Abstract
Background
Little is known about the endoscopic and histologic findings of non-esophageal eosinophilic gastrointestinal diseases (EGID).
Aim
To characterize the presenting endoscopic and histologic findings in patients with eosinophilic gastritis (EG), eosinophilic gastroenteritis (EGE), and eosinophilic colitis (EC) at diagnosis and 6 months after initiating the treatment.
Methods
We conducted a retrospective cohort study at 6 US centers associated with the Consortium of Eosinophilic Gastrointestinal Researchers. Data abstracted included demographics, endoscopic findings, tissue eosinophil counts, and associated histologic findings at diagnosis and, when available, after initial treatment.
Results
Of 373 subjects (317 children and 56 adults), 142 had EG, 123 EGE, and 108 EC. Normal endoscopic appearance was the most common finding across all EGIDs (62% of subjects). Baseline tissue eosinophil counts were quantified in 105 (74%) EG, 36 (29%) EGE, and 80 (74%) EC subjects. The mean peak gastric eosinophil count across all sites was 87 eos/hpf for EG and 78 eos/hpf for EGE. The mean peak colonic eosinophil count for EC subjects was 76 eos/hpf (range 10–500). Of the 29% of subjects with post-treatment follow-up, most had an improvement in clinical, endoscopic, and histologic findings regardless of treatment utilized. Reductions in tissue eosinophilia correlated with improvements in clinical symptoms as well as endoscopic and histologic findings.
Conclusions
In this large cohort, normal appearance was the most common endoscopic finding, emphasizing the importance of biopsy, regardless of endoscopic appearance. Decreased tissue eosinophilia was associated with improvement in symptoms, endoscopic, and histologic findings, showing that disease activity is reversible.
Keywords: Eosinophilic esophagitis, Eosinophilic gastritis, Eosinophilic gastroenteritis, Eosinophilic colitis
Introduction
Eosinophils are constituents of the GI mucosa where they likely have a role in immune surveillance [1]. In the normal state, no eosinophils are present within the esophagus while eosinophils are a normal resident cell in the stomach, small intestine, and colon [2–8]. However, when eosinophils are found in higher numbers, disease pathology may result, especially following eosinophil activation with resulting proinflammatory effects of their granule proteins. Elevated gastrointestinal eosinophils have been associated with parasitic infections, hypereosinophilic syndrome, inflammatory bowel disease, gastroesophageal reflux, and eosinophilic GI diseases (EGIDs) [9, 10].
EGIDs are rare disorders, and those found outside of the esophagus, including eosinophilic gastritis (EG), gastroenteritis (EGE), and colitis (EC), have an estimated prevalence of 2.1–5.1 per 100,000 compared to 10–57 per 100,000 for eosinophilic esophagitis (EoE) [11–13]. The diagnosis is typically made when a patient exhibits symptoms of gastrointestinal dysfunction and is found to have elevated numbers of GI tract eosinophils, and other causes for the eosinophilia have been ruled out. Defining an elevated tissue eosinophil count in these non-esophageal EGIDs remains an area of active investigation. While criteria for diagnosing non-esophageal EGIDs have been proposed, none have been widely validated or had consensus agreement [14].
Given the rarity of these disorders, lack of multicenter studies, and limited knowledge of endoscopic and histologic characteristics, this study sought to characterize the presenting endoscopic and histologic findings of a relatively large, multicenter cohort of non-esophageal EGIDs and to determine changes in these findings after treatment.
Methods
Subject Selection and Areas of Study
For this retrospective cohort study, centers from the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) were asked to participate and 6 centers (4 pediatric and 2 pediatric/adult) submitted subjects for enrollment [15]. Complete methodology and clinical characteristics of the study cohort were described previously [16]. Briefly, subjects were considered eligible for study inclusion if they were seen at enrolling sites between 2005 and 2016, had a clinical diagnosis of EG, EGE (EG and eosinophilic enteritis), and/or EC, and at least one endoscopy with biopsy demonstrating an increase in gastrointestinal eosinophils. Local pathology reports were reviewed by site investigators to confirm that the number of tissue eosinophils reached a threshold level based upon the area of biopsy: stomach ≥ 30 eosinophils/high-power field (hpf); small intestine ≥ 50 eosinophils/hpf; and/or colon ≥ 60 eosinophils/hpf [12]. Not all centers reported a specific number of tissue eosinophils, and in these cases subjects were considered eligible for inclusion if the pathologist’s description of the GI tract biopsy noted increased eosinophils, the investigator excluded other causes of gastrointestinal eosinophilia (including hypereosinophilic syndrome, inflammatory bowel disease, parasite infection, and autoimmune disorders), and the patient had a clinical diagnosis of an EGID.
Using a standardized data collection form, we extracted cohort demographics (age, gender, race), findings during diagnostic endoscopy, as well as histologic findings during pathology review of gastrointestinal biopsies. When available, data were also collected from follow-up endoscopy and histologic analysis of biopsies performed within 6 months of the initial diagnosis, after starting treatment as clinically prescribed by providers at each center. We also extracted outcomes for patients with follow-up data (improvement in tissue eosinophilia [yes/no]; clinical improvement [yes/no]; endoscopic improvement [yes/no]; change in tissue absolute peak eosinophil count, and improvement in other histologic findings [yes/no]) based upon the investigator’s interpretation of changes in these outcome measures before and after treatment. This interpretation was compared to changes in tissue peak eosinophil counts, when possible. Specific endoscopic and histologic findings were coded using dichotomous variables depicting their presence or absence [yes/no, per patient reporting at follow-up].
All data were extracted from site medical records and placed on a standardized spreadsheet. De-identified electronic data from each site were submitted to a central data repository established by the CEGIR Data Management and Coordinating Center (DMCC) after which analysis was performed. This study was approved by NIH, the CEGIR central Institutional Review Board (IRB) at Cincinnati Children’s Hospital Medical Center, as well as each of the participating site’s local IRBs.
Statistical Analysis
The primary aim of this study was to characterize the presenting endoscopic and histologic findings of a cohort of non-esophageal EGID patients. As such, descriptive statistics were utilized to summarize baseline and post-treatment characteristics. Proportions were tabulated for endoscopic findings, which were defined a priori preceding data extraction. Summary statistics were also utilized to describe baseline histologic findings and eosinophil counts (max eosinophils per high-power field [eos/hpf]), respectively. Histologic findings were categorized following data extraction rather than a priori. We also sought to determine whether baseline eosinophil counts predict the presence or absence of baseline endoscopic findings. To this end, logistic regression models were constructed.
We also aimed to assess changes in outcome variables from baseline to follow-up. Bivariate statistics were performed with paired t tests for continuous variables (e.g., eosinophil counts) and McNemar’s Chi-square for categorical variables (e.g., clinical and endoscopic findings). At the time of follow-up, Chi-square testing assessed for associations between dichotomous outcome variables and follow-up endoscopic findings, and outcomes by EGID subtype. All analyses were performed using Stata 14.2 (StataCorp, College Station, TX).
Results
Overall Population
A total of 376 subjects were enrolled including 317 children less than 18 years of age and 56 adults (Fig. 1). Data on baseline population characteristics have been previously reported [16]. In brief, the overall age range of patients was 0.5–77 years. For children, the mean age at diagnosis was 7.3 years (range 0.5–17, median 7) and for adults 36 years (range 18–77, median 32). The overall population was 52% male, and 71% of subjects were Caucasian. There were 142 subjects diagnosed with EG, 123 subjects with EGE, and 108 subjects with EC.
Fig. 1.
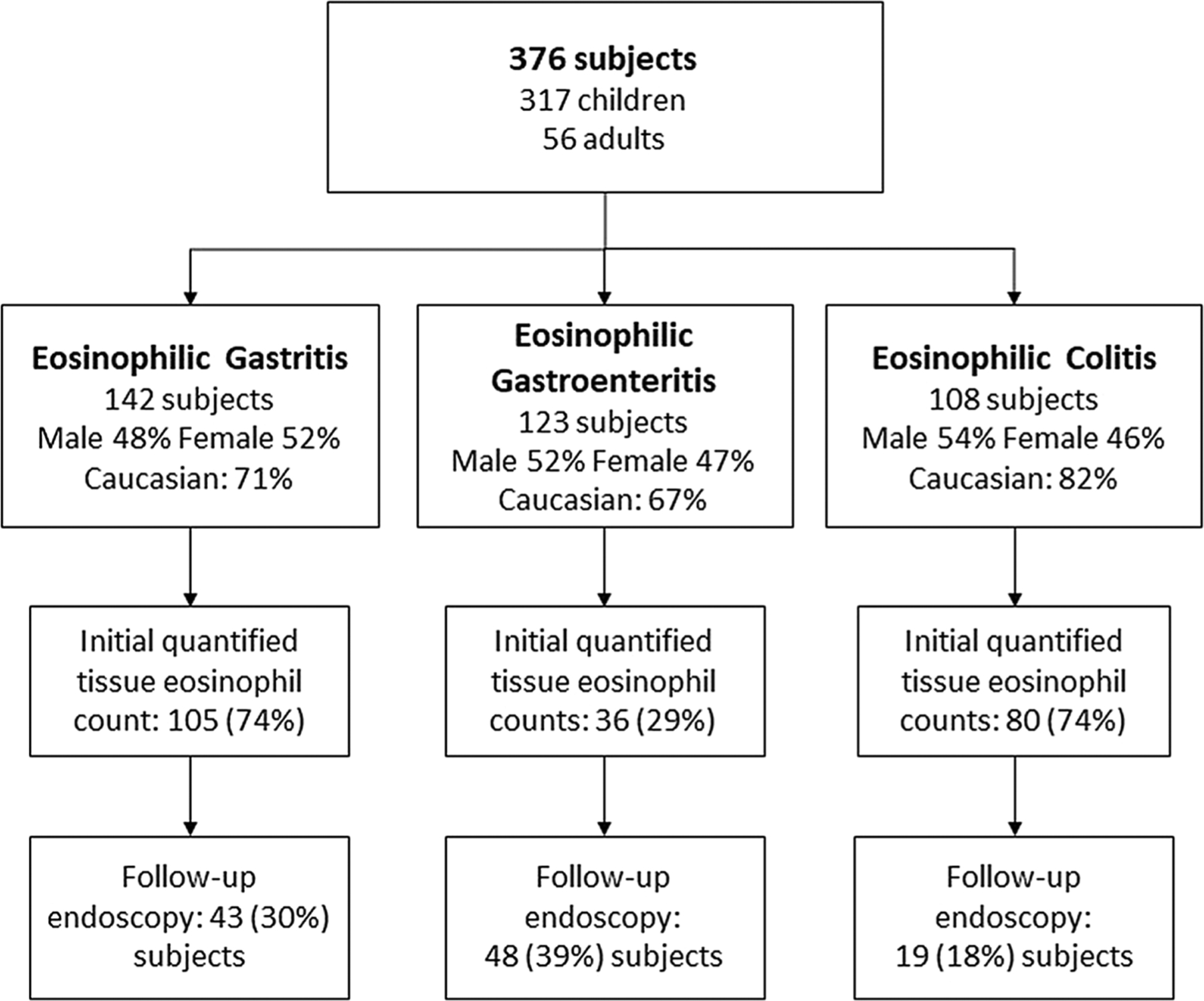
Study population by EGID diagnosis, tissue eosinophil quantification, and follow-up endoscopy rate
Endoscopic Findings
Endoscopic findings were assessed for each EGID diagnosis and by GI segment (Table 1). For the subjects with EG, normal appearance was the most common endoscopic finding and was seen in 62% of subjects. Erythema (24%), ulceration (8%), nodularity (8%), and mucosal friability (6%) were also commonly reported. For EGE, normal appearance was the most common finding in the stomach (66%) as well as in the duodenum (83%). The jejunum and ileum were assessed less frequently, but normal appearance was the most common finding in these segments as well (67% and 81%, respectively). Ulceration (6%), nodularity (3%), erythema (2%), and mucosal friability (2%) were less common findings. In EC, again, normal appearance (64%) was the most common finding, followed by erythema (12%) and friability (11%). Polyps (6%), nodularity (5%), and ulceration (4%) were less common findings.
Table 1.
Baseline endoscopic findings by disease and GI segment
| Eosinophilic gastritis (total N = 142) | ||
|---|---|---|
| Endoscopic finding | N | % |
| Stomach | ||
| Normal appearance | 88 | 62 |
| Erythema | 34 | 24 |
| Friable | 8 | 6 |
| Nodularity | 12 | 8 |
| Polyp | 0 | 0 |
| Stricture | 1 | 1 |
| Ulcer | 12 | 8 |
| Missing description | 2 | 1 |
| Eosinophilic gastroenteritis (total N = 123) | ||
| Endoscopic finding | N | % |
| Stomach | ||
| Normal appearance | 81 | 66 |
| Erythema | 18 | 15 |
| Friable | 7 | 6 |
| Nodularity | 11 | 9 |
| Polyp | 1 | 1 |
| Stricture | 1 | 1 |
| Ulcer | 14 | 11 |
| Missing description | 5 | 4 |
| Duodenum | ||
| Normal appearance | 102 | 83 |
| Bleeding | 0 | 0 |
| Erosions | 0 | 0 |
| Friable | 3 | 2 |
| Erythema | 2 | 2 |
| Nodularity | 4 | 3 |
| Polyp | 0 | 0 |
| Stricture | 1 | 1 |
| Ulcer | 7 | 6 |
| Missing description | 9 | 7 |
| Jejunuma | ||
| Normal | 4/6 | 67 |
| Missing description | 117 | 95 |
| Ileuma | ||
| Normal appearance | 17/21 | 81 |
| Missing description | 91 | 74 |
| Eosinophilic colitis (total N = 108) | ||
| Endoscopic finding | N | % |
| Colon | ||
| Normal appearance | 69 | 64 |
| Bleeding | 1 | 1 |
| Erosions | 2 | 2 |
| Friable | 12 | 11 |
| Erythema | 13 | 12 |
| Nodularity | 5 | 5 |
| Polyp | 7 | 6 |
| Stricture | 0 | 0 |
| Ulcer | 4 | 4 |
| Missing description | 2 | 2 |
Data shown as number (N) and % of specific population
These GI segments were not endoscopically assessed in all subjects
Histologic Findings
Tissue eosinophil counts from diagnostic biopsies were assessed by disease and GI segment. Quantified tissue eosinophil counts were available for 105 (74%) subjects with EG, 36 (29%) with EGE (both stomach and small intestine), and 80 (74%) with EC (Fig. 2, Table 2). For EG subjects, biopsies were most commonly performed in the antrum or body. The mean peak eosinophil count across all stomach biopsy sites was 87 eos/hpf (range 13–500). The highest mean eosinophil counts in EG subjects with biopsies taken from a specific gastric site were in the body (mean peak 87 eos/hpf, range 13–400) followed by the antrum (mean peak 78 eos/hpf, range 13–400). There were 27 subjects who had biopsies taken from an unspecified site (mean peak 120 eos/hpf, range 15–500). In subjects with EGE, gastric biopsies were most commonly performed in the antrum (34 subjects). Small intestinal biopsies were most commonly performed in the duodenum (38 subjects), followed by the ileum (10 subjects) and jejunum (3 subjects). The mean peak eosinophil count in gastric biopsies in EGE subjects was similar to those with isolated EG with a mean peak eosinophil count of 78 eos/hpf (range 6–500). The mean peak eosinophil count in small intestine biopsies across all segments was 65 eos/hpf (range 12–200). In subjects with EC, eosinophil counts were distributed equally across the right, left, and rectosigmoid colon with a mean peak of 76 eos/hpf (range 10–500). A comparison between pediatric and adult subjects was also made in regard to peak eosinophil counts at baseline and after treatment (Table 3). At baseline, adult subjects had significantly more tissue eosinophils in the stomach compared to children but there were no significant differences between small intestine or colon eosinophils. At baseline, endoscopic abnormalities were associated with elevated tissue eosinophils during endoscopy of the duodenum (p = 0.005), but a similar association was not found in biopsies of the stomach or colon (Fig. 3).
Fig. 2.
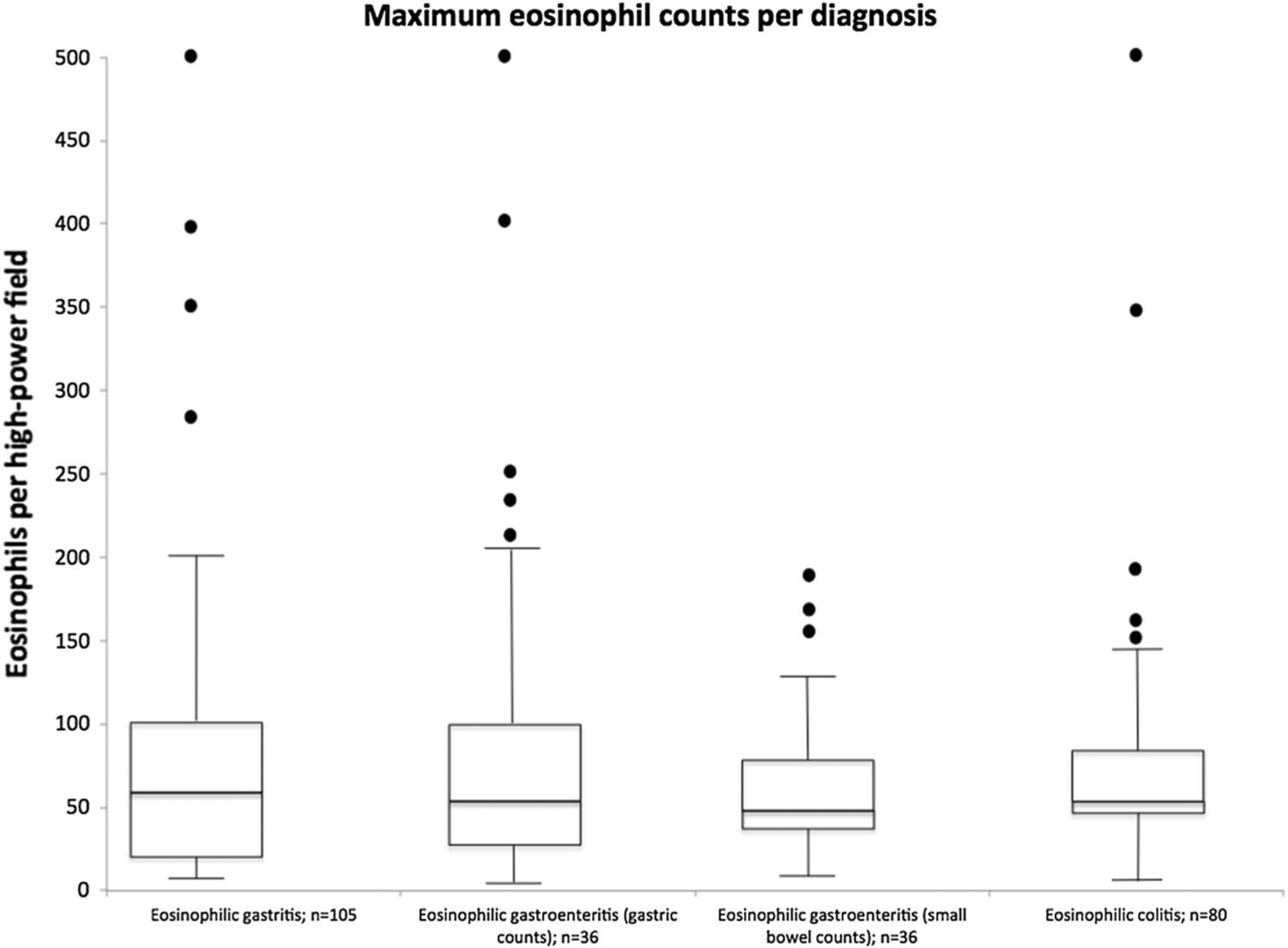
Eosinophil counts by EGID diagnosis. Box and whisker plots—the horizontal sides of the box depict the first and third quartiles for the distributions of eosinophil counts, while the horizontal bar depicts the median. The whiskers extend to the smallest and largest non-outlier data within the set. The dots describe the outliers within the distribution
Table 2.
Mean peak eosinophil counts by disease and site of biopsy at the time of diagnosis
| Eosinophilic gastritis | |||
|---|---|---|---|
| Biopsy site | N | Mean eosinophil count | Range |
| Stomach | |||
| Overall | 105 | 86.7 | 13–500 |
| Fundus | 12 | 48.2 | 20–100 |
| Body | 40 | 86.7 | 13–400 |
| Antrum | 64 | 78.4 | 13–400 |
| Unspecified site | 27 | 120 | 15–500 |
| Eosinophilic gastroenteritis | |||
| Biopsy site | N | Mean eosinophil count | Range |
| Stomach | |||
| Overall | 54 | 78.3 | 6–500 |
| Fundus | 9 | 58.2 | 14–100 |
| Body | 19 | 79 | 6–404 |
| Antrum | 34 | 74.8 | 12–404 |
| Unspecified site | 9 | 112.3 | 23–500 |
| Small intestine | |||
| Overall | 45 | 64.9 | 12–200 |
| Duodenum | 38 | 61.1 | 12–182 |
| Jejunuma | 3 | 76.7 | 25–140 |
| Ileuma | 10 | 73.6 | 32–200 |
| Eosinophilic colitis | |||
| Biopsy Site | N | Mean eosinophil count | Range |
| Colon | |||
| Overall | 80 | 75.6 | 10–500 |
| Right colon | 49 | 75.1 | 9–500 |
| Left colon | 55 | 64.1 | 10–350 |
| Rectosigmoid colon | 37 | 38.9 | 3–135 |
| Unspecified site | 14 | 78.8 | 30–168 |
Data shown as number (N) of population. Peak tissue eosinophil counts are expressed per high-power field (hpf), including mean peak eosinophil count and range of eosinophils/hpf
For subjects with a clinical diagnosis of EGE, 36 (29%) had quantified eosinophils in both the stomach and small intestine
Table 3.
Comparison of adult versus child peak tissue eosinophil counts before and after treatment
| Pre-treatment | Post-treatment | |||||
|---|---|---|---|---|---|---|
| Children | Adults | p value | Children | Adults | p value | |
| Stomach | 75 | 120 | 0.01 | 15 | 45 | 0.01 |
| Small Intestine | 58 | 75 | 0.06 | 3 | 24 | 0.01 |
| Colon | 71 | 72 | 0.95 | Too few to calculate | ||
Data shown as peak eosinophil counts per high-power field (hpf). p value was considered significant if < 0.05
Fig. 3.
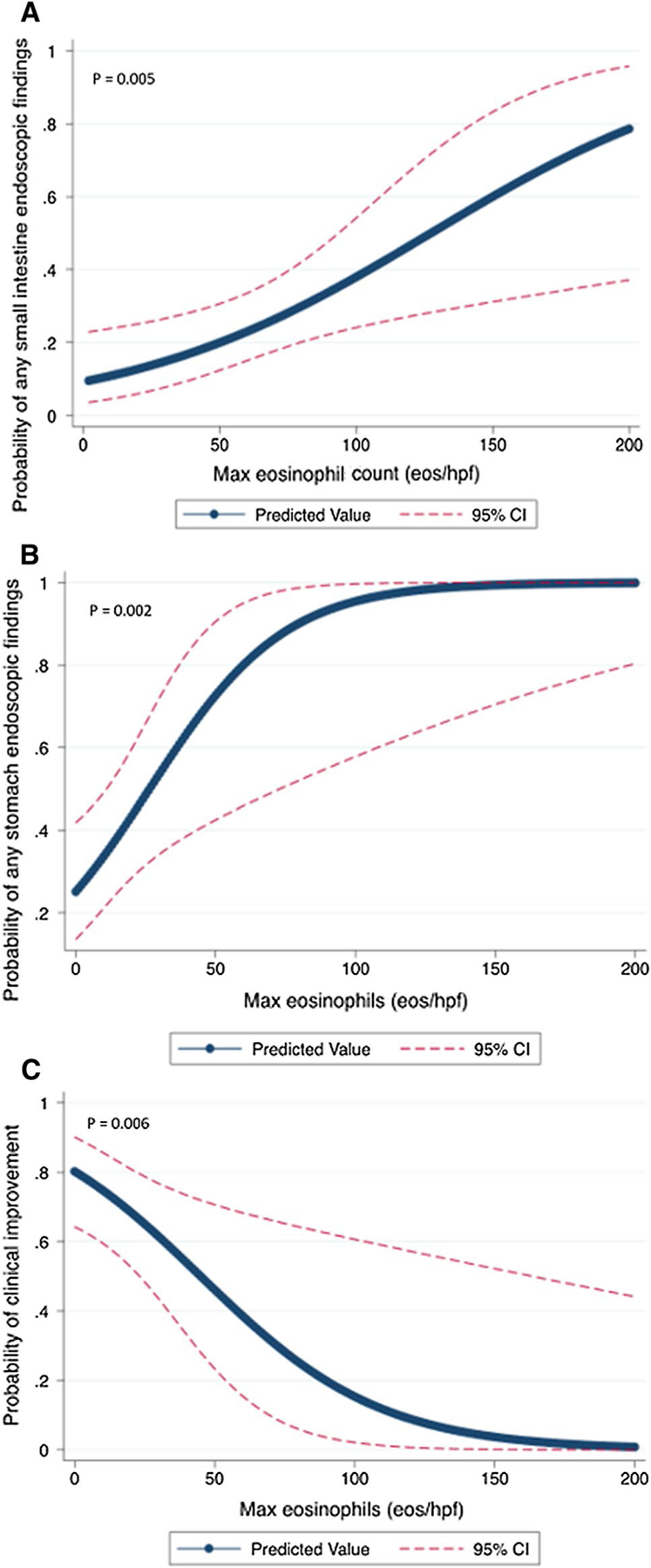
Relationship between pre- and post-treatment eosinophilic count and predicted probabilities of endoscopic and clinical improvement. a Baseline probability of abnormal small bowel endoscopic findings with increasing pre-treatment eosinophil counts. b Probability of persistence of gastric endoscopic findings with decreasing post-treatment eosinophil counts. c Probability of clinical/symptomatic improvement with decreasing post-treatment eosinophil counts
Other associated (but non-eosinophil count) histologic findings were assessed by disease and GI segment at baseline. In EG, normal tissue appearance, outside of elevated tissue eosinophil counts, was described in 9–12% of samples analyzed (Table 4). The most common abnormality was eosinophil degranulation (6%), while cryptitis (1%), crypt abscesses (1%), and lamina propria/muscularis involvement (1%) were uncommonly reported. Other histologic findings including reactive epithelial changes, chronic gastritis, and increased lymphocytes/lymphoid aggregates were recorded in 13% of subjects. Tissue eosinophilia with no additional histologic findings specified in the pathology report (21–47%) was more common in biopsies of the stomach in EGE subjects compared to those with isolated EG (p < 0.05). This was also the most common finding in small intestine biopsies, regardless of segment (24–43%). Less common findings included villous blunting/inflammation (7%) and increased lymphocytes or lymphocyte aggregates (4%). Eosinophilic degranulation was infrequently reported (1%). In EGE subjects, 9% had other histologic findings including reactive epithelial changes, peptic duodenitis with gastric metaplasia, and increased neutrophils. In subjects with EC, tissue eosinophilia with no additional abnormal histologic findings specified in the pathology report was the most common finding (13–30%) in colonic biopsies. Eosinophil degranulation (8%), increased lymphocytes or lymphocyte aggregates (7%), and eosinophilic cryptitis (3%) were the most common abnormal findings. Other histologic findings including lamina propria fibrosis, neutrophilic infiltration, and abnormal crypt architecture were found in 15% of subjects.
Table 4.
Baseline-associated histologic findings by EGID diagnosis and site of biopsy
| Eosinophilic gastritis (n, %) | Eosinophilic gastroenteritis (n, %) | Eosinophilic colitis (n, %) | |
|---|---|---|---|
| Stomach | |||
| Normal appearancea | |||
| Fundus | 1/11 (9%) | 8/17 (47%) | |
| Body | 4/41 (10%) | 6/21 (29%) | |
| Antrum | 9/73 (12%) | 15/72 (21%) | |
| Unspecified site | 0 (0%) | 0 (0%) | |
| Eosinophilic cryptitis | 2/142 (1%) | 0 (0%) | |
| Eosinophilic crypt abscesses | 1/142 (1%) | 1 (1%) | |
| Eosinophil sheets or eosinophilic abscess | 0 (0%) | 1 (1%) | |
| Muscularis involvement | 1/142 (1%) | 0 (0%) | |
| Lamina propria involvement | 2/142 (1%) | 1 (1%) | |
| Eosinophilic degranulation | 8/142 (6%) | 3 (2%) | |
| Other pathologic findings | 18/142 (13%) | 11 (9%) | |
| Small intestine | |||
| Normal appearancea | |||
| Duodenum | 34/79 (43%) | ||
| Jejunum | 0 (0%) | ||
| Ileum | 7/29 (24%) | ||
| Unspecified site | 1/3 (33%) | ||
| Increased lymphocytes or aggregates | 5/123 (4%) | ||
| Villous blunting or inflammation | 9/123 (7%) | ||
| Serosal involvement | 1/123 (1%) | ||
| Crypt hyperplasia | 3/123 (2%) | ||
| Eosinophilic degranulation | 1/123 (1%) | ||
| Other pathologic findings | 11/123 (9%) | ||
| Large intestine | |||
| Normal appearancea | |||
| Right colon | 5 (17%) | ||
| Transverse colon | 4 (13%) | ||
| Rectosigmoid | 9 (30%) | ||
| Unspecified site | 0 (0%) | ||
| Eosinophilic cryptitis | 3/108 (3%) | ||
| Eosinophilic crypt abscesses | 2/108 (2%) | ||
| Eosinophilic degranulation | 9/108 (8%) | ||
| Increased lymphocytes or aggregates | 8/108 (7%) | ||
| Other pathologic findings | 16/108 (15%) |
Data shown as number (n) and % of specific population
Normal appearance denotes no additional histologic findings reported outside of tissue eosinophilia
EGID Treatment and Effects on Endoscopic and Histologic Findings
Of enrolled subjects, 109 (29%) had follow-up within 6 months of initial diagnosis and initiation of treatment. Of these, 40 (32%) EG subjects, 42 (42%) EGE, and 14 (15%) EC subjects underwent repeat endoscopy during the follow-up period while the remaining subjects received clinical follow-up only. As previously published, the majority of subjects had reported clinical, endoscopic, and histologic improvements regardless of treatment utilized (Table 5; Supporting Information) [14]. In the current study, endoscopic and histologic findings after treatment were further analyzed. Among those subjects with improvements in tissue eosinophilia, 79% reported clinical improvement compared to 37% of those without tissue eosinophil reduction (p < 0.001). In regard to histologic findings, 93% of subjects who had reductions in tissue peak eosinophil counts had an improvement in the associated histologic abnormalities found at baseline (Table 4) compared to only 14% of those without such reductions in peak eosinophil counts (p < 0.001). Pediatric subjects had significantly lower levels of stomach and small intestine eosinophils after treatment, compared to adults (p = 0.01) (Table 3). Of all subjects with reductions in tissue peak eosinophil counts, 70% had improvements in endoscopic findings compared to only 28% of those who did not have tissue peak eosinophil count reductions (p = 0.004). While the proportion of subjects with normal endoscopic findings after treatment increased across all diagnoses, the differences were not statistically significant.
Table 5.
Comparison of pre-/post-treatment eosinophil counts by site and disease
| Pre-treatment (n, mean, SD) | Post-treatment (n, mean, SD) | p value | |||||
|---|---|---|---|---|---|---|---|
| All subjects | |||||||
| Stomach | 31 | 119.8 | 136.7 | 31 | 31.5 | 43.8 | 0.001 |
| Small intestine overalla | 15 | 60.5 | 25.7 | 15 | 20.1 | 29 | 0.0003 |
| Duodenum | 12 | 63.1 | 28.1 | 12 | 20.2 | 31.8 | 0.002 |
| Jejunum | N/A | N/A | N/A | ||||
| Ileum | 2 | 47.5 | 10.6 | 2 | 0 | 0 | 0.1 |
| Colon | 6 | 66.3 | 29.3 | 6 | 30.8 | 36.9 | 0.03 |
| EG | |||||||
| Stomach | 22 | 132.6 | 129.5 | 22 | 36.7 | 49 | 0.002 |
| EGE | |||||||
| Stomach | 8 | 93.4 | 166.6 | 18 | 20.9 | 25.6 | 0.27 |
| Duodenum | 7 | 59.6 | 28.4 | 7 | 26.1 | 41 | 0.07 |
| Jejunum | N/A | N/A | N/A | ||||
| Ileum | N/A | N/A | N/A | ||||
| EC | |||||||
| Colon | 4 | 64.5 | 28.6 | 4 | 32.5 | 21.6 | 0.06 |
Data shown as number of subjects with paired pre-/post-treatment tissue eosinophil counts. Eosinophil counts are shown as mean and standard deviation (SD). p value was considered significant if < 0.05. N/A indicates no observations
SI small intestine; including the highest SI value for an individual patient when multiple sections biopsied
After treatment and when analyzing the entire cohort, an improvement in endoscopic findings was associated with improvement in tissue eosinophilia. There were 90% and 65% of patients with and without endoscopic improvement who had documented improvement in tissue peak eosinophil counts, respectively (p = 0.004). There was also a correlation between changes in clinical symptoms and endoscopic findings: 78% of patients with clinical improvement were also found to have endoscopic improvement compared to 55% without such clinical improvement (p = 0.03). Similarly, for the entire cohort, post-treatment gastric eosinophil counts were significantly associated with clinical and endoscopic responses (p = 0.002 and p = 0.006, respectively) (Fig. 3). However, the same analysis could not be performed for post-treatment small bowel and colon eosinophil counts as there were limited post-treatment data for these assessments.
Discussion
Non-esophageal EGIDs are rare disorders and have only been described in small cohorts from single centers. Previous reports have described endoscopic and histologic findings, but small sample sizes have limited the conclusions that can be drawn from these studies. In this study, we describe the endoscopic and histologic findings of a large cohort of non-esophageal EGIDs and characterize changes associated with treatment. We had several notable findings. First, the most common finding during endoscopy, regardless of disease, was normal mucosal appearance. Second, tissue eosinophil counts were only quantified in 59% of the study population, even though they were labeled by EGID diagnosis. Third, treatment improved clinical symptoms as well as endoscopic and histologic findings. Last, declines in tissue eosinophils were associated with improvements in endoscopic findings and in clinical symptoms for those with gastric biopsies. Those with normal endoscopic findings in their stomach and colon after treatment were also more likely to have reduction in tissue eosinophils after treatment.
The finding that the majority of subjects, regardless of disease, had normal mucosal appearance during endoscopy merits particular consideration. Traditionally, EGIDs are considered in patients presenting with GI tract complaints and are found to have abnormalities such as erythema and/or ulceration during endoscopy. While these abnormalities were present in our cohort, normal tissue appearance was most common. This finding has been reported in several previous studies [17–24]. For example, in a study by Reed et al. [19] of 44 EGID subjects, 47% had normal endoscopic exams. Hui et al. [20] evaluated nearly 2500 patients presenting with lower abdominal complaints, 64 of whom were diagnosed with EGE but only 5 (7.8%) of whom had endoscopic abnormalities. Ko et al. [18], in their study of 30 EG children, found similar variability in endoscopic appearance, and Grandinetti et al. [23] found no clear pathognomonic endoscopic findings in their study of 22 EGE patients. When combined with the results from this study, GI tract biopsy is a critical factor in making the diagnosis of non-esophageal EGIDs regardless of endoscopic appearance. However, previous reports have shown marked variability of biopsy practice patterns in EGIDs, so this is an area where future standardization of clinical practice is important [25].
Also of note is that a quantified eosinophil count was only reported in 59% of the study population. This finding may be due to uncertainty regarding the “normal” level of eosinophils throughout the GI tract. Outside of the esophagus, eosinophils are a normal finding in the GI tract. In healthy children, higher numbers of eosinophils can be found in lower segments of the GI tract (cecum and ascending colon) compared to the upper GI tract (stomach and duodenum) [2, 10]. There can also be differences in tissue eosinophilia based on location in the GI tract wall, with higher counts in the lamina propria compared to surface or glandular epithelium [19]. Several studies have suggested histologic criteria for the diagnosis of non-esophageal EGIDs including ≥ 30 eos/hpf in at least 5 hpf of the stomach for EG and > 20 eos/hpf in the duodenum or at least 56 eos/hpf in the ileum for EGE [3, 6, 26]. For EC, ≥ 100 eos/hpf in the cecum/ascending colon, ≥ 84 eos/hpf in the transverse/descending colon, and/or at least 64 eos/hpf in the rectosigmoid colon may indicate disease [14]. In this study, variations on these recommendations were used. For EG diagnosis, a threshold value of 30 eosinophils was required in at least 1 hpf, rather than in 5 hpf because of the unlikely clinical practice of counting eosinophils in more than 1 hpf. For EGE, a threshold value of 50 eos/hpf was chosen which is approximately twice the expected peak count in non-diseased duodenum and ileum. For EC, a threshold value of 60 eos/hpf was used for all sites in the colon. This value provides greater stringency for diagnosing EC in the left colon and rectosigmoid compared to the right colon and may have resulted in under-representation of cases with eosinophilia in those sites compared to the right colon. However, the number of subjects whose biopsies qualified for inclusion in the study are close in number when divided among right and descending colon and rectosigmoid, and the highest peak count at all 3 sites easily exceeds the chosen threshold value. In addition to increased tissue eosinophil counts, findings of tissue pathology including eosinophil sheets, cryptitis or crypt abscesses, muscularis involvement, and/or villous blunting may also be present, helping to make the diagnosis. Regardless, more work is needed to establish appropriate histologic criteria and guidelines for non-esophageal EGIDs which should help pathologists differentiate between normal findings and disease. Education is also likely required about the importance of quantification of GI tract eosinophilia and reporting of associated histologic findings.
We previously reported that treatments such as corticosteroids or food elimination improved clinical, endoscopic, and histologic outcome measures [16]. The current study examined the impact of treatment on specific endoscopic and histologic findings. Treatment led to significant reductions in tissue eosinophilia across all non-esophageal EGIDs as well as improved clinical and endoscopic findings. Subjects with treatment-associated reductions in tissue eosinophilia were more likely to experience clinical and endoscopic improvement compared to those without such reductions. Interestingly, improvements in gastric endoscopic findings were predictive of improvements in gastric tissue eosinophil counts, but this did not hold for the small intestine or colon. This finding again supports the importance of biopsy in assessing disease outcomes.
There are several limitations to this study. First, the study was retrospective and has the inherent limitations of this study design, and endoscopic and pathology findings were not able to be re-reviewed which could have impacted study results. For example, the proportion of normal findings during endoscopy could have been overreported. In the meta-analysis by Kim et al. [22] evaluating the endoscopic findings in EoE, normal findings were reported in 17% of all cases, but this decreased to 7% when only prospective studies were analyzed. Second, eosinophil counts were not quantified in all subjects. However, if eosinophil counts were not present for a specific subject, that subject could still be included if the site investigator confirmed the clinical diagnosis. Biopsy slides of subjects enrolled in this study were not re-reviewed for the study, and the findings reported are based on review by multiple different pathologists with an unknown degree of inter-observer variability. We also relied on investigator interpretation of clinical, endoscopic, and histologic changes after treatment without strict criteria, but specific changes in endoscopic and histologic findings, including tissue eosinophil counts, were also analyzed to reduce bias. Also, changes in endoscopic and histologic findings were not compared to peripheral eosinophil counts, which can serve as a potential biomarker of disease activity. This comparison could be the focus of future studies. Similarly, the low rate of histology findings in addition to peak tissue eosinophil counts could have been due to underreporting. This study included mostly pediatric subjects due to the types of centers that participated. This could limit the impact of results in adult populations. Strengths of the study include the multicenter cohort design, the large number of subjects representing the broadest experience in non-EoE EGIDs yet published, the rigorous data extraction, collection, and sharing protocol, and the comprehensive analysis plan.
In conclusion, the majority of subjects in this study had normal endoscopic findings at the time of presentation, highlighting the importance of having a high level of suspicion for these conditions and obtaining biopsies in order to make an EGID diagnosis. Nearly half of subjects did not have specific numbers of tissue eosinophils reported, possibly due to a lack of consensus regarding threshold levels necessary to diagnosis non-esophageal EGIDs. Finally, treatment improves clinical, endoscopic, and histologic findings and there appears to be a significant association of these processes with gastric eosinophils. These findings suggest that biopsy is not only important in making an EGID diagnosis but in assessing treatment response. Additional work is needed to develop consensus regarding the assessment of endoscopic findings, standardized biopsy protocols, and assessment of associated histologic findings in EGIDs.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10620-019-05961-4) contains supplementary material, which is available to authorized users.
Conflict of interest As a corresponding author, I declare the disclosures and potential conflicts of interest listed below on behalf of all authors. This has been verified to be accurate and up to date at the time of submission of the manuscript. In addition to the disclosures below, this study was funded in part through a research training grant as a part of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) (U54 AI117804). CEGIR is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC. This project also received support from NIH T32 DK007634 (CCR).
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zuo L, Rothenberg ME. Gastrointestinal eosinophilia. Immunol Allergy Clin North Am. 2007;27:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernetsova E, Sullivan K, de Nanassy J, et al. Histologic analysis of eosinophils and mast cells of the gastrointestinal tract in healthy Canadian children. Hum Pathol. 2016;54:55–63. [DOI] [PubMed] [Google Scholar]
- 3.Genta RM, Sonnenberg A, Turner K. Quantification of the duodenal eosinophil content in adults: a necessary step for an evidence-based diagnosis of duodenal eosinophilia. Aliment Pharmacol Ther. 2018;47:1143–1150. [DOI] [PubMed] [Google Scholar]
- 4.Kalach N, Huvenne H, Gosset P, et al. Eosinophil counts in upper digestive mucosa of Western European children: variations with age, organs, symptoms, Helicbacter pylori status, and pathological findings. J Pediatr Gastroenterol Nutr. 2011;52:175–182. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopatho-logical characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011;24:556–563. [DOI] [PubMed] [Google Scholar]
- 7.Silva J, Canao P, Espinheira MC, et al. Eosinophils in the gastrointestinal tract: how much is normal? Virchos Archiv. 2018;473:313–320. [DOI] [PubMed] [Google Scholar]
- 8.DeBrosse CW, Case JW, Putnam P, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–218. [DOI] [PubMed] [Google Scholar]
- 9.Hogan SP, Waddell A, Fulkerson PC. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol. 2013;29:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle B, Collins MH, Wang Z, et al. Histologic correlates of clinical and endoscopic severity in children newly diagnosed with ulcerative colitis. Am J Surg Pathol. 2017;41:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr. 2016;62:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733–1741. [DOI] [PubMed] [Google Scholar]
- 13.Moawad FJ. Eosinophilic esophagitis: incidence and prevalence. Gastrointest Endosc Clin N Am. 2018;28:15–25. [DOI] [PubMed] [Google Scholar]
- 14.Collins MH, Capocelli K, Yang GY. Eosinophilic gastrointestinal disorders pathology. Front Med. 2018;15:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng K, Gupta SK, Kantor S, et al. Creating a multi-center rare disease consortium: The consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Transl Sci Rare Dis. 2017;2:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pesek RD, Reed CC, Muir AB, et al. Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Duan L, Ding S, et al. Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol. 2011;46:1074–1080. [DOI] [PubMed] [Google Scholar]
- 18.Ko HM, Morotti RA, Yershov O, Chehade M. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol. 2014;109:1277–1285. [DOI] [PubMed] [Google Scholar]
- 19.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis. 2015;47:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui CK, Hui NK. A prospective study on the prevalence, extent of disease and outcome of eosinophilic gastroenteritis in patients presenting with lower abdominal symptoms. Gut Liver. 2018;12:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saad AG. Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr Dev Pathol. 2011;14:294–300. [DOI] [PubMed] [Google Scholar]
- 22.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastrenterol Hepatol. 2012;10:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandinetti T, Biedermann L, Bussmann C, et al. Eosinophilic gastroenteritis: clinical manifestation, natural course, and evaluation of treatment with corticosteroids and vedolizumab. Dig Dis Sci. 2019;64:2231–2241 10.1007/s10620-019-05617-3. [DOI] [PubMed] [Google Scholar]
- 24.Ashitani K, Tsuzuki Y, Yamaoka M, et al. Endoscopic features and diagnostic procedures of eosinophilic gastroenteritis. Intern Med. 2019;58:2167–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellon ES, Collins MH, Bonis PA, et al. Substantial variability in biopsy practice patterns among gastroenterologists for suspected eosinophilic gastrointestinal disorders. Clin Gastroenterol Hepatol. 2016;14:1842–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43:257–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


