Abstract
Collagen signaling is critical for proper bone and tooth formation. Discoidin domain receptor 2 (DDR2) is a collagen-activated tyrosine kinase receptor shown to be essential for skeletal development. Patients with loss of function mutations in DDR2 develop spondylo-meta-epiphyseal dysplasia (SMED), a rare, autosomal recessive disorder characterized by short stature, short limbs, and craniofacial anomalies. A similar phenotype was observed in Ddr2-deficient mice, which exhibit dwarfism and defective bone formation in the axial, appendicular, and cranial skeletons. However, it is not known if Ddr2 has a role in tooth formation. We first defined the expression pattern of Ddr2 during tooth formation using Ddr2-LacZ knock-in mice. Ddr2 expression was detected in the dental follicle/sac and dental papilla mesenchyme of developing teeth and in odontoblasts and the periodontal ligament (PDL) of adults. No LacZ staining was detected in wild-type littermates. This Ddr2 expression pattern suggests a potential role in the tooth and surrounding periodontium. To uncover the function of Ddr2, we used Ddr2slie/slie mice, which contain a spontaneous 150-kb deletion in the Ddr2 locus to produce an effective null. In comparison with wild-type littermates, Ddr2slie/slie mice displayed disproportional tooth size (decreased root/crown ratio), delayed tooth root development, widened PDL space, and interradicular alveolar bone defects. Ddr2slie/slie mice also had abnormal collagen content associated with upregulation of periostin levels within the PDL. The delayed root formation and periodontal abnormalities may be related to defects in RUNX2-dependent differentiation of odontoblasts and osteoblasts; RUNX2-S319-P was reduced in PDLs from Ddr2slie/slie mice, and deletion of Ddr2 in primary cell cultures from dental pulp and PDL inhibited differentiation of cells to odontoblasts or osteoblasts, respectively. Together, our studies demonstrate odontoblast- and PDL-specific expression of Ddr2 in mature and immature teeth, as well as indicate that DDR2 signaling is important for normal tooth formation and maintenance of the surrounding periodontium.
Keywords: collagen receptor, periodontal tissues, alveolar bone, cell differentiation, root formation, collagen fiber
Introduction
During tooth development, cell–extracellular matrix (ECM) interactions play an important role in cell adhesion, proliferation, differentiation, and formation of dental structures (Thesleff et al. 1989; Fukumoto and Yamada 2005; Chen et al. 2009). Dysregulation of proteins mediating ECM interactions is implicated in several deformities affecting tooth structure and function (Mardh et al. 2002; Umemoto et al. 2012). Type I collagen is the core structural protein in the tooth dentin and bone where collagen-mediated signaling is critical for tissue differentiation and mineralization (Thesleff and Hurmerinta 1981; Andujar et al. 1991; Nicholls et al. 1996). Type I collagen organized into fiber bundles in periodontal ligaments (PDLs) is important for tooth function during masticatory loads, proprioception, and regulation of alveolar bone volume (Andujar et al. 1991; McCulloch et al. 2000). Defects in PDL collagen fibers contribute to periodontal diseases, alveolar bone resorption, and tooth loss (Uitto and Larjava 1991). Therefore, proper collagen signaling is essential for development and maintenance of dental and periodontal tissues.
Two classes of receptors mediate cell-collagen interactions: β1-integrins and the discoidin domain receptors, DDR1 and DDR2 (Leitinger 2011). Unlike integrins, DDRs have intrinsic tyrosine kinase activity and are selectively activated by triple-helical collagens (Shrivastava et al. 1997; Vogel et al. 1997). In addition, DDR1 preferentially binds to and is activated by type IV collagen while DDR2 is strongly activated by type I and III collagens found primarily in bones and teeth (Shrivastava et al. 1997; Vogel et al. 1997). Unlike DDR1, which is found primarily in epithelia, DDR2 is exclusively expressed in mesenchyme (Alves et al. 1995). DDR2 is essential for skeletal development in humans and mice. Patients carrying loss-of-function mutations in DDR2 develop spondylo-meta-epiphyseal dysplasia (SMED), a rare, autosomal recessive disorder characterized by short stature, short limbs, and craniofacial anomalies (Bargal et al. 2009; Rozovsky et al. 2011; Mansouri et al. 2016). A similar phenotype is observed in Ddr2-deficient mice and associated with impaired postnatal bone growth, osteoblast differentiation, chondrocyte proliferation, and maturation (Labrador et al. 2001; Zhang et al. 2011; Ge et al. 2016). While these studies uncovered a critical role for Ddr2 in postnatal skeletal bone development, possible functions of Ddr2 in teeth and associated structures have not been previously examined.
In this study, we define the temporospatial expression of Ddr2 in dentoalveolar tissues and investigate the functional importance of DDR2 during postnatal development of teeth and surrounding periodontium using a Ddr2-deficient mouse model. These findings provide new insights into the role of cell-ECM interactions in the development of teeth and associated structures and may have important implications for understanding periodontal disease.
Materials and Methods
Mice
Generation and genotyping of Ddr2slie/slie mice and Ddr2-LacZ (Ddr2+/LacZ) knock-in mice were previously described (Ge et al. 2018). Ddr2fl/fl mice in which exon 8 of the Ddr2 gene is flanked by 2 LoxP sequences were generated from “knockout-first” ES cell clone Ddr2tm1a(EUCOMM)Wtsi (EPD0607__B01; European Mutant Mouse Repository) as described in Appendix Figure 1. All mouse experimental procedures conformed to standards for the use of laboratory animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan. This study conforms to the Animal Research: Reporting In Vivo Experiments (ARRIVE) Guidelines.
Detection of β-gal (Lacz) Expression
Samples dissected from heterozygous Ddr2-LacZ (Ddr2+/LacZ) mice were processed for X-gal staining using standard procedures. Frozen sections were stained with freshly prepared X-gal solution and counterstained with Vector Nuclear Fast Red.
Tissue Preparation and Histological Analysis
Whole skulls were fixed in 4% paraformaldehyde and processed for paraffin sections. For immunofluorescence, histological sections were incubated with rabbit antiperiostin polyclonal antibody (ab14041; Abcam) and rabbit anti–RUNX2-S319-P (generated in the project laboratory) (Ge et al. 2012) overnight at 4°C, and then with Alexa Fluor 488–conjugated donkey anti–rabbit IgG (A21206; Invitrogen). For Picro Sirius Red (PSR) staining, paraffin sections were processed as previously described (Coleman 2011) and visualized under polarizing light using an Olympus BX51-P microscope.
Micro–Computed Tomography Analysis of Bone
After fixation in 10% formalin (Fisher), skulls were subjected to micro–computed tomography (CT) analysis using a Scanco Model 100 (Scanco Medical). Scan settings were as follows: voxel size of 12 µm, 70 kVp, 114 µA, 0.5-mm aluminum filter, and integration time of 500 ms. Tooth measurements were made on first molars unless stated otherwise. To avoid examiner bias, the genotype of mice was not specifically highlighted during quantification analysis.
Cell Proliferation and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
For proliferation assays, 2-wk-old mice were intraperitoneally injected with 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen) and sacrificed 4 h after injection. Cells incorporating EdU were detected using the Click-iT EdU Alexa Fluor 488 Imaging Kit (C10337; Invitrogen). For terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays, sections were incubated in TUNEL reaction mixture according to the manufacturer instructions (Calbiochem).
Cell Culture, Transfection, and In Vitro Differentiation
Periodontal ligament (PDL) and dental pulp stromal cells (DPSCs) were isolated as described (Balic and Mina 2010; see Appendix Fig. 5). The adenoviruses Ad5-CMV-LacZ (AdLacZ) and Ad5-CMV-CRE (AdCre), at a titer of 1 × 1011 pfu/mL, were obtained from the Vector Core, University of Michigan, and used at a multiplicity of infection of 100 particles/cell. Viral transduction did not affect cell viability, cell number, or RNA yield (results not shown). Cells were stimulated for 21 d in α–minimum essential medium/10% fetal bovine serum containing 50 µg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone (DPSCs only). For gene expression analysis, total RNA (mRNA) was isolated using TRIzol (Invitrogen), complementary DNA (cDNA) was synthesized, and quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed on the following mRNAs: Runx2, Col1a1, Ibsp, Bglap, and Postn.
Statistical Analysis
All data were analyzed using the GraphPad Prism software version 6.0e (GraphPad Software). Values were reported as mean ± SD. Student’s t test was used for statistical comparison between the 2 experimental groups. Differences were considered significant at P < 0.05. A sample size of 5 to 7 mice (including both sexes) was used for experiments except where indicated. Cell culture experiments were done in triplicate and repeated at least twice.
Results
Ddr2 Is Highly Expressed in Dentin-Forming Odontoblasts, PDL Fibroblasts, and Alveolar Osteoblasts
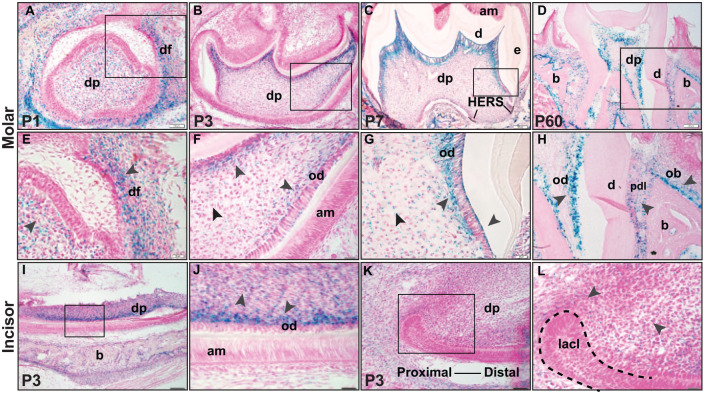
As a prerequisite to examining possible Ddr2 functions in teeth and associated structures, we examined its spatiotemporal expression pattern using a Ddr2-lacZ reporter mouse line. In this mouse, the bacterial LacZ gene is regulated by the endogenous Ddr2 locus (Ge et al. 2018). Ddr2-LacZ expression assessed by X-gal staining was detected in developing and mature teeth of Ddr2+/LacZ mice at postnatal days 1 (P1), 3 (P3), 7 (P7), and 60 (P60) (Fig. 1). However, no X-gal staining was shown in wild-type (WT) littermates (Appendix Fig. 2). At P1, X-gal staining was predominately found in the dental papilla mesenchyme and surrounding dental follicle, which gave rise to cementum, PDL, and alveolar bone (Fig. 1A, magnified inset in Fig. 1E). As the tooth continued to form, strong X-gal staining was observed in dentin-forming odontoblasts of the coronal dental pulp and in differentiating odontoblasts of developing molar roots at P3 and P7 (Fig. 1B, C, magnified insets in Fig. 1F, G). Weak X-gal staining was found in mesenchymal cells around Hertwig’s epithelial root sheath (HERS) of developing molar roots (Fig. 1C). Similar to molars, the mouse incisor also showed strong X-gal staining in dentin-forming odontoblasts along the incisor length with less evident staining in the proximal mesenchyme around the labial cervical loop at the incisor apex (Fig. 1I, K, magnified insets in Fig. 1J, L). Consistent with the exclusive mesenchymal expression of Ddr2, no detectable X-gal staining was seen in dental epithelial cells such as HERS and enamel-secreting ameloblasts. In addition to odontoblast-specific expression, Ddr2-LacZ expression was also evident in PDL-forming cells or fibroblastic cells aligned along PDL collagen fibers and in putative alveolar bone–associated osteoblasts (Fig. 1D, H). The odontoblast- and PDL-specific pattern of Ddr2 expression was maintained after the completion of tooth formation and eruption into the oral cavity (Fig 1D, H). From these results, we conclude that Ddr2 is expressed throughout tooth and PDL development and suggest it may have important functions in these tissues.
Figure 1.
Localization and expression of Ddr2 in dental and periodontal tissues. (A–H) X-gal staining of Ddr2+/LacZ mice reveals Ddr2 expression in the molars during development through adulthood. (A) At P1, Ddr2 expression is observed in dental papilla (dp) and the surrounding dental follicle (df) in developing molars (high magnification in E). (B–D) Ddr2-LacZ expression is selectively high in dentin-producing odontoblasts (od) at P3, P7, and P60. (F, G) High magnification also reveals Ddr2 expression in odontoblasts (od) and a subset of cells in dental pulp (dp). In a fully erupted molar (D, H), Ddr2 is expressed in PDL cells (pdl) and putative alveolar bone–associated osteoblasts (ob). Black arrowheads point to the expression sites. (I–L) Ddr2 expression in odontoblasts (od) of lower incisors, in dental pulp (dp), and around the labial cervical loop (lacl) at the proximal end of the incisor (dashed lines). (J, L) Higher magnification of boxed areas. No Ddr2 expression was seen in molar (F) or incisor (J) ameloblasts (am). Scale bar: 100 µm in A–D, I, K; 20 µm in E–H, J, L.
Ddr2 Is Important for Optimal Development of the Molar Root and Periodontium
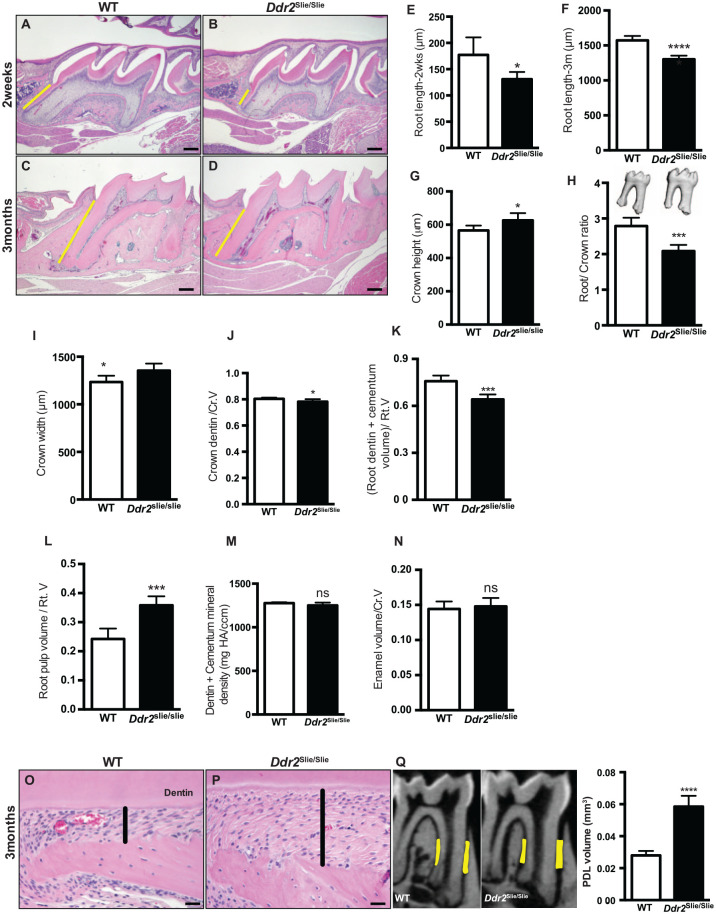
To investigate the functional role of Ddr2 in tooth formation, we used Ddr2slie/slie mice, which contain a spontaneous 150-kb deletion in the Ddr2 locus, resulting in an effective null (Kano et al. 2008). Our results showed that molar roots in Ddr2slie/slie mice were shorter than in their WT littermates (Fig. 2A–F). The mean root length (cementum-enamel junction [CEJ] to root tip) in Ddr2slie/slie mice was reduced by 26% at 2 wk and by 17% at 3 mo of age, suggesting delayed root formation. In addition, crown height (cusp tip to CEJ) and crown width both increased by approximately 11% in 3-mo-old Ddr2slie/slie mice (Fig. 2G, I). When linear tooth measurements were normalized by expressing data as root length/crown height (root/crown ratio), an overall 25% decrease was observed in Ddr2slie/slie mice (Fig. 2H).
Figure 2.
Tooth and periodontal phenotypes in Ddr2-deficient mice. (A–D) Representative hematoxylin and eosin (H&E) staining of first molars showing delayed root formation (yellow lines) in developing (A, B) and adult teeth (C, D) compared with wild-type (WT). (E, F) Quantification of the molar root length at 2 wk and 3 mo of age. (G–I) Quantification of the crown height, root/crown ratio, and crown width in 3-mo-old mice. (J–N) Quantification of the crown dentin volume/total crown volume (Cr.V), root dentin + cementum volume/total root volume (Rt.V), root pulp volume/(Rt.V), dentin + cementum mineral density, and enamel volume/(Cr.V). (O, P) H&E staining shows wide periodontal ligament (PDL) spacing (highlighted in black lines) in Ddr2slie/slie mice compared with WT. (Q) Micro–computed tomography (CT) analysis of PDL spacing in WT and Ddr2slie/slie mice. Region of interest is shown in yellow. Quantitation of micro-CT data (right). *P < 0.05. ***P < 0.001. ****P < 0.0001. ns, not significant. n = 5–7 mice/group. Scale bar: 50 µm in A, B; 200 µm in C, D; 20 µm in O, P.
Crown and root compositional changes were also seen in Ddr2slie/slie molars. These included a 3% decrease in crown dentin/total crown volume (Fig. 2J), a 15% decrease in the mineralized portion of the root (dentin + cementum volume/total root volume, Fig. 2K), and a 46% increase in root pulp volume/total root volume (Fig. 2L). No differences were observed in dentin + cementum mineral density or enamel/total crown volume (Fig. 2M, N). While incisor teeth were shorter in Ddr2slie/slie mice, no differences were observed in enamel volume and mineral density (Appendix Fig. 3). In addition to the tooth phenotypes, Ddr2slie/slie teeth exhibited a widened PDL space around molar roots (Fig. 2O, P). This was quantified by micro-CT analysis in 3-mo-old mice, which showed that the PDL space adjacent to molars of Ddr2slie/slie animals was twice the width of WT controls (Fig. 2Q, right). From these studies, we conclude that DDR2 signaling is required for optimal tooth morphogenesis and periodontal structure.
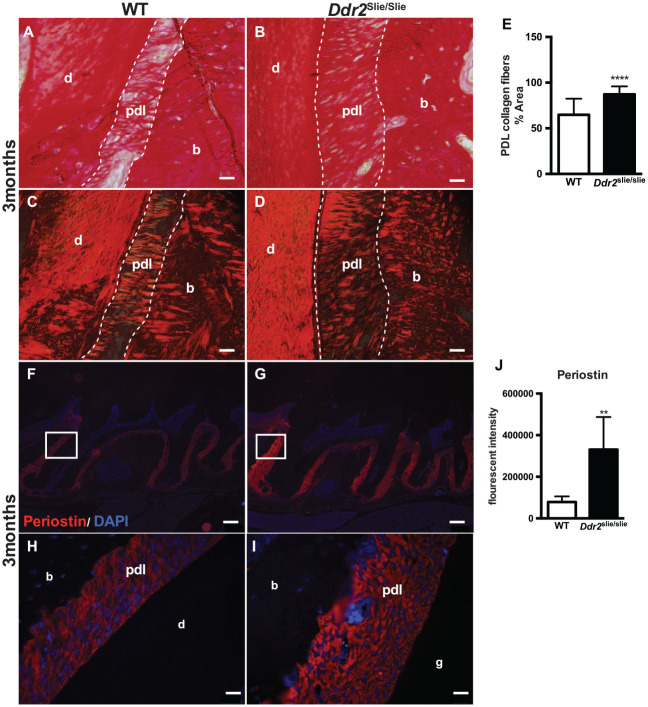
Ddr2 Is Necessary for Proper PDL Collagen Organization
In addition to the wide PDL space, collagen fiber organization was abnormal in PDLs of Ddr2slie/slie teeth. For detailed analysis, we used PSR staining to visualize type I and III collagen fibers under bright-field and polarized light microscopy. This allowed us to assess collagen content, maturation, and polarization (Fig. 3A–D). PSR staining, quantified using NIH ImageJ software, revealed an increase (34%) in PDL collagen area in 3-mo-old Ddr2slie/slie mice compared with WT littermates (Fig. 3E). The data are presented as a percentage of collagen area to total area assessed using the thresholding function of ImageJ. Sharpey’s fiber insertions into alveolar bone were also less evident in Ddr2slie/slie mice. Periostin is an abundant noncollagenous matricellular protein in the PDL matrix that binds type I collagen and is necessary for maintenance of periodontal health (Rios et al. 2008). We conducted immunofluorescence staining to determine if Ddr2 status affected levels of this important PDL component. Periostin immunoreactivity was increased in PDLs of Ddr2slie/slie mice (320% increase, Fig. 3J) and was specifically localized in the PDL space surrounding the molar roots (Fig. 3F, G and magnified insets in Fig. 3H, I). These findings suggest that DDR2 signaling may directly or indirectly regulate periostin expression and thus is important for periodontal integrity and function. Together, our data suggest that DDR2 signaling may determine the amount or integrity of collagen matrix required to maintain periodontal health.
Figure 3.
Ddr2slie/slie teeth had atypical periodontal collagen fibers. (A–D) Picro Sirius Red (PSR) staining of periodontal ligament (PDL) collagen fibers in 3-mo-old mice under bright-field (A, B) and polarizing (C, D) microscopy. (E) Quantification of the PDL collagen area stained with PSR, n = 3 mice, 6 measurements per mouse. (F–I) Immunostaining of periostin (red) with inserts (H, I) showing periostin expression in the PDL space surrounding the molar roots. The cell nuclei were stained with DAPI (blue). (J) Quantification of the fluorescent intensity of periostin immunostaining, n = 3 mice, 5 measurements per mouse. **P < 0.01. ****P < 0.000. Scale bar: 200 µm in F, G; 20 µm in A–D, H, I.
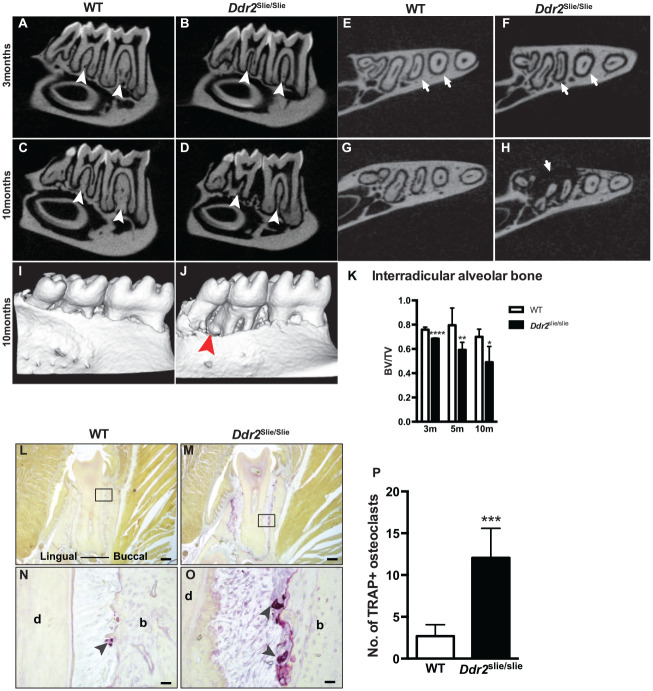
Ddr2 Deficiency Is Associated with Progressive Alveolar Bone Loss
To explore the role of Ddr2 in alveolar bone, micro-CT and MicroView software (Parallax Innovations) were used to assess bone parameters such as bone volume and bone volume fraction. Ddr2slie/slie mice displayed a 10% reduction in interradicular alveolar bone volume at 3 mo that progressively increased as mice aged (Fig. 4A–D, K). With age, Ddr2slie/slie mice showed a progressive bone loss of about 26% after 5 mo and 30% after 10 mo (Fig. 4K). Transverse micro-CT views showed a widened PDL space and severe bone defects around the molar roots (Fig. 4E–H, arrows). However, no sign of spontaneous tooth loss was observed at any of the ages examined. Three-dimensional reconstruction of the lower jaw of 10-mo-old animals clearly showed severe alveolar bone loss in Ddr2slie/slie mice compared with WT (Fig. 4I, J). Given the significant loss of alveolar bone volume, we performed tartrate-resistant acid phosphatase (TRAP) staining to assess whether Ddr2 knockout affected osteoclast activity in the supporting alveolar bone. Our results revealed a significant increase in osteoclast numbers in 10-mo-old Ddr2slie/slie mice (Fig. 4L–P), suggesting that increased osteoclast activity contributes to age-dependent alveolar bone resorption in knockout mice.
Figure 4.
Progressive alveolar bone loss in Ddr2 knockout. (A–H) Representative micro–computed tomography (CT) images showing thin interradicular alveolar bone (white arrowheads) and wide periodontal ligament (PDL) spacing (white arrows) in Ddr2slie/slie mice at 3 mo (B, F) and 10 mo (D, H) of age compared with wild-type (WT). (I, J) Three-dimensional reconstruction of the lower jaw section showing severe bone defects (J, red arrowhead) around lower teeth at 10 mo of age. (K) Micro-CT analysis of bone volume/total volume of interradicular alveolar bone of first and second molars (n = 5–7 mice/group). (L–O) Representative tartrate-resistant acid phosphatase (TRAP) staining images showing osteoclasts (purple color, arrowheads in N–O) along the alveolar bone surface at 10 mo of age. (P) Shows quantification of osteoclast numbers on frontal sections of the second molar alveolar bone (n = 4 mice). *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001. Scale bar: 200 µm in L, M; 20 µm in N, O.
Ddr2 Signaling Regulates Differentiation Potential of Osteogenic and Odontoblastic Lineages of PDL and Dental Pulp Cells, Respectively
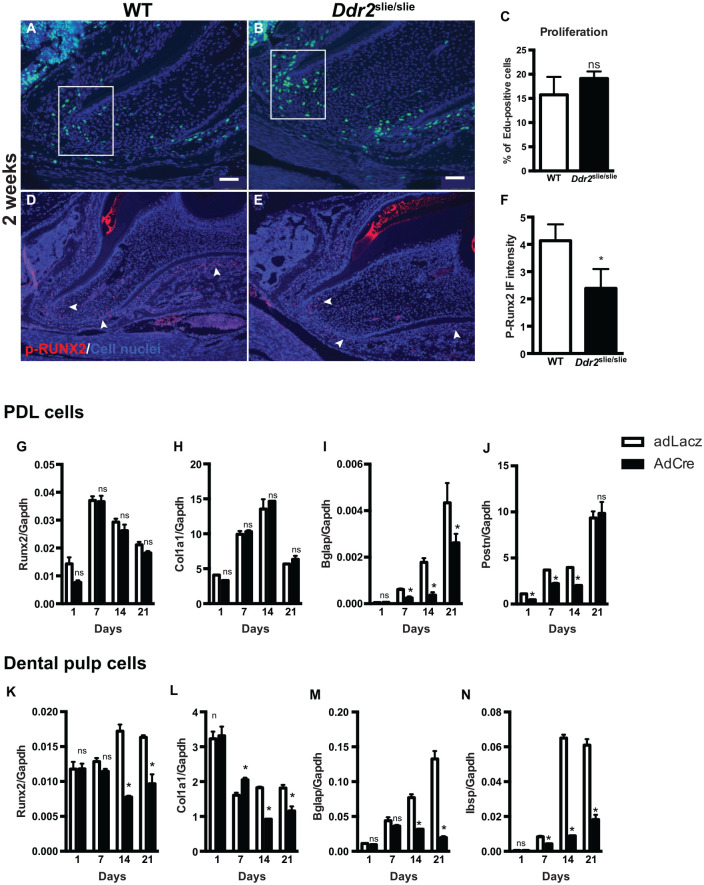
To further understand the function of Ddr2, we sought to elucidate its effects on cellular activities involved in root formation such as proliferation, differentiation, and apoptosis. To measure proliferation, we administered EdU to 2-wk-old Ddr2slie/slie mice, and the number of EdU+ cells was counted and reported as a percentage of EdU+ cells to the total cells (stained blue with DAPI). Our analysis of the first molars revealed positive EdU staining in PDL and mesenchymal cells surrounding the HERS of developing molar roots in Ddr2slie/slie and WT mice (Fig. 5A–C). However, there was no significant difference in the percentage of EdU+ cells, suggesting the proliferation potential of dental cells in Ddr2slie/slie and WT teeth was not affected. In addition, no change in the number of apoptotic cells was seen between Ddr2slie/slie and WT, as measured by TUNEL staining along the root dentin odontoblast and PDL regions (Appendix Fig. 4).
Figure 5.
Ddr2 knockout exhibits decreased osteogenic and odontogenic differentiation of periodontal ligament (PDL) and dental pulp cells, respectively. (A, B) Representative images of EdU staining for labeling proliferating cells (green) and DAPI staining for cell nuclei (blue). (C) Quantification of the percentage of EdU-positive cells. (D, E) Representative images of p-RUNX2 immunostaining (red) in the nuclei of PDL cells and at the apex of the developing root. (F) Quantification of the fluorescent intensity of p-RUNX2 immunostaining, n = 3 mice. (G–J) Quantification of differentiation markers for PDL cells. (K–N) Quantification of differentiation markers for dental pulp stromal cells after 21 d. *P < 0.05. ns, not significant. n = 3 cell cultures/group. Scale bar: 50 µm in A, B; 100 µm in D, E.
To assess the influence of Ddr2 on the differentiation potential of relevant tooth-associated cells, we examined phosphorylation of RUNX2 at Serine 319, which at least in part mediates the effect of DDR2 on osteogenesis (Ge et al. 2016). Immunostaining of p-RUNX2 showed nuclear staining mostly in PDLs and mesenchymal cells around HERS at the apex of the developing roots (Fig. 5D, E). Ddr2slie/slie teeth showed reduced levels of RUNX2 phosphorylation compared with WT controls (Fig. 5D–F), suggesting that RUNX2 activity mediates DDR2 regulation of root development. We also generated cultures of incisor PDL cells (PDLs) and DPSCs from 3-mo-old Ddr2flox/flox mice and examined differentiation after treatment with either AdLacZ or AdCre (Appendix Fig. 5A). PCR analysis of DNA from cultured PDLs and DPSCs treated with AdCre confirmed the deletion of the floxed Ddr2 allele with generation of an approximately 0.7-kb PCR product (Appendix Fig. 5B). In addition, Ddr2 mRNA levels were reduced by >80%. Control cultures of both cell types mineralized and expressed typical osteoblast/odontoblast/PDL cell markers such as Runx2, Col1a1, Bglap, Ibsp, and Postn. For both cell types, Ddr2 loss dramatically reduced differentiation as measured by either expression of the differentiation markers, Bglap, Ibsp, and Postn (Fig. 5I, M, N) or mineralization (von Kossa staining) (Appendix Fig. 5C). Partial reduction of Runx2 and Col1a1 expression was also noted in dental pulp cultures at later culture times (Fig. 5K, L). Taken together, these results show a clear requirement for Ddr2 in the differentiation of PDL and dental pulp cells that may explain observed tooth and alveolar bone defects.
Discussion
Type I collagen, the core structural protein in tooth dentin, PDL, and alveolar bone, plays an important role during tissue differentiation and mineralization (Thesleff and Hurmerinta 1981; Andujar et al. 1991; Nicholls et al. 1996). Previous studies largely focused on collagen-binding integrins as mediators of the response to this ECM component. However, bone-selective knockout of individual integrins resulted in relatively mild phenotypes (Gardner et al. 1996; Holtkotter et al. 2002; Bengtsson et al. 2005), suggesting the involvement of other collagen receptors. DDR2, a nonintegrin collagen-activated receptor tyrosine kinase, is critical for bone growth and development. The aim of this study was to examine the function of Ddr2 in development of the tooth and associated periodontium.
Using LacZ staining, we localized Ddr2 expression in developing and adult fully erupted teeth. Ddr2 was expressed in undifferentiated cells in the dental papilla mesenchyme and subsequently in the dentin-forming odontoblasts and dental pulp. Ddr2 was also highly expressed in the dental follicles during tooth development and in PDL and alveolar bone of adult teeth. This Ddr2 distribution suggests potential functions in the formation of teeth and periodontium. However, the identity of Ddr2-expressing cells in the PDL needs to be determined.
Our analysis of Ddr2-deficient mice indicates that proper DDR2 signaling is required for optimal tooth morphogenesis and periodontal integrity. Ddr2slie/slie mice had short tooth roots and decreased root/crown ratio, resulting in disproportionate tooth size. BMP/TGF-β and Wnt/β-catenin pathways play a critical role in tooth root formation, and their disruption could cause a short root phenotype (Wang and Feng 2017). However, we do not know if either of these pathways is involved in DDR2 signaling. Mechanistically, our analysis suggests that RUNX2 phosphorylation mediates Ddr2 regulation of root development as it does in bone (Zhang et al. 2011; Ge et al. 2016). In addition to the tooth phenotype, Ddr2-knockout mice exhibited a progressive alveolar bone loss first seen in the interradicular bone of 3-mo-old mice and most dramatic after 10 mo. This was accompanied by a dramatic increase in osteoclast numbers. This result was somewhat surprising in view of our previous work, which failed to detect any changes in osteoclast differentiation, tibial osteoclast surface, or serum resorption markers in Ddr2-deficient mice (Ge et al. 2016). These discrepancies may be explained by the different bone regions being examined. For example, it is possible that the altered PDL structure seen with Ddr2 deficiency (Fig. 3) may lead to changes in mechanical stability of the tooth accompanied by increased osteoclast-mediated bone resorption. Changes in periodontal structure could be a primary effect of Ddr2 deficiency or be secondary to defects in alveolar bone formation due to impaired osteogenic differentiation of Dd2-deficient PDL cells. This would be consistent with the requirement for Ddr2 in osteoblast differentiation of bone progenitors previously reported (Zhang et al. 2011; Ge et al. 2016). A direct demonstration that Ddr2 specifically functions in PDL or alveolar bone in vivo will require cell-specific conditional knockout studies.
We also observed a widening of the PDL space in Ddr2-deficient mice; however, there was no evidence of PDL widening before tooth eruption (data not shown), suggesting that the PDL changes may require posteruption occlusal loading. One interpretation of this observation is that loss of Ddr2 may compromise PDL structure, which then fails when exposed to occlusal loads, leading to PDL widening. Interestingly, there are anecdotal reports of tooth abnormalities in some SMED patients, although it is not known if the specific changes in tooth or PDL structure that we described in mice were present (Bargal et al. 2009).
Consistent with its effect on the periodontium, Ddr2slie/slie teeth also had atypical PDL collagen fibers associated with a significant increase in periostin immunostaining. However, periostin mRNA levels were significantly reduced in Ddr2-deficient PDL cells. This inverse correlation between periostin mRNA and protein is not understood. Periostin, a collagen-binding protein, is required to maintain PDL integrity and for proper collagen fibril formation and maturation (Rios et al. 2008). Aberrant PDL collagen fibers in Ddr2 knockout could be due to impaired collagen fiber formation, crosslinking, and/or maturation. A possible role of Ddr2 in collagen crosslinking was previously demonstrated; these studies showed that Ddr2 activation with type I collagen induces lysyl oxidase, the enzyme required for ECM crosslinking (Khosravi et al. 2014). Abnormalities in collagen deposition and/or crosslinking have been reported in Ddr2-deficient heart and postnatal testicular tissues (Cowling et al. 2014; Zhu et al. 2015). Further studies will be required to resolve if Ddr2 also controls collagen fibrillogenesis in dental structures.
Author Contributions
F.F. Mohamed, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; C. Ge, A. Binrayes, contributed to data acquisition, critically revised the manuscript; R.T. Franceschi, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519892563 for The Role of Discoidin Domain Receptor 2 in Tooth Development by F.F. Mohamed, C. Ge, A. Binrayes and R.T. Franceschi in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by a scholarship from the Ministry of Higher Education and Scientific Research, Libyan Transitional Government (F.F. Mohamed), a scholarship from King Saud University (A. Binrayes), National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research grant DE11723, and research funds from the Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry (to R.T. Franceschi) and the Michigan Musculoskeletal Health Core Center (NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases P30 AR069620).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. 1995. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 10(3):609–618. [PubMed] [Google Scholar]
- Andujar MB, Couble P, Couble ML, Magloire H. 1991. Differential expression of type I and type III collagen genes during tooth development. Development. 111(3):691–698. [DOI] [PubMed] [Google Scholar]
- Balic A, Mina M. 2010. Characterization of progenitor cells in pulps of murine incisors. J Dent Res. 89(11):1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen DH, Smithson SF, Borochowitz Z, Belostotsky R, et al. 2009. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet. 84(1):80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T, Aszodi A, Nicolae C, Hunziker EB, Lundgren-Akerlund E, Fassler R. 2005. Loss of alpha10beta1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J Cell Sci. 118(Pt 5):929–936. [DOI] [PubMed] [Google Scholar]
- Chen B, Goodman E, Lu Z, Bandyopadhyay A, Magraw C, He T, Raghavan S. 2009. Function of beta1 integrin in oral epithelia and tooth bud morphogenesis. J Dent Res. 88(6):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. 2011. Picrosirius red staining revisited. Acta Histochem. 113(3):231–233. [DOI] [PubMed] [Google Scholar]
- Cowling RT, Yeo SJ, Kim IJ, Park JI, Gu Y, Dalton ND, Peterson KL, Greenberg BH. 2014. Discoidin domain receptor 2 germline gene deletion leads to altered heart structure and function in the mouse. Am J Physiol Heart Circ Physiol. 307(5):H773–H781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Yamada Y. 2005. Review: Extracellular matrix regulates tooth morphogenesis. Connect Tissue Res. 46(4–5):220–226. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. 1996. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 175(2):301–313. [DOI] [PubMed] [Google Scholar]
- Ge C, Mohamed F, Binrayes A, Kapila S, Franceschi RT. 2018. Selective role of discoidin domain receptor 2 in murine temporomandibular joint development and aging. J Dent Res. 97(3):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Wang Z, Zhao G, Li B, Liao J, Sun H, Franceschi RT. 2016. Discoidin receptor 2 controls bone formation and marrow adipogenesis. J Bone Miner Res. 31(12):2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT. 2012. Interactions between extracellular signal-regulated kinase 1/2 and p38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res. 27(3):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. 2002. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 277(13):10789–10794. [DOI] [PubMed] [Google Scholar]
- Kano K, Marin de, Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. 2008. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, DDR2, in the mouse. Mol Endocrinol. 22(8):1866–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi R, Sodek KL, Faibish M, Trackman PC. 2014. Collagen advanced glycation inhibits its discoidin domain receptor 2 (DDR2)–mediated induction of lysyl oxidase in osteoblasts. Bone. 58:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, et al. 2001. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2(5):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B. 2011. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 27:265–290. [DOI] [PubMed] [Google Scholar]
- Mansouri M, Kayserili H, Elalaoui SC, Nishimura G, Iida A, Lyahyai J, Miyake N, Matsumoto N, Sefiani A, Ikegawa S. 2016. Novel DDR2 mutation identified by whole exome sequencing in a moroccan patient with spondylo-meta-epiphyseal dysplasia, short limb-abnormal calcification type. Am J Med Genet A. 170A(2):460–465. [DOI] [PubMed] [Google Scholar]
- Mardh CK, Backman B, Holmgren G, Hu JC, Simmer JP, Forsman-Semb K. 2002. A nonsense mutation in the enamelin gene causes local hypoplastic autosomal dominant amelogenesis imperfecta (AIH2). Hum Mol Genet. 11(9):1069–1074. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Lekic P, McKee MD. 2000. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000. 24:56–72. [DOI] [PubMed] [Google Scholar]
- Nicholls AC, Oliver J, McCarron S, Winter GB, Pope FM. 1996. Splice site mutation causing deletion of exon 21 sequences from the pro alpha 2(I) chain of type I collagen in a patient with severe dentinogenesis imperfecta but very mild osteogenesis imperfecta. Hum Mutat. 7(3):219–227. [DOI] [PubMed] [Google Scholar]
- Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. 2008. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 79(8):1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky K, Sosna J, Le Merrer M, Simanovsky N, Koplewitz BZ, Bar-Ziv J, Cormier-Daire V, Raas-Rothschild A. 2011. Spondyloepimetaphyseal dysplasia, short limb-abnormal calcifications type: progressive radiological findings from fetal age to adolescence. Pediatr Radiol. 41(10):1298–1307. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, et al. 1997. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1(1):25–34. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Hurmerinta K. 1981. Tissue interactions in tooth development. Differentiation. 18(2):75–88. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vainio S, Jalkanen M. 1989. Cell-matrix interactions in tooth development. Int J Dev Biol. 33(1):91–97. [PubMed] [Google Scholar]
- Uitto VJ, Larjava H. 1991. Extracellular matrix molecules and their receptors: an overview with special emphasis on periodontal tissues. Crit Rev Oral Biol Med. 2(3):323–354. [DOI] [PubMed] [Google Scholar]
- Umemoto H, Akiyama M, Domon T, Nomura T, Shinkuma S, Ito K, Asaka T, Sawamura D, Uitto J, Uo M, et al. 2012. Type vii collagen deficiency causes defective tooth enamel formation due to poor differentiation of ameloblasts. Am J Pathol. 181(5):1659–1671. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. 1997. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1(1):13–23. [DOI] [PubMed] [Google Scholar]
- Wang J, Feng JQ. 2017. Signaling pathways critical for tooth root formation. J Dent Res. 96(11):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Yu J, Bu X, Ren T, Liu X, Yao L. 2011. An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of runx2 activation. J Bone Miner Res. 26(3):604–617. [DOI] [PubMed] [Google Scholar]
- Zhu CC, Tang B, Su J, Zhao H, Bu X, Li Z, Zhao J, Gong WD, Wu ZQ, Yao LB, et al. 2015. Abnormal accumulation of collagen type i due to the loss of discoidin domain receptor 2 (DDR2) promotes testicular interstitial dysfunction. PLoS One. 10(7):e0131947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519892563 for The Role of Discoidin Domain Receptor 2 in Tooth Development by F.F. Mohamed, C. Ge, A. Binrayes and R.T. Franceschi in Journal of Dental Research