Abstract
Background:
Superficial non-ampullary duodenal epithelial tumors (SNADET) are increasingly found during upper endoscopy. Underwater endoscopic mucosal resection (UEMR) is an emerging technique for the endoscopic resection of SNADET. We performed a systematic review and meta-analysis to evaluate the efficacy and safety of this technique.
Methods:
We conducted a comprehensive search of several databases from inception to August 2019, which included Ovid Cochrane Database of Systematic Reviews, Ovid Embase, Scopus, Ovid Cochrane Central Register of Controlled trials, Ovid MEDLINE®, and In-Process and other non-indexed citations. The primary outcome assessed was the pooled clinical success rate of UEMR. Secondary outcomes included rate of en bloc resection, pooled rate of high-grade dysplasia or intramucosal carcinoma (HGIC), and pooled rate of adverse events. Meta-regression analysis was performed based on tumor size.
Results:
A total of 8 study arms were included for analysis with UEMR performed in a total of 258 lesions. The pooled clinical success rate was 89.9% (95% confidence interval [CI] 83.4-94.1). En-bloc removal was achieved in 84.6% of treated lesions (95%CI 75.5-90.7). The pooled rate of HGIC was 24.7% (95%CI 10.3-48.3). The pooled rate of adverse events was 6.9% (95%CI 2.5-17.9). This included 10 total adverse events, with the majority being self-limited delayed bleeding. There were no duodenal perforations.
Conclusions:
UEMR for endoscopic resection of SNADET has a high efficacy. In addition, this technique has a high rate of en bloc resection and an acceptable adverse event profile. Given these data, UEMR should be considered as a method for endoscopic resection of SNADET.
Keywords: Duodenum, non-ampullary adenoma, underwater endoscopic mucosal resection
Introduction
Superficial non-ampullary duodenal epithelial tumors (SNADET) are uncommonly encountered during upper endoscopy as opposed to ampullary tumors [1]. However, the incidence of non-ampullary adenomas and duodenal adenocarcinoma is increasing. A multicenter study conducted in Japan from 2007-2012 showed 396 SNADET resected lesions in 364 patients [2]. The incidence increased 2-fold over the study duration (from first to second half) and the incidence of duodenal adenocarcinoma increased 3-fold.
There is no standardized technique for endoscopic resection of SNADET. Endoscopic mucosal resection (EMR) with submucosal injection and endoscopic submucosal dissection (ESD) are commonly utilized. EMR has been shown to have a superior safety profile; however, this technique has the disadvantage of lower rates of complete initial resection, requiring multiple endoscopy sessions, and a recurrence rate of up to 37% [3]. In contrast, ESD has been shown to be superior regarding the rate of complete resection. However, ESD has limited experience in western countries and is associated with higher rates of perforation [4].
Underwater EMR (UEMR) is an emerging technique for endoscopic resection. This technique was first described by Binmoeller et al for mucosal resection of large colonic polyps [5]. UEMR for colonic polyps has been shown to be superior to traditional EMR with submucosal injection in terms of macroscopic resection and local recurrence [6]. In addition, subsequent studies have shown that UEMR has low rates of adverse events and self-limited delayed bleeding [7]. UEMR has been more recently described for the endoscopic resection of SNADET. The purpose of our study was to perform a systematic review and meta-analysis of the available literature to evaluate the efficacy and safety of UEMR for SNADET.
Materials and methods
Search strategy
We conducted a comprehensive search of several databases from inception to August 2019. An experienced medical librarian assisted with the literature search. The databases searched were as follows: Ovid Cochrane Database of Systematic Reviews, Ovid Embase, Scopus, Ovid Cochrane Central Register of Controlled trials, Ovid MEDLINE®, and In-Process and other non-indexed citations. Controlled vocabulary supplemented with keywords was used to search for studies of interest. The full search strategy is available in Appendix1. The PRISMA and MOOSE checklists were followed and are provided in Appendices 2 and 3 [8,9].
Study selection
In this meta-analysis, we included studies that evaluated the clinical outcomes of UEMR. Studies were included regardless of sample size, study setting or location, as long as the data needed for the analysis were available.
Our exclusion criteria were studies that had pediatric patients (age <18 years old) and studies not published in the English language. If there were multiple publications from the same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained.
Data abstraction and quality assessment
Outcomes data from each study were abstracted onto a standardized form by a minimum of 2 authors, and 2 authors independently completed quality scoring. The Newcastle-Ottawa scale for cohort studies was used to assess the quality of studies [10]. The details of this scoring system can be found in Supplementary Table 1.
Supplementary Table 1.
Study quality assessment
Outcomes assessed
1. Pooled rate of clinical success
2. Pooled rate of en bloc tumor removal
3. Pooled rate of high-grade dysplasia/intramucosal carcinoma (HGIC)
4. Pooled rate of adverse events
Meta-regression analysis based on the tumor size was also performed. Clinical success was defined in 7 studies as complete endoscopic resection without local recurrence on follow-up examination. A single study defined clinical success as complete endoscopic resection and did not have follow up.
Statistical analysis
The meta-analysis was carried out by calculating the pooled estimates following the methods suggested by DerSimonian and Laird. The random-effects model was used [11]. When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis [12]. Heterogeneity was assessed by using the Cochran Q statistical test for heterogeneity, 95% prediction interval (PI) [13-15], and the I2 statistics [16,17]. In the latter, values of <30%, 30-60%, 61-75% and >75% were considered to be of low, moderate, substantial and considerable heterogeneity, respectively [18]. Publication bias was ascertained qualitatively, by visual inspection of a funnel plot, and quantitatively, by the Egger test [19-21]. A P value of <0.05 was used a priori to define significance of differences between groups, as provided by the statistical software. All analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 3 (BioStat, Englewood, NJ).
Results
Search results and population characteristics
From an initial 69 studies, 33 records were screened and 25 full-length articles were assessed. Eight studies were included in the final analysis [22-29]. A schematic diagram of the study selection is provided in Supplementary Fig. 1 and the population characteristics are described in Table 1.
Supplementary Figure 1.

Study flow selection
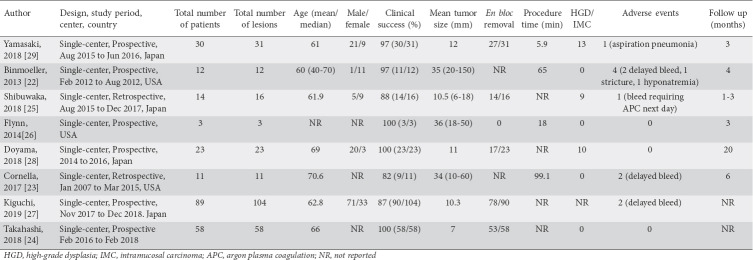
Table 1.
Study and population characteristics
Characteristics and quality of included studies
There were no multicenter or population based studies. Two studies had sample sizes >40. The detailed assessment is summarized in Supplementary Table 1. Overall, 7 studies were considered to be of medium-quality and one study of high-quality. There were no low-quality studies.
Meta-analysis outcomes
Demographics and clinical success
There were 258 SNADET resected using the UEMR technique in 240 patients. The median patient age was 64.5 years. The mean tumor size was 19.4 mm (6-150).
Clinical success was reported in 8 studies that evaluated 240 patients. The pooled clinical success rate was 89.9% (95%CI 83.4-94.1). The I2 heterogeneity was 13% with a 95% PI of 76-96.
En bloc removal was reported in 6 studies that evaluated 221 patients and the achieved pooled rate was 84.6% of treated lesions (95%CI 75.5-90.7), with an I2 heterogeneity of 44% and 95% PI of 55-96. HGIC lesions reported in 7 studies, evaluated 151 patients. The pooled rate of HGIC lesions was 24.7% (95%CI 10.3-48.3), with an I2 heterogeneity of 70% and 95% PI of 2-87.
Adverse events
Adverse events were reported in 8 studies that evaluated 240 patients. The pooled rate of adverse events was 6.9% (95%CI 2.5-17.9), with an I2 heterogeneity of 60% and 95% PI 1-63. This included 10 total adverse events, of which 9 were postoperative. There were no documented duodenal perforations. There were 7 patients with delayed bleeding. Of these patients, 6 were managed conservatively and 1 patient required endoscopic therapy. Other adverse outcomes included 1 patient with aspiration, 1 patient with postprocedural hyponatremia, and 1 patient who developed a duodenal stricture.
Analysis based on tumor size
A meta-regression analysis was performed based on tumor size and the result was not significant, with a 2-sided P value of 0.47. This was performed to assess if there was any difference in clinical success based on tumor size, and no significant difference was found. The pooled results are summarized in Table 2 and Supplementary Fig. 2-5.
Table 2.
Summary of pooled results

Supplementary Figure 2.

Forest plot, clinical success
Supplementary Figure 5.
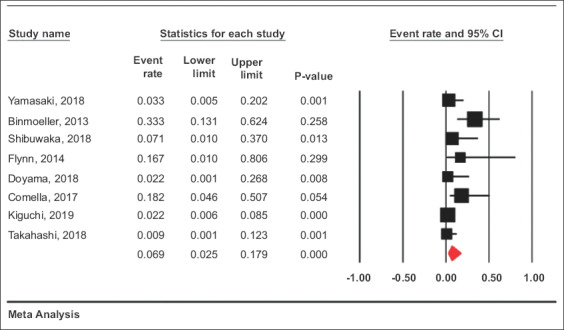
Forest plot, adverse events
Supplementary Figure 3.

Forest plot, en bloc removal
Supplementary Figure 4.

Forest plot, high-grade dysplasia / intramucosal carcinoma
Validation of meta-analysis results
Sensitivity analysis
We excluded each study, one at a time, and analyzed the effect on the main summary estimate. This was to assess whether any single study had a dominant effect on the meta-analysis outcome results. In this analysis, no single study significantly affected the outcome or the heterogeneity. This was performed for all outcomes.
Heterogeneity
We assessed the dispersion of the calculated rates using the PI and I2 percentage values. The PI gives an idea of the range of the dispersion and I2 tell us what proportion of the dispersion is true vs. chance [15]. The calculated PIs are reported with the pooled results in Table 2. The PI was narrow with minimal heterogeneity in the pooled clinical success rate.
Publication bias
Publication bias assessment was carried out in relation to the primary outcomes in hand, which were the pooled rate of clinical success and pooled rate of en bloc removal. Based on visual inspection of the funnel plot, as well as quantitative measurement using the Egger regression test, there was no evidence of publication bias (Supplementary Fig. 6, Eggers 2-tailed P=0.07, 0.6).
Supplementary Figure 6.
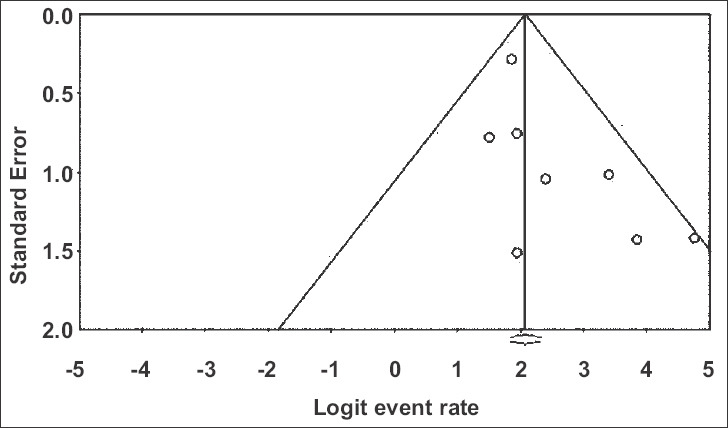
Funnel plot
Quality of evidence
The GRADE working group approach was used to assess the quality of evidence [30]. Observational studies start with a low-quality rating. This meta-analysis would be considered low quality of evidence, based on factors such as publication bias, heterogeneity, risk of bias and indirectness. These results are summarized in Table 3.
Table 3.
Summary of findings, quality of evidence

Discussion
In this study we present a systematic review and meta-analysis of the efficacy and safety of UEMR for SNADET. The pooled clinical success rate was 89.9% and the adverse event rate was 6.9%. The adverse events were generally self-limited, with no documented perforations. In addition, the en bloc resection rate was high at 84.6%. Meta-regression analysis showed no significant difference in clinical success based on tumor size. Based on these results, this technique appears to be a safe and effective method for endoscopic resection in the duodenum.
UEMR is an emerging technique for resection of superficial epithelial tumors in the gastrointestinal tract. The efficacy and safety of this technique is well described for resection of colonic polyps. In a recent systematic review and meta-analysis of UEMR for colorectal lesions, complete resection was achieved in 96.4% of lesions and the adverse event rate was low at 3.3%. As in our analysis, no cases of perforation, a feared complication of both EMR and ESD, were reported [31].
Several factors make complete endoscopic resection of mucosal lesions difficult in the duodenum. These include a relatively narrow lumen, occasional luminal angulation, glandular formation precluding adequate submucosal lifting, and a thin muscular wall, which may increase the risk of perforation [32]. The most commonly described technique is traditional EMR with submucosal injection. A recent systematic review of 10 studies showed a 93% rate of complete resection using traditional EMR. However, this technique was also associated with a pooled bleeding rate of 16%, a perforation rate of 1% and a recurrence rate of 15% [33]. There was a delayed bleeding rate of 5% requiring endoscopic therapy, with one patient requiring surgical intervention.
Although less well studied, ESD is also a commonly utilized technique for the treatment of SNADET. This procedure is associated with less procedural bleeding than EMR; however, it carries a high perforation rate of 6-50%, limiting its utility in this setting [34-36]. In addition, this technique is not frequently employed in western countries, thus limiting its generalizability.
UEMR for SNADET has thus far shown promise. The pooled clinical success rate is comparable to both EMR and ESD. In addition, the adverse event profile appears acceptable, with no documented duodenal perforations and relatively benign post-procedural bleeding in previous studies.
There are several strengths to this study. There were no low-quality studies included. In addition, there was minimal heterogeneity and no publication bias found. However, the study also has limitations. Given the novelty of UEMR for SNADET, only 8 studies were included for analysis. In addition, all of the studies were single-center observational studies, although the majority were prospective. There was also a wide range in the mean size of tumor resection (6-150 mm). Finally, 2 studies did not report on rate of en bloc resection, 1 study did not report on HGIC, and 1 study did not report follow-up endoscopic examinations. Regardless, there is a relative paucity of data regarding endoscopic resection of SNADET and this study supports using UEMR in this setting.
In conclusion, UEMR appears to be an effective technique for SNADET. In addition, the adverse event profile is acceptable, with no documented duodenal perforation. Based on these results, this technique should be considered as a therapeutic option for resection of SNADET.
Summary Box.
What is already known:
• The incidence of superficial non-ampullary duodenal epithelial tumors (SNADET) is increasing
• Commonly used techniques for endoscopic removal including traditional endoscopic mucosal resection (EMR) and endoscopic submucosal dissection both have limitations in this setting
• Underwater EMR (UEMR) is an emerging technique for endoscopic resection; previous studies involving the colorectum have shown excellent efficacy and safety
What the new findings are:
• UEMR has high pooled rates of clinical success and en bloc removal for SNADET
• UEMR has an acceptable pooled rate of adverse events, with no documented duodenal perforations
• UEMR is an effective technique for endoscopic removal of SNADET
Acknowledgments
We would like to acknowledge Emily Glenn in the McGoogan Library at the University of Nebraska, School of Medicine for providing assistance with a literature review. We would also like to acknowledge Kenneth Binmoeller, MD, at California Pacific Medical Center, for pioneering the technique of UEMR.
Biography
University of Nebraska Medical Center, Omaha NE; University of Arizona, Tucson, AZ; Carilion Clinic, Roanoke, VA; University of Utah School of Medicine, Salt Lake City, UT, USA
APPENDIX 1
Literature search strategy:
Strategy:
PubMed (15)
((Underwater AND endoscop*) OR “UW-EMR” OR UEMR) AND (duodenum OR duodenal) Limit to English
Embase (26)
(underwater AND endoscop* OR “UW-EMR” OR uemr) AND (‘duodenum’/exp OR duodenum* OR duodena*)AND [english]/lim
Scopus (16)
(TITLE-ABS-KEY ((underwater AND endoscop*) OR “UW-EMR” OR uemr) AND TITLE-ABS-KEY (duoden*))
APPENDIX 2

APPENDIX 3
MOOSE Checklist for meta-analyses of observational studies
Footnotes
Conflict of Interest: None
References
- 1.Ochiai Y, Kato M, Kiguchi Y, et al. Current status and challenges of endoscopic treatments for duodenal tumors. Digestion. 2019;99:21–26. doi: 10.1159/000494408. [DOI] [PubMed] [Google Scholar]
- 2.Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan:multicenter case series. Dig Endosc. 2014;26(Suppl 2):23–29. doi: 10.1111/den.12277. [DOI] [PubMed] [Google Scholar]
- 3.Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501–12508. doi: 10.3748/wjg.v20.i35.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoteya S, Furuhata T, Takahito T, et al. Endoscopic submucosal dissection and endoscopic mucosal resection for non-ampullary superficial duodenal tumor. Digestion. 2017;95:36–42. doi: 10.1159/000452363. [DOI] [PubMed] [Google Scholar]
- 5.Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. “Underwater”EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086–1091. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Schenck RJ, Jahann DA, Patrie JT, et al. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017;31:4174–4183. doi: 10.1007/s00464-017-5474-4. [DOI] [PubMed] [Google Scholar]
- 7.Uedo N, Nemeth A, Johansson GW, Toth E, Thorlacius H. Underwater endoscopic mucosal resection of large colorectal lesions. Endoscopy. 2015;47:172–174. doi: 10.1055/s-0034-1390749. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology:a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Sutton AJ, Abrams KR, Jones DR, et al. New York: John Wiley &Sons Ltd.; 2000. Methods for meta-analysis in medical research; pp. 205–28. [Google Scholar]
- 13.Higgins J, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 15.Mohan BP, Adler DG. Heterogeneity in systematic review and meta-analysis:how to read between the numbers. Gastrointest Endosc. 2019;89:902–903. doi: 10.1016/j.gie.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, White D. “Systematic reviews and meta-analyses” in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–1186. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines:7 Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill:A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis:Prevention, assessment and adjustments. John Wiley and Sons. 2006 [Google Scholar]
- 22.Binmoeller KF, Shah JN, Bhat YM, Kane SD. “Underwater”EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496–502. doi: 10.1016/j.gie.2013.03.1330. [DOI] [PubMed] [Google Scholar]
- 23.Cornella S, Flynn MM, Strand D, et al. Underwater endoscopic mucosal resection (EMR) appears safe and efficacious compared to conventional EMR for the removal of duodenal adenomas. Gastrointest Endosc. 2017;85:AB298. [Google Scholar]
- 24.Takahashi A, Oyama T. Usefulness of underwater EMR for nonampullary duodenal tumor. United European Gastroenterol J. 2018;6:A723–A724. [Google Scholar]
- 25.Shibukawa G, Irisawa A, Sato A, et al. Endoscopic mucosal resection performed underwater for nonampullary duodenal epithelial tumor:Evaluation of feasibility and safety. Gastroenterol Res Pract. 2018;2018:7490961. doi: 10.1155/2018/7490961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn MM, Wang AY. Underwater endoscopic mucosal resection of large duodenal adenomas (Video) VJGIEN. 2014;2:84–86. [Google Scholar]
- 27.Kiguchi Y, Kato M, Nakayama A, et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor <20 mm. Dig Endosc. 2019 Sep 9; doi: 10.1111/den.13524. [Epub ahead of print]. doi:10.1111/den.13524. [DOI] [PubMed] [Google Scholar]
- 28.Doyama H, Tsuji S, Miyajima S, et al. Clinical outcomes of underwater endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors. J Gastroenterol Hepatol. 2018;33:122. [Google Scholar]
- 29.Yamasaki Y, Uedo N, Takeuchi Y, et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy. 2018;50:154–158. doi: 10.1055/s-0043-119214. [DOI] [PubMed] [Google Scholar]
- 30.Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 31.Spadaccini M, Fuccio L, Lamonaca L, et al. Underwater EMR for colorectal lesions:a systematic review with meta-analysis (with video) Gastrointest Endosc. 2019;89:1109–1116.e4. doi: 10.1016/j.gie.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22:600–617. doi: 10.3748/wjg.v22.i2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navaneethan U, Hasan MK, Lourdusamy V, Zhu X, Hawes RH, Varadarajulu S. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps:a systematic review. Endosc Int Open. 2016;4:E699–E708. doi: 10.1055/s-0042-107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Annals Transl Med. 2017;5:188. doi: 10.21037/atm.2017.03.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133–135. doi: 10.1055/s-0032-1326178. [DOI] [PubMed] [Google Scholar]
- 36.Lim CH, Cho YS. Nonampullary duodenal adenoma:Current understanding of its diagnosis, pathogenesis, and clinical management. World J Gastroenterol. 2016;22:853–861. doi: 10.3748/wjg.v22.i2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]