Abstract
Introduction:
The purpose of this study is to evaluate the diagnostic value of macrophage migration inhibitory factor in patients with nasopharyngeal carcinoma.
Materials and Methods:
The expression levels of macrophage migration inhibitory factor in nasopharyngeal carcinoma cell lines, tumor tissues, and plasma were measured by real-time polymerase chain reaction, Western blotting, enzyme-linked immunosorbent assay, and immunohistochemistry. Plasma Epstein-Barr virus viral capsid antigen was determined by immunoenzymatic techniques.
Results:
Both the messenger RNA and protein expression levels of macrophage migration inhibitory factor were upregulated in nasopharyngeal carcinoma cell lines and nasopharyngeal carcinoma tissues. Macrophage migration inhibitory factor in plasma was significantly elevated in patients with nasopharyngeal carcinoma compared to Epstein-Barr virus viral capsid antigen–negative and Epstein-Barr virus viral capsid antigen–positive healthy donors. The combination of macrophage migration inhibitory factor and Epstein-Barr virus viral capsid antigen was better for diagnosing nasopharyngeal carcinoma (area under receiver operating characteristic curve = 0.925, 95% CI: 0.898-0.951) than macrophage migration inhibitory factor (area under receiver operating characteristic curve = 0.778, 95% CI: 0.732-0.824) and Epstein-Barr virus viral capsid antigen. Combining macrophage migration inhibitory factor and Epstein-Barr virus viral capsid antigen had higher specificity (82.40% vs 69.96%) and higher positive predictive value (79.17% vs 67.44%) without an obvious reduction in sensitivity (95.25%) compared to Epstein-Barr virus viral capsid antigen alone. Macrophage migration inhibitory factor was highly expressed in nasopharyngeal carcinoma cell lines, whereas it was not associated with Epstein-Barr virus infection. The level of macrophage migration inhibitory factor in plasma was not related to the titer of Epstein-Barr virus viral capsid antigen.
Conclusion:
The combination of macrophage migration inhibitory factor and Epstein-Barr virus viral capsid antigen increases the specificity and positive predictive value of detecting nasopharyngeal carcinoma and improves the diagnostic accuracy of nasopharyngeal carcinoma in high-risk individuals.
Keywords: biomarkers, MIF, nasopharyngeal carcinoma, diagnosis, EBV
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor of the head and neck; over 84 000 new patients with NPC are diagnosed per year, with a higher incidence in Southeast Asia and North Africa.1,2 In China, the incidence and mortality of NPC in 2015 were 60 600 and 34 100, respectively.3 The mortality of NPC has decreased due to the improvements in treatment, especially radiotherapy. However, the survival rate is still dissatisfactory. Early diagnosis is important to improve the survival of patients with NPC. Epstein-Barr virus (EBV) infection was strongly associated with the development of the nonkeratinizing and undifferentiated nasopharyngeal carcinoma, which has been postulated since 1966.4-6 Thus, EBV-based biomarkers are useful for NPC diagnosis and continuous surveillance of disease. In recent years, the immunoglobulin antibodies against the EBV viral capsid antigen (VCA-IgA), EBV early antigen (EA/IgA), EBNA1-IgA, Zat-IgA, Rat-IgG, and EBV DNA were commonly used as screening biomarkers for NPC.7-9 As a large number of people have been infected with EBV, these EBV-based biomarkers were not satisfactory for NPC diagnosis, especially for distinguishing patients with NPC from high-risk individuals who have positive anti-EBV antibodies.10,11
The combination of EBV-based biomarkers has been developed to improve diagnostic value of NPC. Yu et al reported that VCA-IgA and EA-IgA are closely associated with NPC, but neither of them is particularly specific.12 Additionally, EBV DNA tests showed limited diagnostic significance for the patients of early-stage and local recurrence NPC.8,13 These results have proven insufficient to accurately diagnose NPC. Therefore, novel biomarkers for increasing the specificity of NPC diagnosis are urgently needed.
Macrophage migration inhibitory factor is a pleiotropic cytokine of the immune response produced by macrophages and cancer cells, which induces expression of inflammatory cytokines including interleukin (IL)-2, tumor necrosis factor-α, interferon (INF)-γ, IL-1β, IL-6, INF-7, IL-12, and so on.14 Macrophage migration inhibitory factor may function to regulate cellular proliferative and differentiation in the epidermis in addition to regulate immunity and inflammation.15 In recent years, MIF has been reported to be overexpressed in many cancers, such as gallbladder cancer, gastric cancer, liver cancer, and lung cancer.16-18 Furthermore, high expression of MIF has been found to be a poor prognosis in NPC, hepatocellular cancer, colorectal cancer, and breast cancer.19-22 In addition, serum MIF has been suggested as a potential biomarker for the diagnosis of these cancer.23-25 Nevertheless, the diagnostic significance of plasma MIF in patients with NPC was unknown. The aim of this study is to investigate the plasma MIF levels in patients with NPC and provide further validation of the diagnostic potency of MIF in detecting NPC. We demonstrated that MIF would be a good supplement marker to improve the specificity and PPV of NPC diagnosis.
Materials and Methods
Cell Lines
The immortalized NPEC1, NPEC2,26,27 and C666 cells were grown in keratinocyte/serum-free medium (Invitrogen). The NPC cell lines (S18, HK1, CNE1, HNE1, SUNE1, CNE2, 6-10B, CNE2-EBV, HNE1-EBV) were grown in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum in a humidified incubator containing 5% CO2 at 37 °C. CNE2-EBV and HNE1-EBV cell lines were created by infecting CNE2 and HNE1 cell lines with EBV-containing green fluorescent protein (GFP). The virus was established as previously described.27 The supernatants of cell lines were collected and centrifuged at 1000g for 5 minutes to eliminate suspended cells and stored at −80 °C until use.
Plasma and Tissue Specimens
The plasma samples were obtained from 147 primary patients with NPC (ages 15-69 years, median 45 years, 107 males and 40 females) at the Sun Yat-sen University Cancer Center from 2011 to 2013. All patients with nasopharyngeal cancer received confirmed diagnoses by pathological examination. Tumor-node-metastasis (TNM) stage was established based on the 7th edition of the UICC/AJCC staging system for NPC. The 127 VN healthy controls (without otolaryngological-related diseases, infection, or other cancer diseases) were collected from Sun Yat-sen University Cancer Center (ages 17-75 years, median 38 years, 59 males and 68 females). The 106 VCA-IgA positive healthy controls (VP) cases (ages 21-88 years, median 53 years, 45 males and 61 females) were confirmed to have non-neoplastic diseases in the nasopharynx by nasopharyngeal biopsy during the 3 years of follow-up. Plasma was separated after centrifugation at 4 °C and stored at −80 °C until use.
A total of 20 formalin-fixed and paraffin-embedded NPC tumor specimens were used for immunohistochemistry. Nine nontumor NP tissues and 9 primary NPC tissues were obtained by biopsies. All these samples were obtained at the Sun Yat-sen University Cancer Center.
Prior to use of these plasma and tissues, informed consent was obtained from each of the participants. All patients provided written informed consent. This experiment was approved by the Institute Research Ethics Committee of the Cancer Center of Sun Yat-sen University, Guangzhou, China.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from the cell lines and tissue specimens and using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription of total RNA (2 μg) was done by a reverse transcriptase kit (Invitrogen).
The messenger RNA (mRNA) levels of target and reference (β-Actin) genes were performed on a LightCycler 480 II (Roche, Applied Science) using a SYBR green-based assay (BioRad). The primers for detecting MIF and EBV as follows:
MIF forward: 5′-CACAGTGCCCAGACCCTACAGC-3’
MIF reverse: 5’-GCTTGCTGTAGGAGCGGTTCTG-3’
BKRF1 forward: 5’-GTAGGGGATGCCGATTATTTTG-3’
BKRF1 reverse: 5’-CTCCTTGACCACGATGCTTTC-3’
EBER1 forward: 5’-GAGGTTTTGCTAGGGAGGAGAC-3’
EBER1 reverse forward: 5’-GAAGACGGCAGAAAGCAGAGT-3’
BMRF1 forward: 5’-TCTCAAGGGAGGAGTGCTGC-3’
BMRF1 reverse forward: 5’-TCTGGGCTCTGGTGATTCTG-3’
BZLF1 forward: 5’-CCCAGTCTCCGACATAACCC-3’
BZLF1 reverse forward: 5’-CAGGCTGTGGAACACCAATG-3’
β-Actin forward: 5’-GTGAAGGTGACAGCAGTCGGT-3’
β-Actin reverse: 5’-AAGTGGGGTGGCTTTTAGGAT-3’
Western Blotting Analysis
Nasopharyngeal carcinoma cells were harvested and lysed in sodium dodecylsulfate (SDS) sample buffer. Cellular proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Then, the membranes were incubated with MIF (1:1000, Abcam, ab65869) and α-tubulin (1:3000, Abcam, ab126165) antibodies at 4 °C overnight. At last, the blots were treated with a horseradish peroxidase (HRP)-conjugated secondary antibody.
Immunohistochemistry
Formalin-fixed, paraffin-embedded NPC tissues were incubated with anti-MIF antibody (1:250, Abcam) overnight at 4 °C. After washing, these slides were incubated with a HRP-conjugated antirabbit secondary antibody at 37 °C for 30 minutes. The tissue sections were immersed in 3-diaminobenzidine tetrahydrochloride for 45 seconds, followed by counterstaining with 10% Mayer’s hematoxylin. All tissues were reviewed by 2 independent observers.
ELISA
Plasma MIF levels were detected using the RayBiotech sandwich ELISA kits (RayBiotech Systems). The test plasma or standard (100 µL/well) was added into 96-well plates and incubated for 2.5 hours. Then, the biotinylated antibody solution (100 µL/well) was added and incubated for 1 hour. After washing, streptavidin solution (100 µL/well) was added at 37 °C for 45 minutes. Finally, The TMB (100 µL/well) was added, and the reaction was terminated with 50 µl stop solution. The absorbance was determined at 450 nm using a microplate reader. Each test included a standard control (coefficient of variation < 10%).
Immunoenzymatic Assay of Plasma EBV VCA-IgA
Immunoenzymatic assay method supplied by the Shanghai Institute of Biological Products was used to determine the plasma EBV titers. B95 cell lines were prepared for test on glass slides. Plasma at a dilution of 1:10 was applied, and this was followed by 2-fold serial dilutions. The reciprocal of the highest dilution showing brown within 15% of the cells was the plasma antibody titer. The levels of EBV VCA-IgA were determined by titration, with cutoff values set at 1:40.
Statistical Analysis
Data analyses were performed using SPSS 20.0 (SPSS Inc). Mann-Whitney U tests were applied to analyze the relationships between MIF expression and the clinicopathological features. Kruskal-Wallis tests were used to analyze the differences in MIF concentration among different groups. The area under the receiver operating characteristic curve (AUC) was used to perform the efficacy of MIF. The cutoff value for MIF was defined as the value with the maximization of Yuden index. A 2-sided P value less than 0.05 was considered to be statistically significant.
Results
Overexpression of MIF in Nasopharyngeal Carcinoma Cells and Tumor Tissues
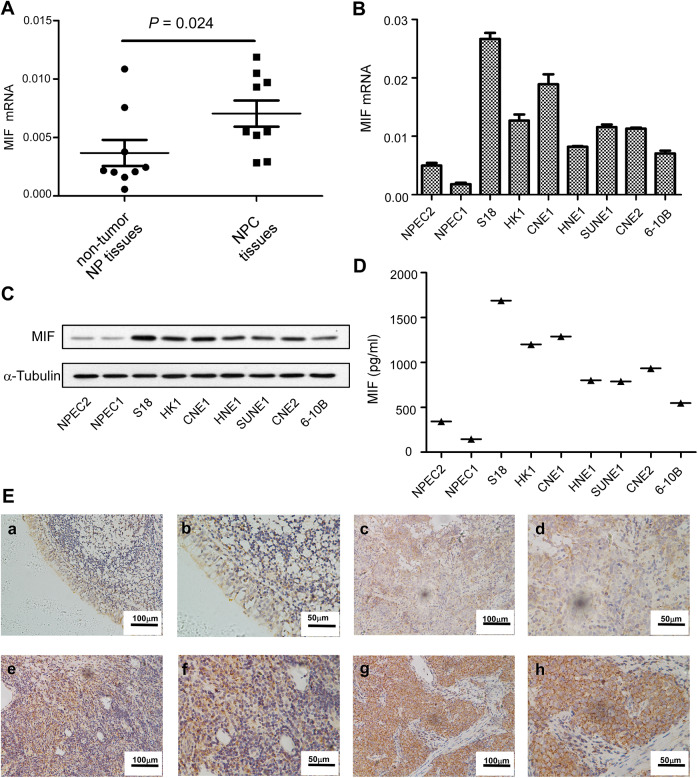
To investigate the expression of MIF in NPC, 9 nontumor NP tissues and 9 NPC tissues were used. Compared to the nontumor NP tissues, MIF mRNA was significantly upregulated in tumor tissues (Figure 1A). Then, we detected the levels of MIF in several NPC cell lines (S18, HK1, CNE1, HNE1, SUNE1, CNE2, 6-10B,) and immortalized nasopharyngeal epithelial cell lines (NEPC1, NEPC2) using real-time -polymerase chain reaction and Western blotting. We demonstrated that tumor cell lines exhibited higher MIF mRNA and protein expression levels (Figure 1B and C). Next, we performed an ELISA to determine the MIF levels in the supernatant of the culture cells. The tumor cell lines also showed higher levels of MIF protein expression in supernatant than that of immortalized nasopharyngeal epithelial cell lines (Figure 1D). Then, a total of 20 NPC tissues were used to detect the expression of MIF. The levels of MIF protein were highly expressed at cytoplasm of tumor cells in 17 of 20 NPC samples (85%). Various levels of MIF protein were observed in tumor cells: low (Figure 1E, c and d), medium (Figure 1E, e and f) and high (Figure 1E, g and f). However, MIF was lower or no expression in the normal nasopharyngeal epithelium (Figure 1E, a and b).
Figure 1.
Expression of MIF messenger RNA (mRNA) or protein in nasopharyngeal carcinoma (NPC) cell lines, and immunohistochemical staining of MIF in NPC tumor tissue. The levels of mRNA in non-tumor NP tissues and NPC tissues (A). The levels of mRNA and protein in the immortalized nasopharyngeal epithelial cell lines (NPEC1, NPEC2) and NPC cell lines were determined by real-time-polymerase chain reaction (PCR) (B), Western Blotting (C) and ELISA (D). Expression level was normalized by β-actin and α-tubulin, respectively. Error bars represent calculated from three parallel experiments. The normal nasopharyngeal epithelial tissue showed lower of MIF (E a-b). Low (E c-d), medium (E e-f), and high (E g-h) expression of MIF were showed in the nasopharyngeal carcinoma (NPC) tissues.
Plasma MIF Levels in NPC and the Association Between Plasma MIF and Clinicopathological Characteristics
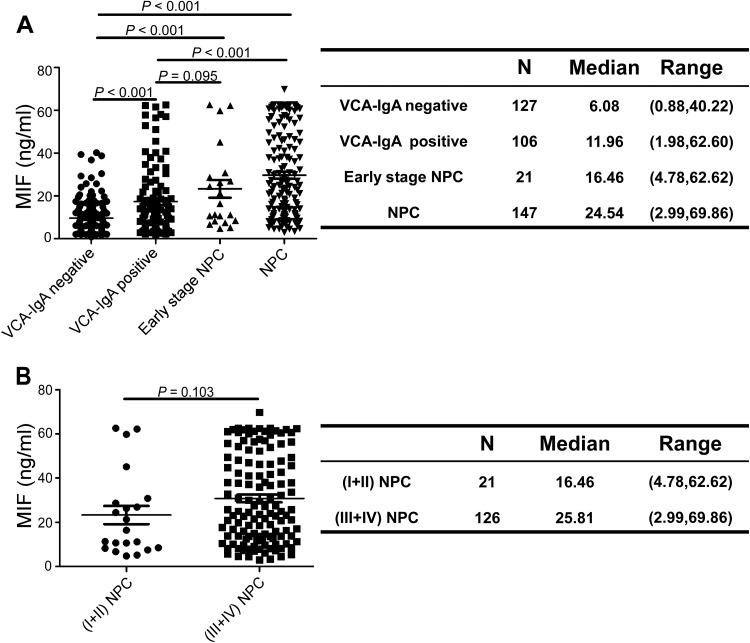
The results in Figure 2A demonstrate the plasma levels of MIF in the VN (n = 127), VP (n = 106), patients with NPC (n = 147), and patients with early-stage NPC (n = 21, 3 cases of stage Ⅰ, 18 cases of stage Ⅱ). The median levels of MIF were 6.08 ng/mL (range, 0.88-40.22) in VN, 11.96 ng/ml (range, 1.98-62.60) in VP, 24.54 ng/ml (range, 2.99-69.86) in patients with NPC, and 16.46 ng/ml (range, 4.78-62.62) in early-stage patients with NPC. The plasma levels of MIF in patients with NPC were significantly higher than VN (p < 0.001) and VP (p < 0.001), the plasma levels of MIF in VP were significantly higher than VN (P < 0.001), and the plasma levels of MIF in the early-stage patients with NPC were also significantly higher than VN (P < 0.001) but similar to VP (P = 0.095) (Figure 2A). The median plasma levels of MIF were 16.46 ng/ml (range, 4.78-62.62) in early-stage patients with NPC (Ⅰ + Ⅱ) and 25.81 ng/ml (range, 2.99-69.86) in advanced-stage patients with NPC (Ⅲ + Ⅳ), which has not a significant difference (P = 0.103) (Figure 2B).
Figure 2.
The plasma concentration of MIF in the test cohort. Plasma levels of MIF were measured in VCA-IgA negative cohort, VCA-IgA positive cohort, patients with early-stage NPC and patients with NPC, left side. Right side, MIF plasma levels in different groups(A). The plasma levels of MIF in patients with early-stage NPC (Ⅰ + Ⅱ) and patients with advanced-stage NPC (Ⅲ + Ⅳ), left side. Right side, MIF plasma levels in two groups (B). P value was obtained by Kruskal-Wallis test.
Table 1 presents the relationship between the plasma levels of MIF and the clinicopathological parameters in patients with NPC. The MIF levels had a significant association with gender (P = 0.007) and T classification (P = 0.016). However, the expression of MIF was not correlated with age, N classification, or overall stage.
Table 1.
Levels of MIF and Clinical Characteristics in 147 Untreated Patients With NPC.
| Characteristics | No. of patients | MIF (ng/mL) | P valuea |
|---|---|---|---|
| Median (range) | |||
| Age | |||
| <45 | 74 | 22.30 (4.78, 62.62) | |
| ≥45 | 73 | 27.50 (2.99, 69.86) | 0.058 |
| Gender | |||
| Female | 40 | 13.80 (3.45, 69.86) | |
| Male | 107 | 27.50 (2.99, 62.63) | 0.007 |
| pT status | |||
| pT1-2 | 34 | 20.91 (2.99, 62.63) | |
| pT3 | 66 | 24.31 (3.45, 69.86) | 0.016 |
| pT4 | 47 | 37.92 (6.17, 62.62) | |
| pN status | |||
| pN 0-1 | 66 | 21.21 (3.45, 62.62) | |
| pN 2-3 | 81 | 29.62 (2.99, 69.86) | 0.115 |
| Overall stage | |||
| Stage I-II | 21 | 16.46 (4.78, 62.62) | |
| Stage III | 65 | 23.66 (2.99, 62.63) | 0.215 |
| Stage IV | 61 | 32.32 (4.47, 69.86) |
Abbreviations: MIF, macrophage migration inhibitory factor; NPC, nasopharyngeal carcinoma.
aKruskal-Wallis test.
Diagnostic Values of Plasma MIF and VCA-IgA for Patients With NPC
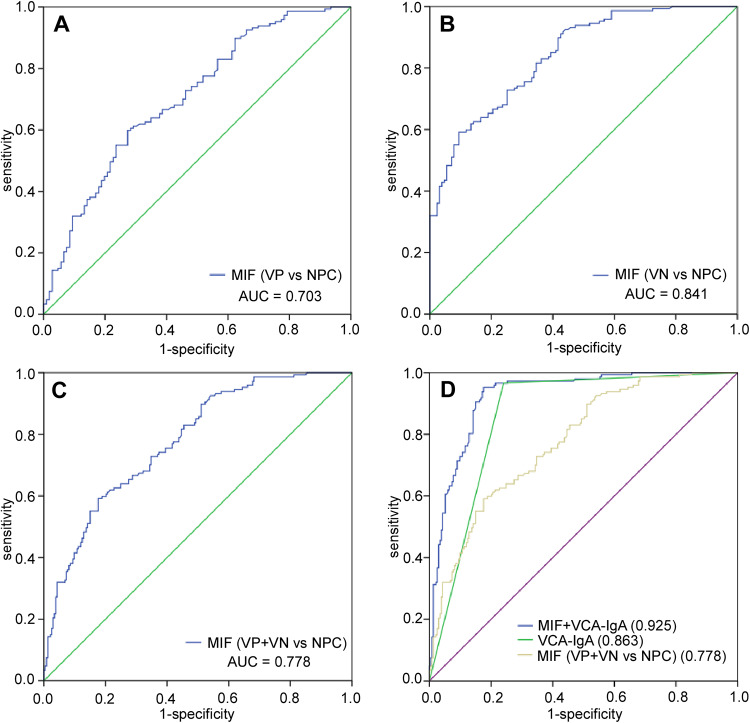
The ROC curve was plotted to identify the optimum diagnostic cut-off value to distinguish 147 patients with NPC from 233 healthy people (127 VN and 106 VP). In Figure 3, we show that the AUC of MIF for distinguishing NPC from the VN cohort or VP cohort was 0.841 (95% CI: 0.796-0.885) and 0.703 (95%CI: 0.638-0.767), respectively. The AUC value of MIF and VCA-IgA combination was 0.925 (95%CI: 0.898-0.951). Table 2 demonstrates that the sensitivity of MIF was much lower, but the specificity of MIF (82.40%) was higher than VCA-IgA (69.96%). More importantly, the combination of MIF and VCA-IgA exhibits a higher specificity (82.40%) without significant decrease in sensitivity (95.25%). In addition, the PPV of the combination (79.17%) was also higher than that of MIF (67.97%) or VCA-IgA (67.44%) alone in the NPC. In conclusion, the specificity and PPV of NPC diagnosis was improved by combination of MIF and VCA-IgA.
Figure 3.
Diagnosis efficacy of MIF and VCA-IgA in the diagnosis of nasopharyngeal carcinoma (NPC). ROC curves for diagnosing NPC from VN cohort (AUC = 0.841) (B), VP cohort (AUC = 0.703) (A) and (VN + VP) cohort (AUC = 0.778) (C). ROC curves for the diagnostic strength to identify NPC using MIF and VCA-IgA (MIF: AUC = 0.778; VCA-IgA: AUC = 0.863; MIF+VCA-IgA: AUC = 0.925) (D).
Table 2.
Diagnostic Values of MIF and VCA-IgA for NPC.
| Optimal cutoff point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive likelihood ratio | Negative likelihood ratio | AUC | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| MIF | |||||||||
| VN vs NPC | 20.92 ng/mL | 59.20 | 90.55 | 87.88 | 65.71 | 6.263 | 0.190 | 0.841 | 0.796-0.885 |
| VP vs NPC | 19.49 ng/mL | 59.90 | 72.64 | 75.21 | 56.61 | 2.188 | 0.377 | 0.703 | 0.638-0.767 |
| (VN + VP) vs NPC | 20.72 ng/mL | 59.20 | 82.40 | 67.97 | 76.19 | 3.363 | 0.214 | 0.778 | 0.732-0.824 |
| VCA-IgA | 1:40 | 96.60 | 69.96 | 67.44 | 98.79 | 4.019 | 0.316 | 0.863 | 0.825-0.901 |
| MIF + VCA-IgA | 0.518 | 95.25 | 82.40 | 79.17 | 96.48 | 5.412 | 0.214 | 0.925 | 0.898-0.951 |
Abbreviations: AUC, area under the curve; CI, confidence interval; MIF, macrophage migration inhibitory factor; NPC, nasopharyngeal carcinoma; NPV, negative predictive value; PPV, positive predictive value; VCA-IgA, viral capsid antigen; VN, VCA-IgA negative healthy controls; VP, VCA-IgA positive healthy controls.
The MIF Levels Were Not Associated With EBV Infection
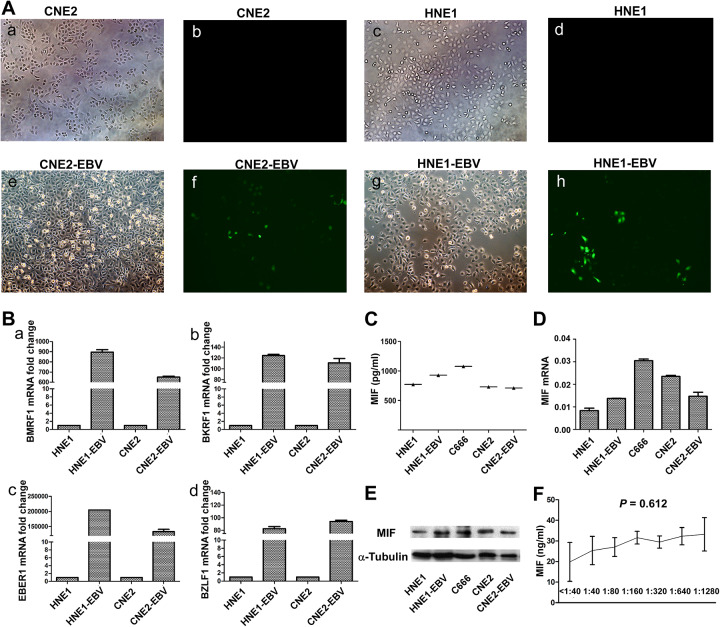
Epstein-Barr virus is a crucial factor in NPC development, so we further investigated whether EBV plays a role in the altered MIF levels of NPC cells. First, we used 2 stable cell lines with persistent GFP-labeled EBV infection, confirmed by immunofluorescence (Figure 4A). We used 4 markers (BMRF1, BKRF1, EBER1, BZLF1) to further verify that CNE2-EBV and HNE1-EBV cell lines were indeed infected with EBV (Figure 4B). The results show us that the expression of MIF protein (Figure 4C and E) and mRNA (Figure 4D) was not significantly different in NPC cell lines with or without EBV infection. Moreover, there was no significant association between the levels of plasma MIF and the levels of serum VCA-IgA (P = 0.612; Figure 4F). All of these results suggest that EBV infection does not have a significant effect on the expression of MIF.
Figure 4.
Green fluorescence of cells post-infection show green fluorescent protein (GFP; A, 200×). Real-time-polymerase chain reaction (RT-PCR) was used to detected four markers (BMRF1, BKRF1, EBER1, BZLF1) in NPC cell lines with (HNE1-EBV, CEN2-EBV, C666) or without EBV (HNE1, CNE2) (B). The level of MIF protein in supernatant in the cell lines with EBV (HNE1-EBV, CEN2-EBV, C666) or without EBV (HNE1, CNE2) was measured by ELISA (C). The levels of messenger RNA (mRNA) and protein were determined by RT-PCR (D) and Western Blotting (E). The correlations between MIF concentrations and VCA-IgA titers (F) by Spearman’s rank correlation test. Expression level was normalized by β-actin and α- tubulin, respectively. Error bars represent standard deviations (SD) calculated from three parallel experiments.
Discussion
Nasopharyngeal carcinoma is a disease different from other cancers, which commonly metastasized to other sides of the body such as the lymph node of the neck.28 The etiology of NPC is complex, containing genetic susceptibility, environmental factors, and EBV infection.29 Usually, nasopharyngeal biopsy examination is the most important tools for NPC diagnosis, which provide the most definitive evidence to determine the clinical stage. However, nasopharyngeal biopsy is an invasive and painful examination, which is not suitable for screening for NPC. Thus, detection of antibodies against EBV antigens, such as VCA-IgA and EA-IgA, has been used as noninvasive tools for NPC diagnosis.9 However, the specificity and PPV of these markers are not satisfactory. Therefore, other non-EBV-related serological biomarkers of NPC have been explored for NPC diagnosis.
In this study, we confirmed that MIF was upregulated at the protein and RNA levels in NPC cell lines and tumor tissues. In addition, MIF expression was observed mostly in the cytoplasm of tumor cells but not in normal nasopharyngeal epithelia. The levels of plasma MIF in the NPC cohort were significantly higher than that of the VP cohort (P < 0.001) and VN cohort (P < 0.001), and the AUC values of MIF for diagnosing NPC from VN and VP were 0.841 and 0.703, respectively. VCA-IgA is one of the most common biomarkers for NPC, which showed limited specificity (69.96%), despite its high sensitivity (96.60%) when 1:40 was used as the reference cutoff value. Our results demonstrate that MIF exhibited low sensitivity but high specificity for NPC detection. When combined with VCA-IgA, the sensitivity was 95.25% and the specificity (82.40%) was much higher than that of VCA-IgA alone (69.96%). Moreover, the combination scheme increased the PPV from 67.44% to 79.17% for detecting NPC, suggesting that the combination of MIF and VCA-IgA could more accurately differentiate patients with NPC from individuals who have anti-EBV antibodies. Macrophage migration inhibitory factor may complement VCA-IgA well in NPC diagnosis.
There was a significant association between plasma MIF concentration and gender (P = 0.007) and T classification (P = 0.016), but not with other clinicopathological characteristics. Compared with the VP and VN group, NPC group had higher expression of MIF (P < 0.001). Our data show that the levels of MIF had no significant differences between the VP cohort and early-stage NPC (P = 0.095). This may be due to the small sample size of patients with early-stage NPC in this study. We need much larger sample scale to assess MIF in early diagnosis of NPC. In addition, we observed no significant differences in plasma MIF levels between patients with early-stage tumors (I-II) and patients with advanced-stage tumors (III-IV) (P = 0.103).
Macrophage migration inhibitory factor is an inflammatory cytokine expressed by macrophage and various organs.14 Macrophage migration inhibitory factor promotes the generation and recruitment of Th17 cells mediated by NPC tumor cells. This effect was dependent on the mammalian target of rapamycin pathway and mediated by the MIF-CXCR4 axis.30 Tumor-derived MIF promotes cell invasion and migration and modulates cell biological behaviors by activating the NF-kB, Erk1/2, AP-1, and PI3K/Akt pathway.31 All these results suggested that MIF play important roles in the processing of NPC. Our study was aimed to explore the application of plasma MIF for NPC diagnosis. We also explored the correlation about the MIF levels of NPC cells and EBV infection. Our results show that EBV infection does not have a significant effect on the expression of MIF.
This study is the first to suggest that MIF represents a potential non-EBV plasma marker for the diagnosis of NPC. Moreover, the specificity and PPV for screening and diagnosis of NPC was increased by combined detection of MIF and VCA-IgA. Our next efforts to increase test specificity for NPC screening would benefit from marker combinations, such as the incorporation of measures of nasopharyngeal EBV DNA levels.
In conclusion, this study evaluated the diagnostic significance of plasma MIF and the traditional NPC tumor marker VCA-IgA for NPC. The results showed that MIF protein was highly expressed in tumor cells, tumor tissues, and plasma of patients with NPC. Moreover, we found that MIF was not associated with EBV infection. The plasma MIF levels were not associated with the levels of VCA-IgA. Combination with MIF and VCA-IgA may significantly improve the specificity and PPV of NPC diagnosis.
Abbreviations
- AUC
area under curve
- BKRF1
Epstein-Barr nuclear antigen 1
- BMRF1
early antigen protein D
- EA/IgA
EBV early antigen
- EBER1
Epstein-Barr virus-encoded RNA
- EBV
Epstein-Barr virus
- ELISA
enzyme linked immunosorbent assay
- GFP
green fluorescence protein
- HRP
horseradish peroxidase
- MIF
macrophage migration inhibitory factor
- NPC
nasopharyngeal carcinoma
- NPV
negative predictive value
- PPV
positive predictive value
- SDS
sodium dodecyl sulfate
- VCA-IgA
EBV viral capsid antigen
- VN
VCA-IgA negative healthy controls
- VP
VCA-IgA positive healthy controls.
Footnotes
Authors’ Note: Ning Xue and Shan Xing are equal contributors. This study was approved by the Institutional Review Board and Ethics Committee of the Cancer Center of Sun-Yat University (approval no. GZR2018-147). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (Grant No. 81472008; NO. 81271902).
ORCID iD: Qingxia Xu  https://orcid.org/0000-0002-8925-1230
https://orcid.org/0000-0002-8925-1230
References
- 1. Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104(3):272–278. doi:10.1016/j.radonc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 2. Razak AR, Siu LL, Liu FF, Ito E, O’Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46(11):1967–1978. doi:10.1016/j.ejca.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4. Mahdavifar N, Ghoncheh M, Mohammadian-Hafshejani A, Khosravi B, Salehiniya H. Epidemiology and inequality in the incidence and mortality of nasopharynx cancer in Asia. Osong Public Health Res Perspect. 2016;7(6):360–372. doi:10.1016/j.phrp.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. doi:10.5732/cjc.014.10197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowe M, Lear AL, Croom-Carter D, Davies AH, Rickinson AB. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng P, Chan SH, Soo MY, et al. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma: a new serologic parameter for diagnosis. Cancer. 2001;92(7):1872–1880. doi:10.1002/1097-0142(20011001)92:7<1872:: aid-cncr1704>3.0.co;2-n [DOI] [PubMed] [Google Scholar]
- 8. Chan KC. Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):598–603. doi:10.5732/cjc.014.10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng Y, Zhang LG, Li HY, et al. Serological mass survey for early detection of nasopharyngeal carcinoma in Wuzhou City, China. Int J Cancer. 1982;29(2):139–141. doi:10.1002/ijc.2910290204 [DOI] [PubMed] [Google Scholar]
- 10. Karray H, Ayadi W, Fki L, et al. Comparison of three different serological techniques for primary diagnosis and monitoring of nasopharyngeal carcinoma in two age groups from Tunisia. J Med Virol. 2005;75(4):593–602. doi:10.1002/jmv.20310 [DOI] [PubMed] [Google Scholar]
- 11. Neel HB, III, Pearson GR, Taylor WF. Antibodies to Epstein-Barr virus in patients with nasopharyngeal carcinoma and in comparison groups. Ann Otol Rhinol Laryngol. 1984;93(5 Pt 1):477–482. doi:10.1177/000348948409300513 [DOI] [PubMed] [Google Scholar]
- 12. Yu KJ, Hsu WL, Pfeiffer RM, et al. Prognostic utility of anti-EBV antibody testing for defining NPC risk among individuals from high-risk NPC families. Clin Cancer Res. 2011;17(7):1906–1914. doi:10.1158/1078-0432.CCR-10-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay JK, Chan SH, Lim CM, Siow CH, Goh HL, Loh KS. The Role of Epstein-Barr Virus DNA Load and Serology as Screening Tools for Nasopharyngeal Carcinoma. Otolaryngology Head Neck Surg. 2016;155(2):274–280. doi:10.1177/0194599816641038 [DOI] [PubMed] [Google Scholar]
- 14. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi:10.1038/nri1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimizu T, Ohkawara A, Nishihira J, Sakamoto W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immunohistochemical localization. FEBS Lett. 1996;381(3):188–202. doi:10.1016/0014-5793(96)00120-2 [DOI] [PubMed] [Google Scholar]
- 16. He LJ, Xie D, Hu PJ, et al. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2015;21(34):9916–9926. doi:10.3748/wjg.v21.i34.9916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Subbannayya T, Leal-Rojas P, Barbhuiya MA, et al. Macrophage migration inhibitory factor - a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843 doi:10.1186/s12885-015-1855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res. 2002;8(12):3755–3760. [PubMed] [Google Scholar]
- 19. Ren Y, Tsui HT, Poon RT. Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int J Cancer. 2003;107(1):22–29. doi:10.1002/ijc.11287 [DOI] [PubMed] [Google Scholar]
- 20. Xu X, Wang B, Ye C, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261(2):147–157. doi:10.1016/j.canlet.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 21. Liao B, Zhong BL, Li Z, Tian XY, Li Y, Li B. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol. 2010;102(7):844–851. doi:10.1002/jso.21728 [DOI] [PubMed] [Google Scholar]
- 22. Pacheco-Fernandez T, Juárez-Avelar I, Illescas O, et al. Macrophage migration inhibitory factor promotes the interaction between the tumor, macrophages, and t cells to regulate the progression of chemically induced colitis-associated colorectal cancer. Mediators Inflamm. 2019;2019:2056085 doi:10.1155/2019/2056085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee H, Rhee H, Kang HJ, et al. Macrophage migration inhibitory factor may be used as an early diagnostic marker in colorectal carcinomas. Am J Clin Pathol. 2008;129(5):772–779. doi:10.1309/GFCLLRH8A68XKMJN [DOI] [PubMed] [Google Scholar]
- 24. DE Souza MB, Curioni OA, Kanda JL, DE Carvalho MB. Serum and salivary macrophage migration inhibitory factor in patients with oral squamous cell carcinoma. Oncol Lett. 2014;8(5):2267–2275. doi:10.3892/ol.2014.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HW. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55(6):797–802. doi:10.1136/gut.2005.078113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–6232. doi:10.1158/0008-5472.CAN-06-0094 [DOI] [PubMed] [Google Scholar]
- 27. Xiong D, Yong D, Bo Wang H, et al. Nonmuscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells. Proc Natl Acad Sci U S A. 2015;112(35):11036–11041. doi:10.1073/pnas.1513359112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee AW, Wai Tong NG, Chan LK, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi:10.1016/j.radonc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 29. Tsao SW, Yim LY, Chi MT, et al. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50(5):330–338. doi:10.1016/j.oraloncology.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 30. Li J, Hao-Yuan M, Geng X, et al. al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem. 2012;287(42):35484–35495. doi:10.1074/jbc.M112.367532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pei XJ, Tong Tong W, Bin L, Xiao-Ying T, Zhi L, Qing XY. Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int J Med Sci. 2013;11(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]