Abstract
Study Objectives
To describe the crude prevalence of rapid eye movement (REM) sleep behavior disorder (RBD) following traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) in Veterans, given potential relationships between TBI, PTSD, RBD, and neurodegeneration.
Methods
Veterans (n = 394; 94% male; 54.4 ± 15.5 years of age) were prospectively/cross-sectionally recruited from the VA Portland Health Care System and completed in-lab video-polysomnography and questionnaires. TBI and PTSD were assessed via diagnostic screening and medical record review. Subjects were categorized into four groups after assessment of REM sleep without atonia (RSWA) and self-reported dream enactment: (1) “Normal,” neither RSWA nor dream enactment, (2) “Other Parasomnia,” dream enactment without RSWA, (3) “RSWA,” isolated-RSWA without dream enactment, and (4) “RBD,” RSWA with dream enactment. Crude prevalence, prevalence odds ratio, and prevalence rate for parasomnias across subjects with TBI and/or PTSD were assessed.
Results
Overall prevalence rates were 31%, 7%, and 9% for Other Parasomnia, RSWA, and RBD, respectively. The prevalence rate of RBD increased to 15% in PTSD subjects [age adjusted POR: 2.81 (1.17–4.66)] and to 21% in TBI + PTSD subjects [age adjusted POR: 3.43 (1.20–9.35)]. No subjects met all diagnostic criteria for trauma-associated sleep disorder (TASD), and no overt dream enactment was captured on video.
Conclusions
The prevalence of RBD and related parasomnias is significantly higher in Veterans compared with the general population and is associated with PTSD and TBI + PTSD. Considering the association between idiopathic-RBD and synucleinopathy, it remains unclear whether RBD (and potentially TASD) associated with PTSD or TBI + PTSD similarly increases risk for long-term neurologic sequelae.
Keywords: RBD, REM sleep without atonia, PTSD, TBI, trauma-associated sleep disorder
Statement of Significance.
Idiopathic REM sleep behavior disorder (RBD) and neuropsychiatric trauma (e.g. traumatic brain injury; TBI and/or post-traumatic stress disorder; PTSD) are both independently associated with subsequent neurodegeneration (e.g. Parkinson’s Disease or a related synucleinopathy). Given the purported relationships between TBI, PTSD, RBD, and neurodegeneration, we sought to determine the crude prevalence and related associations of RBD following TBI and PTSD among Veterans. Our data show that the prevalence of RBD and related parasomnias is significantly higher in Veterans with PTSD and TBI + PTSD compared with Veterans without a history of neuropsychiatric trauma. Accordingly, the present study fills a critical gap in the literature by providing evidence associating neuropsychiatric trauma with RBD and related parasomnias.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) was first described in 1986 by Schenck, Mahowald, and colleagues in five elderly men, 67–72 years of age [1]. On polysomnography, these men had elevated mentalis and limb electromyography (EMG) activity, despite showing polysomnographic evidence of being in REM sleep, a state typically associated with paralysis of the mentalis and limb muscles [1]. Since this time, considerable work has been done (for review [2]) describing the neuroanatomical and physiological basis [3], treatment [4], and association of RBD with neurodegeneration [5–11]. Indeed, it is estimated that 60%–70% (and potentially as high as 92%) of patients with idiopathic RBD will develop Parkinson’s disease or a related synucleinopathy, such as multiple systems atrophy or Dementia with Lewy Bodies [5–8]. REM sleep without atonia (RSWA) but without coincident dream enactment may also be an early indicator for Parkinson’s disease [12, 13].
The prevalence of idiopathic RBD in the general population has been estimated to be ~0.38% [14] (n = 1,034) to ~0.5% [15, 16] (n = 4,972 and 19,961) based on self-reported sleep-related injury and violent sleep behaviors. Other prevalence focused studies, although not population based, have suggested a crude prevalence of ~1% [17], with certain demographic factors being associated with a higher prevalence rate (e.g., >60 years of age, ~2% [18] to ~5.5% [19]; sleep center referrals, 4.8% [20]; Parkinson’s disease, 69% [21]; and brainstem infarct, 10.9% [22]).
There may also be a higher rate of RBD in patients in the chronic phase of recovery from traumatic brain injury (TBI) 13% (7/54 subjects) [23], as well as posttraumatic stress disorder (PTSD) 56% (15/27 subjects) [24]. Given the association between RBD and subsequent synucleinopathy, and the suggested association between TBI/PTSD and eventual neurodegeneration/dementia, additional work assessing larger cohorts is needed to more accurately describe the prevalence of RBD in TBI and PTSD, as well as in co-morbid TBI + PTSD which may potentiate symptoms [25, 26]. Moreover, additional work is needed to clarify whether RBD in Veterans with TBI and/or PTSD is in fact, RBD, and not the related parasomnia, trauma-associated sleep disorder (TASD). Diagnostic criteria overlap such that both RBD and TASD present with self or witnessed dream enactment behavior and polysomnography confirmed evidence of RSWA. Further diagnostic criteria separate TASD from RBD, as well as observational/anecdotal patterns between the two that not explicitly part of the diagnostic criteria yet often contribute to the overall clinical picture.
The Veteran population is particularly at risk for TBI and/or PTSD and, therefore, represents a population enriched for a history of trauma. Thus, the primary purpose of this study was to describe the prevalence rate of RBD and related parasomnias across a large cohort of Veterans who underwent in-lab video-polysomnography, and the association of these parasomnias with TBI, PTSD, and co-morbid TBI + PTSD. We hypothesized that RBD and related parasomnias would be more prevalent in Veterans with TBI and/or PTSD. As an exploratory analysis, we assessed whether Veterans with RBD also fit the diagnostic criteria for trauma-associated sleep disorder (TASD). We hypothesized that Veterans with a history of neuropsychiatric trauma, and meeting clinical criteria for RBD, would also meet diagnostic criteria for TASD.
Methods
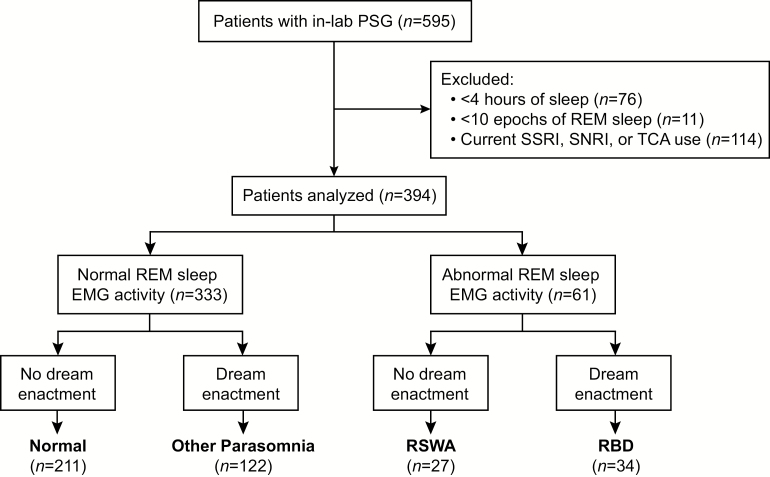
The VA Portland Health Care System (VAPORHCS) approved this study, and each subject gave informed consent prior to participation (IRB #3641). Consecutive Veterans (n = 595) were recruited on random nights of the week for participation in this prospective, cross-sectional study between May 2015 and November 2016 from the VAPORHCS Sleep Clinic. Subjects were excluded from analyses for (1) having <4 hr of recorded sleep (n = 76), (2) having <10 epochs of recorded REM sleep (n = 11), and (3) current selective serotonin reuptake inhibitor (SSRI), selective norepinephrine reuptake inhibitor (SNRI), or tricyclic antidepressant (TCA) usage (n = 114). Thus, the remaining n = 394 subjects were included in the final analyses (Figure 1). Reasons for referral to in-lab video polysomnography by providers included suspected obstructive sleep apnea, excessive daytime sleepiness, hypersomnia, insomnia, restless leg syndrome, and abnormal movements during sleep, with suspected obstructive sleep apnea and excessive daytime sleepiness being the most frequently cited.
Figure 1.
Study overview. Schematic overview of our patient population, exclusion criteria and parasomnia grouping. Of the total n = 595 subjects evaluated with in-lab polysomnography, we excluded n = 76 for having <4 hr of recorded sleep, n = 11 for having <10 epochs of recorded REM sleep, n = 114 for reporting selective serotonin reuptake inhibitor (SSRI), selective norepinephrine reuptake inhibitor (SNRI), or tricyclic antidepressant (TCA) use. Mentalis muscle electromyographic (EMG) activity in the remaining n = 394 subjects was analyzed according to American Academy of Sleep Medicine (AASM) criteria [29] to separate subjects into those with normal (n = 333, 85%) and abnormal (n = 61, 15%) REM sleep EMG activity. Subjects were subsequently further stratified based on their self-reported history of dream enactment [30]. Those with normal REM sleep EMG activity and no history of dream enactment (Normal; n = 211, 53%), those with normal REM sleep EMG activity and a history of dream enactment (Other Parasomnia; n = 122, 31%), those with abnormal REM sleep EMG activity and no history of dream enactment (RSWA; n = 27, 7%), those with abnormal REM sleep EMG activity and a history of dream enactment (RBD; n = 34, 9%). In the analysis of abnormal REM sleep EMG activity, epochs that co-occurred with an apnea or hypopnea were not considered “abnormal.” Thus, only epochs that unambiguously met AASM criteria for abnormal REM sleep EMG activity (i.e. RSWA) contributed to the identification of RSWA and RBD.
Trauma status and medical history
TBI and PTSD status was determined for all subjects using a combination of diagnostic screening, self-report and medical record review. TBI status was assessed using self-report and then confirmed via manual medical record review of relevant clinical notes and ICD-10 codes. PTSD status was assessed via the Post-Traumatic Stress Disorder Checklist DSM-5 (PCL-5). This survey is 20 questions, each a 0 to 4 Likert scale (maximum = 80; higher = worse PTSD) [27], and is subdivided into four clusters: B-Intrusion; 1–5, C-Avoidance; 6–7, D-Mood/Cognition; 8–14, and E-Arousal; 15–20. PTSD was determined by a PCL-5 score ≥33 and positive “cluster criteria” (i.e. rating of ≥2 for 1 B item, 1 C item, 2 D items, and 2 E items), as is standard [28].
Additional medical history was extracted via medical record review, including current ICD-10 coded diagnoses for cognitive impairment, lung disease, heart disease, hypertension, diabetes, and Parkinson’s disease. The presence of sleep apnea was determined using data from the overnight polysomnography.
In-lab video polysomnography
All subjects completed in-laboratory, AASM-accredited polysomnographic technician-attended overnight video-polysomnography recorded using Polysmith (NihonKohden, Japan). Standard AASM [28] parameters were collected, including electroencephalography (6 scalp electrodes), mentalis muscle EMG, bilateral electrooculography, electrocardiography, peripheral blood-oxygen saturation, respiratory movement/effort (thorax and abdominal), airflow (nasal and oral), auditory (snoring), and body positioning (right side, left side, supine, prone). AASM-accredited polysomnographic technicians manually performed standard sleep staging for each 30 s epoch according to standard clinical criteria [29]. Each 30 s epoch was scored as Wake, REM, or NREM stages N1, N2, and N3. All sleep staging was validated by a board-certified sleep physician.
Assessment of REM sleep behavior disorder
Diagnostic criteria for RBD consists of abnormally elevated REM sleep EMG activity (i.e. RSWA) and self or witnessed dream enactment behavior. These criteria were assessed according to standard clinical and published guidelines (described below).
Abnormally elevated REM sleep EMG activity (i.e. RSWA) was manually scored as either phasic (excessive transient EMG activity) or tonic (sustained elevated EMG activity) events according to AASM criteria [29]. RSWA was not assessed using automated computer algorithms, which are not yet recognized by the AASM. Briefly, each 30 s epoch was divided into ten 3 s mini-epochs. An epoch with abnormally high phasic REM sleep EMG activity was required to have at least five 3 s mini-epochs containing EMG bursts lasting 0.1–5 s in duration of at least 4 times the amplitude of background REM sleep EMG activity. An epoch with abnormally high tonic REM sleep EMG activity was required to have at least 50% (15 s) of EMG activity that is greater than the minimum amplitude of EMG activity during NREM sleep. Subjects met criteria for having RSWA if they met phasic or tonic AASM criteria (described above) [29]. A blinded study investigator (D.P.) identified all abnormal REM sleep EMG activity. Studies were verified for accuracy by additional study investigators (J.E.E., T.R., and M.M.L.), through random sampling of ~25% of subjects. Given the high incidence of sleep apnea in our patient population, careful attention was paid to exclude abnormal REM sleep EMG activity coincident with an apnea or hypopnea [29]. Additionally, abnormal REM sleep EMG activity that coincided with marked “snore channel” activity was also excluded.
Subjects’ history of dream enactment behavior was assessed via the single (yes/no) validated screen published by Postuma et al. [30] that reads: “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” This question was reported to be 94% sensitive and 87% specific. All subjects underwent time-synchronized video recordings during polysomnography, and epochs with abnormally elevated REM sleep EMG activity were flagged for manual video review of movement and/or vocalizations. However, no evidence of overt dream enactment was captured on video recording.
Assessment of trauma-associated sleep disorder
TASD shares the previously described diagnostic criteria for RBD, i.e. self or witnessed dream enactment behavior, and polysomnography confirmed evidence of RSWA. Accordingly, only subjects meeting criteria for RBD are potentially eligible to be categorized as having TASD.
The additional distinctive diagnostic criteria separating TASD from RBD include: (1) subjects reporting having an inciting traumatic experience, (2) a history of dream mentation related to this prior traumatic experience, and (3) evidence of autonomic hyperarousal not due to sleep disordered breathing [31]. History of an inciting traumatic experience was assumed positive if subjects screened positive for TBI and/or PTSD. Dream mentation related to this prior experience was assessed by way of a single question administered via the PCL-5 asking subjects to rate, on a scale of 0–4, “In the past month, how much were you bothered by repeated, disturbing dreams of the stressful experience?” [27]. Subjects were required to report a score of 3 or 4 to be considered positive for this criteria. Finally, evidence of autonomic hyperarousal was assessed by analyzing polysomnography for evidence of tachycardia (≥10 bpm increase above baseline) and/or tachypnea (≥2× increase above baseline) [31, 32].
Subject grouping
We categorized subjects into one of four different possibilities based on their self-reported history of dream enactment behavior and polysomnography verified RSWA. (1) Subjects who neither reported a history of dream enactment behavior nor showed RSWA on polysomnography were categorized as “Normal.” (2) Subjects who reported a history of dream enactment behavior but did not show RSWA on polysomnography were categorized as “Other Parasomnia.” (3) Subjects who did not report a history of dream enactment behavior but did show RSWA on polysomnography were categorized as “RSWA.” (4) Subjects who reported a history of dream enactment behavior as well as showed RSWA on polysomnography were categorized as “RBD.” Secondary TASD-specific analyses were conducted only in the RBD specific group, and as will be discussed, no subjects met all additional diagnostic criteria to justify reclassifying any subjects from RBD to TASD.
Statistical analysis
Analyses were performed using R v.3.6.0. Alpha of 0.05, defined a priori, was used for all tests before making appropriate post hoc adjustments when warranted and appropriate. Demographic data were analyzed using one-way analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference post hoc test for normally distributed numeric variables, and either a chi-square test with Bonferroni post hoc test for categorical variables or a Fisher’s exact test for categorical variables with n’s <5. Prevalence odds ratios and prevalence ratios with 95% confidence intervals (with and without age adjustment) were computed via logistic regression for each combination of trauma status and sleep parasomnia. Multinomial logistic regression was used to model the contribution of trauma exposure toward each parasomnia.
Results
Subjects’ demographics and medical history are in Table 1. The cohort (n = 394) was predominately male (94%), middle-aged (54.4 ± 15.5 years of age), and obese (32.7 ± 6.8 kg/m2). No differences across any parameters were observed when analyzing only male subjects, and therefore, female subjects were included in all analyses. Analysis of abnormal REM sleep EMG activity (via polysomnography) and dream enactment behavior (via self-report) identified n = 211 (53%) Normal, n = 122 (31%) Other Parasomnia, n = 27 (7%) RSWA, and n = 34 (9%) RBD subjects (Figure 1). No group differences were found within BMI, sex, and the proportions of obstructive sleep apnea, cognitive impairment, lung disease, heart disease, hypertension, diabetes, Parkinson’s Disease, and melatonin and clonazepam usage (Table 1). However, Other Parasomnia and RBD subjects were on average ~5–10 years of age younger than RSWA and Normal subjects.
Table 1.
Demographic and medical history across subjects
| All Subjects | Normal | Other Parasomnia | RSWA | RBD | |||
|---|---|---|---|---|---|---|---|
| n = 394 | n = 211 (53%) | n = 122 (31%) | n = 27 (7%) | n = 34 (9%) | Stat. | P | |
| Demographics | |||||||
| Age (years) | 54.4 ± 15.5 | 58.6 ± 14.4 | 48.5 ± 15.9* | 54.1 ± 14.4 | 49.6 ± 13.8* | 13.12 | <0.0001 |
| BMI (kg/m2) | 32.7 ± 6.8 | 32.8 ± 6.5 | 32.4 ± 7.5 | 33.4 ± 5.9 | 32.5 ± 6.6 | 0.21 | 0.887 |
| Sex (male) | 369 (94%) | 196 (93%) | 115 (94%) | 25 (93%) | 32 (94%) | 0.17 | 0.982 |
| TBI and/or PTSD | 150 (38%) | 50 (24%) | 69 (57%)* | 10 (37%) | 21 (62%)* | 16.46 | <0.0001 |
| Medical History | |||||||
| OSA | 327 (83%) | 178 (84%) | 95 (78%) | 26 (96%) | 28 (82%) | 5.95 | 0.114 |
| Cognitive Impairment | 6 (2%) | 4 (2%) | 2 (2%) | 0 (0%) | 0 (0%) | - | 1.0 |
| Lung disease | 53 (13%) | 29 (14%) | 20 (16%) | 2 (7%) | 2 (6%) | 3.44 | 0.328 |
| Heart disease | 81 (20%) | 51 (23%) | 18 (15%) | 4 (14%) | 8 (23%) | 4.93 | 0.177 |
| Stroke | 10 (3%) | 8 (4%) | 1 (1%) | 0 | 1 (3%) | 3.52 | 0.345 |
| Hypertension | 178 (45%) | 102 (48%) | 50 (41%) | 9 (32%) | 15 (44%) | 3.85 | 0.284 |
| Diabetes | 88 (22%) | 51 (24%) | 22 (18%) | 7 (25%) | 8 (24%) | 1.94 | 0.585 |
| Parkinson’s Disease | 2 (1%) | 1 (1%) | 1 (1%) | 0 | 0 | - | 1.0 |
| Sleep Drug Use | |||||||
| Melatonin | 17 (4%) | 7 (3%) | 5 (4%) | 1 (4%) | 4 (11%) | 5.11 | 0.163 |
| Clonazepam | 5 (2%) | 2 (1%) | 3 (1%) | 0 | 0 | - | 1.0 |
Data are mean ± standard deviation, or n (% of total).
BMI = body mass index; OSA = obstructive sleep apnea; Cognitive Impairment (history of Alzheimer’s, dementia, or mild cognitive impairment); RSWA = REM sleep without atonia; RBD = REM sleep behavior disorder; Stat = F or chi-square statistic.
*p < 0.05 vs Normal. One-way ANOVA with Tukey HSD post hoc analysis; chi-square with Bonferroni post hoc test or Fisher’s exact test for categorical variables with n’s < 5.
Alpha for these omnibus comparisons has been corrected (i.e. 0.05/14 = 0.003). p values for all significant post hoc multiple comparisons are <0.0006.
Per our inclusion criteria, all subjects had ≥10 epochs (≥5 min) of REM sleep; however, ~90% of subjects within each group had ≥60 epochs (≥30 min) of REM sleep. Only ~1% of subjects per group had between 10 and 30 epochs (5–15 min) of REM sleep. No bias was detected with respect to the likelihood of detecting abnormal REM sleep EMG activity relative to the amount of REM sleep recorded.
There were no differences across groups in the reason for referral to in-lab polysomnography (data not shown), and therefore, no referral bias was detected.
Relative proportions of neuropsychiatric trauma and sleep parasomnias
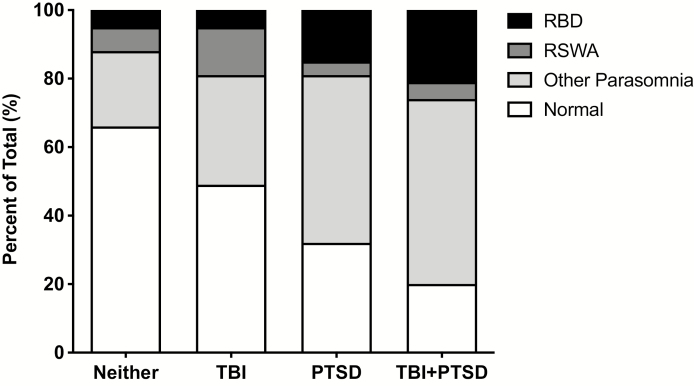
Of the n = 394 Veterans included in our analysis, we identified n = 244 (62%) with neither TBI nor PTSD, n = 37 (9%) with TBI, n = 74 (19%) with PTSD, and n = 39 (10%) with co-morbid TBI + PTSD (Table 2). Within subjects with neither TBI nor PTSD, approximately two-thirds were classified as having Normal sleep (n = 161; 66% of 244), with the remaining one-third having a sleep parasomnia (i.e. either Other Parasomnia, RSWA, or RBD). Interestingly, the relative proportion of these parasomnias increased across subjects with TBI, PTSD, and TBI + PTSD. Subjects with only TBI were fairly evenly split, with 47% being Normal and 53% having a sleep parasomnia. However, subjects with PTSD or comorbid TBI + PTSD showed a significantly increased distribution with only 32% and 20%, respectively, being classified as Normal (i.e. 68% and 80%, respectively, having a sleep parasomnia). This pattern of an increased proportion of sleep parasomnias across subjects with neither TBI nor PTSD, only TBI, only PTSD, or comorbid TBI + PTSD is readily visualized via Figure 2.
Table 2.
Crude prevalence, prevalence odds ratios, and prevalence ratios associating TBI and/or PTSD with sleep parasomnias
| Normal | Other Parasomnia | RSWA | RBD | |
|---|---|---|---|---|
| Neither TBI nor PTSD (n = 244) |
n = 161 (66%) Reference |
n = 53 (22%) Reference |
n = 17 (7%) Reference |
n = 13 (5%) Reference |
| TBI (n = 37) | n = 18 (49%) | n = 12 (32%) | n = 5 (14%) | n = 2 (5%) |
| POR (95% CI) | 0.48 (0.24 – 0.98)* | 1.73 (0.79 – 3.61) | 2.09 (0.65 - 5.70) | 1.01 (0.16 – 3.88) |
| Adj. POR (95% CI) | 0.61 (0.29 – 1.27) | 1.35 (0.60 – 2.90) | 2.19 (0.67 – 6.19) | 0.81 (0.12 – 3.19) |
| PR (95% CI) | 0.69 (0.48 – 1.00) | 1.49 (0.88 – 2.52) | 1.94 (0.76 – 4.94) | 1.01 (0.24 – 4.32) |
| Adj. PR (95% CI) | 0.81 (0.54 – 1.08) | 1.25 (0.66 – 2.01) | 2.01 (0.69 – 4.40) | 0.82 (0.13 – 2.86) |
| PTSD (n = 74) | n = 24 (32%)† | n = 36 (49%)† | n = 3 (4%) | n = 11 (15%)† |
| POR (95% CI) | 0.24 (0.14 – 0.44)* | 3.41 (1.98 – 5.93)* | 0.56 (0.13 - 1.74) | 3.10 (1.30 – 7.27)* |
| Adj. POR (95% CI) | 0.27 (0.15 – 0.47)* | 3.13 (1.79 – 5.49)* | 0.53 (0.12 – 1.68) | 2.81 (1.17 – 4.66)* |
| PR (95% CI) | 0.49 (0.35 – 0.69)* | 2.25 (1.60 – 3.13)* | 0.58 (0.17 – 1.93) | 2.79 (1.30 – 5.96)* |
| Adj. PR (95% CI) | 0.47 (0.30 – 0.68)* | 1.98 (1.46 – 2.43)* | 0.55 (0.13 – 1.61) | 2.47 (1.15 – 4.66)* |
| TBI+PTSD (n = 39) | n = 8 (20%)† | n = 21 (54%)† | n = 2 (5%) | n = 8 (21%)† |
| POR (95% CI) | 0.13 (0.06 – 0.29)* | 4.20 (2.09 – 8.54)* | 0.72 (0.11 – 2.66) | 4.59 (1.70 – 11.80)* |
| Adj. POR (95% CI) | 0.18 (0.07 – 0.40)* | 3.22 (1.55 – 6.71)* | 0.65 (0.10 – 2.55) | 3.43 (1.20 – 9.35)* |
| PR (95% CI) | 0.31 (0.17 – 0.58)* | 2.48 (1.70 – 3.61)* | 0.74 (0.18 – 3.06) | 3.85 (1.71 – 8.68)* |
| Adj. PR (95% CI) | 0.35 (0.16 – 0.62)* | 2.04 (1.35 – 2.69)* | 0.67 (0.10 – 2.31) | 2.90 (1.18 – 5.77)* |
Data are point prevalence rates and prevalence odds ratios (POR) and prevalence ratios (PR) with 95% confidence intervals (CI). Adj. POR and PR are adjusted based on age.
*95% CI does not overlap null value of 1.0.
† p < 0.05 vs. Neither within a given parasomnia; chi-square with Bonferroni post hoc analysis.
Figure 2.
Schematic representation of neuropsychiatric trauma and sleep parasomnia. Subjects with neither a TBI nor PTSD, TBI alone, PTSD alone, and comorbid TBI + PTSD are oriented along the abscissa. For each trauma group, the total percentage of subjects with Normal sleep (clear bar), Other Parasomnia (light fill), RSWA (dark fill), and RBD (totally filled) are shown. Accordingly, the general breakdown between Normal sleep and a parasomnia, within trauma groups, is readily evident. Note the significant percentage of subjects with PTSD or TBI + PTSD that have RBD.
Of particular interest was the crude prevalence of RBD in our sample population, estimated at 9% overall (n = 34 out of 394); markedly higher than previous general population estimates (referenced in the Introduction). The majority of subjects determined to have RBD were those with either PTSD or comorbid TBI + PTSD (n = 19 of 34; 56%). More specifically, the relative proportion of subjects with PTSD or comorbid TBI + PTSD who had RBD was 15% (n = 11 of 74) and 21% (n = 8 of 39), respectively. Accordingly, the combined overall crude prevalence of RBD in subjects with either PTSD alone or TBI + PTSD was 16.8% (n = 19 out of 113).
Prevalence odds ratios and prevalence ratios
Given the cross-sectional nature of these data, we present both prevalence odds ratios and prevalence ratios as measures of association (rather than odds ratios and risk ratios appropriate in other experimental designs). Crude (unadjusted) and age-adjusted values for both are included for comparison (Table 2). For these measures of association, the presence of neuropsychiatric trauma (i.e. TBI, PTSD, or TBI + PTSD) was treated as “exposed” while those with neither TBI nor PTSD were treated as “unexposed,” hence their inclusion as the reference category. Sleep conditions (i.e. Normal, Other Parasomnia, RSWA, or RBD) were treated as the outcome of interest. There are strengths and weaknesses to both measures of association within cross-sectional prevalence studies [33–35]. Prevalence odds ratios better approximate risk factors associated with outcomes of interest and offer consistency between prevalence studies (the present study) and prevalence case-control studies (potential future studies). Prevalence ratios are often viewed as more intelligible/comprehensible and can be superior depending on disease frequency. However, the degree to which these measures of association are intelligible/comprehensible depends on the context, and reader. Furthermore, our primary outcome of interest (i.e. RBD) is classically very uncommon, and therefore, prevalence odds ratios and prevalence ratios should not be expected to significantly differ (i.e. not over- or under-estimating the other; demonstrated in Table 2). Ultimately, we are interested in prevalence in the present study, and therefore, it could be argued that prevalence ratio is the preferred measure of association. However, the analytical benefits (both presently and for future work) of the prevalence odds ratio justify the inclusion of both effect measures. By presenting both measures of association, and fully describing the experimental design herein, the interested reader can choose to interpret both or the measure they feel is most appropriate. Neither prevalence odds ratios nor prevalence ratios imply directionality, a causal relationship (e.g. neuropsychiatric trauma preceding sleep parasomnias), or describe risk.
Significant associations between neuropsychiatric trauma and sleep parasomnias were only detected in subjects with either PTSD or comorbid TBI + PTSD, across Normal sleep, Other Parasomnia, and RBD. There was a clear negative association between PTSD (0.27 times the odds after age-adjustment; 53% lower prevalence ratio) and TBI + PTSD (0.18 times the odds after age-adjustment; 65% lower prevalence ratio) with Normal sleep. Conversely, PTSD and TBI + PTSD were positively associated with Other Parasomnia and RBD, albeit slightly stronger in comorbid TBI + PTSD subjects. Specifically, PTSD subjects showed 3.13 times the odds and a 98% increased prevalence ratio (both after age-adjustment) for Other Parasomnia, and 2.81 times the odds and a 147% increased prevalence ratio (both after age-adjustment) for RBD. However, TBI + PTSD subjects showed 3.22 times the odds and a 104% increased prevalence ratio (both after age-adjustment for Other Parasomnia; and 3.43 times the odds and a 190% increased prevalence ratio (both after age-adjustment) for RBD.
Multinomial logistic regression
The contribution and significance of TBI and/or PTSD on predicting sleep parasomnias were further parsed out via multinomial logistic regression (Table 3). These data generally mirror the prevalence odds ratio and prevalence ratios described above, highlighting the positive predictive contribution of PTSD and comorbid TBI + PTSD for both Other Parasomnia and RBD. Furthermore, age negatively predicted Other Parasomnia and RBD. Our analytical approach treated TBI, PTSD, and TBI + PTSD as separate entities, i.e. without statistically assessing the interactive effect of TBI and PTSD.
Table 3.
Multinomial logistic regression analyses
| Outcome | Predictor | POR (95% CI) | P |
|---|---|---|---|
| Other Parasomnia | |||
| TBI | 1.58 (0.69 – 3.60) | 0.274 | |
| PTSD | 4.21 (2.26 – 7.84) | <0.001 | |
| TBI + PTSD | 5.72 (2.33 – 14.07) | <0.001 | |
| Age | 0.96 (0.94 – 0.98) | <0.001 | |
| RSWA | |||
| TBI | 2.35 (0.76 – 7.25) | 0.137 | |
| PTSD | 1.14 (0.31 – 4.21) | 0.839 | |
| TBI+PTSD | 2.05 (0.39 – 10.64) | 0.393 | |
| Age | 0.98 (0.96 – 1.01) | 0.227 | |
| RBD | |||
| TBI | 1.12 (0.23 – 5.42) | 0.891 | |
| PTSD | 5.31 (2.12 – 13.33) | <0.003 | |
| TBI+PTSD | 9.38 (2.94 – 29.86) | <0.001 | |
| Age | 0.97 (0.94 – 0.99) | <0.01 | |
TBI = traumatic brain injury; PTSD = posttraumatic stress disorder; TBI + PTSD = comorbid TBI and PTSD; RSWA = REM sleep without atonia; RBD = REM sleep behavior disorder.
Trauma-associated sleep disorder
Whether n = 34 subjects that met clinical criteria for RBD showed evidence supporting the presence of TASD was of particular interest, given the high incidence of neuropsychiatric trauma in this population of subjects. Within these RBD subjects, n = 22 had TBI and/or PTSD and thus, evidence for an inciting traumatic event. Of these n = 22 subjects with TBI and/or PTSD and RBD, n = 9 subjects reported evidence of altered dream mentation related to prior traumatic experience. None of these n = 9 subjects with TBI and/or PTSD, RBD, and altered dream mentation showed evidence of autonomic nervous system hyperarousal coincident with epochs of abnormal REM sleep EMG activity. In fact, none of the n = 34 RBD subjects showed evidence of autonomic nervous system hyperarousal coincident with epochs of abnormal REM sleep EMG activity, which is typical of RBD. Of note, this was only true after removing epochs with abnormal REM sleep EMG activity that co-occurred with an apnea or hypopnea. Indeed, in many epochs with an apnea or hypopnea, there was clear tachycardia, presumably associated with the resulting respiratory-related arousal. Epochs with abnormal REM sleep EMG activity that did not co-occur with an apnea or hypopnea were flagged for video review; however, no evidence of overt dream enactment and/or vocalizations were observed.
Discussion
The present study describes the crude prevalence rate of RBD and related parasomnias in Veterans with and without a history of TBI, PTSD, and co-morbid TBI + PTSD. We report an overall crude prevalence rate of Other Parasomnia, RSWA, and RBD of 31%, 7%, and 9% out of n = 394 Veterans, respectively, via in-lab video-polysomnography and self-reported dream enactment. Subjects with TBI, PTSD, or TBI + PTSD accounted for 57% of the subjects with Other Parasomnia (69/122), 37% of the subjects with RSWA (10/27), and 62% of the subjects with RBD (21/34). Of particular interest was the association between neuropsychiatric trauma and RBD, which was strongly associated with both PTSD and comorbid TBI + PTSD, but not TBI alone. The crude prevalence of RBD increased from 9% overall, to 15% and 21% in Veterans with PTSD and TBI + PTSD, respectively. Additionally, there was ~2.8–3.4 times the odds and an ~200% increase in the prevalence ratio for RBD in Veterans with PTSD and TBI + PTSD. Taken together, these data highlight PTSD, with or without comorbid TBI, as a previously underappreciated significant risk factor for RBD.
Association between neuropsychiatric trauma and RBD
PTSD has been suggested to be positively associated with RBD [24], as well as with general REM sleep disturbances [36], including motor dysfunction [37]. Interestingly, the neuropathology underpinning PTSD shares common features with RBD, raising the question as to whether PTSD has a causal role in the development of RBD, or, if a single pathophysiologic process generates two clinical entities. Following an inciting traumatic event, PTSD manifests neurologically via increased norepinephrine turnover, eventual norepinephrine depletion in the locus coeruleus, and progressive locus coeruleus neuronal death [38]. Indeed, neuroimaging studies have shown that PTSD patients have 50% fewer locus coeruleus and peri-locus coeruleus neurons compared with non-PTSD controls [39]. This reduction in locus coeruleus neuronal activity contributes to disinhibition of the pedunculopontine nucleus, which not only produces PTSD-related symptoms [39] but may also be the same neuropathologic process demonstrated in patients with RBD and in patients with Parkinson’s disease and dementia with Lewy body diease [40, 41].
In previous work identifying TBI as a potential risk factor for RBD [23], Verma et al. followed 54 TBI patients in a prospective cohort design and found n = 13 (24%) to have RBD after a 2 year period. Although the potential causal relationship between TBI and RBD remains uncertain, none of the patients from the Verma et al. study showed RBD upon entry leading to the conclusion that head trauma directly or indirectly contributed to the development of RBD. Of note, PTSD was not diagnostically assessed or considered as a covariate, yet it was suggested that posttraumatic anxiety and/or mood disorders were related to the presence of insomnia complaints and RBD. Our cross-sectional analysis showed no association between TBI and RBD or related parasomnias, unless subjects also met diagnostic criteria for PTSD.
Both TBI and PTSD are associated with subsequent neurodegeneration and neurologic disease (e.g. Parkinson’s disease and dementia) [42–44]. Interestingly, few studies attempt to parse out the relative contribution of TBI and/or PTSD, despite frequently being comorbid. Previous work by our lab has shown comorbid TBI + PTSD to be a significant predictor of other common posttraumatic sequela, including sensory sensitivity, insomnia, gait imbalance, and current pain intensity [25, 26, 45]. These present data lend support that comorbid TBI + PTSD may similarly potentiate symptomology related to RBD and sleep parasomnias, given TBI + PTSD was associated with the highest crude prevalence rate for RBD (21%), prevalence odds ratios (4.59 to 3.43 pending age-adjustment), and prevalence ratios (295% to 190%). However, PTSD alone was also a significant risk factor (via logistic regression), and the magnitude of associations was not significantly lower than TBI + PTSD. Accordingly, PTSD alone and TBI + PTSD are largely comparable in their associations with RBD, whereas TBI alone showed no association, suggesting PTSD to be the primary driver behind these observations.
Nevertheless, these data do not conclusively support or refute TBI as an independent risk factor for RBD for several reasons. First, the sample sizes across trauma groups were neither equal nor very large, which could have contributed to the lack of effect seen with TBI. Second, TBI was assessed by a combination of self-report and medical record review, rather than the gold standard, clinician-administered structured diagnostic interview for TBI. Third, the average duration post-TBI in the present data set was ~15 years, which potentially either supports a noncausal link between TBI and RBD, or suggests that a longer duration is required for neurological sequela to manifest.
Trauma-associated sleep disorder vs. RBD
TASD is a recently proposed (as of 2014 by Vincent Mysliwiec and colleagues) [31, 32] phenomenological sleep disorder. Although TASD by definition meets clinical criteria for RBD (i.e. self or witnessed dream enactment behavior, and polysomnography confirmed evidence of RSWA), differentiation comes from several additional diagnostic criteria not traditionally associated with RBD (note: AASM diagnostic criteria do not currently distinguish RBD from TASD). Specifically, TASD is also associated with (1) an inciting traumatic experience, (2) a history of dream mentation related to this prior experience, and (3) evidence of autonomic hyperarousal not due to sleep-disordered breathing [31]. All three additional criteria were assessed, and as described, no subjects fulfilled all of the necessary diagnostic criteria for TASD. An inciting traumatic experience determined by a history of TBI and/or PTSD was present in n = 22 subjects, of which, n = 9 reported evidence of altered dream mentation related to prior trauma, and none showed evidence of autonomic nervous system hyperarousal (assessed by the presence of tachypnea and/or tachycardia not coincident with an apnea or hypopnea). Accordingly, subjects generally fulfilled a partial clinical picture of TASD, while universally lacking the finding of autonomic nervous system hyperarousal. The presence of diaphoresis was not assessed, which remains a possible indicator for autonomic nervous system hyperarousal that could have been present. Our approach to excluding all respiratory related arousals may have also been overly conservative, given the potential that disruptive nocturnal behavior consistent with TASD or RBD may include changes in respiratory flow signals during REM sleep unrelated to sleep-disordered breathing. Interestingly, our data are consistent with a recent case report of a single subject who also met all of the proposed diagnostic criteria for TASD save for evidence of autonomic nervous system hyperarousal [46].
Thus, although our cases of idiopathic/isolated RBD do not appear to be cases of TASD (per the proposed diagnostic criteria), this does not negate the possibility that the two conditions are distinct clinical entities. Furthermore, previous work has shown that capturing overt dream enactment behavior in RBD is often very common [47], whereas it is seemingly uncommon in TASD [32]. This anecdotal observation remains outside of the diagnostic criteria, but would lend support, at a minimum, that these patients may not have “traditional” RBD or “traditional” TASD. Clarifying these disorders remains complex given the overlapping of key diagnostic criteria, but the possibility remains that TASD and RBD are on the same pathological spectrum of disease. Additional work is needed to better understand the relationship between TASD and RBD, and to specifically address whether or not TASD is also associated with an underlying synucleinopathy as is often the case with idiopathic/isolated RBD. Specifically, longitudinal follow-up of these 34 patients and others [46] will add clarity to whether these patients progress to fulfill all diagnostic criteria for TASD, and/or progress to demonstrate overt dream enactment reliably on in-lab video polysomnography.
Rigor/reproducibility, caveats, and limitations
Assessing the presence of RBD in a large sample of subjects is a substantial undertaking, given the extremely resource intensive nature of overnight in-lab video-polysomnography. However, in-lab polysomnography is required to differentiate definite from probable RBD (e.g. only a self-reported history of dream enactment without polysomnographic determined RSWA). Our study, though underpowered to reflect a true epidemiological measure of prevalence, is still one of the largest cohorts of n = 394 Veterans with in-lab video-polysomnography to date. Furthermore, the overall crude point prevalence for RBD of 9% in this study includes a 95% confidence interval of 6.22% to 11.78% (i.e., precision of 2.78%). Thus, the lower end of 6.22% is still higher than prior general population estimates. However, one caveat is that these data are potentially limited in their generalizability beyond the sleep center-referred Veteran population.
Subjects were rigorously assessed for the presence of RSWA and RBD, and potential confounding issues preemptively minimized. First, all subjects using SSRIs, SNRIs or TCAs (n = 114) were excluded, given the established association with secondary RBD [48, 49]. It is still interesting to note that, of the n = 114 subjects reporting SSRI, SNRI, or TCA usage, n = 7 and n = 17 subjects were categorized as RSWA or RBD, respectively. Of these subjects, 1 of the 7 RSWA and 13 of the 17 RBD subjects reported TBI and/or PTSD. Therefore, SSRI, SNRI, or TCA usage was only associated with an increase in RBD, but not RSWA. Inclusion of these subjects in the overall analyses does not change the 7% prevalence of RSWA but does increase the prevalence for RBD to ~10%. Overall, this finding supports the hypothesis that SSRIs, SNRIs, or TCAs may unmask RBD. Second, subjects were required to have ≥10 epochs of REM sleep, and in fact, ~90% of subjects in each group had ≥60 epochs of REM sleep. This mitigates the potential concern that subjects were erroneously categorized due to a limitation of available REM epochs. Third, due to the established association between obstructive sleep apnea and secondary RBD, RSWA events that coincided with marked snoring or with an apnea or hypopnea were not included as contributing toward RSWA or RBD. Additionally, the proportion of subjects with obstructive sleep apnea across groups was not different, nor was there a group difference in the primary reason for referral for sleep testing.
The present study’s cross-sectional design also includes the inherent issue of antecedent-consequent bias, i.e. whether trauma exposure preceded, and contributed to, the development of parasomnias. Resolution of this issue will require a prospective cohort or other similar experimental design. Nevertheless, in the present study, subjects with RBD were on average 50 years of age (range: 27 to 76; n = 18 <50 years of age), and thus, would be categorized as “early-onset” [50]. This early-onset time course shows some similarity with antidepressant associated “psychiatric RBD.” In both cases, it remains to be fully resolved whether the neurodegenerative prognosis mirrors that of idiopathic/isolated RBD. Clearly, longitudinal data will be crucial to gaining clarity on this, and despite an unknown time course, we intend to follow these subjects long-term.
We report both prevalence odds ratios and prevalence ratios, which each have their own relative strengths and weaknesses (discussed in the Results). Although our overall sample size has n = 394 subjects, we have three experimental “exposed” groups (i.e. TBI, PTSD, and TBI + PTSD), with four “outcome” groups (Normal, Other Parasomnia, RSWA, and RBD). The majority of subjects are “unexposed” (i.e. neither have TBI nor PTSD) and have Normal sleep (n = 161), thus, some of the remaining comparisons have relatively small n’s for traditional analyses pertaining to prevalence odds ratios and prevalence ratios. For example, there are an n = 5, 3, and 2 for subjects with TBI, PTSD, and TBI + PTSD, in the RSWA group. These relatively small n’s are likely contributing to the lack of significant associations, but additional work is needed for confirmation. Other comparisons have similarly small n’s, yet remain statistically significant (e.g. an n = 8 subjects with TBI + PTSD and RBD). In these examples, the relatively wide 95% confidence intervals reflect potential imprecision without negating their relative accuracy/statistical significance.
Conclusion
This study reports an overall crude prevalence rate of 9% for RBD in sleep center referred Veterans. Within these subjects, a significant proportion of subjects reported a history of TBI, PTSD, or co-morbid TBI + PTSD, which was most prevalent in patients with RBD. Considering the strong association between idiopathic/isolated RBD and aging-related progressive neurodegeneration, it remains unclear whether the association between RBD and TBI/PTSD increases the risk of similar long-term neurologic sequelae. Future directions will include following the patients in this study longitudinally and additional work should employ prospective cohort experimental designs to better describe the directionality between TBI/PTSD and RBD.
Acknowledgments
The authors would like to express their sincere appreciation and gratitude for the participation of all subjects, to the staff at the VAPORHCS Sleep Disorders Clinic, to Nadir Balba, MS for technical and biostatistical assistance, and to Barbara H. Brumbach, PhD, for biostatistical consulting.
Work Performed: VA Portland Health Care System, 3710 SW US Veterans Hospital Road, Mail code P3-RD42, Portland, OR 97239
Funding
This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, U.S. Department of Veterans Affairs #1K2 BX002712, NIH EXITO Institutional Core, #UL1GM118964, and the Portland VA Research Foundation to M.M.L.; National Institutes of Health T32 AT002688, and U.S. Department of Veterans Affairs #1K2 RX002947 to J.E.E.,; VA OAA Post-doctoral Nursing Research Fellowship to K.B.W. Dr. Lim takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; has full access to all of the data; and has the right to publish any and all data separate and apart from any sponsor. Interpretations and conclusions are those of the authors and do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Authors’ Contributions
Conception and design of the study: J.E.E., R.A.O., K.B.W., and M.M.L. Acquisition and analysis of data: J.E.E., R.A.O., D.P., T.R., A.Q.C., K.B.W., and M.M.L. Drafting a significant portion of the manuscript or figures: J.E.E. and M.M.L.
Conflict of interest statement. None declared.
References
- 1. Schenck CH, et al.. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293–308. [DOI] [PubMed] [Google Scholar]
- 2. Dauvilliers Y, et al. . REM sleep behaviour disorder. Nat Rev Dis Primers. 2018;4(1):19. [DOI] [PubMed] [Google Scholar]
- 3. Jennum P, et al. . Neurophysiological basis of rapid eye movement sleep behavior disorder: informing future drug development. Nat Sci Sleep. 2016;8:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCarter SJ, et al. . Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14(3):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plazzi G, et al. . REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48(4):1094–1097. [DOI] [PubMed] [Google Scholar]
- 6. Marion MH, et al. . Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J Neurol. 2008;255(2):192–196. [DOI] [PubMed] [Google Scholar]
- 7. Gagnon JF, et al. . Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424–432. [DOI] [PubMed] [Google Scholar]
- 8. Schenck CH, et al. . Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):744–748. [DOI] [PubMed] [Google Scholar]
- 9. Schenck CH, et al. . Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46(2):388–393. [DOI] [PubMed] [Google Scholar]
- 10. Galbiati A, et al. . The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta-analysis of longitudinal studies. Sleep Med Rev. 2019;43:37–46. [DOI] [PubMed] [Google Scholar]
- 11. Barone DA, et al. . Rapid eye movement sleep behavior disorder and the link to alpha-synucleinopathies. Clin Neurophysiol. 2018;129(8):1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefani A, et al. . Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a Pilot Study. J Clin Sleep Med. 2015;11(11):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarter SJ, et al. . REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12(2):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiu HF, et al. . Sleep-related injury in the elderly–an epidemiological study in Hong Kong. Sleep. 2000;23(4):513–517. [PubMed] [Google Scholar]
- 15. Ohayon MM, et al. . Violent behavior during sleep. J Clin Psychiatry. 1997;58(8):369–76. [PubMed] [Google Scholar]
- 16. Ohayon MM, et al. . Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep Med. 2010;11(9):941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haba-Rubio J, et al. . Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep. 2018;41(2):1–8. [DOI] [PubMed] [Google Scholar]
- 18. Kang SH, et al. . REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahlknecht P, et al. . Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417–1421. [DOI] [PubMed] [Google Scholar]
- 20. Frauscher B, et al. . REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11(2):167–171. [DOI] [PubMed] [Google Scholar]
- 21. Adler CH, et al. . Probable RBD is increased in Parkinson’s disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011;17(6):456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang WK, et al. . Brainstem infarcts predict REM sleep behavior disorder in acute ischemic stroke. BMC Neurol. 2014;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verma A, et al. . Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med. 2007;3(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- 24. Husain AM, et al. . Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148–157. [DOI] [PubMed] [Google Scholar]
- 25. Elliott JE, et al. . Sleep disturbances in traumatic brain injury: Associations With Sensory Sensitivity. J Clin Sleep Med. 2018;14(7):1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balba NM, et al. . Increased sleep disturbances and pain in Veterans with co-morbid TBI and PTSD. J Clin Sleep Med. 2018;14(11):1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blevins CA, et al. . The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. [DOI] [PubMed] [Google Scholar]
- 28. Weathers FW, et al. . Posttraumatic stress disorder checklist for DSM-5 (PCL-5). 2015;5:4–6. [DOI] [PubMed] [Google Scholar]
- 29. Berry RB, Brooks R, Gamaldo CE, et al. . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Versin 2.0. Darien, IL: American Acadamy of Sleep Medicine; 2012. [Google Scholar]
- 30. Postuma RB, et al. . A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mysliwiec V, et al. . Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mysliwiec V, et al. . Trauma associated sleep disorder: A parasomnia induced by trauma. Sleep Med Rev. 2018;37:94–104. [DOI] [PubMed] [Google Scholar]
- 33. Pearce N. Effect measures in prevalence studies. Environ Health Perspect. 2004;112(10):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamhane AR, et al. . Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med. 2016;35(30):5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt CO, et al. . When to use the odds ratio or the relative risk? Int J Public Health. 2008;53(3):165–167. [DOI] [PubMed] [Google Scholar]
- 36. Ross RJ, et al. . Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35(3):195–202. [DOI] [PubMed] [Google Scholar]
- 37. Ross RJ, et al. . Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. [DOI] [PubMed] [Google Scholar]
- 38. Anisman H, et al. . Coping with stress, norepinephrine depletion and escape performance. Brain Res. 1980;191(2):583–588. [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Rill E. Disorders of the reticular activating system. Med Hypotheses. 1997;49(5):379–387. [DOI] [PubMed] [Google Scholar]
- 40. Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boeve BF, et al. . REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51(2):363–370. [DOI] [PubMed] [Google Scholar]
- 42. Chan YE, et al. . Post-traumatic stress disorder and risk of parkinson disease: a Nationwide Longitudinal Study. Am J Geriatr Psychiatry. 2017;25(8):917–923. [DOI] [PubMed] [Google Scholar]
- 43. Crane PK, et al. . Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73(9):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Camacho-Soto A, et al. . Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using medicare data. Ann Neurol. 2017;82(5):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teutsch P, et al. . Gait and conditioned fear impairments in a mouse model of comorbid TBI and PTSD. Behav Neurol. 2018;2018:6037015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feemster JC, et al. . Trauma-Associated sleep disorder: a posttraumatic stress/REM sleep behavior disorder mash-up? J Clin Sleep Med. 2019;15(2):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández-Arcos A, et al. . The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a Study in 203 Consecutive Patients. Sleep. 2016;39(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Winkelman JW, et al. . Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27(2):317–321. [DOI] [PubMed] [Google Scholar]
- 49. Frauscher B, et al. . Comorbidity and medication in REM sleep behavior disorder: a multicenter case-control study. Neurology. 2014;82(12):1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ju YE, et al. . Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12(3):278–283. [DOI] [PubMed] [Google Scholar]