Abstract
Objective:
To explore the clinical efficacy of HiPorfin photodynamic therapy for advanced esophageal cancer and evaluate its impact on survival.
Methods:
Retrospective analysis of 32 patients with advanced obstructive esophageal cancer at our institution from September 2013 to December 2016. HiPorfin was infused as the photosensitizer at a dose of 5 mg/kg, and after 48 hours, 630-nm laser irradiation was subsequently performed through an optical fiber that passed through the biopsy channel of a flexible endoscope.
Results:
The effectiveness rate was 78.1% (25/32), and the significant efficacy rate was 56.3% (18/32). The dysphagia score decreased from 3.43 ± 0.73 to 1.79 ± 0.53 (P < .05). There was no grade 3 or more toxicity. The median overall survival was estimated to be 16 months. Univariate analysis showed higher overall survival with a Karnofsky Performance Status score ≥80 compared with a Karnofsky Performance Status score <80 (hazard ratio: 2.626; 95% CI: 1.091-6.322; P = .024). Overall survival was higher in patients who had received radiation therapy than in patients who did not receive radiation therapy (hazard ratio: 3.574; 95% CI: 1.501-8.510; P = .002).
Conclusion:
Photodynamic therapy is an effective method for advanced esophageal cancer. The side effects are mild, and the short-term effect is good, especially in the relief of dysphagia. Photodynamic therapy can prolong the survival of patients with advanced esophageal cancer, and the Karnofsky Performance Status score and previous radiation therapy have a significant effect on the overall survival.
Keywords: photodynamic therapy, esophageal cancer, obstruction, clinical efficacy, survival
Introduction
Esophageal cancer is one of the most common malignant tumors of the digestive tract in the world, and it is also one of the most common causes of cancer-related deaths.1,2 Esophageal cancer is more common in people older than 40 years and often occurs in the middle thoracic section of the esophagus. The most common histological type in China is squamous cell carcinoma (up to 90%), followed by adenocarcinoma.2 The disease is highly malignant with poor prognosis. Most patients are already in the advanced stage when diagnosed.3 Although surgery is considered the main treatment, the overall survival (OS) rate after 5 years is less than 30%. Local recurrence and metastasis are the main causes of surgical failure.4 Radiotherapy (RT) and chemotherapy are also traditional treatments, but they easily cause negative side effects and have limited efficacy. For advanced esophageal cancer with obstruction, patients are often ineligible for surgery, which result in patients with dysphagia, poor physical condition, and difficulty tolerating RT or chemotherapy. Stent implantation is an option to alleviate dysphagia, but the possibility exists for tumor growth at the location of the stent, as well as stent expansion.5
Photodynamic therapy (PDT) is a clinically approved, minimally invasive therapeutic procedure. The procedure involves administration of a photosensitizing agent followed by irradiation at a wavelength corresponding to an absorbance band of the sensitizer. In the presence of molecular oxygen, the energy transfer can lead to a series of photochemical reactions and generation of various cytotoxic species and other reactive oxygen species, and consequently induce apoptosis and/or necrosis of targeted lesion.6 Photodynamic therapy received Food and Drug Administration approval for palliative treatment for obstructive esophageal cancer as early as 1995. Recently, PDT has emerged as a safe and effective technique for treating esophageal cancer. Additional benefits are increased survival and improved quality of life.7
Photosensitizer is an important factor in PDT.8-10 HiPorfin is a photosensitizer developed by China, and its efficacy has been confirmed by some reports.10,11 Its molecular weight is 598.7 and the molecular formula is C34H38N4O6 (Figure 1). The main components are hematoporphyrin, porphyrin polymer, hydroxyethyl-vinylporphyrin, and a small amount of protoporphyrin.
Figure 1.
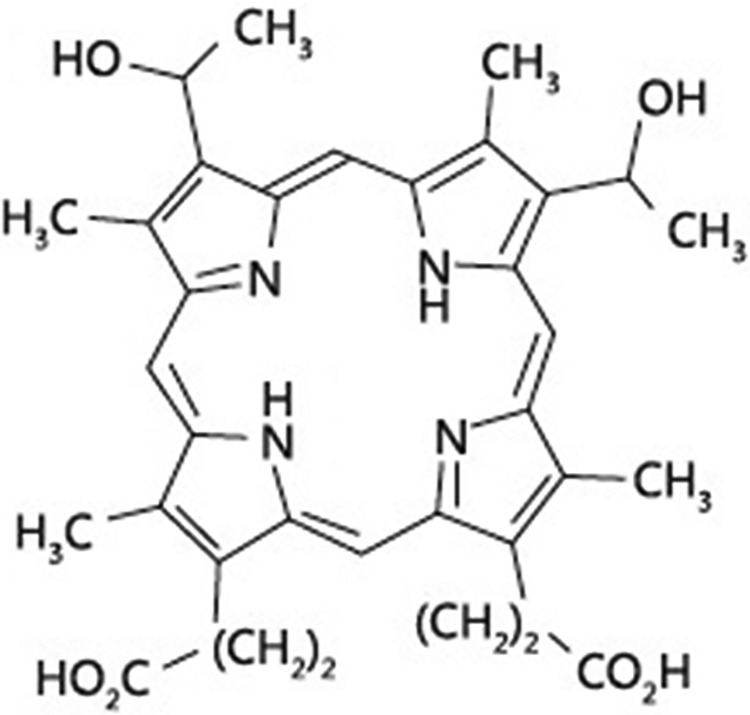
The chemical structure of HiPorfin.
At present, there are few institutions that carry out PDT in China.11-13 This study was designed to retrospectively analyze the short-term efficacy and side effects of PDT in the treatment of advanced esophageal cancer and to evaluate its impact on survival.
Materials and Methods
Patient Selection and Data Collection
Following approval by our Ethics Committee, we identified patients by searching the tumor registry of the cancer center in our institution for pathologically confirmed esophageal cancer from September 2013 to December 2016.
The inclusion criteria (1) ineligible for surgery or RT, (2) recurrence after surgery or RT, (3) any reason for refusing surgery or RT, (4) advanced esophageal cancer (T3-4N1-3M0, Union for International Cancer Control [UICC] seventh), (5) received PDT in our institution, and (6) signed the PDT research consent form.
The exclusion criteria (1) early esophageal cancer (T1-2N0-1M0, UICC seventh), (2) did not receive PDT in our institution, and (3) underwent esophageal stenting.
Charts were reviewed to collect clinical and demographic data, including age, sex, Karnofsky Performance Status (KPS) score, histology information, tumor location and size, symptoms (including dysphagia score), previous treatment (including surgery, RT, chemotherapy, etc), PDT treatment cycle, short-term efficacy and side effects, follow-up, and survival.
Treatment
Equipment and photosensitizer
Treatment light source: DIOMED630 semiconductor laser treatment machine produced by British Laser Instrument Co, Ltd, laser wavelength 630 nm, pulse output;
Optical fiber: Optical fiber of British Laser Instrument Co, Ltd, columnar fiber end light-emitting section 1 to 6 cm;
Electronic endoscope: An electronic fiber endoscope produced by Fujinon, Japan;
Photosensitizer: Trade name: HiPorfin, generic name: Hematoporphyrin Injection, pharmaceutical company name: Chongqing Huading Modern Biopharmaceutical Co, Ltd, origin: China, specification: 100 mg/vial.
Photodynamic therapy preparation
Ward requirements: The doors and windows of the treatment room must be covered with black light-blocking cloth, and the preferred room lighting is from low-power white lights or table lamps;
Use of photosensitizer: HiPorfin was dissolved to 5 mg/mL by 5% glucose injection fluid before intravenous administration at a dose level of 5 mg/kg body weight. The IV infusion tube was rinsed in order to ensure a correct dose.
Note: Underwent the skin test before using HiPorfin. The patients were closely observed for changes in their condition after the injection of the photosensitizer, and 6 hours before the procedure, all patients performed a water fast.
Treatment process
Laser irradiation was performed 48 hours after administration of the photosensitizer. Prior to irradiation, local anesthesia in the form of lidocaine spray was administered. A cylindrical fiber was introduced through an electronic digestive endoscopic biopsy channel to illuminate the lesion. When irradiating, the fiber was placed in the middle of the esophagus as much as possible, and different columnar fiber lengths were used depending on the extent of the lesion. For tumors that completely obstructed the esophagus, we inserted the fiber into the center of the tumor or into the edge of the tumor for laser irradiation. Fiber insertion generally did not cause tumor rupture and bleeding.
Depending on the length of the tumor in the lumen, each treatment consisted of irradiation of 1 to 3 segments of the tumor. The illumination time for each segment was calculated by the laser treatment machine, which determines the times according to the different types of cancer. Generally, the irradiation time was 12 minutes, and the energy density was 200 J/cm. For a tumor in which an optical fiber was inserted, the energy density was 300 J/cm because the increase in the intratumoral dose of light assists the photochemical reactions to maximize tumor killing, while minimizing the effects on normal esophageal mucosa. When the laser was used for irradiation of multiple tumor segments, the laser light needed to exceed at least 0.5 to 1.0 cm past the edge of the lesion in order that the illumination range fully covered the lesion.
Necrotic tissue was removed by bronchoscopy after 72 hours. After that, the necrotic tissue was removed from the lesion according to the specific conditions, and one cycle of treatment was completed.
Postoperative observation
After PDT, the patient’s vital signs were observed. The patients fasted for the next 24 hours and were routinely given acid suppression and nutritional support treatment. Patients were observed so that common complications such as perforation and bleeding could be quickly treated. For the next 30 days after the PDT procedure, patients were instructed to strictly protect themselves from light (mainly sunlight) to avoid any allergic reaction, and allergic treatments were given if necessary.
Efficacy Evaluation
The short-term efficacy was evaluated
One month after PDT, the endoscopic effect was evaluated, and the international standard was adopted.14 Complete response (CR): the tumor completely disappears over a period of 1 month and is negative by pathology biopsy. Significant response (SR): the product of the tumor’s largest diameter and upright diameter (or the height of the tumor) is reduced by over 50% over a period of 1 month. Minor response (MR): the tumor shrinkage is less than 50% and lasts 1 month. No response (NR): the tumor size is the same as that prior to the procedure, with no change or accretion.
The side effects were evaluated
Patient records included data regarding whether the patient had fever, acid reflux, pain, gastrointestinal perforation, or hemorrhage at the end of PDT, and whether there were any photoallergic reactions under strict light protection conditions.
The dysphagia score was recorded
Dysphagia was graded before PDT and 1 month after PDT. The grades for dysphagia: 0—no dysphagia; 1—dysphagia for common food; 2—dysphagia for semiliquid; 3—dysphagia for liquid; and 4—dysphagia for saliva. According to the grades for dysphagia, “no dysphagia” was defined here as the patient can eat liquid, semisolid, and solid food smoothly.
Follow-up
From the end of PDT to the follow-up period, the method was mainly based on the patient’s record of each regular review, supplemented by telephone follow-up, and detailed records of tumor changes and survival. Survival calculations ranged from the start of treatment to death or the last follow-up date.
Statistical Analysis
The primary end points were OS. Overall survival was measured from the date of treatment to the date of death or last follow-up.
The efficacy and side effects were calculated using the direct method. The differences between tables were tested by χ2 or Fisher exact test if appropriate. Dysphagia scores before and after treatment were compared using the paired t test.
Survival rates were analyzed using the Kaplan-Meier method. Univariate analyses of prognostic factors were performed using the log-rank test. All tests were 2 sided, and a P value less than .05 was considered significant. SPSS version 17.0 software was used for the survival analysis.
Results
Data Collection
A total of 288 patients with pathologically confirmed esophageal cancer were diagnosed and treated in our institution from September 2013 to December 2016. Two hundred two early stage patients who underwent surgery and/or RT were excluded. Also excluded were 54 patients who were unwilling to receive and/or could not tolerate PDT. A total of 32 patients met the inclusion criteria.
General information for the patients can be found in Table 1. Of the total, 75% were male, and squamous cell carcinoma (90.6%) was the main pathological type. Tumors were located in the middle thoracic section of the esophagus for 34.4%; tumor length was ≤6 cm for 81.2% of patients. All patients experienced varying degrees of dysphagia. Patients were categorized in stages III to IV, and 68.8% of KPS scores ≥80. There were 8 patients, 20 patients, and 17 patients who underwent previous surgery, RT, and chemotherapy, respectively. Five (15.6%) patients received 2 to 3 cycles of PDT. After the end of PDT, 4 patients underwent esophageal stenting, 2 received 3 courses of platinum-containing dual-drug regimen, and the remaining patients received the best supportive care.
Table 1.
Characteristics of Patients.
| Clinical data | No. (%) or median (range) | Clinical data | No. (%) or median (range) |
|---|---|---|---|
| Age, years | 48 (34-75) | TNM stage | |
| Sex | III | 12 (37.5) | |
| Male | 24 (75.0) | IV | 20 (62.5) |
| Female | 8 (25.0) | KPS score | |
| Histologic type | ≥80 | 22 (68.8) | |
| SCC | 29 (90.6) | <80 | 10 (31.2) |
| Adenocarcinoma | 2 (6.3) | Previous treatment | |
| Undifferentiated | 1 (3.1) | Surgery | 8 (25.0) |
| Location | Radical rsct | 6 (18.8) | |
| Cervical | 6 (18.8) | Palliative rsct | 2 (6.3) |
| Thoracic (upper) | 9 (28.1) | Radiotherapy | 20 (62.5) |
| Thoracic (middle) | 11 (34.4) | PORT | 6 (18.8) |
| Thoracic (lower) | 6 (18.8) | Definitive RT | 10 (31.3) |
| Length(cm) | Palliative RT | 4 (12.5) | |
| ≤4 | 10 (31.2) | Chemotherapy | 17 (53.1) |
| >4, ≤6 | 16 (50.0) | Induction | 2 (6.3) |
| >6 | 6 (18.8) | Concurrent | 6 (18.8) |
| Presenting symptoms | Adjuvant | 9 (28.1) | |
| Dysphagia | 32 (100) | PDT cycle | |
| Pain | 15 (46.9) | 1 | 27 (84.4) |
| Cough | 10 (31.2) | 2 | 4 (12.5) |
| Sonar | 2 (6.3) | 3 | 1 (3.1) |
Abbreviations: KPS, Karnofsky Performance Status; PDT, photodynamic therapy; PORT, postoperative radiotherapy; Rsct, resection; RT, radiotherapy; SCC, squamous cell carcinoma.
Short-Term Efficacy
The short-term effects for the 32 patients are shown in Table 2, with CR in 6 cases, SR in 12 cases, MR in 7 cases, and NR in 7 cases. The significant efficacy rate (CR + SR)/n was 56.3%, and the efficacy rate (CR + SR + MR)/n was 78.1%. Among them, for squamous cell carcinoma, with the tumor located in the cervical segment, tumor length ≤4 cm, stage III patients, KPS score ≥80, and patients receiving 2 to 3 cycles of PDT treatment, the efficacy rates were significantly higher.
Table 2.
Short-Term Efficacy and the Efficacy Comparison of Different Types.
| Types | n | Short-term efficacy | Significant efficacy rate (%) | Efficacy rate (%) | Invalid rate (%) | CR + SR | MR + NR | χ2 value | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | SR | MR | NR | (CR + SR)/n | (CR + SR + MR)/n | NR/n | ||||||
| Histologic type | ||||||||||||
| SCC | 29 | 6 | 10 | 6 | 7 | 55.2 | 75.9 | 24.1 | 16 | 13 | 0.146 | .702 |
| Others | 3 | 0 | 2 | 1 | 0 | 66.7 | 100 | 0 | 2 | 1 | ||
| Location | ||||||||||||
| Cervical | 6 | 1 | 3 | 1 | 1 | 66.7 | 83.3 | 16.7 | 4 | 2 | 0.375 | .945 |
| Thoracic (upper) | 9 | 2 | 3 | 2 | 2 | 55.6 | 77.8 | 22.2 | 5 | 4 | ||
| Thoracic (middle) | 11 | 3 | 3 | 3 | 2 | 54.5 | 81.8 | 18.2 | 6 | 5 | ||
| Thoracic (lower) | 6 | 0 | 3 | 1 | 2 | 50.0 | 66.7 | 33.3 | 3 | 3 | ||
| Length (cm) | ||||||||||||
| ≤4 | 10 | 4 | 4 | 1 | 1 | 80.0 | 90.0 | 10.0 | 8 | 2 | 6.112 | .047 |
| >4, ≤6 | 16 | 2 | 7 | 3 | 4 | 56.3 | 75.0 | 25.0 | 9 | 7 | 1.534 | .216 |
| >6 | 6 | 0 | 1 | 3 | 2 | 16.7 | 66.7 | 33.3 | 1 | 5 | 6.112 | .013 |
| TNM stage | ||||||||||||
| III | 12 | 4 | 4 | 2 | 2 | 66.7 | 83.3 | 16.7 | 8 | 4 | 0.847 | .358 |
| IV | 20 | 2 | 8 | 5 | 5 | 50.0 | 75.0 | 25.0 | 10 | 10 | ||
| KPS score | ||||||||||||
| ≥80 | 22 | 5 | 9 | 5 | 3 | 63.6 | 86.4 | 13.6 | 14 | 8 | 1.561 | .212 |
| <80 | 10 | 1 | 3 | 2 | 4 | 40.0 | 60.0 | 40.0 | 4 | 6 | ||
| PDT cycles | ||||||||||||
| 1 | 27 | 3 | 10 | 7 | 7 | 48.1 | 74.1 | 25.9 | 13 | 14 | 4.609 | .032 |
| 2-3 | 5 | 3 | 2 | 0 | 0 | 100 | 100 | 0 | 5 | 0 | ||
Abbreviations: CR, complete response; KPS, Karnofsky Performance Status; MR, minor response; NR, no response; PDT, photodynamic therapy; SCC, squamous cell carcinoma; SR, significant response.
The factors were compared between the significant efficacy group (CR + SR) and the nonsignificant efficacy group (MR + NR). The χ2 test showed that the difference in tumor length was statistically significant (P = .047). Groups in which tumor length was ≤4 cm were compared with groups in which the tumor length was >6 cm, and the difference was statistically significant (P = .013). Patients who underwent 2 to 3 cycles of PDT exhibited a higher rate of efficacy as compared to patients who underwent 1 cycle of PDT (P = .032).The short-term effects for the 20 patients who received previous RT are shown in Table 3, with CR in 4 cases, SR in 8 cases, MR in 5 cases, and NR in 3 cases. The significant efficacy rate (CR + SR)/n was 60.0%, and the efficacy rate (CR + SR + MR)/n was 85.0%. Patients who received radiation dose >60 Gy exhibited a higher rate of efficacy as compared to patients who received radiation dose 50 to 60 Gy (P = .017).
Table 3.
Short-Term Efficacy and the Efficacy Comparison of Patients Received Previous Radiotherapy.a
| Types | n | Short-term efficacy | Significant efficacy rate (%) | Efficacy rate (%) | Invalid rate (%) | CR + SR | MR + NR | χ2 value | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | SR | MR | NR | (CR + SR)/n | (CR + SR + MR)/n | NR/n | ||||||
| Radiation dose | ||||||||||||
| 50-60 Gy | 14 | 1 | 5 | 5 | 3 | 42.9 | 78.6 | 21.4 | 6 | 8 | 5.714 | .017 |
| >60 Gy | 6 | 3 | 3 | 0 | 0 | 100 | 100 | 0 | 6 | 0 | ||
| Tumor status after RT | ||||||||||||
| Recurrence | 12 | 4 | 4 | 2 | 2 | 66.7 | 83.3 | 16.7 | 8 | 4 | 0.556 | .456 |
| Residual | 8 | 0 | 4 | 3 | 1 | 50.0 | 87.5 | 12.5 | 4 | 4 | ||
| T stage before PDT | ||||||||||||
| T3 | 9 | 3 | 3 | 2 | 1 | 66.7 | 88.9 | 11.1 | 6 | 3 | 0.303 | .582 |
| T4 | 11 | 1 | 5 | 3 | 2 | 54.5 | 81.8 | 18.2 | 6 | 5 | ||
| Interval between RT and PDT | ||||||||||||
| ≤6 months | 7 | 0 | 3 | 3 | 1 | 42.9 | 85.7 | 14.3 | 3 | 4 | 0.319 | .251 |
| >6 months | 13 | 4 | 5 | 2 | 2 | 69.2 | 84.6 | 15.4 | 9 | 4 | ||
Abbreviations: CR, complete response; MR, minor response; NR, no response; PDT, photodynamic therapy; RT, radiation; SR, significant response.
a Recurrence: lesions that relapsed at the primary site after having once achieved a CR after RT; Residual: lesions that remained at the primary site without the achievement of a CR after RT.
Side Effects
There were no serious complications such as perforation or sputum in 32 patients after PDT treatment (Table 4). After treatment, 21 (65.6%) patients experienced grade 1 to 2 local pain or acid reflux discomfort. After symptomatic treatment such as analgesic and acid suppression, the patients were able to tolerate the PDT, and the side effects disappeared after 2 to 7 days. Four (12.5%) patients developed a low fever that spontaneously resolved without any further treatment. There was slight bleeding on the surface of the tumor for 2 (6.3%) patients, and after routine hemostatic treatment was administered, no symptoms of gastrointestinal bleeding occurred. One patient had mild photoallergic symptoms due to lack of strict protection from light. After guidance, the allergic symptoms spontaneously resolved.
Table 4.
Side Effects of Patients.
| Side effects | n | Rate (%) |
|---|---|---|
| Allergy | 1 | 3.1 |
| Pain | 9 | 28.1 |
| Esophageal perforation | 0 | 0 |
| Bleeding | 2 | 6.3 |
| Acid reflux | 12 | 37.5 |
| Fever | 4 | 12.5 |
Dysphagia Improvement
After PDT, 22 (68.8%) patients exhibited significantly improved symptoms of dysphagia. The dysphagia score for all patients decreased from 3.43 ± 0.73 to 1.79 ± 0.53 one month after PDT (P < .05). The median interval between PDT administration and patient reports of no dysphagia was 81.92 (95% CI: 62.56-101.28) days. Patients were allowed to eat semiliquid food 48 hours after the PDT treatment.
Survival
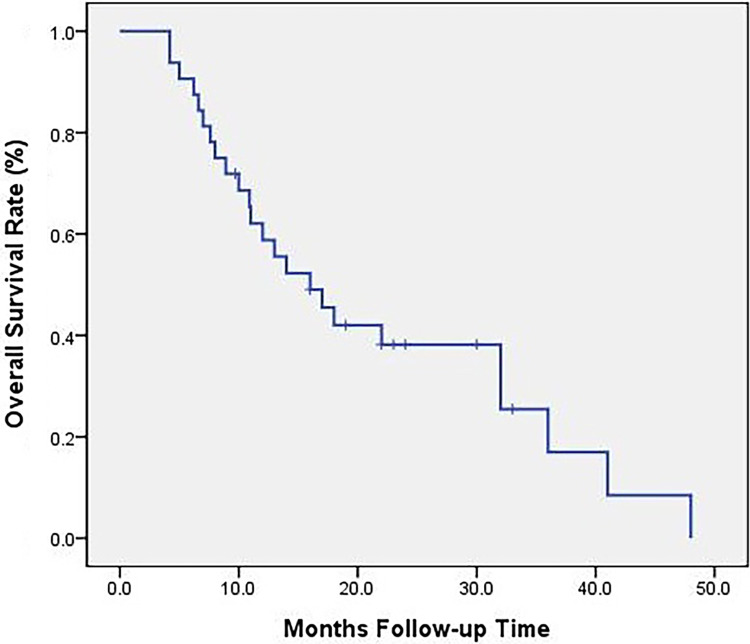
The follow-up date was February 2018, the median follow-up time was 15 months (4-48 months), and one patient was lost to follow-up. The follow-up rate was 96.9%. A total of 24 deaths were included, including 1 loss of follow-up, 1 death from cardiovascular disease, and 22 disease-related deaths. The OS of all patients is shown in Figure 2. The estimated median OS was 16 months.
Figure 2.
Overall survival rate of all patients.
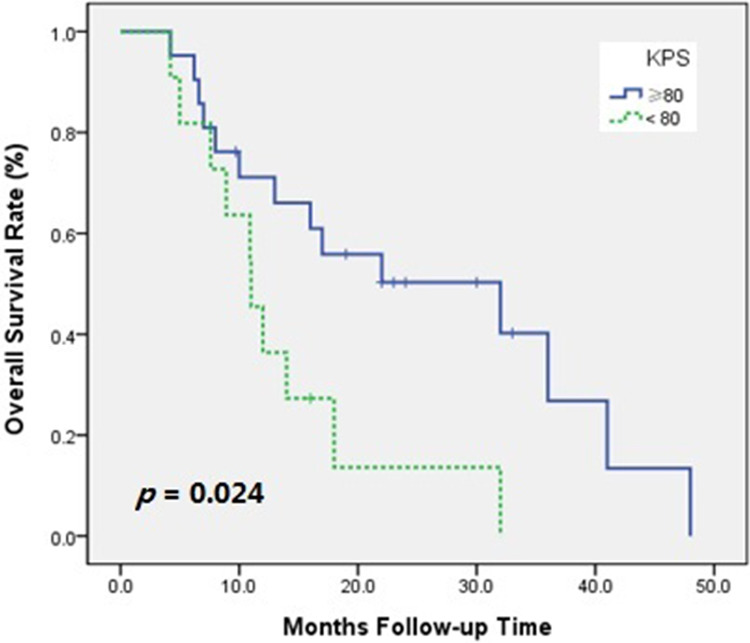
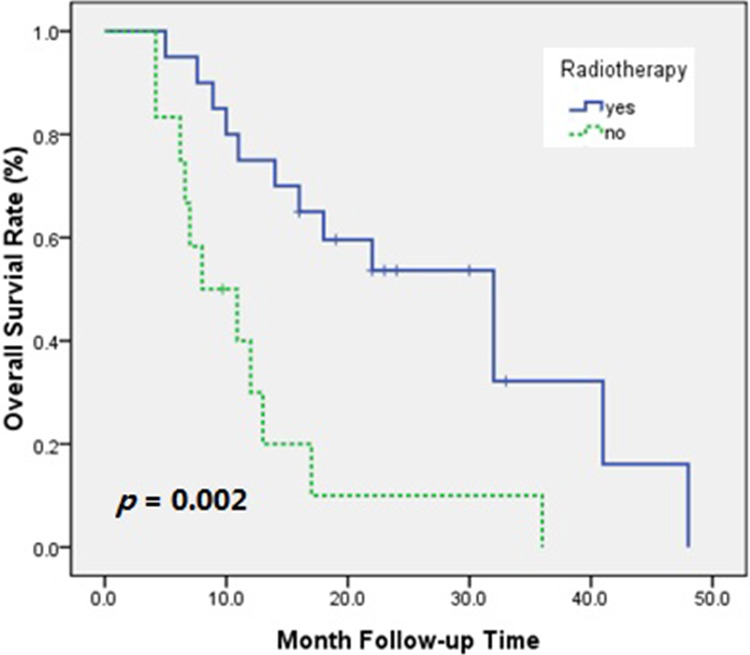
Univariate analysis of OS in all patients was performed with clinically relevant factors and treatment-related factors (Table 5). The results showed that the KPS score and whether or not the patient received RT had an effect on OS. Patients with KPS score ≥80 had greater OS than patients with KPS score <80 (hazard ratio [HR]: 2.626; 95% CI: 1.091-6.322; P = .024; Figure 3). Overall survival was greater in patients who had received previous radiation than in those who did not receive radiation (HR: 3.574; 95% CI: 1.501-8.510; P = .002; Figure 4).
Table 5.
Univariate Analysis of the Relationship of Clinical and Treatment-Related Factors With OS.
| Factors | Comparison | OS | |||
|---|---|---|---|---|---|
| χ2 value | P value | HR | 95% CI | ||
| Clinical factors | |||||
| Sex | Male vs female | 0.165 | 0.684 | 1.216 | 0.470-3.146 |
| Age (years) | ≤40 vs >40 | 3.128 | 0.077 | 5.372 | 0.676-42.688 |
| Histologic type | SCC vs others | 2.220 | 0.136 | 2.564 | 0.708-9.283 |
| Location | Cervical vs thoracic | 0.002 | 0.966 | 1.024 | 0.340-3.087 |
| Length | ≤4 cm vs >4 cm | 0.169 | 0.681 | 1.203 | 0.493-2.937 |
| TNM stage | III vs IV | 1.672 | 0.196 | 1.786 | 0.726-4.393 |
| KPS score | ≥80 vs <80 | 5.060 | 0.024 | 2.626 | 1.091-6.322 |
| Treatment-related factors | |||||
| Previous surgery | Yes vs no | 1.602 | 0.206 | 1.876 | 0.689-5.118 |
| Previous RT | Yes vs no | 9.460 | 0.002 | 3.574 | 1.501-8.510 |
| Previous CT | Yes vs no | 0.041 | 0.839 | 0.917 | 0.396-2.127 |
| PDT cycles | 1 vs 2-3 | 3.917 | 0.141 | 0.289 | 0.073-1.136 |
Abbreviations: CT, chemotherapy; HR, hazard ratio; KPS, Karnofsky Performance Status; PDT, photodynamic therapy; OS, overall survival; RT, radiotherapy; SCC, squamous cell carcinoma.
Figure 3.
Overall survival rate of patients whose Karnofsky Performance Status (KPS) score ≥80 or KPS score <80.
Figure 4.
Overall survival rate of patients who received or did not receive radiotherapy.
Discussion
There have been many basic experiments and clinical studies to explore the use of PDT as an effective adjuvant treatment for digestive tract tumors.15-18 Photodynamic therapy is used for palliative treatment of early and advanced esophageal cancer, and its efficacy has been recognized.16,18 Especially for advanced esophageal cancer, PDT plays an important role.17,19
In our study, the effectiveness rate was 78.1% (25/32), and the significant efficacy rate was 56.3% (18/32). Li et al 13 conducted a controlled study of 90 patients with stage III and IV esophageal cancer, including 27 patients who underwent PDT (group A), 33 patients who were treated with PDT plus chemotherapy (group B), and 30 patients treated with chemotherapy (group C). The PDT was based on Photofrin, and 5-fluorouracil + cisplatin was given as the chemotherapy regimen for 4 cycles, and thus, the combination group was treated with chemotherapy plus PDT. The 3 groups’ effectiveness rates were 85.2%, 90.9%, and 63.3% (A and B, P = .690; A and C, P = .043; B and C, P = .014). Mimura et al 20 performed PDT on 73 patients with gastric cancer. For gastric cancer with different depths of invasion, there were significant differences in the therapeutic effects, where it was observed that the total effectiveness for mucosal cancer, submucosal cancer, and gastric cancer invading the muscle layer was 100%, 75%, and 20%, respectively. This study suggests that the most optimal effect from PDT for upper digestive tract tumors is obtained in early disease. As the disease progresses and the tumor volume increases, the treatment effect can be significantly reduced due to the limitation of laser penetration of the tissue.21 Our study was similar to the above studies in terms of efficacy, suggesting that a more optimal effect will be obtained when PDT is used for short-term treatment.
Our study further showed that increased therapeutic efficacy was obtained with tumor length ≤4 cm and 2 to 3 cycles of PDT, which is consistent with the conclusions of Gahlen et al.21 And, patients who received more than 60 Gy radiation doses previously had better short-term efficacy after PDT. We think that lower doses may not completely kill subclinical lesions, which make endoscopically undetectable microcancer cells cause local residue and recurrence after PDT. But there were only 6 patients (>60 Gy), which could overvalue the efficacy. It requires more data for verification.
The current study showed that the side effects were mild and well tolerated. The main side effects were pain, acid reflux, and mild fever. No photoallergic reaction occurred in patients who strictly adhered to medical advice and protected themselves from light. The observed side effects were similar to the common adverse reactions reported in the literature.13,16,17,19 Perforation did not occur for any of the patients in this study that underwent PDT, which may be related to the depth that was attained when the PDT was performed.
Dysphagia and obstruction are common symptoms of advanced esophageal cancer. Although all the patients in our study exhibited different symptoms of dysphagia, after receiving PDT treatment, the dysphagia was relieved by varying degrees. Yoon et al 19 reported that 4 weeks after PDT, 90% of patients observed a significant improvement in dysphagia grading scores from 2.75 ± 0.91 to 1.05 ± 0.83 (P < .05). Stents were placed for an average of 63 days (range: 37-90) in patients with recurrent dysphagia, which is in accordance with the data from similar reports.5,7,13
Many studies have observed that PDT can improve patients’ quality of life and prolong their survival.5,7,13 McCaughan et al 22 performed PDT on 77 patients with esophageal cancer and evaluated their survival. The study showed that clinical stage is the main factor affecting long-term survival of patients. The median survival for stage I disease was not obtained. For stage II, the median survival was 12 months, for stage III, 6.2 months, and stage IV, 3.5 months. Seven patients with stage I who had not received any other treatment had a 5-year survival rate of 62% after PDT. Lindenmann et al 23 performed a combination of multiple modes of therapy for 640 patients with advanced esophageal cancer, including endoscopically guided dilatation, PDT, intraluminal irradiation, external irradiation, chemotherapy, enteral nutrition tube placement, and palliative resection. The median survival was 34 months. The median survival of patients receiving PDT was 50.9 months. The median survival of patients receiving other treatments was 17.3 months (P = .012).
In our study, the OS was estimated to be 16 months. Univariate analysis showed that the independent prognostic factors affecting survival were KPS score and previous RT. Patients with high KPS score generally have better tolerance to PDT, which thus provides survival benefits. Our study included patients who experienced recurrence after RT. For patients with stage III and stage IV esophageal cancer, it is difficult to perform surgery, and more patients must choose RT. The results indicate that although there may be disease recurrence after RT, there are still survival benefits for those patients who received RT. And, PDT can be a curative intent salvage treatment option for local failure after RT for esophageal cancer. Several reports, including retrospective studies and multi-institutional phase I to II studies, also support the conclusion.24-26
In this study, we excluded patients with metal stents. But metal stents have important value in the treatment of obstructive esophageal cancer.27,28 To prevent severe dysphagia or dyspnea, stents show advantages.27 However, the growth of tumor tissue can flatten the stent and can enter the stent mesh and cause airway restenosis.5,7 Photodynamic therapy relieves obstructions on the basis of killing tumor cells, which can help reduce tumor staging. But necrotic debris formed after PDT is difficult to remove, resulting in increased obstruction. And, PDT using the first-generation photosensitizer has several side effects such as a high occurrence of phototoxicity.10,29 In our study, patients were instructed to strictly protect themselves from light to avoid skin phototoxicity for 4 weeks and one patient developed photoallergic symptoms when exposed to sunlight. Second- or third-generation photosensitizers show advantages in reducing phototoxicity.8-10 In addition, some photosensitizers target to deep lying cancerous lesions, providing new ideas for the treatment of advanced tumors.8
This is a retrospective study with fewer cases. Most patients included were advanced or relapsed patients, and therefore, the patients were generally in poor condition and it was difficult for some of them to undergo the combination of PDT and other treatment methods (such as systemic chemotherapy). Therefore, the results have certain limitations. The current study did not compare PDT with stent implantation, and we expect that further clinical trials will be conducted to gather additional data. The new generation of photosensitizers is also worthy of further study, especially for deep lying tumors and subclinical lesions.
Conclusion
Photodynamic therapy is an effective method of treatment for advanced esophageal cancer. The side effects are mild, and the short-term effect is good, especially in the relief of dysphagia. Photodynamic therapy can prolong the survival of patients with advanced esophageal cancer, and the KPS score and previous RT have a significant effect on the OS. In many instances, different therapeutics (PDT and/or stent implantation, new generation of photosensitizers) show great potential.
Abbreviations
- CR
complete response
- HR
hazard ratio
- KPS
Karnofsky Performance Status
- MR
minor response
- NR
no response
- OS
overall survival
- PDT
photodynamic therapy
- PORT
postoperative radiotherapy
- Rsct
resection
- RT
radio therapy
- SCC
squamous cell carcinoma
- SR
significant response
Footnotes
Authors’ Note: The first 2 authors contributed equally to this work. This study was approval by Ethics Committee of Integrated Hospital of Traditional Chinese Medicine (No.NFZXY20130919). The data sets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Planning Project of Guangdong Province of China [Grant No. 2017ZC0059]; the Medical Scientific Research Foundation of Guangdong Province of China (General Program) [Grant No. A2017553]; and the Professor Academic Development Fund of Fujian Medical University [Grant No. JS06050, JB06256].
ORCID iD: Ruifang Zeng, MD  https://orcid.org/0000-0001-6657-1330
https://orcid.org/0000-0001-6657-1330
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Tieulent JL, Jemal A. Global cancer statistics. 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Molnarova A. Advanced esophageal carcinoma recanalization [in Slovak]. Klin Onkol. 2008;21(5):309–312. [PubMed] [Google Scholar]
- 4. Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–95. [DOI] [PubMed] [Google Scholar]
- 5. Luketich JD, Christie NA, Buenaventura PO, Weigel TL, Keenan RJ, Nguyen NT. Endoscopic photodynamic therapy for obstructing esophageal cancer: 77 cases over a 2-year period. Surg Endosc. 2000;14(7):653–657. [DOI] [PubMed] [Google Scholar]
- 6. Rogers L, Sergeeva NN, Paszko E, Vaz GM, Senge MO. Lead structures for applications in photodynamic therapy. 6. temoporfin anti-inflammatory conjugates to target the tumor microenvironment for in vitro PDT. PLoS One. 2015;10(5):e0125372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg. 2003;76(5):1687–1692. discussion 92-93. [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal A, Samaroo D, Jovanovic IR, Singh S, Paola TM, Rampersad MM. Porphyrinoid-based photosensitizers for diagnostic and therapeutic applications: an update. J Porphyr Phthalocyanines. 2019; 23(07n08):729–765. [Google Scholar]
- 9. Frochot C, Mordon S. Update of the situation of clinical photodynamic therapy in Europe in the 2003-2018 period. J Porphyrins Phthalocyanines. 2019;23(04n05):347–357. [Google Scholar]
- 10. Huang Z, Heping X, Meyers A, et al. Photodynamic therapy for treatment of solid tumors—potential and technical challenges. Technol Cancer Res Treat. 2008;7(4):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang Z. An update on the regulatory status of PDT photosensitizers in China. Photodiagnosis Photodyn Ther. 2008;5(4):285–287. [DOI] [PubMed] [Google Scholar]
- 12. Huang Z. Photodynamic therapy in China: 25 years of unique history-Part two: clinical experience. Photodiagnosis Photodyn Ther. 2006;3(2):71–84. [DOI] [PubMed] [Google Scholar]
- 13. Li LB, Xie JM, Zhang XN, et al. Retrospective study of photodynamic therapy vs photodynamic therapy combined with chemotherapy and chemotherapy alone on advanced esophageal cancer. Photodiagnosis Photodyn Ther. 2010;7(3):139–143. [DOI] [PubMed] [Google Scholar]
- 14. Jin ML, Yang BQ, Zhang W, Ren P. Evaluation of photodynamic therapy in advanced gastrointestinal cancer. J Clin Laser Med Surg. 1991;91(1):45–48. [DOI] [PubMed] [Google Scholar]
- 15. Saczko J, Chwilkowska A, Kulbacka J, et al. Photooxidative action in cancer and normal cells induced by the use of photofrin® in photodynamic therapy. Folia Biol (Praha). 2008;54(1):24–29. [PubMed] [Google Scholar]
- 16. Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc. 2011;73(1):1–6. [DOI] [PubMed] [Google Scholar]
- 17. Maier A, Tomaselli F, Gebhard F, Rehak P, Smolle J, Smolle Jüttner FM. Palliation of advanced esophageal carcinoma by photodynamic therapy and irradiation. Ann Thorac Surg. 2000;69(4):1006–1009. [DOI] [PubMed] [Google Scholar]
- 18. Cheon YK, Kim WJ, Cho JY, Lee JS, Lee MS, Shim CS. Outcome of photodynamic therapy for early esophageal cancer. Gut Liver. 2007;1(2):126–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon HY, Cheon YK, Choi HJ, Shim CS. Role of photodynamic therapy in the palliation of obstructing esophageal cancer. Korean J Intern Med. 2012;27(3):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mimura S, Hiroyuki N, Toshio H, et al. Cooperative clinical trial of photodynamic therapy for early gastric cancer with photofrininjectionand YAG-OPO laser. Diagn Ther Endosc. 1998;4(4):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gahlen J, Prosst RL, Stern J. Photodynamic therapy in the gastrointestinal tract. possibilities and limits. Der Chirurg. 2002;73(2):122–131. [DOI] [PubMed] [Google Scholar]
- 22. McCaughan JS, Jr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg. 1996;62(4):1005–1009. [DOI] [PubMed] [Google Scholar]
- 23. Lindenmann J, Matzi V, Neuboeck N, et al. Individualized, multimodal palliative treatment of inoperable esophageal cancer: clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg Med. 2012;44(3):189–198. [DOI] [PubMed] [Google Scholar]
- 24. Hatogai K, Yano T, Kojima T, et al. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc. 2016;83(6):1130–1139. [DOI] [PubMed] [Google Scholar]
- 25. Yano T, Muto M, Yoshimura K, et al. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat Oncol. 2012;117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yano T, Kasai H, Horimatsu T, et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget. 2017;8(13):22135–22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund ME, Garland R, Ernst A. Airway stenting: applications and practice management considerations. Chest. 2007;131(2):579–587. [DOI] [PubMed] [Google Scholar]
- 28. Chang YC, Lee JM, Ko WJ, Lee YC. Airway obstruction following bronchoscopic photodynamic therapy in early centrally located lung cancer requiring extracorporeal membrane oxygenation. J Formos Med Assoc. 2013;112(1):54–56. [DOI] [PubMed] [Google Scholar]
- 29. Yano T, Muto M, Hattori S, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40(9):717–721. [DOI] [PubMed] [Google Scholar]