Abstract
Background
While a majority of cigarette smokers who use electronic cigarettes (e-cigarettes) choose to continue using cigarettes, completely switching to e-cigarettes is necessary to reduce tobacco-related harm. Whether specific subjective responses to e-cigarettes are associated with extent of smoking reduction and complete switching from cigarettes to e-cigarettes is unclear. This study determined whether initial subjective responses to e-cigarettes related to the successful substitution of e-cigarettes for cigarettes and extent of cigarette and e-cigarette use.
Methods
Adult cigarette smokers (N=58) uninterested in quitting were asked to completely substitute their cigarettes with an e-cigarette (Vuse V2) for 8 weeks. At week 1, subjective responses to e-cigarettes were measured using the Product Evaluation Scale and Drug Effects/Liking Survey. A Poisson regression examined whether any of these initial subjective responses were associated with smoke-free days, e-cigarette puffs, and cigarettes smoked between weeks 6 and 8 after adjustment for potential confounders. A logistic regression examined the relationship between subjective measures and exhaled carbon monoxide (CO) verified 7-day abstinence at week 8 after adjustment for potential confounders.
Results
Following Holm’s p-value adjustment, e-cigarette liking and desire were associated with increased e-cigarette use (adjusted p<0.01) and decreased cigarette use (adjusted p<0.05). Measures of psychological reward and drug liking were associated with 7-day abstinence, however this association was no longer significant following p-value adjustment.
Conclusions
Initial subjective responses were related to cigarette and e-cigarette use at weeks 6–8, but not smoke-free days or CO-verified 7-day abstinence.
Keywords: abuse liability, electronic cigarettes, tobacco regulatory science, smoking, subjective responses
1. INTRODUCTION
Electronic cigarettes (e-cigarettes) have gained substantial popularity in the last decade (US DHHS, 2016). Many cigarette smokers who use e-cigarettes choose to continue smoking cigarettes (i.e., dual use; Coleman et al., 2017; Jaber et al., 2018). However, to experience the potential reduced harm from using e-cigarettes, it is best for smokers to completely switch to e-cigarettes (Goniewicz et al., 2018; NASEM, 2018), as dual use may lead to similar if not higher levels of toxicant and carcinogen exposures (Goniewicz et al., 2018; Hatsukami et al., in press). Product uptake is influenced by subjective responses to a product, which may also be relevant in assessing a product’s abuse liability—or the likelihood that the product will be used and its addiction potential (Carter et al., 2009; deWit et al., 2012; Rees et al., 2009) and appeal (Henningfield et al., 2011). Previous research suggests subjective responses play a role in short-term use of tobacco products including cigarettes with varying nicotine levels (Bergeria et al., 2019), oral tobacco (Hatsukami et al., 2013), and e-cigarettes (Tucker et al., 2017). However, subjective responses to e-cigarettes and their association with complete switching and longer-term use have not been extensively examined.
This secondary data analysis (Hatsukami et al., in press) examined which initial subjective responses are associated with complete switching to e-cigarettes, number of smoke free days and the extent of e-cigarette and cigarettes use in smokers who were instructed and incentivized to completely switch to e-cigarettes in a non-cessation study.
2. METHODS
A complete description of the study procedures can be found in Hatsukami et al (in press). Details specific to the current investigation are described below.
2.1. Participant Recruitment
Smokers were recruited from the University of Minnesota-Twin Cities (lead site), the Ohio State University, and Columbus and Roswell Park Comprehensive Cancer Center, Buffalo, NY. Eligible participants were daily cigarette smokers (≥5 cigarettes per day i.e., CPD) 18 years and older with stable mental and physical health status, who used other tobacco products ≤ 9 days in a month and who were uninterested in quitting in the next 3 months. Institutional Review Board approval of the study was obtained from each academic institution and a Data and Safety Monitoring Board (DSMB) met annually to monitor study progress and adverse events.
2.2. Design
2.2.1. Baseline
This study had a two-week baseline. Participants self-reported the number of cigarettes smoked and number of e-cigarette puffs taken during the last 24 hours using an Interactive Voice Recording (IVR) that called them on a daily basis. At clinic visits, participants were asked about other tobacco or nicotine use and completed subjective measures related to tobacco use.
2.2.2. Groups
The groups in this study were a combination of two sets of studies, which used almost identical study procedures. Participants who were analyzed for this study were randomized to complete substitution with electronic cigarettes (i.e. “you will stop smoking cigarettes and use only electronic cigarettes”).
2.2.3. Clinical Trial Phase
Participants were assigned to a product for eight weeks and continued to complete daily diaries on the IVR for cigarettes smoked and number of e-cigarette puffs. During study visits at weeks 1, 2, 4, 6, and 8, participants completed measures obtained during baseline (e.g. subjective measures related to tobacco use). All used and unused e-cigarette cartridges were collected at each visit.
2.2.4. Compensation
Participants were compensated for attending each clinic visit, submitting biological samples (not analyzed here), completing daily diaries, and protocol compliance. To maximize complete substitution, smokers in this group were also monetarily incentivized to be abstinent (CO of 4 ppm or less).
2.3. Products
Initially, Blu Rechargeable (marketed by Imperial Brands; purchased 2014–2015) and Fin Advanced Tank Vaping System (manufactured by Fin Branding Group; purchased 2015–2016) were offered as choices. Over the course of the study, we switched to Vuse Solo with 4.8% nicotine (manufactured by RJ Reynolds, Inc. purchased 2015–2017) because it exceeded the market share of Blu and because of leakage observed with Fin. In total, three participants used Blu, six participants started with Fin but switched early in the study to Vuse due to leakage issues, and 67 participants used Vuse only. Participants chose one of four flavors: tobacco, mint, menthol, or berry. Participants were provided seven cartridges per week. All products were purchased at each of the sites and provided free to the participants.
2.4. Measures
Week 1 subjective measures that were administered and analyzed for this study included the Product Evaluation Scale (PES) that assessed subjective responses to e-cigarettes (Hatsukami et al., 2013; modified from Cappelleri et al., 2007), including four sub-scales of satisfaction (4 questions), psychological reward (5 questions), craving relief (5 questions), and aversion (4 questions) that are rated from 1–7 and averaged; and a modified Drug Effects/Liking Survey (Zacny et al., 1997), of which two single-item 0–10 point scales were used for drug liking (“How much do you like the study product?”) and desire for drug (“How much do you desire the study product?”). Included baseline covariates were Wisconsin Inventory of Smoking Dependence Motives (WISDM) Primary Dependence subscale (Smith et al., 2010), comprised of sixteen 1–7 point questions that are averaged; Questionnaire of Smoking Urges (QSU) (Cox et al., 2001) for cigarettes, comprised of ten 1–7 point questions that are summed; and the Center for Epidemiological Studies—Depression (CES-D) (Lewinsohn et al., 1997), comprised of twenty questions with a 1–4 scale that are averaged.
2.5. Data Analysis
Dependent variables of interest for this study were week 8 CO-verified (<4ppm) 7-day abstinence and week 6–8 smoke-free days, cigarette use, and e-cigarette use among study completers. Independent variables of interest included week 1 Product Evaluation Scale for e-cigarettes (satisfaction, psychological reward, craving relief, and aversion) and drug effects/liking (e-cigarette liking and desire for e-cigarette). As the baseline time point happened prior to any e-cigarette use, week 1 measures of subjective responses were the earliest time point following initial use. Preselected baseline covariates included WISDM primary dependence score, gender, employment status (part/full time or not), race (white or non-white), site, study protocol, use of other combustible tobacco products, Questionnaire of Smoking Urges total score, CES-D, and age.
A multivariable logistic regression model was fit for the outcome of CO-verified 7-day abstinence at week 8. Multivariable Poisson regression models using sandwich variance were fit for the outcomes of the number of cigarettes, smoke-free days, and e-cigarette puffs at week 8. An offset variable was included in the model to adjust for the number of days between visits since the time interval was not consistent across visits and varied among participants. Coefficients from the Poisson and logistic regression models were exponentiated and represent the estimated mean ratios and odds ratios, respectively, of the outcome between levels of the predictor variables. To account for multiple testing, we used Holm’s method to generate adjusted p-values (Aickin & Gensler, 1996). In particular, the p-values were adjusted to control the family-wise error rate at 0.05 for the testing of each set of predictors in association with a single outcome.
All statistical analysis was performed using R version 3.6.1 (R Core Team, 2013) and adjusted p-values that were <0.05 were considered statistically significant.
3. RESULTS
3.1. Participant Characteristics
Detailed participant characteristics can be found in Table 1. Seventy-six participants (36 female) were randomized to complete substitution with e-cigarettes. Of these, 58 (27 female) completed the study and were included in analyses. The flavor choices for electronic cigarettes (N, %) at week 1 were tobacco (15, 23%), menthol (17, 26%) mint (12, 19%), berry (18, 28%). Of the 58 completers, 28 (17 female) were “switchers”, defined as having 7-day CO-verified cigarette abstinence at week 8, and the other 30 (10 female) were “non-switchers.”
Table 1.
Participant Characteristics
| Variable | Total Enrolled (N = 76) | Completer (N=58) | Switcher (N=28) | Non-Switcher (N=30) |
|---|---|---|---|---|
| Study site, N (%) | ||||
| UMN | 37 (48.7) | 28 (48.3) | 12 (42.9) | 16 (53.3) |
| OSU | 30 (39.5) | 21 (36.2) | 15 (53.6) | 6 (20) |
| Roswell | 9 (11.8) | 9 (15.5) | 1 (3.5) | 8 (26.7) |
| Study Protocol, N (%) | ||||
| Study B | 21 (27.6) | 16 (27.6) | 10 (35.7) | 6 (20) |
| Study C | 55 (72.4) | 42 (72.4) | 18 (64.3) | 24 (80) |
| Age | 45 | 46.5 | 45 | 49 |
| Median [min/max] | [20/71] | [21/71] | [21/67] | [25/71] |
| {IQR} | {36/56} | {37/58} | {35/60} | {40/56} |
| Sex, Female, N (%) | 36 (47.4) | 27 (46.6) | 17 (60.7) | 10 (33.3) |
| Race, N (%) | ||||
| White | 48 (63.2) | 38 (65.5) | 21 (75) | 17 (56.7) |
| Nonwhite | 28 (36.8) | 20 (34.5) | 7 (25) | 13 (43.3) |
| Current employment, full/part-time, N (%) | 28 (36.8) | 21 (36.2) | 11 (39.3) | 10 (33.3) |
| WISDM Primary | ||||
| Dependence score | 5.00 | 5.03 | 4.32 | 5.72 |
| Median [min/max] | [1.44/7] | [1.69/7] | [1.69/7] | [3.06/6.63] |
| {IQR} | {4.13/5.81} | {4.14/5.97} | {3.81/5.63} | {4.53/6.20} |
| CES-D (depression) | 6.5 | 7.5 | 6 | 8 |
| Median [min/max] | [0/31] | [0/31] | [0/16] | [0/31] |
| {IQR} | {4/11} | {4/10} | {1/9} | {5/11} |
| Cigarettes per day* | 16.5 | 15.3 | 13.3 | 17.6 |
| Median [min/max] | [6/35] | [6/31] | [7/31] | [6/31) |
| {IQR} | {12/19} | {11/19} | {10/17} | {14/22} |
| QSUC total score | 24 | 26 | 24 | 27 |
| Median [min/max] | [10/70] | [10/70] | [10/70] | [10/67] |
| {IQR} | {17/41} | {18/41} | {15/41} | {18/43} |
UMN = University of Minnesota. OSU = Ohio State University. IQR = Interquartile range. WISDM = Wisconsin Inventory of Smoking Dependence Motives. CES-D = Center for Epidemiological Studies—Depression. QSUC = Questionnaire of Smoking Urges Cigarettes.
Cigarettes per day from the Tobacco history questionnaire.
3.2. Regression Analyses
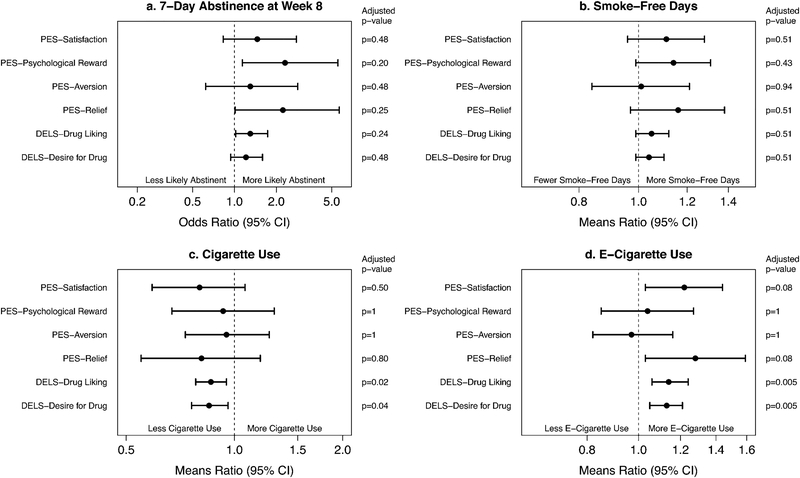
The primary goal of these analyses was to examine the association between initial subjective responses to an e-cigarette and complete switching to an e-cigarette and associated measures (cigarette and e-cigarette use) over the course of the 8-week clinical trial. As shown in Figure 1, there were significant relationships between week 1 subjective responses and certain outcome measures.
Figure 1.
Forest plots for (a) 7-day abstinence, (b) smoke-free days, (c) cigarette use, and (d) e-cigarette use showing effects (odds or means ratios) of increase in each subjective response variable. CI=Confidence Interval. PES=Product Evaluation Scale. DELS=Drug Effects and Liking Survey.
3.2.1. CO-Verified 7-day Abstinence
CO-verified 7-day abstinence at week 8 was significantly related to psychological reward and e-cigarette liking (Figure 1a). However, following p-value adjustment, results were no longer significant at the 0.05 level. On average, the odds of 7-day abstinence were 2.31 times higher (95% confidence interval [CI]: 1.14–5.54 times higher) for each 1-point increase in e-cigarette psychological reward (unadjusted p-value = 0.03; adjusted p-value = 0.20). On average, the odds of 7-day abstinence were 1.30 times higher (95% CI: 1.02–1.73 times higher) for each 1-point increase in drug liking for e-cigarette (unadjusted p-value =0.048; adjusted p-value = 0.24).
3.2.2. Smoke-Free Days
There were no significant relationships between any of the examined subjective responses and smoke-free days between weeks 6 and 8 (p > 0.05; Figure 1b).
3.2.3. Cigarette Use
E-cigarette liking and desire for e-cigarette were significantly associated with cigarettes smoked since the previous visit (Figure 1c). Specifically, a 1-point increase in e-cigarette drug liking was associated with a 14% decrease (95% CI: 5–22% decrease) in cigarettes smoked since the previous visit (unadjusted p-value = 0.004; adjusted p-value=0.02) and a 1-point increase in desire for e-cigarette was associated with a 13% decrease (95% CI: 4–24% decrease) in cigarettes smoked since the previous visit (unadjusted p-value = 0.009; adjusted p-value = 0.04).
3.2.4. E-Cigarette Use
E-cigarette puffs taken since the previous visit was associated with satisfaction, craving relief, e-cigarette liking, and desire for e-cigarettes (Figure 1d). Following p-value adjustment, e-cigarette liking and desire for e-cigarette remained significant at the 0.05 level. On average, e-cigarette since the previous visit increased 22% (95% CI: 3–44% increase) for each 1-point increase in e-cigarette satisfaction (unadjusted p-value = 0.02; adjusted p-value = 0.08). E-cigarette puffs increased 28% (95% CI: 3–59% increase) for each 1-point increase in craving relief (unadjusted p-value = 0.03; adjusted p-value = 0.08). E-cigarette puffs increased 14% (95% CI: 6–24% increase) for each 1-point increase in drug liking for e-cigarette (unadjusted p-value = 0.0008; adjusted p-value = 0.005). E-cigarette puffs increased 13% (95% CI: 5–21% increase) for each 1-point increase in desire for drug (e-cigarette) (unadjusted p-value = 0.0009; adjusted p-value = 0.005).
4. DISCUSSION
This study examined the role of initial subjective responses to an e-cigarette in the ability of non-treatment seeking cigarette smokers to successfully switch to an e-cigarette for eight weeks—measured using CO-verified 7-day abstinence and related measures (cigarette use, e-cigarette use, and smoke-free days). Subjective measures, specifically liking and desire for drug, were significantly associated with the extent of uptake of e-cigarettes and decrease in cigarette smoking. Satisfaction and craving relief were associated with the extent of e-cigarette use, however this association only trended toward statistical significance following p-value adjustment. Psychological reward and drug liking were associated with 7-day abstinence, however this association was no longer significant following p-value adjustment.
The results from “e-cigarette liking” and “desire for e-cigarette” mirror those showing that positive subjective responses relate to increased use of tobacco product (Bergeria et al., 2019; Hatsukami et al., 2013; O’Connor et al., 2018; Tucker et al., 2017), similar to other drugs of abuse (deWit et al., 2012). However, satisfaction only trended towards significance after adjustment. This may be a reflection of the device itself not being sufficiently satisfying to maintain significant use.
4.1. Limitations
This study has several limitations. First, the early-generation e-cigarettes used in this study may not have a sufficiently high abuse liability which led to low uptake, perhaps due to low nicotine delivery (Hajek et al., 2017; Vansickel et al., 2010). Low nicotine delivery is associated with less satisfaction (Norton et al., 2014). Indeed, the Vuse Solo e-cigarettes used in this study have abuse liability closer to nicotine gum than to cigarettes (Stiles et al., 2017). More novel high-nicotine e-cigarettes (e.g., JUUL) may have more similar abuse liability to cigarettes and therefore could be better for switching (Hajek et al., 2020). In further analysis of our data using Kruskall-Wallis tests, we found that non-switchers were more dependent on cigarettes (p=0.02) at baseline than switchers, supporting the need to examine higher nicotine content e-cigarettes that better mimic cigarettes. Second, our population was limited to those who completed the study, which decreased our sample size and excluded individuals who dropped out. However, an ad-hoc sensitivity analysis of our logistic regression with dropouts (following week 1; total N=67) imputed as “non-switchers” supported our results that no subjective responses related to CO-verified 7-day abstinence (p>0.05). Third, our population was uninterested in quitting smoking and was also incentivized to avoid cigarettes, which decreased generalizability of findings. Finally, this was not a classical abuse liability study as defined by the US Food and Drug Administration (2017) and cannot directly speak to the abuse liability of the product, although it does examine subjective measures such as drug liking, which are outlined as an important outcome measure for human abuse liability studies (FDA, 2017).
4.2. Conclusion
Subjective responses to a drug are one part of assessing their abuse liability (Carter et al., 2009; deWit et al., 2012). This study found that subjective measures of initial response to e-cigarettes, particularly liking and desire for e-cigarettes, might contribute to determining the extent of uptake of this product and hence its effectiveness in helping to achieve complete abstinence from cigarette smoking.
Highlights.
Product liking and desire associated with subsequent e-cigarette and cigarette use
Subjective measures may not accurately predict ability to abstain from cigarettes
Subjective measures may be used to assess likelihood and extent of product use
Acknowledgements
The authors would like to acknowledgement the work of the many research assistants who contributed to the conduct of this study.
Funding
Research reported in this publication was supported by grants U19CA157345 from the National Cancer Institute (DKH/PS), UL1 TR000062 from the National Center for Advancing Translational Science of the National Institutes of Health, and T32 DA007097 from the National Institute of Drug Abuse (MSG; EM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest
RJC was a member of the FDA Tobacco Products Scientific Advisory Committee.
PGS serves or has served as an expert witness in tobacco company litigation on behalf of plaintiffs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aickin M, Gensler H, 1996. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am. J. Public Health 86, 726–728. 10.2105/AJPH.86.5.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeria CL, Heil SH, Davis DR, Streck JM, Sigmon SC, Bunn JY, Tidey JW, Arger CA, Reed DD, Gallagher T, Hughes JR, Gaalema DE, Stitzer ML, Higgins ST, 2019. Evaluating the utility of the modified cigarette evaluation questionnaire and cigarette purchase task for predicting acute relative reinforcing efficacy of cigarettes varying in nicotine content. Drug Alcohol Depend. 197, 56–64. 10.1016/J.DRUGALCDEP.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG, 2007. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict. Behav. 32, 912–23. 10.1016/j.addbeh.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK, 2009. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 18, 3241–62. 10.1158/1055-9965.EPI-09-0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BN, Rostron B, Johnson SE, Ambrose BK, Pearson J, Stanton CA, Wang B, Delnevo C, Bansal-Travers M, Kimmel HL, Goniewicz ML, Niaura R, Abrams D, Conway KP, Borek N, Compton WM, Hyland A, 2017. Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Tob. Control 26, e117–e126. 10.1136/tobaccocontrol-2016-053462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG, 2001. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 3, 7–16. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ, 2012. Do initial responses to drugs predict future use or abuse? Neurosci. Biobehav. Rev. 36, 1565–1576. 10.1016/J.NEUBI0REV.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, Cder Bonson, & Katherine R (2017). Assessment of Abuse Potential of Drugs Guidance for Industry. Retrieved from http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
- Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, Wang L, Christensen C, Ambrose B, Borek N, van Bemmel D, Konkel K, Erives G, Stanton CA, Lambert E, Kimmel HL, Hatsukami D, Hecht SS, Niaura RS, Travers M, Lawrence C, Hyland AJ, 2018. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 1, e185937 10.1001/jamanetworkopen.2018.5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, Przulj D, 2020. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction add.14936 10.1111/add.14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H, 2017. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl)., Psychopharmacologia 234, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Meier E, Lindgren BR, et al. A Randomized Clinical Trial Examining the Effects of Instructions for Electronic Cigarette Use on Smoking-Related Behaviors, and Biomarkers of Exposure. Nicotine Tob. Res. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Zhang Y, O’Connor RJ, Severson HH, 2013. Subjective Responses to Oral Tobacco Products: Scale Validation. Nicotine Tob. Res. 15, 1259–1264. 10.1093/ntr/nts265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Hatsukami DK, Zeller M, Peters E, 2011. Conference on abuse liability and appeal of tobacco products: conclusions and recommendations. Drug Alcohol Depend. 116, 1–7. 10.1016/j.drugalcdep.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber RM, Mirbolouk M, DeFilippis AP, Maziak W, Keith R, Payne T, Stokes A, Benjamin E, Bhatnagar A, Blankstein R, Saxena A, Blaha MJ, Nasir K, 2018. Electronic Cigarette Use Prevalence, Associated Factors, and Pattern by Cigarette Smoking Status in the United States From NHANES (National Health and Nutrition Examination Survey) 2013–2014. J. Am. Heart Assoc. 7, e008178 10.1161/JAHA.117.008178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB, 1997. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12, 277. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and M., 2018. Public Health Consequences of E-Cigarettes. National Academies Press, Washington, D.C. 10.17226/24952 [DOI] [PubMed] [Google Scholar]
- Norton KJ, June KM, O’Connor RJ, 2014. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob. Induc. Dis. 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Lindgren BR, Schneller LM, Shields PG, Hatsukami DK, 2018. Evaluating the utility of subjective effects measures for predicting product sampling, enrollment, and retention in a clinical trial of a smokeless tobacco product. Addict. Behav 76, 95–99. 10.1016/J.ADDBEH.2017.07.025 [DOI] [PubMed] [Google Scholar]
- Rees VW, Kreslake JM, Cummings KM, O’Connor RJ, Hatsukami DK, Parascandola M, Shields PG, Connolly GN, 2009. Assessing consumer responses to potential reduced-exposure tobacco products: a review of tobacco industry and independent research methods. Cancer Epidemiol. Biomarkers Prev. 18, 3225–3240. 10.1158/1055-9965.EPI-09-0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, Baker TB, 2010. Development of the brief Wisconsin inventory of smoking dependence motives. Nicotine Tob. Res. 12, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE, 2017. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 234, 2643–2655. 10.1007/s00213-017-4665-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MR, Laugesen M, Bullen C, Grace RC, 2018. Predicting Short-Term Uptake of Electronic Cigarettes: Effects of Nicotine, Subjective Effects, and Simulated Demand. Nicotine Tob. Res. 20, 1265–1271. 10.1093/ntr/ntx269 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2016. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE, 2010. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. 19, 1945–53. https://doi.org/10.n58/1055-9965.EPI-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J, 1997. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J. Pharmacol. Exp. Ther. 282, 1187–1197. [PubMed] [Google Scholar]