations led to the use of such biomarkers to drive treatment decisions, firstly in IBD, due to limited biological drug choice, and then in RA and SpA. Algorithms have been developed to identify TNFi poor responders and to drive treatment decisions, especially after the failure of a TNFi.90,91 In some studies, in patients with ADAbs and suboptimal treatment response the drug dosage was increased successfully to achieve a better treatment effectiveness.88 More recently, the detection of ADAbs was used to identify patients more prone to successfully taper the biological treatment.92 In patients with insufficient response, detectable ADAbs and low TNFi levels, we can assume that the drug effect is impaired by ADAbs (Figure 1). Thus, switching to another TNFi may be beneficial as it restores the TNF-α blockade. Nonetheless, if ADAbs are absent or low and the adequate therapeutic levels of the TNFi are achieved, inefficacy is not due to the neutralization of the therapeutic effect, but due to the fact that probably in that patient, TNF-α is not the main pathogenetic cytokine.90 Therefore, in the latter, patients switching to another mode of action might be the best treatment choice. The large experience in RA confirms these observations, and also suggests that testing drug levels might be sufficient.91,175,176 One study on a small cohort of patients with SpA also found that in patients starting a second TNFi, those who had developed ADAbs on the first TNFi showed a better ASDAS response.93
Figure 1.
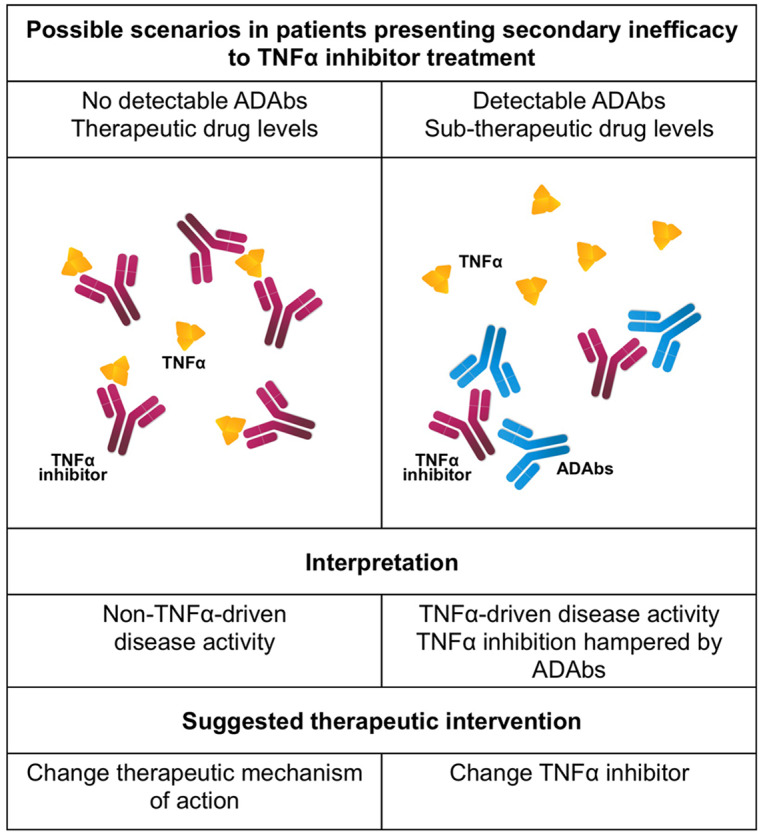
Possible pathogenetic mechanisms of secondary inefficacy to TNFα treatment. ADAbs, anti-drug antibodies; TNFα, tumor necrosis factor α
In addition to ADAbs, there are other factors that affects the pharmacokinetics of TNFi, such as concomitant use of conventional DMARDs, especially methotrexate, the degree of systemic inflammation, body weight, and sex.174 Combination therapy with biologic agent and DMARDs is used to prevent the development of ADAbs in patients with RA and IBD,86,89,177 but there is no evidence to support their use in axSpA.178,179 Noteworthy, some studies also evaluated immunogenicity of switching from innovator to biosimilar infliximab in patients with SpA180 and, as expected, it was not associated with any significant difference in the anti-drug antibody level.180
Combination of biomarkers
In rheumatic diseases, the need for biomarkers has led to several attempts to identify an index including a panel of biomarkers to be used for clinical assessment. In SpA, only two studies are available to date. The first was conducted in a large cohort of 356 patients with SpA treated with golimumab. The study considered 73 molecules potentially associated with structural damage as assessed with mSASSS over a 2-year follow up, and found that only IL-6 was correlated with MRI changes.45 Rademacher and colleagues,77 considered 117 patients treated with nonsteroidal anti-inflammatory drugs in the ENRADAS trial. Only 10 biomarkers were investigated: VEGF, leptin and HMW-ADN were associated with mSASSS changes after 2 years (the latter two were inversely correlated with mSASSS). The combination of these three biomarkers with clinical characteristics proved to be superior than the clinical variables alone, but with a small added value in predicting structural damage.
Conclusion
Numerous studies have been conducted to seek biomarkers to guide treatment in SpA and to monitor the disease. Currently, no new biomarkers satisfy the characteristics for use in clinical practice and CRP remains the most relevant biomarker in axSpA. Grouping and understanding the role of all other potential biomarkers is challenging. Inflammatory markers, such as CPT, have been investigated but standardization is lacking and they mirror CRP. Markers of bone metabolism, such as MMP and Dkk-1, have shown diverging results on disease activity and progression. The reason could be that biomarkers implicated in the axSpA pathogenetic process are restricted to specific tissues and endothelial compartments and do not migrate to the systemic circulation and/or lymphoid organs.127,146 ADAbs and therapeutic drug monitoring are adequate tools to drive treatment decision but are not useful to characterize the patient and identify those prone to more rapid structural damage. Eventually, a combination of biomarkers may be promising, nevertheless association with clinical characteristics is necessary to increase their predictive value. Further research should focus on the most promising biomarkers in order to reduce heterogeneity of observations and better define their role and cut-offs either alone or in combination.
Acknowledgments
The authors would like to express their appreciation to Eric Frank Nde for his assistance in editing the English version of this manuscript.
Footnotes
Author contributions: RR and AD contributed to study design and critical revision. ML and FO contributed to data collection and manuscript drafting. AO, MF and MF contributed to literature revision.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mariagrazia Lorenzin  https://orcid.org/0000-0002-5082-3043
https://orcid.org/0000-0002-5082-3043
Contributor Information
Mariagrazia Lorenzin, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Francesca Ometto, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Augusta Ortolan, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Mara Felicetti, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Marta Favero, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Andrea Doria, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Padova, Italy.
Roberta Ramonda, Rheumatology Unit, Department of Medicine –DIMED, University of Padova, Via Giustiniani 2, Padova, 35128, Italy.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390: 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 3. Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011; 70: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao SS, Radner H, Siebert S, et al. Comorbidity burden in axial spondyloarthritis: a cluster analysis. Rheumatology (Oxford) 2019; 58: 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Hooge M, Ramonda R, Lorenzin M, et al. Work productivity is associated with disease activity and functional ability in Italian patients with early axial spondyloarthritis: an observational study from the SPACE cohort. Arthritis Res Ther 2016; 18: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubash S, Bridgewood C, McGonagle D, et al. The advent of IL-17A blockade in ankylosing spondylitis: secukinumab, ixekizumab and beyond. Expert Rev Clin Immunol 2019; 15: 123–134. [DOI] [PubMed] [Google Scholar]
- 7. Blair HA. Secukinumab: a review in ankylosing spondylitis. Drugs 2019; 79: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 258–270. [DOI] [PubMed] [Google Scholar]
- 9. Poddubnyy D, Hermann KGA, Callhoff J, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014; 73: 817–823. [DOI] [PubMed] [Google Scholar]
- 10. Maksymowych WP, van der Heijde D, Baraliakos X, et al. Tofacitinib is associated with attainment of the minimally important reduction in axial magnetic resonance imaging inflammation in ankylosing spondylitis patients. Rheumatology (Oxford) 2018; 57: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017; 76: 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Vries MK, van Eijk IC, van der Horst-Bruinsma IE, et al. Erythrocyte sedimentation rate, C-reactive protein level, and serum amyloid a protein for patient selection and monitoring of anti-tumor necrosis factor treatment in ankylosing spondylitis. Arthritis Rheum 2009; 61: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 13. Poddubnyy DA, Rudwaleit M, Listing J, et al. Comparison of a high sensitivity and standard C-reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis 2010; 69: 1338–1341. [DOI] [PubMed] [Google Scholar]
- 14. Visvanathan S, Wagner C, Marini JC, et al. Inflammatory biomarkers, disease activity and spinal disease measures in patients with ankylosing spondylitis after treatment with infliximab. Ann Rheum Dis 2008; 67: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenzin M, Ortolan A, Vio S, et al. Biomarkers, imaging and disease activity indices in patients with early axial spondyloarthritis: the Italian arm of the spondyloarthritis caught early (SPACE) study. Reumatismo 2017; 69: 65–74. [DOI] [PubMed] [Google Scholar]
- 16. Prajzlerová K, Grobelná K, Pavelka K, et al. An update on biomarkers in axial spondyloarthritis. Autoimmun Rev 2016; 15: 501–509. [DOI] [PubMed] [Google Scholar]
- 17. Mohan C, Assassi S. Biomarkers in rheumatic diseases: how can they facilitate diagnosis and assessment of disease activity? BMJ 2015; 351: h5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen SJ, Sørensen IJ, Garnero P, et al. ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFα inhibitors. Ann Rheum Dis 2011; 70: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 19. Pedersen SJ, Sørensen IJ, Lambert RGW, et al. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor α inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum 2011; 63: 3789–3800. [DOI] [PubMed] [Google Scholar]
- 20. Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin Rheumatol 2015; 34: 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 22. Cantini F, Niccoli L, Nannini C, et al. ; Italian board for the TAilored BIOlogic therapy. Tailored first-line biologic therapy in patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum 2016; 45: 519–532. [DOI] [PubMed] [Google Scholar]
- 23. Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum 2017; 47: 183–192. [DOI] [PubMed] [Google Scholar]
- 24. Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken) 2019; 71: 2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015; 74: 52–59. [DOI] [PubMed] [Google Scholar]
- 26. Poddubnyy D, Haibel H, Listing J, et al. Cigarette smoking has a dose-dependent impact on progression of structural damage in the spine in patients with axial spondyloarthritis: results from the GErman SPondyloarthritis inception cohort (GESPIC). Ann Rheum Dis 2013; 72: 1430–1432. [DOI] [PubMed] [Google Scholar]
- 27. Zhao S, Jones GT, Macfarlane GJ, et al. Associations between smoking and extra-axial manifestations and disease severity in axial spondyloarthritis: results from the BSR biologics register for ankylosing spondylitis (BSRBR-AS). Rheumatology (Oxford) 2019; 58: 811–819. [DOI] [PubMed] [Google Scholar]
- 28. van Tubergen A, Ramiro S, van der Heijde D, et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012; 71: 518–523. [DOI] [PubMed] [Google Scholar]
- 29. Machado PM, Baraliakos X, van der Heijde D, et al. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016; 75: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 30. Lorenzin M, Ortolan A, Frallonardo P, et al. Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet Disord 2015; 16: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Yin Z, Song G, et al. Discriminating value of calprotectin in disease activity and progression of nonradiographic axial spondyloarthritis and ankylosing spondylitis. Dis Markers 2017; 2017: 7574147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oktayoglu P, Bozkurt M, Mete N, et al. Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Investig Med 2014; 62: 880–884. [DOI] [PubMed] [Google Scholar]
- 33. Turina MC, Sieper J, Yeremenko N, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis 2014; 73: 1746–1748. [DOI] [PubMed] [Google Scholar]
- 34. Turina MC, Yeremenko N, Paramarta JE, et al. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther 2014; 16: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turina MC, Yeremenko N, van Gaalen F, et al. Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: results from the spondyloarthritis caught early (SPACE) cohort. RMD Open 2017; 3: e000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 37. Lange U, Boss B, Teichmann J, et al. Serum amyloid A–an indicator of inflammation in ankylosing spondylitis. Rheumatol Int 2000; 19: 119–122. [DOI] [PubMed] [Google Scholar]
- 38. van Eijk IC, de Vries MK, Levels JHM, et al. Improvement of lipid profile is accompanied by atheroprotective alterations in high-density lipoprotein composition upon tumor necrosis factor blockade: a prospective cohort study in ankylosing spondylitis. Arthritis Rheum 2009; 60: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 39. Bal A, Unlu E, Bahar G, et al. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol 2007; 26: 211–215. [DOI] [PubMed] [Google Scholar]
- 40. Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 1995; 38: 499–505. [DOI] [PubMed] [Google Scholar]
- 41. Musacchio E, Valvason C, Botsios C, et al. The tumor necrosis factor-α-blocking agent infliximab inhibits interleukin 1β (IL-1β) and IL-6 gene expression in human osteoblastic cells. J Rheumatol 2009; 36: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 42. Romero-Sanchez C, Jaimes DA, Londoño J, et al. Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin Exp Rheumatol 2011; 29: 828–834. [PubMed] [Google Scholar]
- 43. Xueyi L, Lina C, Zhenbiao W, et al. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-α therapy. J Clin Immunol 2013; 33: 151–161. [DOI] [PubMed] [Google Scholar]
- 44. Sveaas SH, Berg IJ, Provan SA, et al. Circulating levels of inflammatory cytokines and cytokine receptors in patients with ankylosing spondylitis: a cross-sectional comparative study. Scand J Rheumatol 2015; 44: 118–124. [DOI] [PubMed] [Google Scholar]
- 45. Inman RD, Baraliakos X, Hermann KGA, et al. Serum biomarkers and changes in clinical/MRI evidence of golimumab-treated patients with ankylosing spondylitis: results of the randomized, placebo-controlled GO-RAISE study. Arthritis Res Ther 2016; 18: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Appel H, Maier R, Bleil J, et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum 2013; 65: 1522–1529. [DOI] [PubMed] [Google Scholar]
- 47. Chen WS, Chang YS, Lin KC, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc 2012; 75: 303–308. [DOI] [PubMed] [Google Scholar]
- 48. Kim HR, Lee SH, Kim HY. Elevated serum levels of soluble receptor activator of nuclear factors-kappaB ligand (sRANKL) and reduced bone mineral density in patients with ankylosing spondylitis (AS). Rheumatology (Oxford) 2006; 45: 1197–1200. [DOI] [PubMed] [Google Scholar]
- 49. Nocturne G, Pavy S, Boudaoud S, et al. Increase in Dickkopf-1 serum level in recent spondyloarthritis. Data from the DESIR cohort. PLoS ONE 2015; 10: e0134974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Andrade KR, de Castro GRW, Vicente G, et al. Evaluation of circulating levels of inflammatory and bone formation markers in axial spondyloarthritis. Int Immunopharmacol 2014; 21: 481–486. [DOI] [PubMed] [Google Scholar]
- 51. Taylan A, Sari I, Akinci B, et al. Biomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitis. BMC Musculoskelet Disord 2012; 13: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saad CGS, Ribeiro ACM, Moraes JCB, et al. Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res Ther 2012; 14: R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heiland GR, Appel H, Poddubnyy D, et al. High level of functional Dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis 2012; 71: 572–574. [DOI] [PubMed] [Google Scholar]
- 54. Yucong Z, Lu L, Shengfa L, et al. Serum functional Dickkopf-1 levels are inversely correlated with radiographic severity of ankylosing spondylitis. Clin Lab 2014; 60: 1527–1531. [DOI] [PubMed] [Google Scholar]
- 55. Genre F, López-Mejías R, Miranda-Filloy JA, et al. Osteoprotegerin correlates with disease activity and endothelial activation in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin Exp Rheumatol 2014; 32: 640–646. [PubMed] [Google Scholar]
- 56. Franck H, Meurer T, Hofbauer LC. Evaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitis. J Rheumatol 2004; 31: 2236–2241. [PubMed] [Google Scholar]
- 57. Moz S, Aita A, Basso D, et al. Spondyloarthritis: matrix metalloproteinasesas biomarkers of pathogenesis and response to tumor necrosis factor (TNF) inhibitors. Int J Mol Sci 2017; 18: 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fiocco U, Sfriso P, Oliviero F, et al. Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-α blockade in the knee joint. Arthritis Res Ther 2010; 12: R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramonda R, Modesti V, Ortolan A, et al. Serological markers in psoriatic arthritis: promising tools. Exp Biol Med (Maywood) 2013; 238: 1431–1436. [DOI] [PubMed] [Google Scholar]
- 60. Mattey DL, Packham JC, Nixon NB, et al. Association of cytokine and matrix metalloproteinase profiles with disease activity and function in ankylosing spondylitis. Arthritis Res Ther 2012; 14: R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maksymowych WP, Fitzgerald O, Wells GA, et al. Proposal for levels of evidence schema for validation of a soluble biomarker reflecting damage endpoints in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, and recommendations for study design. J Rheumatol 2009; 36: 1792–1799. [DOI] [PubMed] [Google Scholar]
- 62. van Kuijk AWR, DeGroot J, Koeman RC, et al. Soluble biomarkers of cartilage and bone metabolism in early proof-of-concept trials in psoriasic arthritis: effects of adalimumab versus placebo. PLoS One 2010; 5: e12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arends S, van der Veer E, Groen H, et al. Serum MMP-3 level as a biomarker for monitoring and predicting response to etanercept treatment in ankylosing spondylitis. J Rheumatol 2011; 38: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 64. Appel H, Ruiz-Heiland G, Listing J, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 2009; 60: 3257–3262. [DOI] [PubMed] [Google Scholar]
- 65. Siebuhr AS, Hušáková M, Forejtová S, et al. Metabolites of C-reactive protein and vimentin are associated with disease activity of axial spondyloarthritis. Clin Exp Rheumatol 2019; 37: 358–366. [PubMed] [Google Scholar]
- 66. Bay-Jensen AC, Karsdal MA, Vassiliadis E, et al. Circulating citrullinated vimentin fragments reflect disease burden in ankylosing spondylitis and have prognostic capacity for radiographic progression. Arthritis Rheum 2013; 65: 972–980. [DOI] [PubMed] [Google Scholar]
- 67. Siebuhr AS, van der Heijde D, Bay-Jensen AC, et al. Is radiographic progression in radiographic axial spondyloarthritis related to matrix metalloproteinase degradation of extracellular matrix? RMD Open 2018; 4: e000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hušáková M, Bay-Jensen AC, Forejtová Š, et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci Rep 2019; 9: 11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang X, Lin Z, Wei Q, et al. Expression of IL-23 and IL-17 and effect of IL-23 on IL-17 production in ankylosing spondylitis. Rheumatol Int 2009; 29: 1343–1347. [DOI] [PubMed] [Google Scholar]
- 70. Vosse D, Landewé R, Garnero P, et al. Association of markers of bone and cartilage degradation with radiological changes at baseline and after 2 years follow-up in patients with ankylosing spondylitis. Rheumatology (Oxford) 2008; 47: 1219–1222. [DOI] [PubMed] [Google Scholar]
- 71. Maksymowych WP, Rahman P, Shojania K, et al. ; M03-606 Study Group. Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. J Rheumatol 2008; 35: 2030–2037. [PubMed] [Google Scholar]
- 72. Park MC, Chung SJ, Park YB, et al. Bone and cartilage turnover markers, bone mineral density, and radiographic damage in men with ankylosing spondylitis. Yonsei Med J 2008; 49: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kang KY, Jung JY, Hong YS, et al. Positive correlation between inflammation on sacroiliac joint MRI and serum C-terminal telopeptide of type-I collagen in ankylosing spondylitis but not in non-radiographic axial spondyloarthritis. Clin Exp Rheumatol 2017; 35: 415–422. [PubMed] [Google Scholar]
- 74. Kwon SR, Lim MJ, Suh CH, et al. Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol Int 2012; 32: 2523–2527. [DOI] [PubMed] [Google Scholar]
- 75. Yilmaz N, Ozaslan J. Biochemical bone turnover markers in patients with ankylosing spondylitis. Clin Rheumatol 2000; 19: 92–98. [DOI] [PubMed] [Google Scholar]
- 76. Poddubnyy D, Conrad K, Haibel H, et al. Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 2014; 73: 2137–2143. [DOI] [PubMed] [Google Scholar]
- 77. Drouart M, Saas P, Billot M, et al. High serum vascular endothelial growth factor correlates with disease activity of spondylarthropathies. Clin Exp Immunol 2003; 132: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rademacher J, Tietz LM, Le L, et al. Added value of biomarkers compared with clinical parameters for the prediction of radiographic spinal progression in axial spondyloarthritis. Rheumatology (Oxford) 2019; 58: 1556–1564. [DOI] [PubMed] [Google Scholar]
- 79. Sari I, Demir T, Kozaci LD, et al. Body composition, insulin, and leptin levels in patients with ankylosing spondylitis. Clin Rheumatol 2007; 26: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 80. Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 2016; 75: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 81. Hartl A, Sieper J, Syrbe U, et al. Serum levels of leptin and high-molecular-weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: results from the ENRADAS trial. Arthritis Res Ther 2017; 19: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ivanova M, Manolova I, Miteva L, et al. Genetic variations in the IL-12B gene in association with IL-23 and IL-12p40 serum levels in ankylosing spondylitis. Rheumatol Int 2019; 39: 111–119. [DOI] [PubMed] [Google Scholar]
- 83. Maksymowych WP. Biomarkers for diagnosis of axial spondyloarthritis, disease activity, prognosis, and prediction of response to therapy. Front Immunol 2019; 10: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Torres L, Klingberg E, Nurkkala M, et al. Hepatocyte growth factor is a potential biomarker for osteoproliferation and osteoporosis in ankylosing spondylitis. Osteoporos Int 2019; 30: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin Rheumatol 2013; 32: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 86. Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs 2015; 29: 241–258. [DOI] [PubMed] [Google Scholar]
- 87. Bornstein G, Lidar M, Langevitz P, et al. The prevalence and clinical effect of immunogenicity of TNF-α blockers in patients with axial spondyloarthritis. Clin Exp Rheumatol 2018; 36: 228–232. [PubMed] [Google Scholar]
- 88. Arstikyte I, Kapleryte G, Butrimiene I, et al. Influence of immunogenicity on the efficacy of long-term treatment with TNF α blockers in rheumatoid arthritis and spondyloarthritis patients. Biomed Res Int 2015; 2015: 604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013; 72: 1947–1955. [DOI] [PubMed] [Google Scholar]
- 90. Sirotti S, Generali E, Ceribelli A, et al. Personalized medicine in rheumatology: the paradigm of serum autoantibodies. Auto Immun Highlights 2017; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. l’Ami MJ, Krieckaert CL, Nurmohamed MT, et al. Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann Rheum Dis 2018; 77: 484–487. [DOI] [PubMed] [Google Scholar]
- 92. Almirall M, Gimeno R, Salman-Monte TC, et al. Drug levels, immunogenicity and assessment of active sacroiliitis in patients with axial spondyloarthritis under biologic tapering strategy. Rheumatol Int 2016; 36: 575–578. [DOI] [PubMed] [Google Scholar]
- 93. Plasencia C, Pascual-Salcedo D, García-Carazo S, et al. The immunogenicity to the first anti-TNF therapy determines the outcome of switching to a second anti-TNF therapy in spondyloarthritis patients. Arthritis Res Ther 2013; 15: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Spoorenberg A, van der Heijde D, de Klerk E, et al. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol 1999; 26: 980–984. [PubMed] [Google Scholar]
- 95. Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol 1999; 26: 966–970. [PubMed] [Google Scholar]
- 96. Rudwaleit M, Landewé R, van der Heijde D, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009; 68: 770–776. [DOI] [PubMed] [Google Scholar]
- 97. Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009; 60: 717–727. [DOI] [PubMed] [Google Scholar]
- 98. Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012; 64: 1388–1398. [DOI] [PubMed] [Google Scholar]
- 99. Ometto F, Friso L, Astorri D, et al. Calprotectin in rheumatic diseases. Exp Biol Med (Maywood) 2017; 242: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013; 2013: 828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Duran A, Kobak S, Sen N, et al. Fecal calprotectin is associated with disease activity in patients with ankylosing spondylitis. Bosn J Basic Med Sci 2016; 16: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010; 341: c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Klingberg E, Carlsten H, Hilme E, et al. Calprotectin in ankylosing spondylitis–frequently elevated in feces, but normal in serum. Scand J Gastroenterol 2012; 47: 435–444. [DOI] [PubMed] [Google Scholar]
- 104. Levitova A, Hulejova H, Spiritovic M, et al. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res Ther 2016; 18: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Angel K, Provan SA, Fagerhol MK, et al. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens 2012; 25: 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramonda R, Lo Nigro A, Modesti V, et al. Atherosclerosis in psoriatic arthritis. Autoimmun Rev 2011; 10: 773–778. [DOI] [PubMed] [Google Scholar]
- 107. Ramonda R, Puato M, Punzi L, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor-alpha blockers: a two-year prospective observational study. Joint Bone Spine 2014; 81: 421–425. [DOI] [PubMed] [Google Scholar]
- 108. Aita A, Basso D, Ramonda R, et al. Genetics in TNF-TNFR pathway: a complex network causing spondyloarthritis and conditioning response to anti-TNFα therapy. PLoS One 2018; 13: e0194693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Francois RJ, Neure L, Sieper J, et al. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis 2006; 65: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu W, Wu YH, Zhang L, et al. Elevated serum levels of IL-6 and IL-17 may associate with the development of ankylosing spondylitis. Int J Clin Exp Med 2015; 8: 17362–17376. [PMC free article] [PubMed] [Google Scholar]
- 111. Pedersen SJ, Hetland ML, Sørensen IJ, et al. Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFα inhibitors. Clin Rheumatol 2010; 29: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 112. Rosine N, Etcheto A, Hendel-Chavez H, et al. Increase in Il-31 serum levels is associated with reduced structural damage in early axial spondyloarthritis. Sci Rep 2018; 8: 7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Baeten D, Sieper J, Braun J, et al. ; MEASURE 1 Study Group, MEASURE 2 Study Group. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015; 373: 2534–2548. [DOI] [PubMed] [Google Scholar]
- 114. Braun J, Baraliakos X, Deodhar A, et al. ; MEASURE 1 study group. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017; 76: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 115. Sieper J, Deodhar A, Marzo-Ortega H, et al. ; MEASURE 2 Study Group. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 study. Ann Rheum Dis 2017; 76: 571–592. [DOI] [PubMed] [Google Scholar]
- 116. Jansen DTSL, Hameetman M, van Bergen J, et al. IL-17-producing CD4+ T cells are increased in early, active axial spondyloarthitis including patients without imaging abnormalities. Rheumatology (Oxford) 2015; 54: 728–735. [DOI] [PubMed] [Google Scholar]
- 117. Raychaudhuri SK, Saxena A, Raychaudhuri SP. Role of IL-17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin Rheumatol 2015; 34: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 118. Duvallet E, Semerano L, Assier E, et al. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med 2011; 43: 503–511. [DOI] [PubMed] [Google Scholar]
- 119. Andersen T, Rasmussen TK, Hvid M, et al. Increased plasma levels of IL-21 and IL-23 in spondyloarthritis are not associated with clinical and MRI findings. Rheumatol Int 2012; 32: 387–393. [DOI] [PubMed] [Google Scholar]
- 120. Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xiao F, Zhang HY, Liu YJ, et al. Higher frequency of peripheral blood interleukin 21 positive follicular helper T cells in patients with ankylosing spondylitis. J Rheumatol 2013; 40: 2029–2037. [DOI] [PubMed] [Google Scholar]
- 122. Zhang L, Li YG, Li YH, et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One 2012; 7: e31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li XL, Lin TT, Qi CY, et al. Elevated serum level of IL-33 and sST2 in patients with ankylosing spondylitis: associated with disease activity and vascular endothelial growth factor. J Investig Med 2013; 61: 848–851. [DOI] [PubMed] [Google Scholar]
- 124. Li GX, Wang S, Duan ZH, et al. Serum levels of IL-33 and its receptor ST2 are elevated in patients with ankylosing spondylitis. Scand J Rheumatol 2013; 42: 226–231. [DOI] [PubMed] [Google Scholar]
- 125. Han GW, Zeng LW, Liang CX, et al. Serum levels of IL-33 is increased in patients with ankylosing spondylitis. Clin Rheumatol 2011; 30: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 126. Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells 2017; 40: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen CH, Chen HA, Liao HT, et al. Soluble receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in ankylosing spondylitis: OPG is associated with poor physical mobility and reflects systemic inflammation. Clin Rheumatol 2010; 29: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 128. Perpétuo IP, Caetano-Lopes J, Vieira-Sousa E, et al. Ankylosing spondylitis patients have impaired osteoclast gene expression in circulating osteoclast precursors. Front Med (Lausanne) 2017; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 2011; 6: e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Luchetti MM, Ciccia F, Avellini C, et al. Sclerostin and antisclerostin antibody serum levels predict the presence of axial spondyloarthritis in patients with inflammatory bowel disease. J Rheumatol 2018; 45: 630–637. [DOI] [PubMed] [Google Scholar]
- 131. Tsui FWL, Tsui HW, Las Heras F, et al. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann Rheum Dis 2014; 73: 1873–1879. [DOI] [PubMed] [Google Scholar]
- 132. Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007; 13: 156–163. [DOI] [PubMed] [Google Scholar]
- 133. Daoussis D, Liossis SNC, Solomou EE, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum 2010; 62: 150–158. [DOI] [PubMed] [Google Scholar]
- 134. Jadon DR, Sengupta R, Nightingale A, et al. Serum bone-turnover biomarkers are associated with the occurrence of peripheral and axial arthritis in psoriatic disease: a prospective cross-sectional comparative study. Arthritis Res Ther 2017; 19: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013; 65: 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Maas F, Arends S, Wink FR, et al. Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-α inhibitors. PLoS One 2017; 12: e0177231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Molnar C, Scherer A, Baraliakos X, et al. ; Rheumatologists of the Swiss Clinical Quality Management Program. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss clinical quality management cohort. Ann Rheum Dis 2018; 77: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Klingberg E, Nurkkala M, Carlsten H, et al. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol 2014; 41: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 139. Chen CH, Lin KC, Yu DTY, et al. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology (Oxford) 2006; 45: 414–420. [DOI] [PubMed] [Google Scholar]
- 140. Vandooren B, Kruithof E, Yu DTY, et al. Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum 2004; 50: 2942–2953. [DOI] [PubMed] [Google Scholar]
- 141. Yang C, Gu J, Rihl M, et al. Serum levels of matrix metalloproteinase 3 and macrophage colony-stimulating factor 1 correlate with disease activity in ankylosing spondylitis. Arthritis Rheum 2004; 51: 691–699. [DOI] [PubMed] [Google Scholar]
- 142. Lorenzin M, Ortolan A, Felicetti M, et al. Serological biomarkers in early axial spondyloarthritis during 24-months follow up (Italian arm of space study). Front Med (Lausanne) 2019; 6: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Maksymowych WP, Landewé R, Conner-Spady B, et al. Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum 2007; 56: 1846–1853. [DOI] [PubMed] [Google Scholar]
- 144. Soliman E, Labib W, el-Tantawi G, et al. Role of matrix metalloproteinase-3 (MMP-3) and magnetic resonance imaging of sacroiliitis in assessing disease activity in ankylosing spondylitis. Rheumatol Int 2012; 32: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 145. Wendling D, Cedoz JP, Racadot E. Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist therapy. Joint Bone Spine 2008; 75: 559–562. [DOI] [PubMed] [Google Scholar]
- 146. Neidhart M, Baraliakos X, Seemayer C, et al. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Ann Rheum Dis 2009; 68: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 147. Munk HL, Gudmann NS, Christensen AF, et al. Cartilage collagen type II seromarker patterns in axial spondyloarthritis and psoriatic arthritis: associations with disease activity, smoking and HLA-B27. Rheumatol Int 2016; 36: 541–549. [DOI] [PubMed] [Google Scholar]
- 148. Veidal SS, Larsen DV, Chen X, et al. MMP-mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin Biochem 2012; 45: 541–546. [DOI] [PubMed] [Google Scholar]
- 149. Huvelle S, Bothy A, Lepoutre T, et al. Measurement of C-terminal cross-linking telopeptide of type I collagen: evaluation of a new automated assay. Clin Biochem 2013; 46: 1778–1779. [DOI] [PubMed] [Google Scholar]
- 150. Choi ST, Kim JH, Kang EJ, et al. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford) 2008; 47: 1775–1779. [DOI] [PubMed] [Google Scholar]
- 151. Franck H, Keck E. Serum osteocalcin and vitamin D metabolites in patients with ankylosing spondylitis. Ann Rheum Dis 1993; 52: 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Favero M, Belluzzi E, Frallonardo P, et al. Synovial fluid fetuin-A levels in patients affected by osteoarthritis with or without evidence of calcium crystals. Rheumatology (Oxford) 2019; 58: 729–730. [DOI] [PubMed] [Google Scholar]
- 153. Tuylu T, Sari I, Solmaz D, et al. Fetuin-A is related to syndesmophytes in patients with ankylosing spondylitis: a case control study. Clinics (Sao Paulo) 2014; 69: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Miranda-Filloy JA, López-Mejías R, Genre F, et al. Adiponectin and resistin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin Exp Rheumatol 2013; 31: 365–371. [PubMed] [Google Scholar]
- 155. Kim KJ, Kim JY, Park SJ, et al. Serum leptin levels are associated with the presence of syndesmophytes in male patients with ankylosing spondylitis. Clin Rheumatol 2012; 31: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 156. Syrbe U, Callhoff J, Conrad K, et al. Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis Rheumatol 2015; 67: 678–685. [DOI] [PubMed] [Google Scholar]
- 157. Toussirot E, Saas P, Deschamps M, et al. Increased production of soluble CTLA-4 in patients with spondylarthropathies correlates with disease activity. Arthritis Res Ther 2009; 11: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Baerlecken NT, Nothdorft S, Stummvoll GH, et al. Autoantibodies against CD74 in spondyloarthritis. Ann Rheum Dis 2014; 73: 1211–1214. [DOI] [PubMed] [Google Scholar]
- 159. Baraliakos X, Baerlecken N, Witte T, et al. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann Rheum Dis 2014; 73: 1079–1082. [DOI] [PubMed] [Google Scholar]
- 160. de Winter JJ, van de Sande MG, Baerlecken N, et al. Anti-CD74 antibodies have no diagnostic value in early axial spondyloarthritis: data from the spondyloarthritis caught early (SPACE) cohort. Arthritis Res Ther 2018; 20: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wendling D, Cedoz JP, Racadot E. Serum and synovial fluid levels of p40 IL12/23 in spondyloarthropathy patients. Clin Rheumatol 2009; 28: 187–190. [DOI] [PubMed] [Google Scholar]
- 162. Rosas J, Martín-López M, Otón T, et al. Practical aspects of biological throughput levels and antidrug antibodies in rheumatoid arthritis and spondyloarthritis. Reumatol Clin. Epub ahead of print 29 October 2018. DOI: 10.1016/j.reuma.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 163. Balsa A, Lula S, Marshall L, et al. The comparative immunogenicity of biologic therapy and its clinical relevance in psoriatic arthritis: a systematic review of the literature. Expert Opin Biol Ther 2018; 18: 575–584. [DOI] [PubMed] [Google Scholar]
- 164. de Vries MK, Wolbink GJ, Stapel SO, et al. Inefficacy of infliximab in ankylosing spondylitis is correlated with antibody formation. Ann Rheum Dis 2007; 66: 133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Balsa A, Sanmarti R, Rosas J, et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study. Rheumatology (Oxford) 2018; 57: 688–693. [DOI] [PubMed] [Google Scholar]
- 166. Spadaro A, Punzi L, Marchesoni A, et al. Switching from infliximab or etanercept to adalimumab in resistant or intolerant patients with spondyloarthritis: a 4-year study. Rheumatology (Oxford) 2010; 49: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 167. Matsumoto Y, Maeda T, Tsuboi R, et al. Anti-adalimumab and anti-infliximab antibodies developed in psoriasis vulgaris patients reduced the efficacy of biologics: report of two cases. J Dermatol 2013; 40: 389–392. [DOI] [PubMed] [Google Scholar]
- 168. Ordás I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012; 91: 635–646. [DOI] [PubMed] [Google Scholar]
- 169. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 170. Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006; 54: 711–715. [DOI] [PubMed] [Google Scholar]
- 171. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72: 165–178. [DOI] [PubMed] [Google Scholar]
- 172. Deodhar A, Gladman DD, McInnes IB, et al. Secukinumab immunogenicity over 52 weeks in patients with psoriatic arthritis and ankylosing spondylitis. J Rheumatol. Epub ahead of print 15 June 2019. DOI: 10.3899/jrheum.190116. [DOI] [PubMed] [Google Scholar]
- 173. Poor SM, Ulshöfer T, Gabriel LA, et al. Immunogenicity assay development and validation for biological therapy as exemplified by ustekinumab. Clin Exp Immunol 2019; 196: 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hwang J, Kim HM, Jeong H, et al. Higher body mass index and anti-drug antibodies predict the discontinuation of anti-TNF agents in Korean patients with axial spondyloarthritis. Rev Bras Reumatol Engl Ed 2017; 57: 311–319. [DOI] [PubMed] [Google Scholar]
- 175. Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis 2010; 69: 817–821. [DOI] [PubMed] [Google Scholar]
- 176. Jamnitski A, Bartelds GM, Nurmohamed MT, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis 2011; 70: 284–288. [DOI] [PubMed] [Google Scholar]
- 177. Jani M, Barton A, Warren RB, et al. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford) 2014; 53: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Krzysiek R, Breban M, Ravaud P, et al. ; French Ankylosing Spondylitis Infliximab Network. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum 2009; 61: 569–576. [DOI] [PubMed] [Google Scholar]
- 179. Marzo-Ortega H, McGonagle D, Jarrett S, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis 2005; 64: 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Benucci M, Gobbi FL, Bandinelli F, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res 2017; 65: 419–422. [DOI] [PubMed] [Google Scholar]


