Abstract
Study Objectives
To examine associations between cumulative socio-demographic risk factors, sleep health habits, and sleep disorder symptoms in young children.
Methods
Two hundred five caregiver-child dyads (child mean age ± SD: 3.3 ± 1.1 years; 53.7% girls; 62.9% black, 22.4% non-Hispanic/Latinx white, 4.4% Hispanic/Latinx; 85.4% maternal caregiver reporter) completed caregiver-rated sleep measures (Brief Child Sleep Questionnaire [BCSQ]; Pediatric Sleep Questionnaire [PSQ] snoring subscale), which were used to generate indexes of poor sleep health habits, pediatric insomnia symptoms, and obstructive sleep apnea (OSA) symptoms. A cumulative risk index was created reflecting caregiver, family, and neighborhood risks.
Results
Overall, 84.5% of children had ≥ 1 poor sleep health habit, 62.9% had ≥ 1 insomnia symptom, and 40.0% had ≥ 1 OSA symptom. Poisson regression indicated that each increase in the number of cumulative risk factors was associated with a 10% increase in poor sleep health habits, a 9% increase in insomnia symptoms, and an 18% increase in OSA symptoms. Specific caregiver risks (depressive symptoms, lower educational attainment) and family risks (single caregiver, crowded home) were most predictive of poor sleep outcomes.
Conclusions
Poor sleep health habits and sleep disorder symptoms are highly prevalent in early childhood, particularly among families experiencing cumulative socio-demographic risks. Findings underscore the need for targeted screening and prevention for modifiable sleep behaviors and efforts to tailor such strategies for at-risk children and families, especially those living in crowded conditions, or with caregivers who are single or have a lower educational attainment or depressive symptoms.
Keywords: cumulative risk, early childhood, insomnia, obstructive sleep apnea, preschoolers, prevention, sleep, sleep health, sleep hygiene, toddlers
Statement of Significance.
Study findings indicate that poor sleep health habits (insufficient sleep; inconsistent bedtime routine; bedroom electronics; bedtime later than 9:00 pm; caffeine consumption) and symptoms of pediatric insomnia and obstructive sleep apnea (OSA) impact over one-third of children ages 2 to 5 years. Increased cumulative socio-demographic risk factors are associated with worse sleep health and greater sleep disorder symptoms. As sleep is a modifiable behavior contributing to child development, findings highlight the need for enhanced screening and preventive intervention, especially in at-risk families. Such services should be tailored to address malleable risk factors, such as having a caregiver with lower educational attainment or clinically significant depressive symptoms. Additional mechanistic research is needed to better understand how cumulative risk impacts sleep over time.
Introduction
Early childhood is a critical period for preventing and treating sleep problems given the rapid neurobehavioral growth and development that occurs during this time [1] and the deleterious effects of untreated sleep concerns [2–6]. Sleep disorders such as pediatric insomnia and obstructive sleep apnea (OSA) are highly prevalent in toddlers and preschoolers [2, 7–10] and have been linked to a number of adverse developmental outcomes, including both concurrent [9, 11] and longitudinal [2, 3, 6, 12] impairments in social-emotional, academic, and neurobehavioral functioning. Obtaining insufficient sleep, which is one of the primary indicators of poor sleep health [13], in early childhood has also been linked to diminished neurobehavioral functioning in middle childhood [14]. Additional, modifiable poor sleep behaviors that are incorporated within the broader concept of sleep health, often referred to as sleep hygiene practices [15], include the use and presence of electronics items at bedtime and caffeine consumption, and have been associated with worse sleep quality and quantity [16–21]. Furthermore, a consistent bedtime routine in early childhood can have benefits beyond sleep, including maternal and child wellbeing [20].
Socio-demographic risk factors such as caregiver depression [22–24] and socio-economic disadvantage [25–28] have been linked to poor sleep quality and increased sleep problems in childhood. However, few studies have examined the cumulative, or additive, effects of multiple socio-demographic risk factors in relation to early childhood sleep. Studies that use a cumulative risk index approach [29] sum or average a number of risk factors to reflect the aggregate effects of multiple and typically interrelated risks. Such research has found that increased cumulative risk exposure is predictive of poor long-term mental and physical health [30–33], particularly when risk exposure occurs in early childhood [34]. Unfortunately, there is no consensus about the specific socio-demographic risk factors included in the cumulative risk index approach [35], although previous cumulative risk research often examines indexes consisting of caregiver (e.g. educational attainment; occupational status; stress exposure; depressive symptoms) and family (e.g. size and number of children in the home; income) factors [30, 31, 36, 37].
With regard to sleep research, one study of school-aged children found that a cumulative risk score consisting of four risk factors (marital status, maternal education, family poverty, and stressful life events) was associated with decreased actigraphy-derived sleep duration and moderated the link between sleep parameters and zBMI, such that children with the highest levels of risk demonstrated the strongest association between poor sleep (short duration and poor quality) and increased zBMI [38]. Another study of 5-year-old Latinx children showed that an index of family adversity was positively associated with a measure of broad sleep problems, although this link was moderated by child autonomic reactivity, with children experiencing the most adversity and low autonomic reactivity showing the greatest odds of having sleep problems [39]. More recently, a study of child protective service-involved toddlers [40] found that a cumulative risk index reflecting child adversity, which included caregiver depressive symptoms, caregiver divorce or separation, and potential maltreatment, among other risks, was associated with greater odds of having a caregiver-identified sleep problem, although sleep problems were measured using a single item. McQuillan et al. additionally found that a cumulative risk index representing socio-demographic factors and parenting stress was associated with worse maternal sleep [35]. To date, however, studies have yet to examine relations among cumulative risk factors and specific symptoms of early childhood sleep disorders and aspects of poor sleep health habits.
Thus, the goal of this study was to examine associations between cumulative risk factors and sleep outcomes in a socio-demographically diverse group of young children. Cumulative risk factors included in this study were summed to index the additive effects of multiple risk exposure. Risk factors were selected on the basis of previous cumulative risk studies and sleep research showing the impact of caregiver (e.g. marital status, educational attainment, depressive symptoms) [22, 38, 40–42], family (e.g. household overcrowding, income) [25, 26, 31, 36, 43], and neighborhood (e.g. disadvantage) [28, 44] factors on child outcomes. Sleep outcomes included poor sleep health habits and symptoms of pediatric insomnia and OSA, as these are the two most common early childhood sleep issues [2, 7]. The poor sleep health index was created to reflect commonly recommended guidelines for healthy pediatric sleep habits [16] and to encompass aspects of sleep health [13], which included sleep duration and sleep hygiene practices [15, 20]. We hypothesized that increased exposure to cumulative socio-demographic risk factors would be associated with poorer sleep health and greater symptoms of pediatric insomnia and OSA.
Methods
Participants
A total of 205 caregiver-child dyads with a young child participated in this study. An additional 15 dyads consented to the study, but either did not initiate study procedures (n = 4) or discontinued prior to completing study questionnaires (n = 11) due to lack of time. Socio-demographic characteristics for participants who did not complete the study did not significantly differ from those who completed study questionnaires, p > .05. Study eligibility criteria included: (1) child age 2 to 5 years; (2) absence of child acute (e.g. influenza; cold) or chronic medical condition (e.g. sickle cell disease; epilepsy) that could impact sleep; (3) absence of a diagnosed neurodevelopmental (e.g. autism) or behavioral health condition (attention-deficit/hyperactivity disorder); (4) absence of a diagnosed sleep disorder (e.g. OSA); (5) no current use of medications that would impact sleep (e.g. diphenhydramine; clonidine). Demographic information for the sample is provided in Table 1.
Table 1.
Caregiver-reported child and caregiver socio-demographic information
| Variables | Mean (SD)/ % |
|---|---|
| Child age | 3.3 years (1.1 years) |
| Child female sex | 53.7 |
| Child race/ethnicity | |
| Black | 62.9 |
| Non-Hispanic/Latinx white | 22.4 |
| Other or multiple races | 7.4 |
| Hispanic/Latinx | 4.4 |
| Asian | 2.9 |
| Child history of prematurity | 4.9 |
| Child developmental delays | 7.8 |
| Caregiver | |
| Mother | 85.4 |
| Father | 12.1 |
| Grandmother | 2.0 |
| Other relative/legal guardian | 0.5 |
| Caregiver age (years) | |
| 18–24 | 16.6 |
| 25–29 | 25.4 |
| 30–39 | 45.9 |
| ≥40 | 12.1 |
| Caregiver race/ethnicity | |
| Black | 57.6 |
| Non-Hispanic/Latino white | 27.3 |
| Other or multiple races | 4.8 |
| Asian | 4.9 |
| Hispanic/Latino | 5.4 |
| Primary care site | |
| Urban | 74.1 |
| Suburban | 25.9 |
Procedure
Participants attending a well child or follow-up visit were recruited from the waiting rooms of three urban and two suburban pediatric primary care clinics operated by a large tertiary-care children’s hospital in southeast Pennsylvania. A mix of urban and suburban clinics were selected to include a diverse set of families that represented a broad socio-economic spectrum, with an overrepresentation of families from lower-income families intentionally included in order to obtain a sufficient sample of children exposed to a range of cumulative socio-demographic risk factors. Recruitment and data collection occurred over a 1-year period, from November 2016 through November 2017. Consent was provided by the accompanying caregiver, who was also the child’s legal guardian. After consent, a review of the child’s medical record was conducted to ensure study eligibility and to obtain child socio-demographic data. Caregivers then completed study questionnaires. Caregiver-child dyads received a $25.00 gift card following completion of study procedures. This study was approved by the Children’s Hospital Institutional Review Board.
Measures
Sleep
Caregivers completed the expanded Brief Child Sleep Questionnaire (BCSQ), a widely used measure of early childhood sleep [45–47] that has shown good reliability and moderate correspondence with actigraphy [48, 49]. The BCSQ includes 30 questions about a child’s typical sleep patterns over the last 2 weeks (i.e. child bed and wake times, sleep onset latency, nighttime and daytime sleep duration, frequency and duration of nighttime awakenings), aspects of the sleep environment (i.e. sleep location, bedtime routine), and perceived sleep problems (i.e. bedtime resistance, difficulty falling asleep, overall sleep problems). Information about the scaling and structure of BCSQ items is reported in detail in previous research [10, 45, 47–49]. Caregivers are directed to estimate average child bedtime and waketime by inputting clock times (e.g. 08:00 pm) and to estimate sleep onset latency, duration of night awakenings, and duration of nighttime and daytime (nap) sleep duration in hours and minutes. Caregivers select among various options to identify the child sleep arrangement (sleep space, location, caregiver presence; Table 2) and elements of the bedtime routine. Likert scale items ranging from never to every night are used to assess the consistency of the bedtime routine and the frequency of difficulty falling asleep and night awakenings. Likert scales are also used for caregivers to rate the degree of bedtime resistance (ranging from very easy to very difficult) and the severity of the child’s sleep problem (ranging from no problem to a severe problem).
Table 2.
Descriptive statistics for caregiver-reported child sleep patterns and environment
| Variables | Mean (SD)/ % |
|---|---|
| Sleep location and space | |
| Own room, own bed | 52.2% |
| Shared room with caregiver(s), shared bed with caregiver(s) | 25.9% |
| Shared room with caregiver(s), own bed | 12.7% |
| Shared room with sibling(s) or relative(s), own bed | 6.3% |
| Shared room, shared bed/couch with sibling(s) or relative(s) | 2.9% |
| Falls asleep independently | 51.2% |
| Number of electronics in bedroom | 1.01 (1.01) |
| Type of electronics itema | |
| Television | 46.3% |
| Tablet | 34.6% |
| Smartphone/cellphone | 18.0% |
| Gaming device | 5.4% |
| Computer | 3.4% |
| Bedtime | 08:47 pm (51.2 minutes) |
| Sleep onset latency (minutes) | 35.82 (36.12) |
| Number of night awakenings per night | 0.79 (0.91)b |
| Duration of nighttime awakenings (minutes) | 14.32 (27.57) |
| Wake time | 07:22 am (67.2 minutes) |
| Nighttime sleep opportunity (hours) | 10.57 (1.08) |
| Nighttime sleep duration (hours) | 9.08 (1.51) |
| Takes naps | 68.6% |
| Nap duration (minutes) | 84.43 (65.60)b |
| Total (24-hour) sleep duration (hours) | 10.53 (1.70)b |
| Number of caffeinated beverages/day | 0.33 (0.77) |
| Number of cumulative risk factors | 2.96 (1.83) |
aCategories of electronics items are not mutually exclusive.
bIncreased in toddler (age 2 years) versus preschool (ages 3 to 5 years) age groups based on preliminary t-test and chi-square comparisons (p < .05).
An additional sleep opportunity variable reflecting child time in bed was calculated by adding the number of hours between caregiver-reported child bed and wake times on the BCSQ. BCSQ caregiver estimates of total nighttime and daytime sleep were also added to create a total (24-hour) sleep duration variable. To further assess aspects of sleep health, caregivers also responded to two questions drawn from previous research [15, 50] that asked caregivers to identify the number and types of electronics items in the child’s bedroom and to indicate the number of caffeinated beverages that the child consumes per day, on average.
Poor sleep health index
A poor sleep health index was constructed based on commonly recommended sleep health habits for young children [15, 16, 20]. The following items from those described above were each dichotomously coded and summed to generate an index in which higher scores indicated worse sleep health: bedtime routine implemented ≤ 4 nights per week (=1); bedtime later than 09:00 pm (=1); any electronics items in the child’s bedroom (=1); insufficient sleep (caregiver-reported total sleep duration ≤ the recommended [51] 11 hours total 24-hour sleep duration for 2-year-olds and 10 hours total 24-hour sleep duration for 3- to 5-year-olds = 1); and child consumption of ≥ 1 caffeinated beverages per day.
Pediatric insomnia index
A pediatric insomnia symptoms index was generated based on diagnostic criteria for pediatric insomnia [52, 53]. The following BCSQ items were each coded dichotomously and summed to generate an insomnia index, with higher scores indicated greater insomnia symptoms: bedtime resistance (somewhat difficult, difficult, or very difficult = 1); difficulty falling asleep ≥3 nights per week (=1); a sleep onset latency of ≥ 30 minutes (=1); night awakenings ≥ 3 nights per week (=1); and a caregiver-perceived sleep problem (small problem, moderate problem, or severe problem = 1) [10].
OSA symptom index
The 8-item snoring subscale of the Sleep-Related Disordered Breathing Scale (SRDB) from the Pediatric Sleep Questionnaire [54, 55] was used to index breathing symptoms suggestive of OSA, including the frequency and quality of snoring and observed apneas (“have you ever seen your child stop breathing overnight?”). Validation research on the snoring subscale has demonstrated strong reliability and validity in predicting an OSA diagnosis on polysomnogram [54, 55]. Items were rated as Yes, No, or I don’t know. Yes responses were coded as 1 and summed to generate a total subscale score.
Child demographic variables
Child demographic information, including child age, sex, race/ethnicity, history of prematurity (<37 weeks gestation), and presence of developmental delays (e.g. speech; motor; language) was drawn from a review of the child’s electronic medical record.
Caregiver and family variables
Caregivers self-reported their age, sex, racial/ethnic background, employment, marital status, and educational level, number of adults and children in the family home, number of times the family moved within the last year, and family income. Family income, total family household size, and US federal poverty guidelines [56] were used to identify families living ≤ 125% of the federal poverty level. Caregivers also completed the 10-item Center for Epidemiological Studies Depression Scale (CES-D-10) [57, 58] to report on depressive symptoms. The CES-D-10 has shown good reliability and validity in diverse community samples, with a cutoff score of 10 used to indicate clinically significant depressive symptoms [58, 59]. Items were rated on a 4-point Likert scale ranging from Rarely or none of the time to All of the time and summed to create a total score.
Neighborhood context
As a neighborhood-level indicator of exposure to adversity, we used zip codes drawn from the child’s medical record to obtain the Economic Innovation Group’s (www.eig.org/dci) Distressed Communities Index (DCI) for 2012–2016. The DCI is generated by combining seven economic indicators at the zip-code level from the US Census Bureau’s American Community Survey 5-Year Estimates and Business Patterns Datasets. These indicators are the percent of the population without a high school diploma or equivalent, the percent housing vacancy rate, the percent of the population ages 25–64 who are without work, the percent of the population living under the poverty line, the median income ratio as a percentage of the state’s median income, the percent change in the number of jobs from the previous Census period, and the percent change in the number of business establishments from the previous Census period. The DCI is calculated by ranking zip codes on each of these indicators, averaging the ranked indicators, and then normalizing the averages [60]. The resulting DCI ranges from 0 to 100, with higher scores indicating a greater level of distress relative to other US zip codes. To facilitate interpretation of the DCI, it has been grouped into the following quintiles: prosperous (lowest quintile), comfortable, mid-tier, at-risk, and distressed (highest quintile, or most distressed community). Previous research has indicated that increased DCI scores are associated with worse healthcare quality and health outcomes [60].
Cumulative risk index
As described above, the cumulative risk index included nine variables selected on the basis of previous cumulative risk and sleep research [25, 30, 31, 34, 38, 40, 44]. Items were coded dichotomously and summed to generate a risk index, with higher scores indicating greater cumulative risk exposure. Items were: caregiver unemployment (=1); caregiver high school level education or less (=1); single caregiver household (=1); caregiver clinically significant depressive symptoms (CES-D-10 score of ≥ 10 = 1); household includes ≥3 children (=1); household includes ≥5 people total; family moved ≥1 time in the last year (=1); family income ≤ 125% of the US federal poverty guidelines; and family living in a distressed community (=1), based on zip-code level DCI score category quintiles (see above).
Statistical analyses
All analyses were conducted using IBM SPSS software version 24. Means for continuous variables and proportions for categorical variables were used to generate descriptive statistics for sleep and cumulative risk variables. Toddlers (age 2 years) and preschoolers (ages 3 to 5 years) were combined in all analyses to maximize statistical power and given few differences in sleep patterns by age (see below), with age included as a covariate in all analyses. Poisson regression models were used to examine associations between the cumulative risk index and sleep outcomes (poor sleep health, pediatric insomnia symptoms, and OSA symptoms). Whereas using linear regression to examine count outcomes violates model assumptions, Poisson models use a count distribution, resulting in more appropriate standard errors and less biased significance tests [61, 62]. Poisson models provide an exponentiated regression coefficient that is interpreted as the percent increase or decrease in the outcome given a one-unit shift in the predictor variable. Covariates in each model included child sex, age, and racial/ethnic background (non-Latinx/Hispanic white = 1), and child history of prematurity or developmental delays. Given the low numbers of children with a history of prematurity (4.8%) or developmental delays (7.8%) and overlap between these characteristics, these indicators were collapsed into a single prematurity or developmental delay covariate. Post-hoc analyses were conducted to identify the relative contribution of each risk factor included in the cumulative risk index to the sleep outcomes. These Poisson models regressed sleep outcomes on covariates and each of the nine cumulative risk factors.
Results
Sleep patterns and sleep environment
Descriptive statistics for caregiver-reported child sleep patterns and the sleep environment appear in Table 2. On average, children had a bedtime of 8:47 PM (SD = 51.2 minutes) and a wake time of 7:22 AM (SD = 67.2 minutes). Based on these data, sleep opportunity (time in bed, calculated by counting the hours between bed and wake times) averaged 10.57 hours per night (SD = 1.08 hours), which was more than caregiver-estimated total nighttime sleep (9.08 hours, SD = 1.51 hours). Sleep onset latency was markedly prolonged in the sample, averaging about 36 minutes. Children had about one waking per night, with a night awakening duration of close to 15 minutes on average. The majority of children (68.6%) napped during the day, in line with age-related expectations, for an average of about an hour and a half (84.43 minutes, SD = 65.60 minutes).
Child sleep patterns showed minimal differences by age and were in line with age-related expectations, in that 2-year-olds had a significantly longer total sleep duration (24-hour; daytime plus nighttime sleep duration) by about an hour (M = 11.18 hours, SD = 1.68 hours) compared to 3- to 5-year-old children (M = 10.24 hours, SD = 1.63 hours; t(203) = 3.63, p < .001). This is likely due to 2-year-olds having a longer nap by about an hour (M = 127.78 minutes, SD = 54.17 minutes) compared to older children (M = 64.64 minutes, SD = 60.79 minutes; t(203) = 7.37, p < .001). Night awakenings were also more frequent in 2-year-olds (M = 1.05, SD = 0.93; 3- to 5-year-olds M = 0.67, SD = 0.88, t(203) = 7.37, p = .007).
Nearly half of the children shared a room (47.8%). Of room-sharing children, one-quarter co-slept with a caregiver (25.9%) or a sibling or relative (2.9%). Fewer children slept in their own bed in a room shared with a caregiver (12.7%) or sibling (6.3%). It is unclear in these data whether co-sleeping with a caregiver was in response to a child sleep problem, due to economic constraints, or an intentional choice. Although children who co-slept were significantly less likely to fall asleep independently (χ 2 (1, N = 205) = 36.72, p < .001), the prevalence of co-sleeping did not significantly differ according to whether caregivers perceived their child’s sleep to be problematic (χ 2 (1, N = 205) = 2.83, p = .13) or whether families resided in an impoverished home (χ 2 (1, N = 205) = 0.21, p = .76).
Poor sleep health
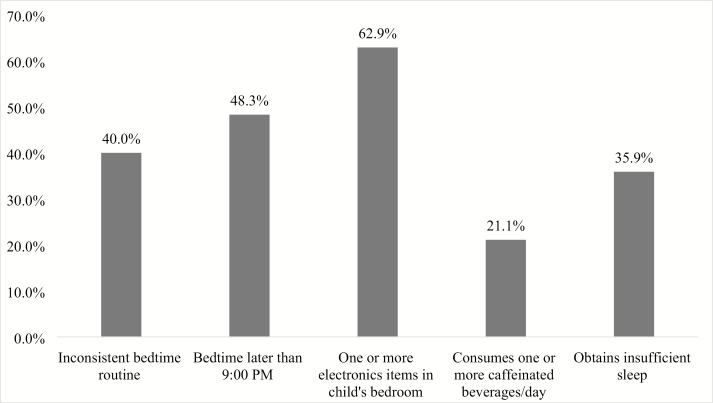
Poor sleep health habits were highly prevalent, with 84.5% of children engaging in one or more unhealthy sleep habits. Twenty percent of caregivers reported one unhealthy habit, 26.2% two unhealthy habits, 24.1% three unhealthy habits, 12.8% four unhealthy habits, and 1.6% noted all 5 unhealthy habits. The most common poor sleep health habit (Figure 1) was the presence of electronics in the child’s bedroom, with the majority of children (62.9%) having one or more items in their bedroom. The most common electronics items were a television (46.3%) or a tablet device (34.6%; Table 2). Nearly half (48.3%) of children had a bedtime later than 09:00 pm. A substantial proportion of children (35.9%) also obtained insufficient sleep for their age (<11 hours in a 24-hour period for 2-year-olds; <10 hours in a 24-hour period for 3- to 5-year-olds). In addition, many children consumed one or more caffeinated beverages per day (21.1%).
Figure 1.
Prevalence of each poor sleep health habit. Note. Inconsistent routine = ≤ 4 nights/week; insufficient sleep = <11 hours total (24-hour) sleep duration for 2-year-olds; <10 hours total (24-hour) sleep duration for 3- to 5-year-olds.
Pediatric insomnia symptoms
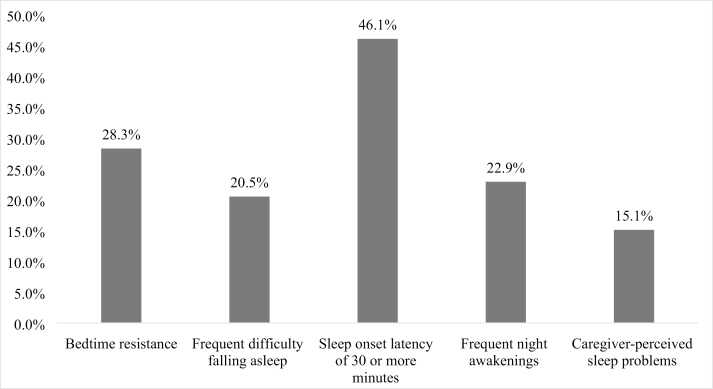
A total of 62.9% of children experienced at least one pediatric insomnia symptom—28.8% experienced one symptom, 12.2% experienced two symptoms, 10.7% experienced three symptoms, 6.3% experienced four symptoms, and 4.4% experienced all five symptoms. The most common insomnia symptom (Figure 2) was a prolonged sleep onset latency of 30 minutes or greater, although only 20.5% of caregivers rated their child as having frequent sleep onset difficulties (≥ 3 nights per week). Bedtime resistance was common and found in 28.3% of children. Overall, approximately one in seven caregivers (15.1%) rated their child’s overall sleep as being problematic.
Figure 2.
Prevalence of each pediatric insomnia symptom. Note. Frequent = ≥3 nights per week.
Diagnostic criteria for pediatric insomnia [52, 53] include one or more difficulties related to initiating sleep, maintaining sleep, difficulty sleeping alone or bedtime resistance and either concern about the child’s sleep or one or more sleep-related impairments in functioning (e.g. energy/alertness; mood; behavior) with concerns occurring at least 3 times per week over the last 3 months. The symptoms cannot occur in the context of inadequate sleep opportunity or environment, or be better explained by another sleep disorder. Some of these criteria were not assessed in this study. However, extrapolating from these diagnostic criteria, results indicate that at least 14.6% of children would potentially meet criteria on the basis of a having a caregiver-reported sleep problem in addition to either a prolonged sleep onset latency, frequent difficulty falling asleep, frequent night awakenings, or bedtime resistance.
OSA symptoms
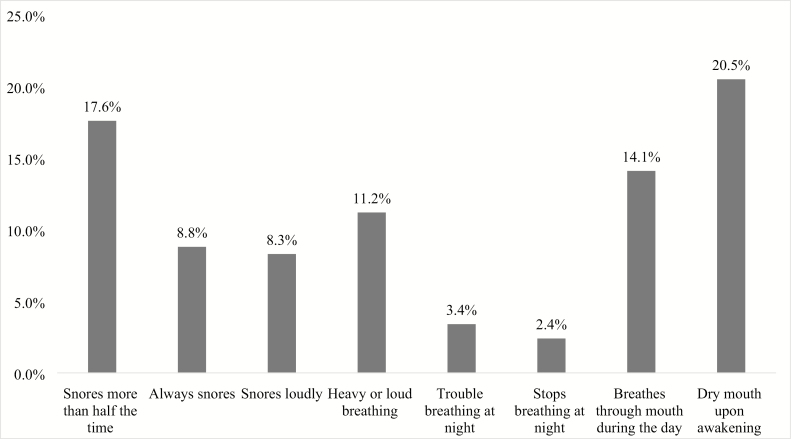
A substantial portion (40%) of the children exhibited OSA symptoms. A total of 19.5% exhibited one symptom followed by 7.8% with two symptoms, 5.4% with three symptoms, 4.4% with four symptoms, and 3% with five or more symptoms. The most common OSA symptom (Figure 3) was having a dry mouth upon awakening (20.5%). Habitual snoring (more than half of the time) was also common (17.6%), with 8.8% of children “always” snoring and 8.3% snoring loudly. Witnessed apneas (seeing the child stop breathing overnight) were the least common symptom (2.4%).
Figure 3.
Prevalence of each OSA symptom.
Cumulative risk exposure
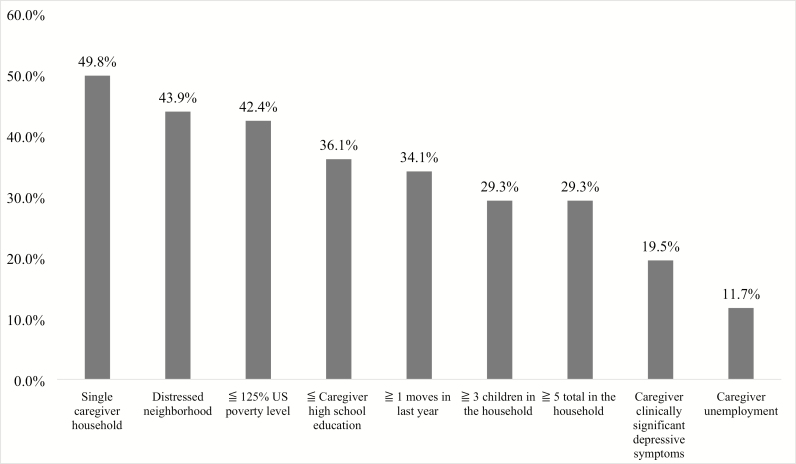
The most common cumulative risk factor (Figure 4) was the presence of a single caregiver household (49.8%; of these, 45.9% were single/never married and 3.9% were single/previously married). The remaining sample of caregivers were married (42.4%) or unmarried but cohabitating with a partner (7.8%). The second most common cumulative risk factor was residing in a distressed neighborhood (43.9%) according to the DCI score, with a total of 21.5% living in an at-risk community, 16.1% living in a mid-tier community, 8.3% living in a comfortable community, and 10.2% living in a prosperous community. Almost half (42.4%) of families resided in a home with a family income of ≤125% of the US federal poverty guidelines. Thirty-six percent of caregivers had a high school education or less, while 28.8% had completed junior college, 22.4% had completed college, and 12.7% held postgraduate degrees. Approximately one-third (29.3%) of families lived in a home with ≥3 children and ≥5 people total. A total of 19.5% of caregivers had clinically significant depressive symptoms. Few caregivers (11.7%) were currently unemployed. The remainder of caregivers identified as being a full-time employee (48.3%), a part-time employee (20.5%), a homemaker (16.6%), or a student (2.9%).
Figure 4.
Prevalence of each cumulative risk index variable.
The majority of the sample (90.7%) had been exposed to one or more cumulative risk factors. Relatively similar numbers of children had been exposed to between one and five cumulative risk factors, with 15.6% exposed to one risk, 17.6% exposed to two risks, 18.5% exposed to three risks, 16.6% exposed to four risks, and 15.1% exposed to five risks. Few children had been exposed to six (3.9%), seven (2.9%), or eight (0.5%) risks, and no children experienced all nine risks.
Associations between cumulative risk index exposure and sleep outcomes
Results of Poisson regression models appear in Table 3. Increased exposure to cumulative risk factors was associated with a significantly greater likelihood of having increased poor sleep health habits and symptoms of pediatric insomnia and OSA, controlling for child sex, age, racial/ethnic background, and history of prematurity and/or developmental delays. With each additional cumulative risk factor, there was a 10% increase in poor sleep health habits, Exp(B) = 1.10 (95% CI 1.04, 1.17), p = .002, a 9% increase in pediatric insomnia symptoms, Exp(B) = 1.09 (95% CI 1.02, 1.17), p = .013, and an 18% increase in OSA symptoms, Exp(B) = 1.18 (95% CI 1.08, 1.29), p < .001.
Table 3.
Associations between cumulative risk exposure index and child sleep outcomes
| Predictors | Poor sleep health habits | |||
|---|---|---|---|---|
| B | SE | p | Exp(B) (95% CI) | |
| Cumulative risk index | 0.10 | 0.03 | .002 | 1.10 (1.04, 1.17) |
| Prematurity and/or developmental delays | 0.27 | 0.15 | .072 | 1.31 (0.98, 1.75) |
| Child female sex | 0.10 | 0.11 | .361 | 1.10 (0.90, 1.35) |
| Child age | 0.03 | 0.05 | .585 | 1.03 (0.94, 1.13) |
| Child non-Latinx/Hispanic white background | −0.67 | 0.17 | <.001 | 0.51 (0.37, 0.71) |
| Pediatric insomnia symptoms | ||||
| Predictors | B | SE | p | Exp(B) (95% CI) |
| Cumulative risk index | 0.09 | 0.04 | .013 | 1.09 (1.02, 1.17) |
| Prematurity and/or developmental delays | −0.15 | 0.22 | .492 | 0.86 (0.57, 1.32) |
| Child female sex | 0.10 | 0.12 | .429 | 1.11 (0.87, 1.41) |
| Child age | −0.13 | 0.06 | .028 | 0.88 (0.79, 0.99) |
| Child non-Latinx/Hispanic white background | 0.21 | 0.16 | .204 | 1.24 (0.91, 1.69) |
| OSA symptoms | ||||
| Predictors | B | SE | p | Exp(B) (95% CI) |
| Cumulative risk index | 0.17 | 0.04 | <.001 | 1.18 (1.08, 1.29) |
| Prematurity and/or developmental delay | 0.42 | 0.21 | .044 | 1.52 (1.01, 2.29) |
| Child female sex | 0.29 | 0.15 | .057 | 1.34 (0.99, 1.82) |
| Child age | −0.21 | 0.07 | .004 | 0.81 (0.70, 0.93) |
| Child non-Latinx/Hispanic white background | −1.31 | 0.35 | <.001 | 0.27 (0.14, 0.54) |
Exp (B) = exponentiated coefficient; SE = standard error.
Associations between individual cumulative risk factors and sleep outcomes
Table 4 provides the results of post hoc Poisson regression models, which evaluated associations between each individual cumulative risk factor in the risk index and sleep outcomes (continuing to control for covariates noted above). In the model for poor sleep health, living in a single caregiver household was associated with a 31% increase in these habits Exp(B) = 1.31 (95% CI 1.03, 1.66), p = .026. In addition, having a caregiver with lower educational attainment (high school diploma or less) was linked to a 26% increase in poor sleep health [Exp(B) = 1.26 (95% CI 1.01, 1.58), p = .045], while living in a crowded home with five or more people was linked to a 40% increase in these habits [Exp(B) = 1.40 (95% CI 1.03, 1.90), p = .030]. Having a caregiver with clinically significant depressive symptoms was the only cumulative risk factor associated with greater pediatric insomnia symptoms, by 93%, Exp(B) = 1.93 (95% CI 1.48, 2.51), p < .001. Caregiver clinically significant depressive symptoms was also associated with a 181% increase in OSA symptoms, Exp(B) = 2.81 (95% CI 2.06, 3.84), p < .001. In this model, the only other significantly associated cumulative risk factor was living in a single caregiver household, which was linked to a 69% increase in OSA symptoms, Exp(B) = 1.69 (95% CI 1.18, 2.41), p = .004.
Table 4.
Associations between individual cumulative risk factors and child sleep outcomes
| Predictors | Poor sleep health habits | |||
|---|---|---|---|---|
| B | SE | p | Exp(B) (95% CI) | |
| Cumulative risk index items | ||||
| Single caregiver household | 0.27 | 0.12 | .026 | 1.31 (1.03, 1.66) |
| Distressed neighborhood | −0.07 | 0.11 | .537 | 0.93 (0.75, 1.16) |
| ≤125% US poverty level | 0.02 | 0.13 | .851 | 1.02 (0.80, 1.31) |
| ≤Caregiver high school education | 0.23 | 0.12 | .045 | 1.26 (1.01, 1.58) |
| ≥1 moves in the last year | 0.03 | 0.11 | .785 | 1.03 (0.83, 1.28) |
| ≥3 children in the household | −0.08 | 0.15 | .614 | 0.93 (0.69, 1.25) |
| ≥5 total in the household | 0.34 | 0.15 | .030 | 1.40 (1.03, 1.90) |
| Caregiver clinically significant depressive symptoms | 0.18 | 0.13 | .171 | 1.19 (0.93, 1.53) |
| Caregiver unemployment | −0.10 | 0.17 | .546 | 0.90 (0.65, 1.26) |
| Prematurity and/or developmental delays | 0.19 | 0.16 | .231 | 1.21 (0.89, 1.65) |
| Child female sex | 0.09 | 0.11 | .425 | 1.09 (0.88, 1.34) |
| Child age | 0.02 | 0.05 | .725 | 1.02 (0.93, 1.12) |
| Child non-Latinx/Hispanic white background | −0.74 | 0.18 | <.001 | 0.48 (0.34, 0.67) |
| Pediatric insomnia symptoms | ||||
| Predictors | B | SE | p | Exp(B) (95% CI) |
| Cumulative risk index items | ||||
| Single caregiver household | 0.16 | 0.14 | .262 | 1.18 (0.89, 1.56) |
| Distressed neighborhood | −0.01 | 0.14 | .473 | 0.91 (0.70, 1.18) |
| ≤125% US poverty level | 0.25 | 0.16 | .119 | 1.28 (0.94, 1.75) |
| ≤ Caregiver high school education | −0.16 | 0.14 | .270 | 0.85 (0.65, 1.13) |
| ≥1 moves in the last year | 0.002 | 0.14 | .990 | 1.00 (0.77, 1.30) |
| ≥3 children in the household | 0.31 | 0.18 | .085 | 1.36 (0.96, 1.93) |
| ≥5 total in the household | −0.10 | 0.18 | .578 | 0.90 (0.63, 1.29) |
| Caregiver clinically significant depressive symptoms | 0.66 | 0.13 | <.001 | 1.93 (1.48, 2.51) |
| Caregiver unemployment | −0.28 | 0.21 | .180 | 0.76 (0.50, 1.14) |
| Prematurity and/or developmental delays | −0.30 | 0.22 | .179 | 0.74 (0.48, 1.15) |
| Child female sex | 0.03 | 0.13 | .840 | 1.03 (0.80, 1.31) |
| Child age | −0.14 | 0.06 | .025 | 0.87 (0.78, 0.98) |
| Child non-Latinx/Hispanic white background | 0.10 | 0.17 | .545 | 1.11 (0.80, 1.54) |
| OSA symptoms | ||||
| Predictors | B | SE | p | Exp(B) (95% CI) |
| Cumulative risk index items | ||||
| Single caregiver household | 0.52 | 0.18 | .004 | 1.69 (1.18, 2.41) |
| Distressed neighborhood | 0.11 | 0.16 | .502 | 1.12 (0.81, 1.53) |
| ≤125% US poverty level | 0.18 | 0.19 | .340 | 1.20 (0.83, 1.73) |
| ≤Caregiver high school education | −0.18 | 0.17 | .307 | 0.84 (0.60, 1.18) |
| ≥1 moves in the last year | −0.02 | 0.17 | .903 | 0.98 (0.70, 1.36) |
| ≥3 children in the household | −0.14 | 0.22 | .542 | 0.87 (0.56, 1.35) |
| ≥5 total in the household | −0.04 | 0.25 | .869 | 0.96 (0.59, 1.55) |
| Caregiver clinically significant depressive symptoms | 1.03 | 0.16 | <.001 | 2.81 (2.06, 3.84) |
| Caregiver unemployment | 0.32 | 0.21 | .135 | 1.37 (0.91, 2.07) |
| Prematurity and/or developmental delay | 0.22 | 0.22 | .318 | 1.25 (0.81, 1.91) |
| Child female sex | 0.23 | 0.16 | .147 | 1.26 (0.92, 1.73) |
| Child age | −0.20 | 0.08 | .008 | 0.82 (0.71, 0.95) |
| Child non-Latinx/Hispanic white background | −1.40 | 0.36 | <.001 | 0.25 (0.12, 0.50) |
Exp (B) = exponentiated coefficient; SE = standard error.
Discussion
This study found associations between increased cumulative risk factors and greater sleep disturbances—specifically, poor sleep health, pediatric insomnia symptoms, and OSA symptoms—in a socio-demographically diverse group of young children. Overall, poor sleep health habits and sleep disorder symptoms were extremely common. Over 80% of preschoolers exhibited one or more unhealthy sleep habits, while over 60% of caregivers reported one or more symptoms of pediatric insomnia and 40% noted one or more sleep disordered breathing symptoms suggestive of child OSA. Each additional cumulative risk factor was associated with a 9%–18% increase in likelihood of a sleep concern, with individual caregiver risks (lower educational attainment, depressive symptoms) and household risks (single caregiver household, crowded home) most predictive of poor sleep outcomes.
The most common poor sleep health habit was having one or more electronics items in the child’s bedroom, which impacted nearly two-thirds of the sample. Whereas previous studies have shown that 17%–37% of toddlers and preschoolers have a television in the bedroom [15, 18, 63], 46% of children in the present study had a bedroom television. Modifiable poor sleep health habits assessed in this study, such as having bedroom electronics, an inconsistent bedtime routine, a later bedtime, and consuming caffeine, have each been associated with diminished sleep quality and quantity [15, 17, 19, 21, 64]. In addition, as in other studies [15, 43], over one-third of young children in this study exhibited insufficient sleep, which confers increased risk for poor developmental outcomes [11, 65]. The increased prevalence of poor sleep health habits such as bedroom electronics could be due to the greater number of families sharing a sleep space, as almost half of children in this sample shared a room and over one-quarter shared a bed with a caregiver. A shared sleep space, particularly with an older sibling or caregiver, could lead to later bedtimes and disrupted routines, as well as more limited nighttime sleep and increased access to electronic items, given the substantial proportion of older children and adults who obtain insufficient sleep and use electronic devices at bedtime [66]. Previous research has primarily examined the presence of bedroom electronics, usually televisions [15], and 24-hour television viewing in relation to early childhood sleep [17, 18]; additional research is needed on the use of televisions and other devices specifically at bedtime and overnight in young children.
Room-sharing or household overcrowding and related increased light or noise could also contribute to a prolonged (≥30 minutes) sleep onset latency, which was the most common pediatric insomnia symptom in this sample, impacting 46% of children. Prolonged sleep onset latency could also be a result of bedtime resistance or due to having electronic items in the bedroom, although having a television in the bedroom was not significantly linked to sleep onset latency in a national study on healthy sleep practices [15]. Interestingly, despite a prolonged sleep onset latency and between 20% and 28% of caregivers reporting other seemingly problematic insomnia symptoms such as bedtime resistance and difficulty falling asleep, fewer (15%) caregivers endorsed a global child sleep problem although this represents one in seven families.
While the rate of a global sleep problem is comparable to other early childhood sleep research [7, 15, 45], study findings indicate a need for a more in-depth investigation into caregiver definitions of what behaviors constitute a child sleep problem [10]. Caregiver expectations for and beliefs about normative versus problematic sleep behaviors may vary by socio-demographic factors, such as culture and context, which may be better identified in qualitative research endeavors [67, 68]. Nonetheless, findings suggest that providers inquiring about global sleep problems in early childhood may miss intervention targets if they do not ask caregivers about specific child sleep behaviors, such as resisting bedtime and having a prolonged sleep onset latency.
The high number of caregivers endorsing habitual child snoring, which aligns with previous research [2], highlights the importance of consistently screening for this particular OSA symptom at child well visits. This screening can be accomplished with a single item, automated into the medical record [69], and will conform with the American Academy of Pediatrics guidelines for OSA management [70]. While snoring and mouth breathing, the most common symptoms of OSA in this sample, do not necessarily confirm an OSA diagnosis, which requires assessment via polysomnography, regular screening for these concerns is important given the negative impacts of OSA on child neurobehavioral functioning [2–4, 70] and limited parental knowledge about snoring as a sign of an underlying sleep disorder [7, 71].
Consistent with study hypotheses, increased cumulative risk exposure was associated with poorer sleep health and increased pediatric insomnia and OSA symptoms. With each increase in the number of cumulative risk factors, there was a 9%–18% increase in problematic sleep outcomes. These findings extend previous pediatric sleep research by linking multiple child risk factors with specific sleep health habits and sleep disorder symptoms. Notably, the cumulative risk index was significantly linked to sleep outcomes over and above other child factors that have been independently associated with variation in sleep health and sleep disorder symptoms in previous research, including child racial/ethnic background [28, 42, 43, 72–74]. When examined separately as correlates of sleep outcomes, several individual variables comprising the cumulative risk index were significantly linked to outcomes, including caregiver depressive symptomatology, lower caregiver educational attainment, a single-caregiver household, and living in a crowded home. These are relatively easily-identified risk factors that should lead to increased screening for sleep concerns, as discussed below. It is important to note that the null findings for other cumulative risks such as neighborhood context and family income, which have been linked to sleep outcomes in other studies [25, 27, 28], maybe due to limited power to detect small effects given the study sample size and the high number of predictors included in the analytic models.
Collectively these results provide important implications for targeted sleep problem screening and preventive intervention in primary care and in other child outpatient settings, including early intervention, childcare centers and services (e.g. Head Start and early Head Start [75]; Nurse-Family Partnership), and health services settings (e.g. pediatric dental offices) [76]. The poor sleep health habits assessed in this study are modifiable and could be the focus of early childhood anticipatory guidance and broad psychoeducation in these contexts, although study findings suggest that recommendations may require tailoring for families facing increased adversity. For example, living in a single caregiver household, having a caregiver with lower educational attainment, and living in a crowded home were each associated with increased poor sleep health habits in this study. Thus, sleep health education efforts targeting the habits described here and others identified in the literature [16] should utilize materials appropriate for lower levels of health-related literacy, which corresponds with educational attainment [41], and should consider methods to incorporate sleep health into families with a single caregiver or living in a crowded home. This may mean problem-solving with families to identify how to avoid having the television or other electronics items present in shared sleeping spaces and how to best implement a bedtime routine—which can encompass and promote many positive health behaviors in addition to sleep [20]—when only one caregiver is available to manage multiple children.
In light of the emerging research on associations between behavioral child sleep problems and caregiver depressed mood [22, 24], it was not surprising that this cumulative risk factor was significantly and independently linked to increased child insomnia symptoms. It could be that caregivers with increased depressive symptoms are less tolerant of child bedtime resistance and other insomnia symptoms and, drawing on bidirectional research [24], that these child behaviors exacerbate low caregiver mood. However, the association between caregiver depressive symptoms and increased OSA symptoms was surprising. Both caregiver depressed mood and OSA symptoms are linked to broad child behavioral impairments, specifically child hyperactivity and inattention [2–4, 77]. These OSA-related behavioral impairments, as well as parenting stress [35], which could emerge as a byproduct of managing difficult child behaviors and contending with increased depressed mood, could be third variables explaining this relationship. Single caregiver household was also associated with increased OSA symptoms, which is consistent with other studies [28] and also represents the potential influence of other variables. In this case, a single caregiver household may be a more proximal measure of an impoverished family environment than family income or neighborhood distress, which have been linked to increased OSA [27, 28]. Single caregiver status could also be a proxy for parenting stress that is related to the noted child behavioral difficulties that could manifest as a result of OSA. Additional research that replicates these results and assesses potential third variables is necessary to better understand this finding.
Overall, caregiver depressive symptoms appear to be a salient factor in identifying children with sleep disorder symptoms and in conceptualizing preventive intervention strategies to mitigate the onset or worsening of sleep problems. Unfortunately, research on implementing behaviorally-based sleep treatment for families exposed to similar socio-demographic risks is limited [50, 75], particularly among families with a depressed primary caregiver. Likewise, there is a paucity of research on factors related to engagement in follow-up care (i.e. polysomnography; otolaryngology; treatment consultation) for children who screen positive for OSA symptoms in outpatient settings. Underscoring the importance of caregiver mood in the context of sleep interventions, in a follow-up evaluation of a behavioral sleep treatment program for children with attention-deficit hyperactivity disorder, child outcomes were diminished in the context of parental depressed mood [78]. Research also indicates that behavioral sleep treatment in early childhood can benefit maternal mood [46, 79], although studies to date have been conducted using predominantly higher-income samples that likely experience fewer cumulative risks. In one high-risk sample, a parenting intervention was found to moderate the link between cumulative risk and caregiver endorsement of a child sleep problem, suggesting that broad family-based interventions may buffer against the development of sleep problems in children exposed to adversity [40]. Future research on innovative methods to either incorporate caregiver mood management into behavioral sleep interventions or to deliver stand-alone intervention to caregivers with low mood to prevent behavioral child sleep problems is critical. Additional research that considers the previously mentioned child and caregiver factors, such as child temperament [80] and behavioral functioning (i.e. comorbid diagnosed behavioral health conditions) [81] as well as parenting stress, is also necessary to better understand the associations found in this study between caregiver mood and symptoms of both insomnia and OSA.
This study is limited by the nature of the cross-sectional data, which do not allow for an examination of casual relations. The interplay between cumulative risk factors and child sleep outcomes is likely dynamic and complex, requiring multiple assessment methods over a longitudinal period. This study relied on one caregiver reporter, which could contribute to shared method variance, and utilized subjective, as opposed to objective, estimates of child sleep and behavior. Objective measurement of sleep, and especially OSA symptoms, would strengthen future studies on this topic. Considering sleep patterns longitudinally, sleep duration on weekdays versus weekends, and the impact of daycare or preschool schedules on sleep outcomes are other important directions for future work. Data for this study were collected over a 1-year period across all seasons, with caregivers reporting on their child’s sleep over the previous 2 weeks. It should be noted that the season and timing of caregiver report on child sleep could also impact sleep patterns.
Future studies should additionally consider expanding sleep health indicators to include sleep time variability and caregiver-perceived child sleep quality [13, 15], as well as the use of electronics at sleep onset and overnight. This study’s use of dichotomous items to capture insomnia symptoms conforms with the dichotomous nature of pediatric insomnia diagnostic criteria [52, 53], but does not substitute for a formal diagnosis, given that all diagnostic criteria (e.g. duration of symptoms over 3 months; other daytime impairments) were not measured in this study. In addition, dichotomizing these data do not address nuanced differences in the continuum of insomnia symptom severity (i.e. degree of sleep problem; frequency of night awakenings), which should be addressed in future work.
As noted previously, future research that considers unmeasured child and family factors related to child sleep outcomes, such as child behavior and temperament [80, 81], household chaos [82], and other aspects of family functioning, is needed. Although we included a number of cumulative risk factors, future research could examine additional risks that have been found to impact child outcomes, such as caregiver convictions [30], stress [35], and adverse childhood experiences [83]. Research should also examine factors that theoretically have a direct impact on early childhood sleep and related routines, such as caregiver shift work or number of jobs, or whether single caregivers have additional support from relatives living outside the home or from another caregiver sharing child custody. In addition, as discussed above, the small sample size relative to the high number of predictors included in the post hoc Poisson models examining individual cumulative risk factors in relation to sleep outcomes could have contributed to null findings in this regard. Future research on cumulative risks and child sleep with a larger sample using multiple measurement methods and reporters is warranted. Finally, this study focused only on risk factors; future research should examine family resilience and other factors that may buffer against sleep problems in high-risk families.
Although additional research is needed to better understand linkages between cumulative risk factors and poor sleep health habits and sleep disorder symptoms in early childhood, this study extends existing pediatric sleep research by examining cumulative risk factors in relation to these sleep outcomes in a socio-demographically diverse sample. Findings underscore the importance of integrating routine screening of modifiable child sleep behaviors as well as specific sleep disorder symptoms in child outpatient settings, especially in families contending with socio-demographic risk factors. Study findings also highlight the need for preventive interventions to improve child sleep that are tailored for families experiencing less modifiable aspects of adversity, such as single parenthood and overcrowded housing, and for caregivers who also experience depressive symptoms. Caregiver education about what constitutes healthy sleep habits in early childhood may be necessary. Enhanced screening and, more importantly, effective preventive interventions for early childhood sleep problems that consider these contextual risk factors are critical for improving child outcomes and for potentially reducing the well-documented sleep health disparities that occur in later childhood and adulthood [84].
Funding
A.A.W. was supported during this study by postdoctoral fellowship T32HL007953-17 from the National Heart, Lung, and Blood Institute, a career development award from the Sleep Research Society Foundation, and K23HD094905 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Acknowledgments
We are indebted to our colleague Carole L. Marcus, MBBCh, FAASM, who provided mentorship on this study’s design and procedures prior to her unexpected passing. We also want to thank the network of primary care clinicians, their patients, and families for their contribution to this project and clinical research facilities through the Pediatric Research Consortium at Children’s Hospital of Philadelphia. We also thank Nicholas Ambrulavage, Esha Bhandari, Olivia Cicalese, Julia Krasny, and Kristen Lanzilotta for their contribution to study data collection. Finally, we thank the Economic Innovation Group for the use of the Distressed Communities Index data. The findings expressed in this publication are solely those of the authors and not necessarily those of the Economic Innovation Group. The Economic Innovation Group does not guarantee the accuracy or reliability of, or necessarily agree with, the information provided herein.
Conflict of interest statement. None declared.
References
- 1. Black MM, et al. ; Lancet Early Childhood Development Series Steering Committee. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonuck K, et al. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129(4):e857–e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonuck K, et al. Pediatric sleep disorders and special educational need at 8 years: a population-based cohort study. Pediatrics. 2012;130(4):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134. [DOI] [PubMed] [Google Scholar]
- 5. Gregory AM, et al. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41(8):964–971. [DOI] [PubMed] [Google Scholar]
- 6. Williams KE, et al. Early childhood profiles of sleep problems and self-regulation predict later school adjustment. Br J Educ Psychol. 2016;86(2):331–350. [DOI] [PubMed] [Google Scholar]
- 7. Byars KC, et al. Prevalence, patterns, and persistence of sleep problems in the first 3 years of life. Pediatrics. 2012;129(2):e276–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hiscock H, et al. Adverse associations of sleep problems in Australian preschoolers: national population study. Pediatrics. 2007;119(1):86–93. [DOI] [PubMed] [Google Scholar]
- 9. Hysing M, et al. Pediatric sleep problems and social-emotional problems. A population-based study. Infant Behav Dev. 2016;42:111–118. [DOI] [PubMed] [Google Scholar]
- 10. Sadeh A, et al. “My child has a sleep problem”: a cross-cultural comparison of parental definitions. Sleep Med. 2011;12(5):478–482. [DOI] [PubMed] [Google Scholar]
- 11. Reynaud E, et al. Sleep and its relation to cognition and behaviour in preschool-aged children of the general population: a systematic review. J Sleep Res. 2018;27(3):e12636. [DOI] [PubMed] [Google Scholar]
- 12. Sivertsen B, et al. Later emotional and behavioral problems associated with sleep problems in toddlers: a longitudinal study. JAMA Pediatr. 2015;169(6):575–582. [DOI] [PubMed] [Google Scholar]
- 13. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taveras EM, et al. Prospective study of insufficient sleep and neurobehavioral functioning among school-age children. Acad Pediatr. 2017;17(6):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mindell JA, et al. Developmental aspects of sleep hygiene: findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009;10(7):771–779. [DOI] [PubMed] [Google Scholar]
- 16. Allen SL, et al. ABCs of SLEEPING: a review of the evidence behind pediatric sleep practice recommendations. Sleep Med Rev. 2016;29:1–14. [DOI] [PubMed] [Google Scholar]
- 17. Brockmann PE, et al. Impact of television on the quality of sleep in preschool children. Sleep Med. 2016;20:140–144. [DOI] [PubMed] [Google Scholar]
- 18. Cespedes EM, et al. Television viewing, bedroom television, and sleep duration from infancy to mid-childhood. Pediatrics. 2014;133(5):e1163–e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mindell JA, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mindell JA, et al. Benefits of a bedtime routine in young children: sleep, development, and beyond. Sleep Med Rev. 2018;40:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owens JA, et al. Effect of energy drink and caffeinated beverage consumption on sleep, mood, and performance in children and adolescents. Nutr Rev. 2014;72(Suppl 1):65–71. [DOI] [PubMed] [Google Scholar]
- 22. de Jong DM, et al. Maternal depressive symptoms and household income in relation to sleep in early childhood. J Pediatr Psychol. 2016;41(9):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warren SL, et al. Maternal depressive symptoms and child sleep: models of mutual influence over time. Dev Psychopathol. 2006;18(1):1–16. [DOI] [PubMed] [Google Scholar]
- 24. Ystrom H, et al. Sleep problems in preschoolers and maternal depressive symptoms: an evaluation of mother- and child-driven effects. Dev Psychol. 2017;53(12):2261–2272. [DOI] [PubMed] [Google Scholar]
- 25. El-Sheikh M, et al. Economic adversity and children’s sleep problems: multiple indicators and moderation of effects. Health Psychol. 2013;32(8):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jarrin DC, et al. Objective and subjective socioeconomic gradients exist for sleep in children and adolescents. Health Psychol. 2014;33(3):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spilsbury JC, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347. [DOI] [PubMed] [Google Scholar]
- 28. Wang R, et al. Associations among neighborhood, race, and sleep apnea severity in children. A six-city analysis. Ann Am Thorac Soc. 2017;14(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rutter M. Protective factors in children’s responses to stress and disadvantage. Ann Acad Med Singapore. 1979;8(3):324–338. [PubMed] [Google Scholar]
- 30. Atkinson L, et al. Cumulative risk, cumulative outcome: a 20-year longitudinal study. PLoS One. 2015;10(6):e0127650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans GW, et al. Cumulative risk and child development. Psychol Bull. 2013;139(6):1342–1396. [DOI] [PubMed] [Google Scholar]
- 32. Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–933. [DOI] [PubMed] [Google Scholar]
- 33. Laucht M, et al. Developmental outcome of infants born with biological and psychosocial risks. J Child Psychol Psychiatry. 1997;38(7):843–853. [DOI] [PubMed] [Google Scholar]
- 34. Appleyard K, et al. When more is not better: the role of cumulative risk in child behavior outcomes. J Child Psychol Psychiatry. 2005;46(3):235–245. [DOI] [PubMed] [Google Scholar]
- 35. McQuillan ME, et al. Maternal stress, sleep, and parenting. J Fam Psychol. 2019;33(3):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ofonedu ME, et al. Understanding barriers to initial treatment engagement among underserved families seeking mental health services. J Child Fam Stud. 2017;26(3):863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Popp TK, et al. The relation of cumulative demographic risk to mothers’ responsivity and control: examining the role of toddler temperament. Infancy. 2008;13(5):496–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bagley EJ, et al. Familial risk moderates the association between sleep and zBMI in children. J Pediatr Psychol. 2013;38(7):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alkon A, et al. Children’s autonomic nervous system reactivity moderates the relations between family adversity and sleep problems in Latino 5-year olds in the CHAMACOS Study. Front Public Health. 2017;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hash JB, et al. Impact of a home visiting program on sleep problems among young children experiencing adversity. Child Abuse Negl. 2019;89:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bathory E, et al. Infant Sleep and Parent Health Literacy. Acad Pediatr. 2016;16(6):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hale L, et al. Social and demographic predictors of preschoolers’ bedtime routines. J Dev Behav Pediatr. 2009;30(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crabtree VM, et al. Cultural influences on the bedtime behaviors of young children. Sleep Med. 2005;6(4):319–324. [DOI] [PubMed] [Google Scholar]
- 44. Bagley EJ, et al. What keeps low-SES children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. 2015;16(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mindell JA, et al. Cross-cultural differences in the sleep of preschool children. Sleep Med. 2013;14(12):1283–1289. [DOI] [PubMed] [Google Scholar]
- 46. Mindell JA, et al. Efficacy of an internet-based intervention for infant and toddler sleep disturbances. Sleep. 2011;34(4):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadeh A, et al. Sleep and sleep ecology in the first 3 years: a web-based study. J Sleep Res. 2009;18(1):60–73. [DOI] [PubMed] [Google Scholar]
- 48. Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics. 2004;113(6):e570–e577. [DOI] [PubMed] [Google Scholar]
- 49. Kushnir J, et al. Correspondence between reported and actigraphic sleep measures in preschool children: the role of a clinical context. J Clin Sleep Med. 2013;9(11):1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mindell JA, et al. Sleep well!: a Pilot Study of an education campaign to improve sleep of socioeconomically disadvantaged children. J Clin Sleep Med. 2016;12(12): 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hirshkowitz M, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 52. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 53. American Academy of Sleep Medicine. International Classification of Sleep Disorders–Third Edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 54. Chervin RD, et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. [DOI] [PubMed] [Google Scholar]
- 55. Chervin RD, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222. [DOI] [PubMed] [Google Scholar]
- 56. United States Department of Health and Human Services. U.S. Federal Poverty Guidelines Used to Determine Financial Eligibility for Certain Federal Programs 2018. https://aspe.hhs.gov/poverty-guidelines. Accessed on 30 August 2018.
- 57. Andresen EM, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 58. Cheng ST, et al. Factorial structure of a short version of the Center for Epidemiologic Studies Depression Scale. Int J Geriatr Psychiatry. 2006;21(4):333–336. [DOI] [PubMed] [Google Scholar]
- 59. Cheung YB, et al. Performance of the CES-D and its short forms in screening suicidality and hopelessness in the community. Suicide Life Threat Behav. 2007;37(1):79–88. [DOI] [PubMed] [Google Scholar]
- 60. Weeks WB, et al. Association between a measure of community economic distress and medicare patients’ health care utilization, quality, outcomes, and costs. J Gen Intern Med. 2018;33(9):1433–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Atkins DC, et al. Rethinking how family researchers model infrequent outcomes: a tutorial on count regression and zero-inflated models. J Fam Psychol. 2007;21(4):726–735. [DOI] [PubMed] [Google Scholar]
- 62. Coxe S, et al. The analysis of count data: a gentle introduction to poisson regression and its alternatives. J Pers Assess. 2009;91(2):121–136. [DOI] [PubMed] [Google Scholar]
- 63. Moorman JD, et al. Beyond access and exposure: implications of sneaky media use for preschoolers’ sleep behavior. Health Commun. 2019;34(5):529–536. [DOI] [PubMed] [Google Scholar]
- 64. Schneider MB, et al. Sports drinks and energy drinks for children and adolescents: are they appropriate? Pediatrics. 2011;127(6):1182–1189. [DOI] [PubMed] [Google Scholar]
- 65. Maski KP, et al. Sleep deprivation and neurobehavioral functioning in children. Int J Psychophysiol. 2013;89(2):259–264. [DOI] [PubMed] [Google Scholar]
- 66. Buxton OM, et al. Sleep in the modern family: protective family routines for child and adolescent sleep. Sleep Health. 2015;1(1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lindsay A, et al. Exploring Brazilian immigrant mothers’ beliefs, attitudes, and practices related to their preschool-age children’s sleep and bedtime routines: a Qualitative Study Conducted in the United States. Int J Environ Res Public Health. 2018;15(9):1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sviggum G, et al. Parents’ experiences with sleep problems in children aged 1-3 years: a qualitative study from a health promotion perspective. Int J Qual Stud Health Well-being. 2018;13(1):1527605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Honaker SM, et al. Unexplained practice variation in primary care providers’ concern for poediatric obstructive sleep apnea. Acad Pediatr. 2018;18(4):418–424. [DOI] [PubMed] [Google Scholar]
- 70. Marcus CL, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. [DOI] [PubMed] [Google Scholar]
- 71. McDowall PS, et al. Parent knowledge of children’s sleep: a systematic review. Sleep Med Rev. 2017;31:39–47. [DOI] [PubMed] [Google Scholar]
- 72. Peña MM, et al. Racial/ethnic and socio-contextual correlates of chronic sleep curtailment in childhood. Sleep. 2016;39(9):1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patrick KE, et al. Sleep Differences by race in preschool children: the roles of parenting behaviors and socioeconomic status. Behav Sleep Med. 2016;14(5):467–479. [DOI] [PubMed] [Google Scholar]
- 74. Redline S, et al. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. [DOI] [PubMed] [Google Scholar]
- 75. Wilson KE, et al. Evaluation of a sleep education program for low-income preschool children and their families. Sleep. 2014;37(6):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sevecke J, et al. It takes a village: multidisciplinary approach to screening and prevention of pediatric sleep issues. Med Sci. 2018;6(3):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goodman SH, et al. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14(1):1–27. [DOI] [PubMed] [Google Scholar]
- 78. Sciberras E, et al. Sustained impact of a sleep intervention and moderators of treatment outcome for children with ADHD: a randomised controlled trial. Psychol Med. 2019;1–10. doi:10.1017/S0033291718004063. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79. Mindell JA, et al. A nightly bedtime routine: impact on sleep in young children and maternal mood. Sleep. 2009;32(5):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cremone A, et al. Sleep tight, act right: negative affect, sleep and behavior problems during early childhood. Child Devel. 2017;17(6):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Van Dyk TR, et al. Mental health diagnoses and symptoms in preschool and school age youth presenting to insomnia evaluation: prevalence and Associations with Sleep Disruption. Behav Sleep Med. 2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boles RE, et al. Family chaos and child functioning in relation to sleep problems among children at risk for obesity. Behav Sleep Med. 2017;15(2):114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schickedanz A, et al. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics. 2018;142(2):e20180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jean-Louis G, et al. Importance of recognizing sleep health disparities and implementing innovative interventions to reduce these disparities. Sleep Med. 2016;18:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]