Abstract
Purpose of the review:
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) are recommended for eligible patients with type 2 diabetes for the secondary prevention of adverse cardiovascular and kidney disease outcomes. Patients with type 2 diabetes and albuminuric chronic kidney disease, a history of atherosclerotic cardiovascular disease, and/or heart failure with reduced ejection fraction should be assessed for the use of these therapies.
Sources of information:
The sources include published clinical trials with SGLT2is, with a focus on cardiovascular safety studies and kidney protection trials.
Methods:
Information was gathered via a review of relevant literature and clinical practice guidelines, incorporated with real-life clinical experience.
Key findings:
Clinicians prescribing these agents must be familiar with the benefits of SGLT2is on cardiovascular and renal endpoints, and with adverse effects of SGLT2is, including mycotic genital infections and diabetic ketoacidosis. Primary care physicians and specialists should know how to adjust antihypertensive, antiglycemic, and diuretic agents. With the results of completed cardiovascular outcome trials and the Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy trial, nephrologists specifically have a unique opportunity to impact the safe, effective, and equitable implementation of SGLT2is into clinical practice.
Limitations:
Further work is needed in specific patient subgroups, including patients with chronic kidney disease stages IV and V, patients with kidney disease but lower levels of albuminuria, and in patients without diabetes.
Keywords: sodium-glucose cotransporter-2 inhibitors, SGLT2-inhibitors, chronic kidney disease, secondary prevention, diabetic kidney disease, type 2 diabetes
Abrégé
Justification:
Les inhibiteurs du co-transporteur sodium-glucose de type 2 (iSGLT2) sont recommandés pour la prévention secondaire des effets indésirables de nature cardiovasculaire ou rénale chez les patients diabétiques de type 2 qui sont admissibles. Les patients diabétiques de type 2 en insuffisance rénale chronique albuminurique et ayant des antécédents de maladie cardiovasculaire athérosclérotique et/ou d’insuffisance cardiaque avec fraction d’éjection réduite devraient être évalués pour l’utilisation de ces traitements.
Sources:
Les essais cliniques publiés portant sur les iSGLT2, avec une attention particulière pour les études traitant de tolérance cardiovasculaire et de protection rénale.
Méthodologie:
L’information a été recueillie par l’entremise d’une revue de la littérature pertinente et des guides de pratique clinique, intégrés à l’expérience clinique réelle.
Principaux résultats:
Les médecins qui prescrivent des iSGLT2 doivent connaître les bienfaits de ces agents sur les résultats cardiovasculaires et rénaux, tout comme leurs effets indésirables, notamment les infections mycosiques de l’appareil génital et l’acidocétose diabétique. Les spécialistes et les médecins de première ligne doivent être en mesure d’ajuster la posologie des agents antihypertenseurs, des diurétiques et des médicaments contrôlant la glycémie. Grâce aux résultats d’essais portant sur les résultats cardiovasculaires et des conclusions de l’essai CREDENCE (Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy), les néphrologues ont une occasion unique d’influer sur une mise œuvre sûre, efficace et équitable des iSGLT2 dans la pratique clinique.
Limites:
Des études supplémentaires portant sur des sous-groupes particuliers de patients sont nécessaires, notamment sur des sujets atteints d’insuffisance rénale chronique de stade IV et V, des patients atteints de néphropathie avec de faibles niveaux d’albuminurie et des sujets non-diabétiques.
What was known before
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) are safe and effective anti-glycemic agent for patients with type 2 diabetes. Several large cardiovascular outcome trials and one major renal outcome trial have shown significant improvements in cardiac and renal outcomes with SGLT2is, leading to expanding criteria for patients who may benefit from SGLT2i therapy.
What this adds
Using evidence from large clinical trials, we outline the characteristics of patients who are eligible for SGLT2i therapy and provide a clinical framework to prescribe and monitor patients while on therapy. This review serves as a practical guide for nephrologists to safely incorporate SGLT2i therapy into clinical practice for cardiac and renal protection.
Introduction
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) are recommended for eligible patients with type 2 diabetes for the secondary prevention of cardiovascular (CV) and/or kidney disease.1 As with all new therapies, it is crucial to address potential barriers to changes in clinical practice, such as lack of clinician familiarity with the medication and with the adverse effect profile.2,3 A “risk-treatment paradox” has been described with SGLT2is, whereby the highest risk patients may be under prescribed these therapies in clinical practice, despite benefits reported in clinical trials in patients with the same risk profiles.2,4 While the reasons for this “risk-treatment paradox” remain speculative, potential explanations include a lack of knowledge of the evidence on the part of physicians, or a reluctance to take a “new” medication on the part of older, higher risk patients.2 Disparities in prescribing patterns by ethnicity and socioeconomic status have also been described with this drug class, which may further limit appropriate use of these therapies.4 With the results of completed cardiovascular outcome trials (CVOTs) and the Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy (CREDENCE) trial, nephrologists have an opportunity to impact the safe, effective, and equitable implementation of SGLT2is into clinical practice.3
Review
What Are Indications for SGLT2i Use Beyond Glucose Lowering?
Clinical trials including patients at various levels of cardiorenal risk have been completed and reported in the last 5 years, and have been summarized in detail elsewhere.5 Patients with type 2 diabetes and a known history of CV disease (coronary artery disease, stroke, or peripheral vascular disease), with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2, benefit from SGLT2is for secondary prevention of atherosclerotic cardiovascular disease (ASCVD). Specifically, the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) and Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes (CANVAS Program) trials each demonstrated a 14% reduction in the primary endpoint of major adverse cardiac event outcome of CV death, nonfatal myocardial infarction, or nonfatal stroke in the group randomized to SGLT2is compared with placebo.6,7 Similarly, the Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes (DECLARE-TIMI 58) trial demonstrated a 17% reduction in CV death or hospitalization for heart failure.8
Patients randomized to SGLT2is had a 46%, 40%, and 47% reduction in the secondary renal endpoints of doubling of serum creatinine, 40% eGFR decline, end-stage kidney disease (ESKD), or renal death in the EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58 trials, respectively.6-8 Furthermore, in patients with type 2 diabetes and chronic kidney disease (CKD), defined as eGFR 30 to 90 mL/min/1.73 m2 and albuminuria (>300 mg/day), on maximum tolerated renin-angiotensin-aldosterone system (RAAS) blockade, SGLT2i therapy is increasingly being recommended in clinical practice guidelines to reduce the risk of CKD progression.9,10 This change in practice is based on the results from the CREDENCE trial that demonstrated patients randomized to canagliflozin compared with placebo had a 30% reduction in the primary renal endpoint of doubling of the serum creatinine level, ESKD, or renal or CV death.11 The number needed to treat with canagliflozin to prevent 1 occurrence of the primary outcome is 22 patients over 2.5 years.11 Based on the available evidence, patients with type 2 diabetes and CKD with eGFR 30 to 90 mL/min/1.73 m2 and albuminuria <300 mg/day are not yet eligible for SGLT2i initiation specifically for kidney protection based on the results from the CREDENCE trial. However, SGLT2is can be initiated in patients with an eGFR ≥30 mL/min/1.73 m2 for CV protection in patients with established ASCVD. While American, European, and Canadian Cardio-vascular Society clinical practice guidelines now recommend SGLT2is for kidney protection, Diabetes Canada Clinical Practice Guidelines have not yet been revised since the completion of the CREDENCE trial.9,10,12,13
SGLT2i therapy has been tested in patients with and without type 2 diabetes, in the setting of heart failure and reduced ejection fraction <40% and eGFR ≥30 mL/min/1.73 m2, for the secondary prevention of hospitalization for heart failure. In the Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) trial, patients had a 26% reduction in the primary outcome of worsening heart failure or CV death with dapagliflozin versus placebo treatment.14 These results were similar in patients with and without diabetes, thereby providing exciting new evidence that SGLT2i-related benefits may extend to patients even in the absence of hyperglycemia (Table 1).
Table 1.
Clinical Indications for Sodium-Glucose cotransporter 2i Based on Large Clinical Trials (Approved Indications Vary by Jurisdiction).
| Type 2 diabetes and history of atherosclerotic cardiovascular disease (empagliflozin to reduce cardiovascular death, canagliflozin to reduce the risk of major adverse cardiac events, and dapagliflozin to reduce the risk of hospitalization for heart failure) |
| Type 2 diabetes and chronic kidney disease with estimated glomerular filtration rate 30 to 90 mL/min/1.73 m2 and macroalbuminuria (canagliflozin) |
| Heart failure with reduced ejection fraction <40%, with or without type 2 diabetesa |
| Use for control of hyperglycemia with metformin unless metformin is contraindicated (empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin) |
Not yet approved in Canada for this indication, dapagliflozin is recommended in 2020 Canadian Cardiovascular Society/Canadian Heart Failure Society Heart Failure Guidelines update.
Who Is Not Eligible for SGLT2i Use Currently?
Despite the strength of evidence described above, there are specific clinical circumstances where there is either no data, or insufficient evidence for the use of SGLT2is. First, safety and efficacy data in kidney transplant recipients with type 2 diabetes are limited to case series and 1 small controlled trial.15-17 Accordingly, SGLT2is should not yet be used in these patients. In addition, although the mechanisms for kidney protection may overlap significantly in type 1 and type 2 diabetes, SGLT2is are not currently recommended in Canada for use in patients with type 1 diabetes due to concerns related to an increased risk of diabetic ketoacidosis (DKA) in this population.18,19 Although SGLT2i use in patients with type 1 diabetes is approved in Europe and Japan, even in the hands of specialized endocrinology clinics, significant education is required to mitigate the risk of DKA, especially around ketone monitoring and sick day advice.20-22
Compared with the overall literature with these agents, there are less data for SGLT2i therapy in some higher risk groups, including elderly individuals.23,24 Accordingly, individualized consideration of risks and benefits is warranted. Sodium-glucose cotransporter-2 inhibitors should be avoided during pregnancy and breastfeeding, given the lack of safety data, and women of child-bearing age should be counseled accordingly.25 While initiating therapy with SGLT2is is not currently recommended if eGFR <30 mL/min/1.73 m2, continuation of therapy may be reasonable until renal replacement therapy initiation, as was done in the CREDENCE trial.11 It is prudent to avoid initiating SGLT2i therapy in patients with known bilateral renal artery stenosis or those with severe liver disease, given the lack of safety data in these groups. In addition, when considering use in patients with a single kidney, consultation with a nephrologist should be considered due to possible hemodynamic issues that may arise, especially when combined with a RAAS inhibitor.
Prior to initiating SGLT2i therapy, consideration should be given to the patient’s current medication regimen. Patients should be on the maximum tolerated dose of a single RAAS-inhibiting agent, which is based on data from published kidney and CV endpoint trials. For example, 99.9% of patients in the CREDENCE trial were taking a RAAS inhibitor at baseline, as were 80% of participants in the CVOTs.6-8,11 Mineralocorticoid receptor antagonist (MRA) use may be considered in appropriate cases, with the caveat that MRA use was excluded in the CREDENCE trial but permitted in published CVOTs and DAPA-HF, with 71% of patients taking MRA therapy in the latter trial.11,14 A final consideration is immunosuppressive therapies. Patients taking systemic immunosuppressive therapies including chronic corticosteroids were excluded from the clinical trials. Use of SGLT2is with immunosuppressive therapies should therefore be considered on an individualized basis.
What Are the Known and Suspected Adverse Effects of SGLT2is?
Patients taking SGLT2is have a 3- to 4-fold increased risk of mycotic genital infections, which are usually not severe and rarely necessitate cessation of therapy.26 There is no increased risk of urinary tract infection (UTI) with SGLT2is, with the caveat that this risk is not well defined in “high-risk” patients such as those with a history of recurrent UTI or catheter use.27 It remains unknown whether SGLT2i is associated with an increased risk of necrotizing fasciitis of the perineum (“Fournier’s gangrene”),26 although the risk was actually lower in patients who received dapagliflozin in the DECLARE-TIMI 58 trial. Accordingly, using objective data, this no longer appears to be an important clinical consideration.
Patients taking SGLT2is are at increased risk of symptomatic hypovolemic events,7,28 and it is prudent to counsel patients to hold therapy in the event of a surgery or acute illness. Temporarily stopping treatment in this context may help prevent rare but serious side effects, including volume depletion and DKA. Patients with type 2 diabetes taking SGLT2is are at an increased risk of DKA, with a reported incidence of 0.16 to 0.76 events per 1000 patients years.26 Serum glucose levels may be normal in the setting of DKA with SGLT2is (“euglycemic DKA”). Therefore, a high index of suspicion is required in patients on SGLT2is who present with an elevated anion gap metabolic acidosis.29 Patients with type 2 diabetes and a history of DKA are likely at risk of recurrent DKA, and SGLT2i therapy should be avoided in these individuals.
Less well understood was the observation that there was an increased risk of bone fractures and lower extremity amputations in patients receiving canagliflozin versus placebo in the CANVAS Program trial.7 Importantly, this signal was not seen in the CREDENCE trial or in any of the other CV safety trials.11 The true nature of the risk remains undefined, and it may be prudent to avoid canagliflozin specifically in patients at very high risk of lower extremity amputation or fractures.20 A different issue that arose in postmarketing surveillance data was a suggestion of an increased risk of acute kidney injury (AKI) with SGLT2is. Subsequent “real world” health care database analyses have convincingly reported that this AKI signal was likely confounded by the lack of the control group, and this concern has not been substantiated in large clinical trials or propensity-matched observational data.28,30 To the contrary, large clinical trials have reported an opposite reduction in AKI risk with SGLT2is, for reasons that are not yet well understood,31 and a similar reduced AKI risk has been reported in administrative database analyses.32
How Do I Initiate and Titrate SGLT2i Therapy?
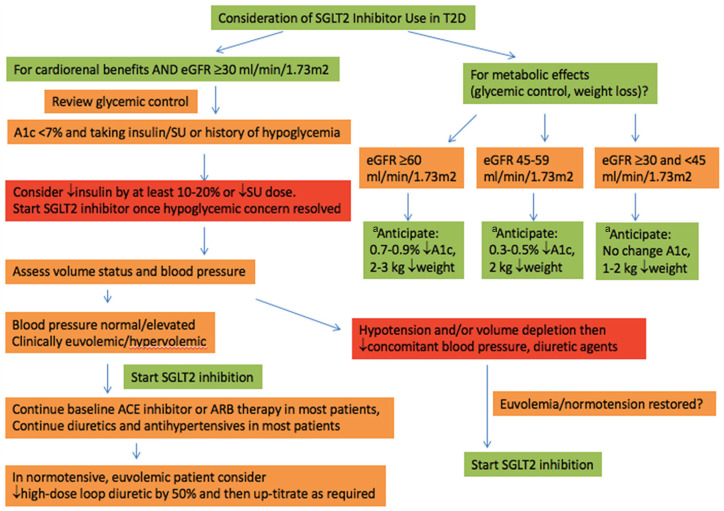
Once a patient is identified as eligible for SGLT2i therapy, the lowest evidence-based dose that provides cardiorenal benefit should be initiated: empagliflozin 10 mg, canagliflozin 100 mg, or dapagliflozin 10 mg once daily (Table 2). At the time of SGLT2i initiation, the patient’s current glycemic control, blood pressure, and volume status should be assessed (Figure 1). The risk of hypoglycemia is generally not increased when SGLT2i is used alone or with metformin monotherapy.1 Patients with recurrent hypoglycemia, or with a hemoglobin A1C <7% on insulin or sulfonylurea therapy should have their insulin dose reduced by approximately 10% to 20%, and sulfonylurea dose reduced or stopped at the time of SGLT2i initiation. Sodium-glucose cotransporter-2 inhibitors should be avoided in hypotensive and hypovolemic patients, based on the judgment of the clinician. Antihypertensive medications may need to be adjusted if blood pressure is already at target because systolic and diastolic blood pressures decrease with SGLT2is by approximately 3 to 5/1 to 2 mm Hg, respectively.33 Importantly, in patients with diabetes (especially with cardiorenal complications), preference should be given to maintaining stable RAAS inhibitor doses and reducing other antihypertensives. While the natriuresis seen with SGLT2i initiation is modest, adjustment of loop diuretic dosing may also be required in patients who are already euvolemic and normotensive at baseline.33-35 For example, we tend to reduce loop diuretic dose by 50% in patients initiating SGLT2i agents when blood pressure and volume status are in the normal or lower range at baseline.
Table 2.
Currently Available Sodium-Glucose Cotransporter-2 Inhibitor in Canada.
| Drug name | Major clinical trials with each agent | Available dosage and recommendations | Health Canada approved eGFR cutoff |
|---|---|---|---|
| Empagliflozin | EMPA-REG OUTCOME, NCT01131676 EMPA-KIDNEYa, NCT03594110 (ongoing) |
10 mg, 25 mgb | eGFR ≥30 mL/min/1.73 m2 |
| Canagliflozin | CANVAS Program, NCT01032629, NCT01989754 CREDENCE, NCT02065791 |
100 mg, 300 mgb
100-mg dose used in CREDENCE |
eGFR ≥30 mL/min/1.73 m2
100-mg dose can be continued until dialysis initiation |
| Dapagliflozin | DECLARE-TIMI 58, NCT01730534 DAPA-HF, NCT03036124 DAPA-CKDa, NCT03036150 (stopped in March 2020 due to clinical benefit) |
5 mg, 10 mg | eGFR ≥45 mL/min/1.73 m2 |
| Ertugliflozin | VERTIS CV (completed in December 2019, results pending) | 5 mg, 15 mg | eGFR ≥45 mL/min/1.73 m2 |
Note. eGFR= estimated glomerular filtration rate; NCT = ClinicalTrials.gov Identifier; VERTIS CV = Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease; EMPA-REG = Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes; EMPA-KIDNEY = Study of Heart and Kidney Protection With Empagliflozin; CANVAS = Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes; CREDENCE = Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy; DECLARE-TIMI 58 = Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes; DAPA-HF = Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction; DAPA-CKD = Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease.
Primary composite renal endpoint.
Cardiovascular benefits seen at both doses.
Figure 1.
Proposed algorithm for SGLT2i initiation.
Note. Check electrolytes, creatinine after initiation of treatment according to local guidelines/practice. SGLT2i = sodium-glucose cotransporter-2 inhibitor; T2D = type 2 diabetes; eGFR= estimated glomerular filtration rate; SU = sulfonylurea; ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker.
aAlso consider following guidance around adjustment of insulin and SU therapies, diuretics, and antihypertensives prior to initiation of therapies.
How Do I Monitor SGLT2i Therapy?
Recommendations for biochemical monitoring after SGLT2i initiation mirror those following initiation of RAAS inhibitors. Specifically, kidney function should be checked 7 to 14 days after initiation of therapy in patients with kidney function impairment or in patients with concerns around circulating volume or hemodynamics. Clinicians should anticipate an acute dip in baseline eGFR of 3 to 4 mL/min/1.73 m2 with SGLT2i initiation.33 Repeat testing and close follow-up is recommended if eGFR declines by more than 20% to 25% with drug initiation, with dose reduction or discontinuation of therapy if eGFR drops by >30%.5 Importantly, after 4 weeks on SGLT2i therapy, the decline in eGFR of 3 to 4 mL/min/1.73 m2 should stabilize, and in many cases returns back to baseline.36
Beyond expected changes in kidney function, patients should be counseled on good perineal hygiene habits to reduce the risk of genital mycotic infections26 and advised to stop the drug during episodes of acute illness or surgery to reduce the risk of symptomatic hypovolemia and DKA.20
Are There Upcoming Studies Relevant to My Nephrology Practice?
SGLT2i therapies are being investigated for potential renal benefits in an expanded population beyond patients with type 2 diabetes and albuminuria. The Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial enrolled patients with and without type 2 diabetes, with eGFR between 25 and 75 mL/min/1.73 m2 and albuminuria (>200 mg/day). The Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease trial was stopped early in March of 2020 due to overwhelming efficacy in the primary composite outcome of time to 50% eGFR decline, ESRD, and renal or CV death,37 which could lead to major changes in clinical practice in nephrology once the full results are available. Furthermore, the Study of Heart and Kidney Protection With Empagliflozin is enrolling patients with eGFR as low as 20 mL/min/1.73 m2 with normoalbuminuria, microalbuminuria, or macroalbuminuria, to evaluate similar composite renal outcomes.3 While the underlying mechanisms for the kidney protective properties of SGLT2is are not entirely understood, the proposed mechanisms are well described in other reviews,5,33 which include attenuation of tubuloglomerular feedback and hyperfiltration, suppression of proinflammatory pathways, and protection against renal ischemia.5
How Do I Integrate Findings From CREDENCE With My Current Practice? Can the Results Be Extrapolated to Any SGLT2i, or Should I Switch My Patients With CKD to Canagliflozin?
In light of the substantive cardiorenal benefits observed in the CVOTs and in the CREDENCE trial, SGLT2is are poised to have a more prominent role in clinical practice as medicines that reduce the risk of significant cardiac and renal complications, independent of glucose lowering. In patients at high risk for CKD progression, SGLT2is should be initiated in patients meeting inclusion and exclusion criteria from the CREDENCE trial: patients with type 2 diabetes, eGFR 30 to 90 mL/min/1.73 m2, and macroalbuminuria. Our practice is to initiate canagliflozin 100 mg in eligible patients who are not yet on SGLT2is as per the CREDENCE trial, but not to switch to this agent if the patient has already been prescribed empagliflozin or dapagliflozin for cardioprotection or glucose-lowering reasons. The rationale for this practice is that the renoprotective effects of SGLT2is are likely a class-effect based on the secondary renal outcomes of the CVOTs and emerging data from DAPA-CKD.31,37
To avoid low rates of usage seen with RAAS inhibitors when these agents first became available, potential barriers need to be addressed to ensure that SGLT2is and other new kidney protective agents are used appropriately and safely. First, a lack of comfort and familiarity for many internists, family doctors, cardiologists, and nephrologists may slow the uptake of these agents for appropriate patients who meet the criteria for the CVOTs and CREDENCE trial. Closer collaboration and communication in particular between endocrine, cardiology, and nephrology specialists might help achieve better uptake of SGLT2is in appropriate patients. Second, financial cost for these therapies remains a significant barrier in many jurisdictions, despite evidence that they reduce the risk of developing expensive complications requiring extended hospital stays. Third, patients need to be identified appropriately in health care systems, where routine blood and urine screening and clinic visits may either be too infrequent or completely absent. As a consequence, for example, patients may not have albuminuria quantified, thereby missing the opportunity to identify patients who would otherwise be eligible for SGLT2i therapy. Finally, community outreach programs are needed to help with awareness around diabetes, complications, and the role of new therapies to increase the uptake, use, and continuation of new treatment options.
Conclusion
SGLT2is represent an important advancement for the prevention of CV disease and treatment of heart failure, and a transformational change in the way diabetic kidney disease is managed. It is of particular importance for the cardiology and nephrology communities to become familiar and comfortable with these agents—both their benefits and side effect profiles—and to be comfortable prescribing them. Furthermore, it is of additional importance to open and maintain a dialogue with clinicians, patients, payers, and regulators to make sure that these and other agents are accessible to patients and thereby yield a maximal benefit to individual patients and to society.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.S. received honoraria from Horizon Pharma, PLC, and AstraZeneca and consulting fees from CVS Caremark. D.Z.C has received honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, Abbvie, Janssen, Bayer, Prometic, BMS, and Novo-Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, and Novo-Nordisk. The other authors have no disclosures.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David Z. Cherney  https://orcid.org/0000-0003-4164-0429
https://orcid.org/0000-0003-4164-0429
References
- 1. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tuttle KR, Cherney DZ, Diabetic Kidney Disease Task Force of the American Society of Nephrology. Sodium glucose cotransporter 2 inhibition heralds a call-to-action for diabetic kidney disease. Clin J Am Soc Nephrol. 2020;15:285-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkinson S, Douglas IJ, Williamson E, et al. Factors associated with choice of intensification treatment for type 2 diabetes after metformin monotherapy: a cohort study in UK primary care. Clin Epidemiol. 2018;10:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cherney DZ, Odutayo A, Aronson R, Ezekowitz J, Parker JD. Sodium glucose cotransporter-2 inhibition and cardiorenal protection: JACC review topic of the week. J Am Coll Cardiol. 2019;74:2511-2524. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes A. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S124-S138. [DOI] [PubMed] [Google Scholar]
- 10. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [DOI] [PubMed] [Google Scholar]
- 11. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306. [DOI] [PubMed] [Google Scholar]
- 12. Diabetes Canada Clinical Practice Guidelines Expert Committee, Lipscombe L, Booth G, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018;42(suppl 1):S88-S103. [DOI] [PubMed] [Google Scholar]
- 13. O’Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36(2):159-169. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. [DOI] [PubMed] [Google Scholar]
- 15. Rajasekeran H, Kim SJ, Cardella CJ, et al. Use of canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: a case series. Diabetes Care. 2017;40(7):e75-e76. [DOI] [PubMed] [Google Scholar]
- 16. Halden TAS, Kvitne KE, Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care. 2019;42(6):1067-1074. [DOI] [PubMed] [Google Scholar]
- 17. Hecking M, Jenssen T. Considerations for SGLT2 inhibitor use in post-transplantation diabetes. Nat Rev Nephrol. 2019;15(9):525-526. [DOI] [PubMed] [Google Scholar]
- 18. Diabetes Canada Clinical Practice Guidelines Expert Committee, McGibbon A, Adams L, Ingersoll K, Kader T, Tugwell B. Glycemic management in adults with type 1 diabetes. Can J Diabetes. 2018;42(suppl 1):S80-S87. [DOI] [PubMed] [Google Scholar]
- 19. van Raalte DH, Bjornstad P, Persson F, et al. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care. 2019;42(10):1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neuen BL, Cherney DZ, Jardine MJ, Perkovic V. Sodium-glucose cotransporter inhibitors in type 2 diabetes: thinking beyond glucose lowering. CMAJ. 2019;191:E1128-E1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boeder S, Edelman SV. Sodium-glucose co-transporter inhibitors as adjunctive treatment to insulin in type 1 diabetes: a review of randomized controlled trials. Diabetes Obes Metab. 2019;21(suppl 2):62-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J, Tang L, Meng H, Zhao J, Liang Y. Effects of sodium-glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2019;35(7):e3169. [DOI] [PubMed] [Google Scholar]
- 23. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardio-vascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol;5:709-717. [DOI] [PubMed] [Google Scholar]
- 24. Yokote K, Terauchi Y, Nakamura I, Sugamori H. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17(15):1995-2003. [DOI] [PubMed] [Google Scholar]
- 25. Diabetes Canada Clinical Practice Guidelines Expert Committee, Feig DS, Berger H, et al. Diabetes and pregnancy. Can J Diabetes. 2018;42(suppl 1):S255-S282. [DOI] [PubMed] [Google Scholar]
- 26. Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab. 2019;21(suppl 2):34-42. [DOI] [PubMed] [Google Scholar]
- 27. Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium-glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: a population-based cohort study. Ann Intern Med. 2019;171:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menne J, Dumann E, Haller H, Schmidt BMW. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. Plos Med. 2019;16:e1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diaz-Ramos A, Eilbert W, Marquez D. Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitor use: a case report and review of the literature. Int J Emerg Med. 2019;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care. 2017;40(11):1479-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845-854. [DOI] [PubMed] [Google Scholar]
- 32. Iskander C, Cherney D, Clemens K, et al. Use of sodium–glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192:E351-1E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098-2107. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Fagbote CO, Zhuo M, Hawley CE, Paik JM. Sodium-glucose cotransporter 2 inhibitors for diabetic kidney disease: a primer for deprescribing. Clin Kidney J. 2019;12:620-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Triantafylidis LK, Hawley CE, Fagbote C, Li J, Genovese N, Paik JM. A Pilot Study Embedding Clinical Pharmacists Within an Interprofessional Nephrology Clinic for the Initiation and Monitoring of Empagliflozin in Diabetic Kidney Disease. J Pharm Pract 2019:897190019876499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752-772. [DOI] [PubMed] [Google Scholar]
- 37. Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35:274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]