Abstract
IMPORTANCE
There is limited evidence regarding how patients make choices in advance directives (ADs) or whether these choices influence subsequent care.
OBJECTIVE
To examine whether default options in ADs influence care choices and clinical outcomes.
DESIGN, SETTING, AND PARTICIPANTS
This randomized clinical trial included 515 patients who met criteria for having serious illness and agreed to participate. Patients were enrolled at 20 outpatient clinics affiliated with the University of Pennsylvania Health System and the University of Pittsburgh Medical Center from February 2014 to April 2016 and had a median follow-up of 18 months. Data analysis was conducted from November 2018 to April 2019.
INTERVENTIONS
Patients were randomly assigned to complete 1 of the 3 following ADs: (1) a comfort-promoting plan of care and nonreceipt of potentially life-sustaining therapies were selected by default (comfort AD), (2) a life-extending plan of care and receipt of potentially life-sustaining therapies were selected by default (life-extending AD), or (3) no choices were preselected (standard AD).
MAIN OUTCOMES AND MEASURES
This trial was powered to rule out a reduction in hospital-free days in the intervention groups. Secondary outcomes included choices in ADs for an overall comfort-oriented approach to care, choices to forgo 4 forms of life support, patients’ quality of life, decision conflict, place of death, admissions to hospitals and intensive care units, and costs of inpatient care.
RESULTS
Among 515 patients randomized, 10 withdrew consent and 13 were later found to be ineligible, leaving 492 (95.5%) in the modified intention-to-treat (mITT) sample (median [interquartile range] age, 63 [56–70] years; 279 [56.7%] men; 122 [24.8%] black; 363 [73.8%] with cancer). Of these, 264 (53.7%) returned legally valid ADs and were debriefed about their assigned intervention. Among these, patients completing comfort ADs were more likely to choose comfort care (54 of 85 [63.5%]) than those returning standard ADs (45 of 91 [49.5%]) or life-extending ADs (33 of 88 [37.5%]) (P = .001). Among 492 patients in the mITT sample, 57 of 168 patients [33.9%] who completed the comfort AD, 47 of 165 patients [28.5%] who completed the standard AD, and 35 of 159 patients [22.0%] who completed the life-extending AD chose comfort care (P = .02), with patients not returning ADs coded as not selecting comfort care. In mITT analyses, median (interquartile range) hospital-free days among 168 patients assigned to comfort ADs and 159 patients assigned to life-extending default ADs were each noninferior to those among 165 patients assigned to standard ADs (standard AD: 486 [306–717] days; comfort AD: 554 [296–833] days; rate ratio, 1.05; 95% CI, 0.90–1.23; P < .001; life-extending AD: 550 [325–783] days; rate ratio, 1.03; 95% CI, 0.88–1.20; P < .001). There were no differences among groups in other secondary outcomes.
CONCLUSIONS AND RELEVANCE
In this randomized clinical trial, default options in ADs altered the choices seriously ill patients made regarding their future care without changing clinical outcomes.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT02017548
Graphical Abstract
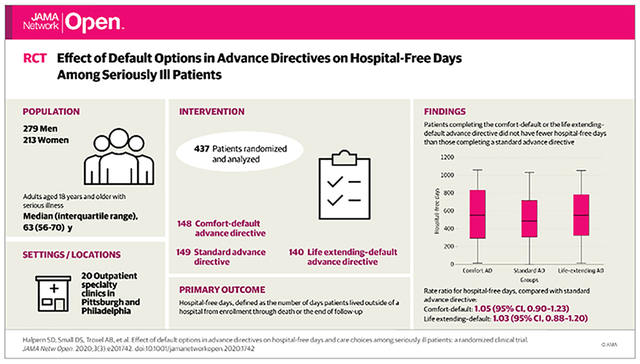
Effect of Default Options in Advance Directives on Hospital-Free Days Among Seriously Ill Patients
Introduction
Seriously ill patients are often hospitalized and receive life-sustaining therapies by default, ie, unless patients or their caregivers have specifically requested otherwise.1,2 Advance directives (ADs) were created to enable the many patients who wish to forgo such aggressive care near the end of life3,4 to set limits on their future therapies.5 However, despite national policies and practices that increasingly encourage AD completion,6–10 evidence regarding the benefits of AD completion, or of making certain choices within ADs, is limited.
The 2 randomized clinical trials11,12 (RCTs) showing benefits of ADs included only very elderly patients who were already in nursing homes or hospitals. Observational studies have shown that patients who complete ADs in the community less commonly die in a hospital,13–16 more often receive care consistent with their preferences,15 and, in certain regions, receive less costly care.16 However, the likelihood of unmeasured differences between patients who do and do not choose to complete ADs precludes inferences regarding whether AD completion or the choices made in ADs cause such benefits.17,18
Given the logistical and ethical difficulties of randomly assigning patients to complete ADs, we sought to determine whether the choices made in ADs could be altered using default options19 and, if so, whether these experimentally induced differences in choices caused different patient outcomes. In a pilot RCT,20 we randomly assigned 132 patients with advanced thoracic illnesses to complete 3 versions of real ADs with default options set to nudge certain treatment choices. These default options significantly influenced the treatments patients selected in the ADs without altering their satisfaction with advance care planning.20 In the present trial, we sought to determine whether default options similarly influenced the AD choices made by a new and larger population of patients with a more diverse range of chronic serious illnesses. We then tested the hypotheses that assignment to complete ADs with default options for overall care and for receiving 4 forms of life support would not reduce the primary outcome of days alive and outside of a hospital (ie, hospital-free days) and would improve secondary patient-reported and clinical outcomes. Finally, we sought to prospectively examine how often seriously ill patients make changes in legally valid ADs.
Methods
Trial Design
We conducted a 3-group RCT comparing patients who were encouraged to complete a standard AD or 1 of 2 ADs with different default options for overall goals of care and preferences to receive 4 forms of life support (ie, cardiopulmonary resuscitation, mechanical ventilation, dialysis, and a surgical feeding tube). The study protocol and statistical analysis plan have been described previously (Supplement 1)21 and were approved by the institutional review boards at the University of Pennsylvania, the University of Pittsburgh, and Rowan University. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
Eligible patients were aged 18 years and older with 1 of the serious illnesses summarized in Table 1. They were recruited from 20 specialty clinics affiliated with the University of Pennsylvania Health System and the University of Pittsburgh Medical Center. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes were used to identify serious illnesses, and the limited exclusion criteria we applied have been described previously.21
Table 1.
Characteristics of the 492 Participants in the Modified Intention-to-Treat Sample
| No. (%) | |||
|---|---|---|---|
| Characteristic | Comfort AD (n = 168) | Standard AD (n = 165) | Life-extending AD (n = 159) |
| Age, mean (SD), y | 62.16 (11.65) | 62.93 (9.79) | 61.92 (11.45) |
| Sex | |||
| Men | 102 (60.7) | 88 (53.3) | 89 (56.0) |
| Women | 66 (39.3) | 77 (46.7) | 70 (44.0) |
| Race | |||
| White | 116 (69.0) | 115 (69.7) | 116 (73.0) |
| Black or African American | 45 (26.8) | 42 (25.5) | 35 (22.0) |
| Other | 6 (3.6) | 7 (4.2) | 7 (4.4) |
| Missing or unknown | 1 (0.6) | 1 (0.6) | 1 (0.6) |
| Ethnicity | |||
| Not Hispanic or Latino | 143 (85.1) | 142 (86.1) | 142 (89.3) |
| Hispanic or Latino | 4 (2.4) | 2 (1.2) | 2 (1.3) |
| Missing or unknown | 21 (12.5) | 21 (12.7) | 15 (9.4) |
| Marital status | |||
| Currently married or living with partner | 99 (58.9) | 107 (64.8) | 109 (68.6) |
| Divorced or separated | 26 (15.5) | 25 (15.2) | 22 (13.8) |
| Never married | 30 (17.9) | 21 (12.7) | 17 (10.7) |
| Widowed | 13 (7.7) | 8 (4.8) | 10 (6.3) |
| Missing | 0 | 4 (2.4) | 1 (0.6) |
| Education | |||
| <High school | 8 (4.8) | 10 (6.1) | 7 (4.4) |
| High school or GED | 48 (28.6) | 54 (32.7) | 48 (30.2) |
| Some college | 40 (23.8) | 33 (20.0) | 44 (27.7) |
| College degree | 37 (22.0) | 41 (24.8) | 37 (23.3) |
| >College | 32 (19.0) | 24 (14.5) | 22 (13.8) |
| Missing | 3 (1.8) | 3 (1.8) | 1 (0.6) |
| Income, $ | |||
| <30 000 | 53 (31.5) | 37 (22.4) | 38 (23.9) |
| 30 000–69 999 | 57 (33.9) | 60 (36.4) | 57 (35.8) |
| 70 000–99 999 | 22 (13.1) | 25 (15.2) | 24 (15.1) |
| ≥100 000 | 32 (19.0) | 32 (19.4) | 32 (20.1) |
| Missing | 4 (2.4) | 11 (6.7) | 8 (5.0) |
| Religion | |||
| Catholic | 58 (34.5) | 49 (29.7) | 65 (40.9) |
| Protestant | 62 (36.9) | 60 (36.4) | 53 (33.3) |
| Other Christian | 19 (11.3) | 21 (12.7) | 8 (5.0) |
| Jewish | 7 (4.2) | 8 (4.8) | 4 (2.5) |
| Other faiths | 4 (2.4) | 6 (3.6) | 6 (3.8) |
| Unaffiliated | 13 (7.7) | 15 (9.1) | 17 (10.7) |
| Missing | 5 (3.0) | 6 (3.6) | 6 (3.8) |
| Diagnosis | |||
| Cancer | 121 (72.0) | 127 (77.0) | 115 (72.3) |
| COPD and other incurable lung disease | 19 (11.3) | 15 (9.1) | 11 (6.9) |
| End-stage renal disease | 11 (6.5) | 9 (5.5) | 14 (8.8) |
| Congestive heart failure | 4 (2.4) | 4 (2.4) | 3 (1.9) |
| Amyotrophic lateral sclerosis | 13 (7.7) | 10 (6.1) | 16 (10.1) |
Abbreviation: AD, advance directive; COPD, chronic obstructive pulmonary disease; GED, general education diploma.
From February 6, 2014, to April 15, 2016, recruiters screened electronic health records (EHRs) to identify eligible patients scheduled for outpatient visits the following week. When patients screened eligible, we notified the outpatient physician by email that we would recruit the patient at the next visit unless the physician requested otherwise by reply email. In the clinics, 1 of 6 recruiters approached each preliminarily eligible patient, described the rationale for AD completion, and sought patients’ written consent to participate in a study comparing 3 different versions of legally valid ADs.21 All consenting patients were given 1 of 3 randomly assigned AD versions, an informational brochure about ADs, the recruiter’s contact information, and a stamped envelope addressed to study staff for returning the AD.
Recruiters encouraged patients to complete their ADs at home with their caregivers and/or with physicians and to return completed ADs, with the signatures of 2 witnesses or a notary, within 10 days. If a returned AD was not received within 10 days, research staff called patients weekly for up to 1 month to answer questions and remind patients to return their ADs (eFigure 1 in Supplement 2).
Randomization and Interventions
Patients were randomized individually to receive 1 of 3 versions of the AD with equal probabilities using electronic random number generation. Randomization was stratified by the 6 research coordinators who recruited patients from different clinics, and we used variable block sizes of 3 and 6 within each recruiter to promote balance. Each AD version was based on the professionally endorsed AD published by the Allegheny County Medical Society and aligned with Pennsylvania statutory guidance. We added 1 question to each AD version asking patients to choose an overall plan of care focused on life extension or comfort if those 2 goals were to come into conflict. Language from the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) trial was used for this question.22,23
The differences in the 3 AD versions are described in eTable 1 in Supplement 2. In the standard AD, patients were asked to actively choose an overall goal of care and whether to receive each of 4 forms of life support. The comfort AD was identical to the life-extending AD, except the comfort plan of care (as opposed to the life-extension plan of care) was listed first and preselected, as were choices to forgo rather than receive the 4 life-sustaining interventions. Patients were informed by research staff and written instructions that other choices could be made by crossing out the preselected options and selecting alternatives, which were identical to those in the standard AD. Patients receiving standard ADs were secondarily randomized to receive a version with either comfort or life-extending options listed first. Because this was merely intended to mitigate ordering effects, all patients in the standard AD group were analyzed together. Thus, there were 3 groups with a total of 4 versions of the AD (eAppendix in Supplement 2).
Debriefing and Processing of ADs
Patients who returned ADs were called by a research coordinator who debriefed them about the precise differences between their assigned AD version and the versions received by other patients. Using an institutional review board–approved debriefing script also employed in our pilot study,20 the coordinator ensured that patients understood the use of default options in the 3 AD versions. The coordinator then reviewed each choice the patient made and encouraged patients to make any changes to these choices before considering the AD complete. We then asked clinic staff to scan the completed ADs into the patient EHR and mailed completed versions to the patient, any identified surrogate, and other requested caregivers.
Outcomes
The primary outcome was hospital-free days, defined as the number of days patients lived outside of a hospital from enrollment through death or the end of follow-up on December 31, 2016. Research staff masked to the patients’ assigned groups attempted to contact patients 2, 6, and 12 months after AD completion to assess secondary, patient-reported outcomes and to help patients make desired changes to their ADs. Decision conflict24 was assessed immediately after patients completed their ADs. Satisfaction with advance care planning, measured with a global satisfaction question,25,26 was assessed as close as possible to 2 months after AD completion. Quality of life was measured using the McGill Quality of Life instrument27,28 as close as possible to 6 months after AD completion. Other secondary outcomes included the choices patients made in their original ADs and choices to modify their AD selections during follow-up.
We used state databases capturing all admissions and inpatient procedures in Pennsylvania and New Jersey to measure survival, hospital and intensive care unit admissions, inpatient costs of care, and receipt of the previously described forms of life support. We had planned to measure surrogates’ perceptions of the quality of death, bereavement outcomes, and health system distrust.21 However, we abandoned this plan after roughly 100 patients were enrolled because of difficulties reaching surrogates within 3 months of patients’ deaths. Owing to delays in obtaining finalized data from the state registries, the data set was considered final in November 2018, and analyses were conducted from November 2018 to April 2019.
Statistical Analysis
We used negative binomial regression to compare the number of hospital-free days among groups, and we used logistic, linear, negative binomial, or Cox proportional hazards models, as appropriate based on outcome parameterizations and distributions, for all secondary outcomes. In all analyses, we first modeled the patient’s recruiter as a fixed effect to adjust for potential clustering. However, because recruiters worked in specific clinics, patient diagnosis and recruiter were collinear. Therefore, we calculated models with recruiter or with diagnosis (ie, cancer vs other) and report the latter because the coefficients for the intervention variables were nearly identical (Statistical Analysis Plan in Supplement 1).
Primary analyses of clinical outcomes were conducted in a modified intention-to-treat (mITT) sample, including all patients who were randomized, were not subsequently found to be ineligible, and did not withdraw consent. Analyses of patient-reported outcomes were conducted among patients who completed ADs and were debriefed. Choices made in ADs were analyzed in both groups. In the mITT sample, patients not completing ADs were considered to have not chosen comfort-oriented approaches to care and to have not chosen to forgo life support, consistent with the underlying defaults in clinical practice. We also evaluated how making certain choices in ADs affected hospital-free days using complier average treatment effect analyses,29,30 which use the randomization ground as an instrumental variable to account for the AD noncompletion that we anticipated in advance.21
We decided a priori that if default options connoted harm, they would be unlikely to be used in ADs outside of research, even if they produced other benefits. Thus, although we were motivated to learn whether changing choices in ADs could improve a variety of patient-centered outcomes and used traditional superiority tests to compare these outcomes among arms, we sought to enroll enough patients to rule out, using noninferiority tests, the possibility that assignment to either of the default ADs would reduce patients’ hospital-free days.21 Specifically, we sought to rule out a rate ratio of less than 0.85, which corresponds to a 15% reduction in hospital-free days associated with use of a default AD compared with the standard AD. Simulations that conservatively assumed substantial data dispersion showed that enrolling 270 patients who completed ADs would enable a noninferiority test with 80% power at this prespecified margin, accounting for the 2 primary hypothesis tests using a Bonferroni correction.
Data analysis was conducted using R version 3.4.2 (R Project for Statistical Computing) and Stata version 15 (StataCorp). Statistical significance was set at P < .05. The noninferiority tests were 1-tailed, and all other tests were 2-tailed.
Results
Among 917 patients who were eligible to participate and were approached, 515 (56.2%) consented and were randomized (Figure 1). Of these, 10 patients withdrew their consent and 13 were determined to be ineligible shortly after randomization, leaving 492 patients in the mITT sample. These patients had a median (interquartile range [IQR]) age of 63 (56–70) years, with 279 (56.7%) men, 122 (24.8%) black participants, 175 participants (35.6%) with a high school education or less, and 363 participants (73.8%) with an advanced cancer as their primary illness (Table 1).
Figure 1. Patient FlowDiagram.
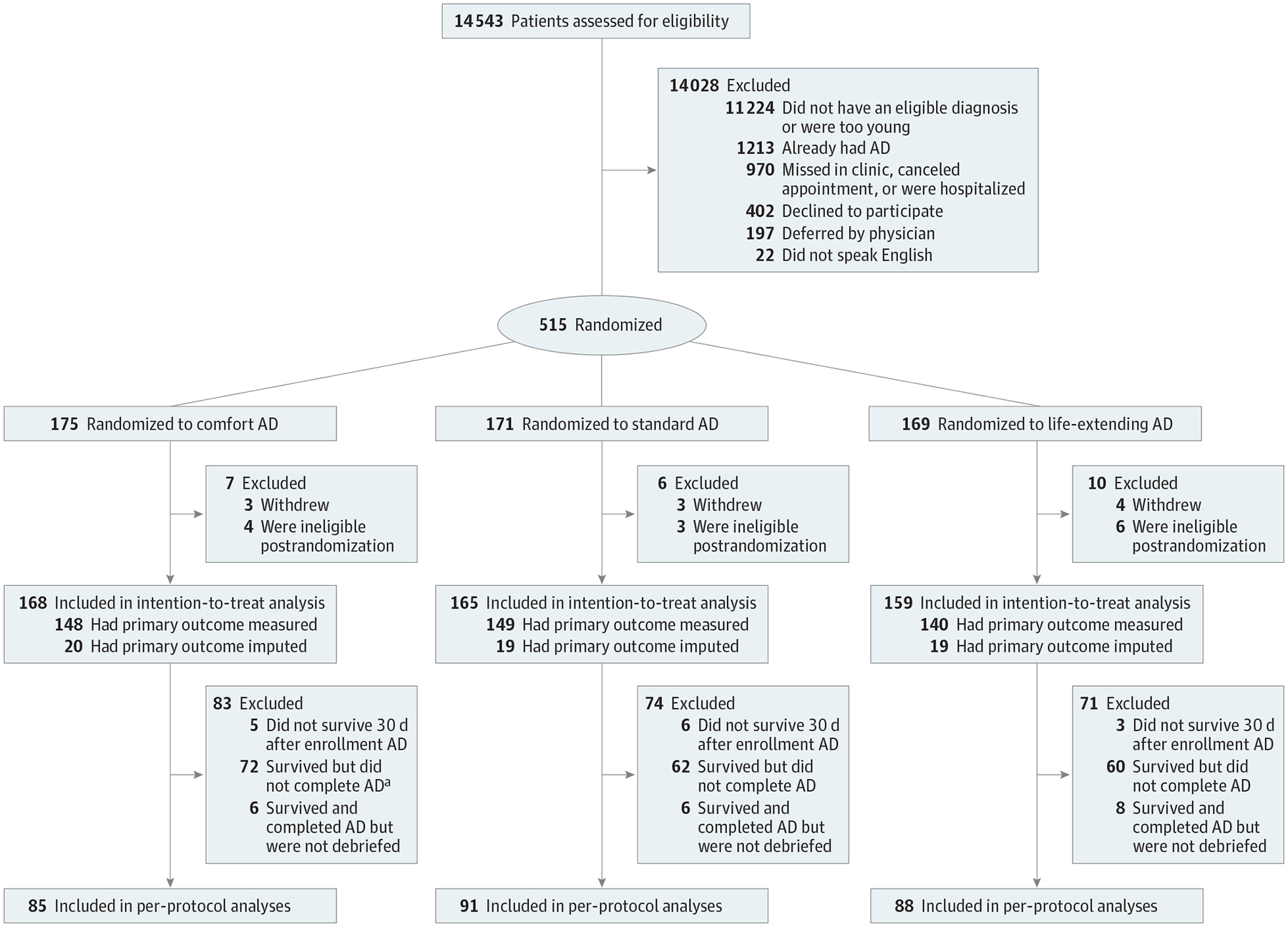
AD indicates advance directive.
a P for AD completion and debriefing by treatment group = .62
Of these 492 patients, 14 (2.8%) did not survive for 30 days, 284 (59.4%) of the remaining 478 patients completed ADs, and 264 (93.0%) of these were successfully debriefed (median [IQR] 14 [5–35] days after returning the AD). The 264 patients who completed ADs and were debriefed were similar to the other 228 patients across all measured variables (eTable 2 in Supplement 2). The proportions of patients who completed ADs and were debriefed were similar across groups (Figure 1). Advance directives were scanned into the EHR for 186 of 264 patients (70.5%) a median (IQR) of 20 (9–47) days after debriefing. Final ADs were mailed to all patients and identified surrogates within a week of debriefing.
Choices Made in AD
Among the 264 patients who completed ADs and were debriefed, only 1 (0.4%) changed the choice they made regarding overall goals of care during debriefing and 10 or fewer (≤3.8%) changed their preferences regarding whether or not to forgo each form of life support (eTable 3 in Supplement 2). After incorporating these changes into the final ADs, patients completing comfort ADs were more likely to choose a comfort-oriented plan of care (54 of 85 [63.5%]) than patients completing standard ADs (45 of 91 [49.5%]) or life-extending ADs (33 of 88 [37.5%]) (P = .001) (Figure 2). This pattern was preserved among all 492 patients in the mITT sample after coding patients who had not returned ADs as having not chosen a comfort-oriented plan of care (comfort AD: 57 of 168 [33.9%]; standard AD: 47 of 165 [28.5%]; life-extending AD: 35 of 159 [22.0%]; P = .02) (Figure 2).
Figure 2. Selected Goals of Care Chosen.
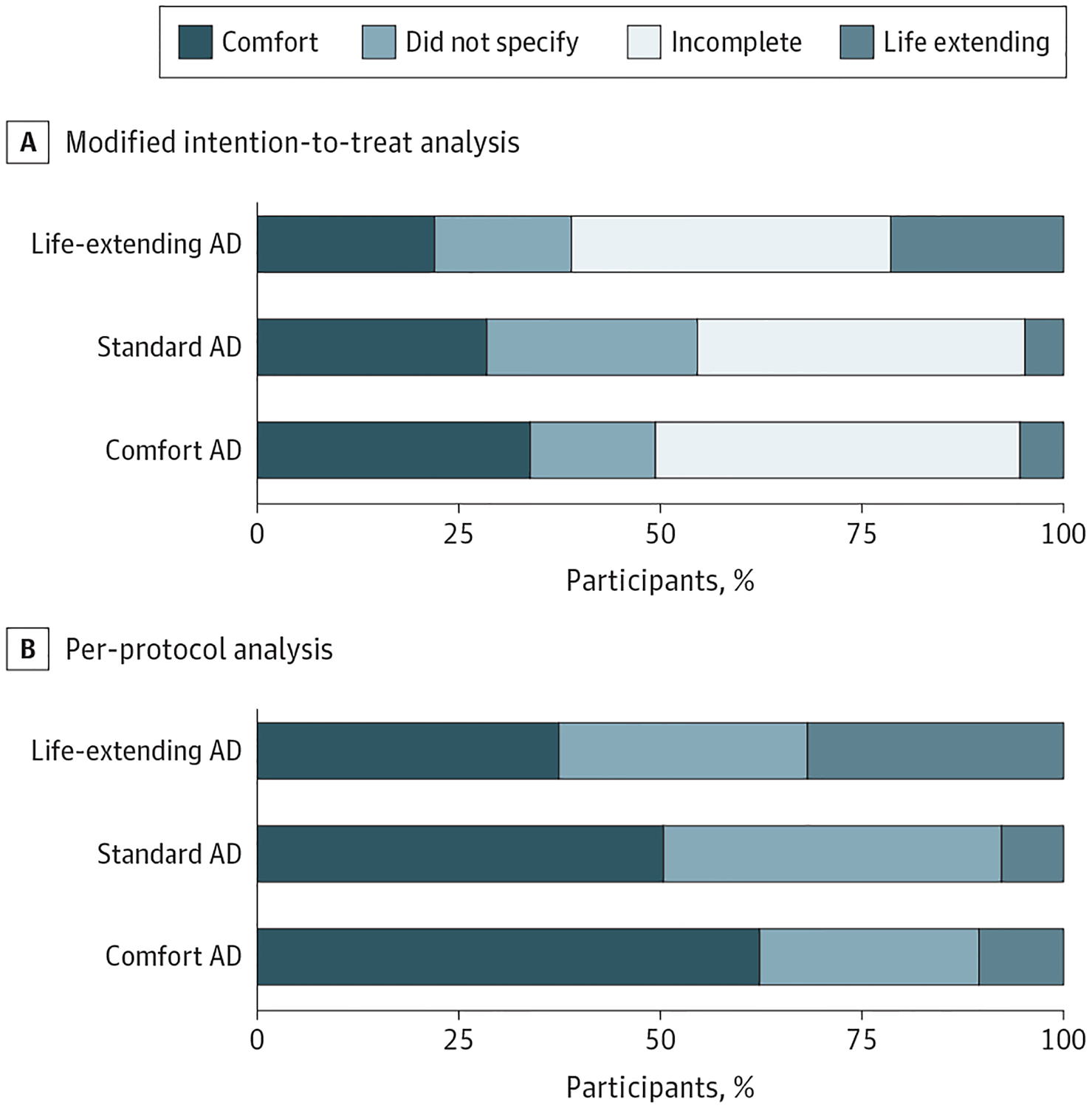
A, The first panel plots the proportions of the 492 patients in the modified intention-to-treat analysis who chose comfort-oriented care, life-extending care, and choices not to specify a preference. The 228 patients who did not complete an advance directive (AD) and/or were not debriefed are coded as incomplete. B, The second panel plots the same proportions among the 264 patients with completed ADs who were also debriefed.
Similarly, in the per-protocol analysis, patients completing comfort ADs were more likely than patients completing other ADs to make choices to forgo mechanical ventilation (comfort AD: 47 [55.3%]; standard AD: 34 [37.4%]; life-extending AD: 34 [38.6%]; P = .03), dialysis (comfort AD: 46 [54.1%]; standard AD: 25 [27.5%]; life-extending AD: 29 [33.0%]; P = .004), and feeding tube insertion (comfort AD: 42 [49.4%]; standard AD: 34 [37.4%]; life-extending AD: 28 [31.8%]; P = .02), without corresponding differences for cardiopulmonary resuscitation (comfort AD: 27 [31.8%]; standard AD: 22 [24.2%]; life-extending AD: 19 [21.6%]; P = .13) (eFigure 2 in Supplement 2). In the mITT sample, similar patterns were observed, but the difference was only statistically significant for forgoing dialysis (comfort AD: 49 [29.2%]; standard AD: 28 [17.0%]; life-extending AD: 32 [20.1%]; P = .046) (eFigure 3 in Supplement 2).
Clinical Outcomes
Of 492 patients in the mITT sample, 55 (11.2%) failed to provide valid social security numbers (SSNs), precluding linkages necessary to measure utilization outcomes. The 55 patients who did not provide valid SSNs were evenly distributed among the 3 groups (eTable 4 in Supplement 2). Compared with patients who returned valid SSNs, these patients were better educated (college and postcollege degree: 162 [37.1%] vs 31 [56.4%]; P = .02) and reported higher incomes (≥$100 000: 75 [17.2%] vs 21 [38.2%]; P = .003) but were otherwise similar (eTable 4 in Supplement 2). Median (IQR) follow-up for the 437 patients with valid SSNs was 18 (11–27) months.
The median (IQR) hospital-free days observed among patients assigned to the comfort AD and to the life-extending AD were not less than the median (IQR) number of hospital-free days among patients assigned to the standard AD (standard AD: 486 [306–717] days; comfort AD: 554 [296–833] days; rate ratio, 1.05; 95% CI, 0.90–1.23; P < .001; life-extending AD: 550 [325–783] days; rate ratio, 1.03; 95% CI, 0.88–1.20; P < .001) (Figure 3; eTable 5 in Supplement 2). A sensitivity analysis, in which hospital-free days were generated using multiple imputation for the 55 patients who did not provide valid SSNs produced similar results (eTable 6 in Supplement 2). Additionally, complier average treatment effect analyses revealed that choices to promote comfort care did not alter the number of hospital-free days (eTable 7 in Supplement 2).
Figure 3. Hospital-Free Days.
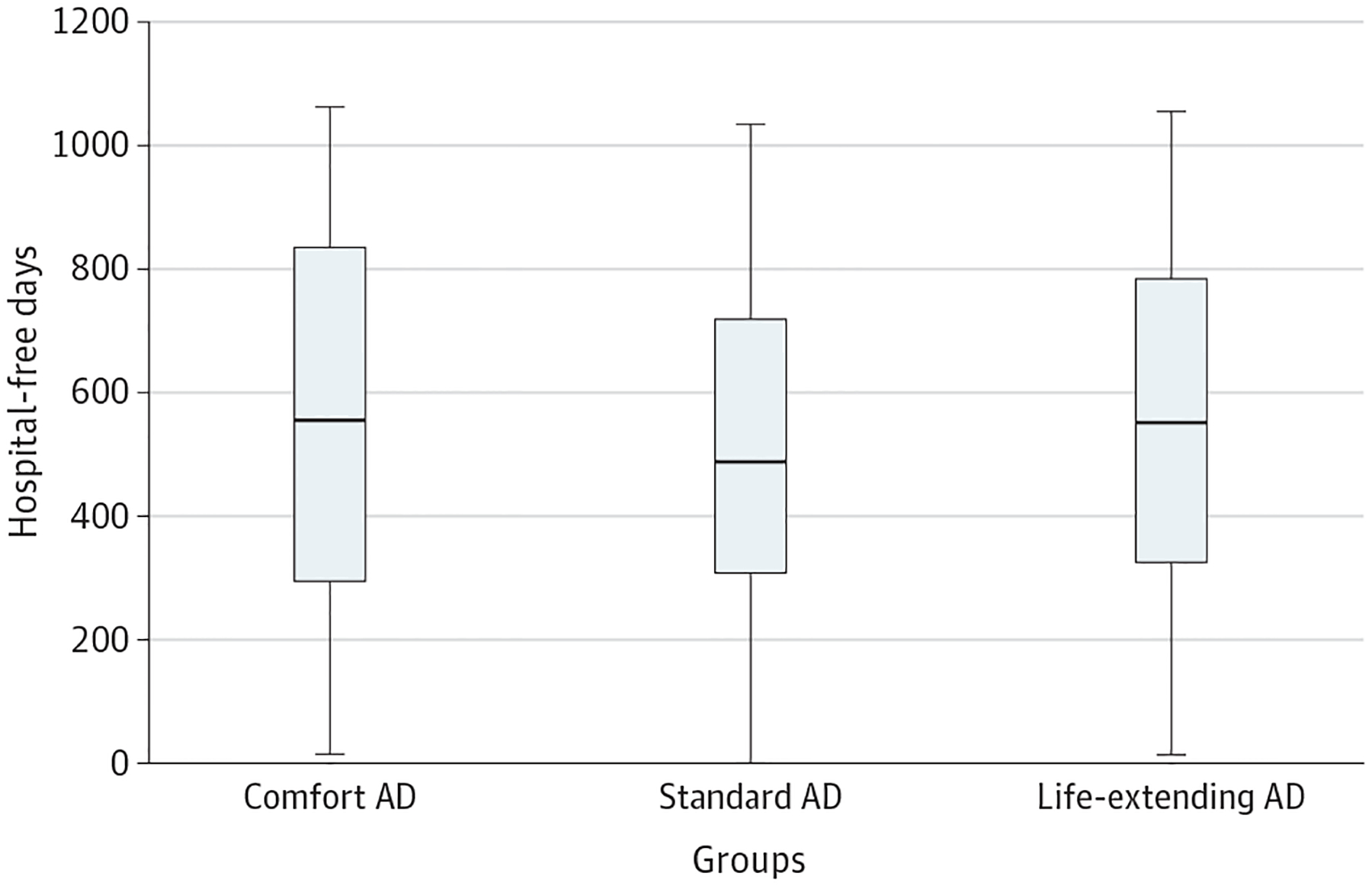
This figure plots hospital-free days among the 492 patients in the modified intention-to-treat sample across the 3 intervention arms. The horizontal line represents the median; the height of the box, the interquartile range; and the vertical lines, the range.
In mITT analyses, the assigned AD version was not significantly associated with survival, hospital or intensive care admissions, place of death, receipt of any form of life support during follow-up, or costs of inpatient care (Table 2; eTable 8 and eTable 9 in Supplement 2). Because these analyses may be biased toward the null by including all randomized and eligible patients, we performed a post hoc analysis limited to the 179 patients who completed ADs, were debriefed, had their ADs uploaded into the EHR, and provided a valid SSN. These patients were equally represented among the 3 trial groups (eTable 10 in Supplement 2). In this restricted sample, patterns of choices made in the 3 AD versions were similar to those observed among all patients completing ADs (eTable 11 in Supplement 2), and there remained no significant associations of AD version with hospital-free days, days in the hospital, or receipt of any life-sustaining therapy (eTable 11 in Supplement 2).
Table 2.
Summary of Adjusted Comparisons of Secondary Outcomes Across Groups
| Outcome | Raw scorea | Estimation method | Adjusted effect estimate (95% CI) | Comfort AD vs standard | Life-extending AD vs standard | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comfort AD | Standard AD, reference | Life-extending AD | Comfort AD | Life-extending AD | Unadjusted P valueb |
Adjusted P value |
Unadjusted P valueb |
Adjusted P value |
||
| Decision conflict total score, mean (SD)c | 17 (12.4) | 16.2 (13.8) | 17.4 (13.9) | Linear change from reference | 0.51 (−3.83 to 4.85) | 1.5 (−2.91 to 5.9) | .73 | .82 | .60 | .50 |
| Very satisfied with advance care planning, No./total No. (%)d | 68/71 (95.8) | 69/76 (90.8) | 62/69 (89.8) | Odds ratio | 2.31 (0.60 to 11.17) | 0.95 (0.30 to 2.97) | .24 | .25 | .85 | .93 |
| McGill Quality of Life, mean (SD)e | 6.2 (3.2) | 6.4 (3.2) | 5.9 (3.1) | Linear change from reference | 0.11 (−0.85 to 1.07) | −0.04 (−0.99 to 0.92) | .82 | .82 | .38 | .94 |
| Survival, median (iQR), df | 564 (302.8 to 843.2) | 494 (717 to 316) | 553.5 (325.8 to 791.2) | Hazard ratio | 0.66 (0.37 to 1.19) | 0.83 (0.45 to 1.51) | .04 | .17 | .39 | .54 |
| Died in hospital, No./Total No. (%)g | 13/148 (8.8) | 23/149 (15.4) | 14/140 (10) | Odds ratio | 0.52 (0.24 to 1.05) | 0.61 (0.29 to 1.23) | .08 | .08 | .15 | .17 |
| Hospital admissions, median (IQR), No.h | 1 (0 to 2) | 1 (0 to 3) | 1 (0 to 2) | Incident rate ratio | 0.98 (0.72 to 1.33) | 0.87 (0.63 to 1.18) | .57 | .88 | .24 | .37 |
| ICU admissions, median (IQR), No.h | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | Incident rate ratio | 0.91 (0.46 to 1.81) | 0.84 (0.4 to 1.73) | .72 | .79 | .61 | .64 |
| Total cost of inpatient care, median (IQR), thousands of $i | 28.7 (0 to 188.1) | 60.7 (0 to 267.8) | 31.9 (0 to 162.2) | Log linear change from reference | −1.09 (−2.43 to 0.25) | −0.78 (−2.14 to 0.58) | .11 | .11 | .22 | .26 |
| Cost of inpatient care per hospitalization day, median (IQR), thousands of $i | 6.7 (0 to 3.2) | 9.4 (0 to 15.0) | 7.1 (0 to 16.0) | Log linear change from reference | −0.9 (−1.97 to 0.16) | −0.52 (−1.6 to 0.56) | .09 | .97 | .30 | .35 |
| Receipt of life-sustaining therapy, No./Total No. (%)j | 16/148 (10.8) | 20/149 (13.4) | 15/140 (10.7) | Odds ratio | 0.64 (0.3 to 1.33) | 0.72 (0.34 to 1.51) | .49 | .48 | .24 | .39 |
Abbreviations: AD, advance directive; ICU, intensive care unit; IQR, interquartile range.
The number of responses is different for different outcomes, given that patient-reported outcomes were collected only for per-protocol sample and there are some missing responses.
Unadjusted P values are reported from univariate analysis. The adjustments include patient characteristics such as age, gender, race, and education.
The estimate for patients’ decision conflict scale is the ordinary least square estimate. A total of 71 patients in the comfort AD group, 75 patients in the standard AD group, and 68 patients in the life-extending AD group completed this measure.
Satisfaction scale analyzed as binary variable with levels very satisfied and not very satisfied. The data used in the model were responses of the patients after approximately 2 months of AD completion.
McGill Quality of Life is reported for 247 patients and imputed for 17 patients, for a total of 264 patients (85 in the comfort AD group, 91 in the standard AD group, and 88 in the life-extending AD group). The regression table and imputation method are described in eTable 12 in Supplement 2. The reported estimate is the mean response from linear regression model. Among these 247 scores, 3 are calculated from a surrogate’s response
Survival data were available for all participants. Survival was analyzed using Cox proportional hazards model.
Place of death categorized as death at the hospital and other. The other category includes death at other places and patients who were still alive.
Hospital admissions and ICU admissions are treated as counts, and suitable count models have been used to model those outcomes. The reported estimates are incident rates. Hospital admission data were available for all participants. For ICU admissions, data were available only from Pennsylvania database, representing 143 patients in the comfort AD group, 134 patients in the standard AD group, and 124 patients in the life-extending AD group.
Total cost of inpatient care and cost of inpatient care per day were available for all participants. The cost analysis was done by log transforming inpatient care charges. The reported estimates are βs. The decrease of hospital-free days in the intervention arm = (ex (β)−1) × 100%.
Percentage of patients receiving any 1 of cardiopulmonary resuscitation, mechanical ventilation, dialysis, or surgical feeding tube.
Patient-Reported Outcomes
Among patients who completed ADs, the version they were assigned to complete was not associated with their levels of decision conflict or satisfaction with advance care planning (Table 2). Quality of life was reported by 247 patients and imputed for the remaining 17 patients. Median (IQR) quality of life scores were 8.34 (5.80–8.97), 8.43 (7.00–8.92), and 7.67 (6.28–8.48) among patients who completed the comfort AD, standard AD, and life-extension AD, respectively (adjusted P values among groups were all >.20) (eTable 12 in Supplement 2).
Discussion
In this RCT of different types of legally valid ADs, we found that default options strongly influenced the choices that seriously ill patients made regarding their overall goals of care and often their expressed preferences to receive life support. Despite this effect on patients’ choices, default options in ADs did not reduce the primary outcome of hospital-free days nor did they yield improvements in any secondary patient outcomes.
The effects of default options on patients’ choices for their future care were observed despite only considering choices final after we described the defaults to patients and reassessed their choices. Thus, these results are unlikely to be due to inattention or misunderstanding and are instead consistent with how defaults have been shown to be interpreted as recommendations and thereby influence many clinical and nonclinical decisions.19,31–37 Choices regarding end-of-life care are conventionally thought to reflect personal and deep-seated values. The present RCT, coupled with the parallel results from our prior RCT among a smaller and less diverse sample of patients,20 challenges this standard view. Further challenging this view are data from a 2019 RCT,38 in which encouraging seriously ill patients to deliberate on their preferences for end-of-life care did not yield choices that differed from those made by patients who were forced to choose quickly and intuitively. Together, these results support the view that, for many seriously ill patients, end-of-life care choices do not stem from patients’ underlying values but rather are constructed, at least in part, during the process of elicitation.1,39,40
This RCT confirmed our hypothesis that, despite encouraging more seriously ill patients to choose comfort-oriented goals of care and to forgo forms of life support, comfort-oriented default options would not reduce these patients’ hospital-free days. However, contrary to our hypotheses, the use of default options did not meaningfully change any secondary clinical or patient-reported outcomes. Specifically, in the full sample, no differences were found for quality of life, satisfaction with advance care planning, decisional conflict, survival, place of death, hospitalizations, intensive care unit admissions, or costs of inpatient care. Similarly, in the sample restricted to patients who completed all elements of the protocol, no differences among groups were identified in any of the analyzed outcomes. Although this study was not specifically powered to identify differences in these outcomes, the consistency of the results suggests limits to the benefits of conventional ADs as deployed in general practice.
This RCT also provides prospective evidence that seriously ill patients rarely choose to modify their ADs, even when actively prompted to do so. This supports and extends prior work using retrospective designs or hypothetical ADs that also found preferences to be largely stable over time.23
Limitations
This study has limitations. First, we were unable to obtain sufficient measures of quality of life among patients who did not return ADs to conduct a prespecified21 complier average treatment effect analysis assessing the effect of choosing comfort care on this outcome. Second, due to low rates of caregiver response to our bereavement assessments following patients’ deaths, we could not achieve our goal of examining how AD defaults affected caregiver outcomes.21 Third, due to limitations in the administrative data, we could not reliably measure hospice use or duration of hospice use before death.
Fourth, despite frequently encouraging patients to complete the ADs that were given to them, more than 40% of enrolled patients did not do so. Despite frequently asking clinic staff to upload completed ADs to the EHR, nearly 30% of such ADs were not uploaded. These limitations in AD completion and accessibility as well as the relatively low rates of hospitalization and life-sustaining therapy use in this sample could have biased the RCT to confirming noninferiority on the primary outcome and contributed to the null effects observed among secondary outcomes. However, the observations that the randomly assigned AD version significantly influenced choices without altering any outcomes among the restricted sample with full protocol adherence provides confidence that these results were not spurious. Furthermore, although default options altered patients’ choices in the mITT and per-protocol samples, complier average treatment effect analyses that accounted for protocol nonadherence provided no evidence that these altered choices led to differences in hospital-free days. These observed limitations in intended care processes reflect the reality of current policies and practices regarding the completion of ADs outside of research. In fact, the rates of AD completion and EHR accessibility observed in this trial were greater than the corresponding rates observed in clinical settings.41–43
Conclusions
This RCT showed that the choices made by many seriously ill patients in legally valid ADs are influenced by how options are framed, suggesting that such patients’ preferences do not stem solely from deeply held values or goals. Although helping seriously ill patients endorse limitations on the aggressiveness of their future care did not worsen any outcomes measured, the absence of clear benefits precludes a recommendation that default options be routinely implemented in ADs. Rather, the observation that randomly assigned default options influenced choices without changing outcomes suggests that current policies and practices that encourage the completion of conventional ADs6–10 may not improve the quality of end-of-life care.
Supplementary Material
Key Points.
Question
What effects do default options on advance directives have on the choices made by seriously ill patients and their future outcomes?
Findings
In this randomized clinical trial of 492 seriously ill patients, default options in advance directives strongly influenced patients’ goals of care and preferences for receiving life support, even though patients were told of these defaults. Advance directives with defaults did not reduce the primary outcome of hospital-free days during a median follow-up of 18 months compared with advance directives without defaults, nor did they improve other patient-reported, clinical, or economic outcomes.
Meaning
The findings of this study suggest that seriously ill patients’ end-of-life care choices are strongly influenced by the way choices are framed, but changing choices in conventional advance directives is unlikely to change patient outcomes.
Funding/Support:
This work was supported by grants from the Gordon and Betty Moore Foundation and the Otto Haas Charitable Trust, both to Dr Halpern.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Angus reported receiving personal fees from Bristol-Myers Squibb, Bayer, and Ferring Pharmaceuticals outside the submitted work; owning stock in Alung Technologies outside the submitted work; and having a patent to Selepressin compounds, compositions, and methods for treating sepsis pending and to Proteomic biomarkers of sepsis in elderly patients pending. Dr Arnold reported receiving personal fees from UpToDate outside the submitted work and being the founder of VitalTalk. Dr Volpp reported receiving grants from the National Institutes of Health and the Otto Haas Foundation during the conduct of the study; receiving grants from Oscar, Vitality/Discovery, Humana, Hawaii Medical Services Association, and Weight Watchers; receiving personal fees from the Center for Corporate Innovation; and receiving personal fees and being the part owner of VAL Health outside the submitted work. Dr White reported receiving grants from the National Institutes of Health during the conduct of the study and receiving personal fees from UpToDate and the American Journal of Respiratory and Critical Care Medicine outside the submitted work. No other disclosures were reported.
Publisher's Disclaimer: Disclaimer: This analysis was not prepared by Pennsylvania Health Care Cost Containment Council. This analysis was done by Dr Halpern and colleagues at the Palliative and Advanced Illness Research Center. The Pennsylvania Health Care Cost Containment Council, its agents, and staff bear no responsibility or liability for the results of the analysis, which are solely the opinion of this entity.
Data Sharing Statement: See Supplement 3.
Additional Information: The Pennsylvania Health Care Cost Containment Council is an independent state agency responsible for addressing the problem of escalating health costs, ensuring the quality of health care, and increasing access to health care for all citizens regardless of ability to pay. The Pennsylvania Health Care Cost Containment Council has provided data to the Palliative and Advanced Illness Research Center in an effort to further Pennsylvania Health Care Cost Containment Council’s mission of educating the public and containing health care costs in Pennsylvania. Pennsylvania Health Care Cost Containment Council, its agents, and staff have made no representation, guarantee, or warranty, express or implied, that the data—financial, patient, payor, and physician specific information—provided to this entity are error-free or that the use of the data will avoid differences of opinion or interpretation.
SUPPLEMENT 1.
Trial Protocol
SUPPLEMENT 2.
eAppendix. Advance Directives Tested
eTable 1. Differences Among the 3 Versions of the Advance Directive
eTable 2. Comparison of Patients Who Were and Were Not in the Per-Protocol Sample
eTable 3. Changes to Choices for Goals of Care and for Life Support at Debriefing and During Follow-up
eTable 4. Comparison of Patients Who Did and Did Not Provide Valid Social Security Numbers
eTable 5. Regression Analysis of Hospital-Free Days, Measured Outcomes Only
eTable 6. Regression Analysis of Hospital-Free Days, Missing Outcomes Imputed
eTable 7. Complier Average Treatment Effect Analysis of Hospital-Free Days
eTable 8. Regression Analysis of Receipt of at Least 1 Form of Life Support
eTable 9. Regression Analysis of Death in a Hospital vs Death Elsewhere or Alive
eTable 10. Distributions of Patients Whose Completed ADs Were Scanned in the Electronic Health Record
eTable 11. Choices and Outcomes Among Patients in the Per-Protocol Sample
eTable 12. Regression Analysis of McGill Quality of Life Score
eFigure 1. Flow of Patient Accrual and Protocol Completion
eFigure 2. Proportions of Patients Choosing to Forgo Each Form of Life Support by Trial Group, Per-Protocol Sample
eFigure 3. Proportions of Patients Choosing to Forgo Each Form of Life Support by Trial Group, Modified Intention-to-Treat Sample
SUPPLEMENT 3.
Data Sharing Statement
REFERENCES
- 1.Halpern SD. Shaping end-of-life care: behavioral economics and advance directives. Semin Respir Crit Care Med. 2012;33(4):393–400. doi: 10.1055/s-0032-1322403 [DOI] [PubMed] [Google Scholar]
- 2.White DB, Arnold RM. The evolution of advance directives. JAMA. 2011;306(13):1485–1486. doi: 10.1001/jama.2011.1430 [DOI] [PubMed] [Google Scholar]
- 3.Fields MJ, Cassel CK. Approaching Death, Improving Care at the End of Life. National Academies Press; 1997. [PubMed] [Google Scholar]
- 4.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 5.Emanuel LL, Emanuel EJ. The medical directive: a new comprehensive advance care document. JAMA. 1989; 261(22):3288–3293. doi: 10.1001/jama.1989.03420220102036 [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. National Academies Press; 2015. [PubMed] [Google Scholar]
- 7.Fried TR, Drickamer M. Garnering support for advance care planning. JAMA. 2010;303(3):269–270. doi: 10.1001/jama.2009.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Puma J, Orentlicher D, Moss RJ. Advance directives on admission: clinical implications and analysis of the Patient Self-Determination Act of 1990. JAMA. 1991;266(3):402–405. doi: 10.1001/jama.1991.03470030102032 [DOI] [PubMed] [Google Scholar]
- 9.Aging with Dignity. Five wishes: changing the way we talk about and plan for care at the end of life. Accessed July 2, 2018 https://fivewishes.org/five-wishes
- 10.Centers for Medicare & Medicaid Services. Physician fee schedule final rule with comment period. Accessed July 2, 2018 https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1631-FC.html
- 11.Molloy DW, Guyatt GH, Russo R, et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444. doi: 10.1001/jama.283.11.1437 [DOI] [PubMed] [Google Scholar]
- 12.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degenholtz HB, Rhee Y, Arnold RM. Brief communication: the relationship between having a living will and dying in place. Ann Intern Med. 2004;141(2):113–117. doi: 10.7326/0003-4819-141-2-200407200-00009 [DOI] [PubMed] [Google Scholar]
- 14.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007;55(2):189–194. doi: 10.1111/j.1532-5415.2007.01045.x [DOI] [PubMed] [Google Scholar]
- 15.Silveira MJ, Kim SYH, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218. doi: 10.1056/NEJMsa0907901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA. 2011;306(13):1447–1453. doi: 10.1001/jama.2011.1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halpern SD. Toward evidence-based end-of-life care. N Engl J Med. 2015;373(21):2001–2003. doi: 10.1056/NEJMp1509664 [DOI] [PubMed] [Google Scholar]
- 18.Halpern SD, Emanuel EJ. Advance directives and cost savings: greater clarity and perpetual confusion. Arch Intern Med. 2012;172(3):266–268. doi: 10.1001/archinternmed.2011.1399 [DOI] [PubMed] [Google Scholar]
- 19.Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340–1344. doi: 10.1056/NEJMsb071595 [DOI] [PubMed] [Google Scholar]
- 20.Halpern SD, Loewenstein G, Volpp KG, et al. Default options in advance directives influence how patients set goals for end-of-life care. Health Aff (Millwood). 2013;32(2):408–417. doi: 10.1377/hlthaff.2012.0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabler NB, Cooney E, Small DS, et al. Default options in advance directives: study protocol for a randomised clinical trial. BMJ Open. 2016;6(6):e010628. doi: 10.1136/bmjopen-2015-010628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parr JD, Zhang B, Nilsson ME, et al. The influence of age on the likelihood of receiving end-of-life care consistent with patient treatment preferences. J Palliat Med. 2010;13(6):719–726. doi: 10.1089/jpm.2009.0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner GJ, Riopelle D, Steckart J, Lorenz KA, Rosenfeld KE. Provider communication and patient understanding of life-limiting illness and their relationship to patient communication of treatment preferences. J Pain Symptom Manage. 2010;39(3):527–534. doi: 10.1016/j.jpainsymman.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 24.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 25.Heyland DK, Cook DJ, Rocker GM, et al. ; Canadian Researchers at the End of Life Network. The development and validation of a novel questionnaire to measure patient and family satisfaction with end-of-life care: the Canadian Health Care Evaluation Project (CANHELP) Questionnaire. Palliat Med. 2010;24(7):682–695. doi: 10.1177/0269216310373168 [DOI] [PubMed] [Google Scholar]
- 26.Heyland DK, Frank C, Tranmer J, et al. Satisfaction with end-of-life care: a longitudinal study of patients and their family caregivers in the last months of life. J Palliat Care. 2009;25(4):245–256. doi: 10.1177/082585970902500402 [DOI] [PubMed] [Google Scholar]
- 27.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11(1):3–20. doi: 10.1177/026921639701100102 [DOI] [PubMed] [Google Scholar]
- 28.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease—a preliminary study of validity and acceptability. Palliat Med. 1995; 9(3):207–219. doi: 10.1177/026921639500900306 [DOI] [PubMed] [Google Scholar]
- 29.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33(13): 2297–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sussman JB, Hayward RA. An IV for the RCT: using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ. 2010;340:c2073. doi: 10.1136/bmj.c2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quill CM, Halpern S. Deciphering the appropriateness of defaults: the need for domain-specific evidence. J Med Ethics. 2012;38(12):721–722. doi: 10.1136/medethics-2012-100724 [DOI] [PubMed] [Google Scholar]
- 32.Chapman GB, Li M, Colby H, Yoon H. Opting in vs opting out of influenza vaccination. JAMA. 2010;304 (1):43–44. doi: 10.1001/jama.2010.892 [DOI] [PubMed] [Google Scholar]
- 33.Choi JJ, Laibson D, Madrian BC, Metrick A. Defined contribution pensions: plan rules, participant decisions, and the path of least resistance In: Poterba JM, ed. Tax Policy and the Economy. Vol 16 MIT Press; 2002:67–113. [Google Scholar]
- 34.Johnson EJ, Goldstein D. Medicine: do defaults save lives? Science. 2003;302(5649):1338–1339. doi: 10.1126/science.1091721 [DOI] [PubMed] [Google Scholar]
- 35.Johnson EJ, Hershey JC, Meszaros J, Kunreuther H. Framing, probability distortions, and insurance decisions. J Risk Uncertain. 1993;7:35–51. doi: 10.1007/BF01065313 [DOI] [Google Scholar]
- 36.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298 (20):2415–2417. doi: 10.1001/jama.298.20.2415 [DOI] [PubMed] [Google Scholar]
- 37.Madrian BC, Shea DF. The power of suggestion: inertia in 401(k) participation and savings behavior. Q J Econ. 2001;116(4):1149–1187. doi: 10.1162/003355301753265543 [DOI] [Google Scholar]
- 38.Rubin EB, Buehler AE, Cooney E, Gabler NB, Mante AA, Halpern SD. Intuitive vs deliberative approaches to making decisions about life support: a randomized clinical trial. JAMA Netw Open. 2019;2(1):e187851. doi: 10.1001/jamanetworkopen.2018.7851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnato AE. Challenges in understanding and respecting patients’ preferences. Health Aff (Millwood). 2017;36 (7):1252–1257. doi: 10.1377/hlthaff.2017.0177 [DOI] [PubMed] [Google Scholar]
- 40.Slovic P The construction of preference. Am Psychol. 1995;50:364–371. Accessed February 20, 2020 http://bear.warrington.ufl.edu/brenner/mar7588/Papers/slovic-ampsy1995.pdf [Google Scholar]
- 41.Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36(7):1244–1251. doi: 10.1377/hlthaff.2017.0175 [DOI] [PubMed] [Google Scholar]
- 42.Platts-Mills TF, Richmond NL, LeFebvre EM, et al. Availability of advance care planning documentation for older emergency department patients: a cross-sectional study. J Palliat Med. 2017;20(1):74–78. doi: 10.1089/jpm.2016.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turley M, Wang S, Meng D, Kanter M, Garrido T. Impact of a care directives activity tab in the electronic health record on documentation of advance care planning. Perm J. 2016;20(2):43–48. doi: 10.7812/TPP/15-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


