Abstract
Background
To compare the effects of chemotherapy dose escalation on survival and prognosis of nasopharyngeal carcinoma (NPC) patients who developed bone-only metastasis.
Material/Methods
Between October 2000 to March 2017, 58 NPC patients with initial bone-only metastasis were retrospectively analyzed. Patients who received <6 or ≥6 cycles of chemotherapy were matched and grouped using receiver operating characteristic curve (ROC) analysis. Overall survival (OS) was assessed using the Kaplan-Meier method, log-rank test, and Cox regression analysis.
Results
The median OS for the entire group was 24 months, while the 1-, 2-, and 3-year OS rates were 78.5%, 49.4%, and 26.8%, respectively. The median OS for patients who received <6 cycles of chemotherapy was 21 months, with 1-, 2-, and 3-year OS rates of 64.8%, 34.3%, and 17.2%, respectively. The median OS of patients who received ≥6 cycles of chemotherapy was 26 months, with 1-, 2-, and 3-year OS rates of 92.6%, 54.9%, and 30.9%, respectively. Multivariate analysis showed that the number of metastatic sites (≥3 vs. <3) and chemotherapy cycles (<6 vs. ≥6) were independent prognostic factors for OS.
Conclusions
NPC patients who had less than 3 bone metastatic sites and who received ≥6 cycles of chemotherapy had better survival and prognosis.
MeSH Keywords: Antineoplastic Combined Chemotherapy Protocols, Nasopharyngeal Neoplasms, Neoplasm Metastasis, Prognosis
Background
Nasopharyngeal carcinoma (NPC) is a common malignant tumor in China. Due to the physiological structure of the nasopharyngeal cavity, the site of occurrence is obscured. Radiation therapy is currently the most effective treatment strategy for NPC. Due to the rapid development of imaging technologies, radiation therapy for NPC has progressed from the conventional two-dimensional method to the present intensity-modulated radiotherapy method (IMRT). This has led to the 5-year patient survival rate increasing from 15% to as high as 90%. In addition, treatment-related adverse effects have been significantly reduced [1–4].
However, distant metastasis is still the major reason for treatment failure. Even after IMRT treatment, some patients are still susceptible to multiple distant metastases [5]. The liver, lungs, and bones are the most common metastatic sites, with bone metastases being predominant [6,7]. Bone metastases are commonly observed before and after treatment of the primary tumor. However, several studies have reported that about 4% to 10% of advanced NPC recur at the primary tumor site [8,9]. Distant metastasis, location, size, and the number of metastases were associated with prognosis [10]. Survival is significantly reduced in NPC patients with distant metastasis. Several studies have shown that the median survival time could be reduced by as much as 9 to 20 months for patients diagnosed with metastasis during initial treatment [11,12]. The prognosis for patients with metastasis is generally poor. Serious clinical complications often significantly affect prognosis and patient quality of life.
The treatment for metastatic NPC involves systemic chemotherapy and treatment of the primary lesion, usually in the nasopharynx and neck. A combination of 2 to 3 platinum-based drugs together with radiotherapy (chemoradiation) is often administered as first-line treatment, and have been shown to have survival benefits [13–15]. However, the optimal number of chemotherapy cycles that are required to treat NPC patients is still controversial, and whether high-intensity chemotherapy is more beneficial for patients is yet to be determined [11,16–18]. This retrospective study compared the survival and prognosis of NPC patients with bone-only metastasis who were administered various numbers of chemotherapy cycles. This study will help determine the appropriate number of cycles that are required to treat NPC patients with bone-only metastasis.
Material and Methods
Patients’ characteristics
A retrospective analysis was performed on clinical data from patients diagnosed with NPC and initial bone-only metastases. NPC was pathologically confirmed in patients admitted from October 2000 to March 2017 at the Affiliated Tumor Hospital of Guangxi Medical University. Patients were restaged based on the 7th edition of AJCC/UICC staging published in 2010 [19,20]. Patient inclusion criteria were: (1) pathologically proven NPC, the pathological type confirmed according to the 2005 World Health Organization (WHO) classification of tumors; (2) Confirmation of bone metastasis was based on comprehensive isotope bone scans, MRI, or CT scans, (3) KPS scores >70 points, (4) Patients diagnosed for the first time with no previous radio-chemotherapy performed; and (5) no previous history of organ transplantation. Patient exclusion criteria were: (1) age ≥75 or ≤18 years, (2) serious cardiovascular, cerebrovascular, liver, or kidney disease; (3) additional malignancies; and (4) recurrent or other organ distant metastasis NPC. All patients had bone metastases at the time of initial diagnosis, and no patients with bone metastasis underwent radiation therapy.
From October 2000 to March 2017, a total of 125 patients with bone-only metastasis were enrolled, of which 59 did not meet the inclusion criteria. The remaining 58 patients were included in the study. All patients were pathologically diagnosed and underwent nasopharyngoscopy, neck magnetic resonance imaging (MRI), chest computed tomography (CT), and whole-body bone scans prior to treatment.
Radiotherapy
A total of 33 patients underwent IMRT for their primary lesion in the nasopharynx. IMRT treatment was based on the International Commission on Radiation Units and Measurements Report 50 and 62 guidelines. Gross tumor volume (GTVnx) and cervical lymph node tumor volume (GTVnd) were determined using CT/MRI. Clinical target volume (CTV) included GTV with a 1-cm to 1.5-cm margin, the entire nasopharyngeal space, and the positive lymph node regions. Five daily fractions were administered per week as follows: 70.4–72.6Gy/32–33f for GTV, 60.8–62.7Gy/32-33f for CTV1, and 54.4–56Gy/32–33f for CTV2.
Chemotherapy
Most of the patients were treated with platinum-based chemotherapy drugs, combined with fluorouracil, docetaxel, and an additional 1 to 2 chemotherapeutic drugs for systemic chemotherapy. At the end of chemotherapy, patients were evaluated for effective treatment outcomes. Concurrent chemoradiotherapy was performed on the primary nasopharyngeal lesion. Fifty-one patients received chemotherapy using TPF regimens consisting of docetaxel (once a day of 75mg/m2), cisplatin (once a day of 75 mg/m2) and 5-fluorouracil (120 h of continuous intravenous infusion of 750 mg/m2). One patient received a PF regimen consisting of cisplatin (once a day of 70–100 mg/m2) and 5-fluorouracil (120 h of continuous intravenous infusion of 750–1000 mg/m2). One patient received a TP regimen consisting of docetaxel (once a day of 75 mg/m2) and cisplatin (70–100 mg/m2 on day 1). Five patients received other chemotherapy regimens.
Concurrent chemotherapy was scheduled on days 1, 22, and 43 with 80 to 100 mg/m2 of cisplatin for 1 or 3 days per cycle during radiotherapy. Chemotherapy was postponed or discontinued for patients who experienced serious adverse events and could not recover before the next scheduled cycle. All chemotherapies were performed based on a 21-day chemotherapy cycle.
Endpoints and follow-up
The primary endpoint was overall survival (OS). OS refers to the duration from the date of treatment to the date of death. Patients were followed up every 3 months for the first 2 years and every 6 months for the next 3 years, and then annually. Physical examinations, nasopharyngoscopy with/without biopsy, MRI, or CT scans of the nasopharynx and neck, chest radiography, or CT scan, and abdominal sonography or CT were performed on follow-up. Bone scans were performed if required.
Statistical analysis
SPSS22.0 (SPSS Inc, Chicago, IL) software was used for data analysis. Receiver operating characteristic (ROC) curve analysis was used to determine the cut-off scores for chemotherapy cycles. The cut-off score was determined using the corresponding parameter value when the Youden index was maximum. Youden index=(sensitivity+specificity)−1. Only the integer value of the corresponding chemotherapy cycle parameter value was considered (decimal values were excluded). This did not affect the grouping. Continuous data were analyzed using the t test, and categorical variables were analyzed using the chi-squared or Fisher’s exact test. Survival was assessed using Kaplan-Meier plots with log-rank test statistics. Cox regression analysis was used for univariate and multivariate analysis. Two-tailed P < 0.05 was considered statistically significant.
Results
Patients
A total of 58 NPC patients with initial bone-only metastasis were enrolled in this study and consisted of 52 males and 6 females, aged 19–68 years (median 48 years). The enrolled patient groups completed a total of 329 cycles of chemotherapy. The median number of chemotherapy cycles was 5 (range 1 to 13) (Table 1).
Table 1.
Characteristics of the patient population.
| Variables | N (%) | |
|---|---|---|
| Age | >60 | 10 (17.2%) |
| ≤60 | 48 (82.8%) | |
| Sex | Male | 52 (89.7%) |
| Female | 6 (10.3%) | |
| Karnofsky performance status | ≤80 | 10 (17.2%) |
| >80 | 48 (82.8%) | |
| Histology (WHO) | Type I/II | 18 (31.0%) |
| Type III | 40 (69.0%) | |
| T stage | T1–2 | 18 (31.0%) |
| T3–4 | 40 (69.0%) | |
| N stage | N0–1 | 10 (17.2%) |
| N2–3 | 48 (82.8%) | |
| Number of bone metastatic sites | 1 | 16 (27.6%) |
| 2 | 10 (17.2%) | |
| 3 | 7 (12.1%) | |
| >3 | 25 (43.1%) | |
| Nasopharyngeal radiotherapy | Yes | 33 (56.9%) |
| No | 25 (43.1%) | |
| Chemotherapy regimen | TPF | 51 (88.0%) |
| PF | 1 (1.7%) | |
| TP | 1 (1.7%) | |
| Others | 5 (8.6%) |
TPF – docetaxel, cisplatin and 5-fluorouracil; PF – cisplatin and 5-fluorouracil; TP – docetaxel and cisplatin.
We established a prognostic model for patient OS based on the number of different chemotherapy cycles administered. ROC curves are shown in Figure 1. The area under the curve (AUC) was 0.773, the cut-off value was 6.5, sensitivity was 0.619, and the specificity was 0.838. Taking only the integer value into the calculations did not affect the grouping, which was performed by comparing the area under the ROC curve when the integers were 6 and 7. The results showed that the AUC value was the largest when the integer 6 was considered (Figure 1).
Figure 1.
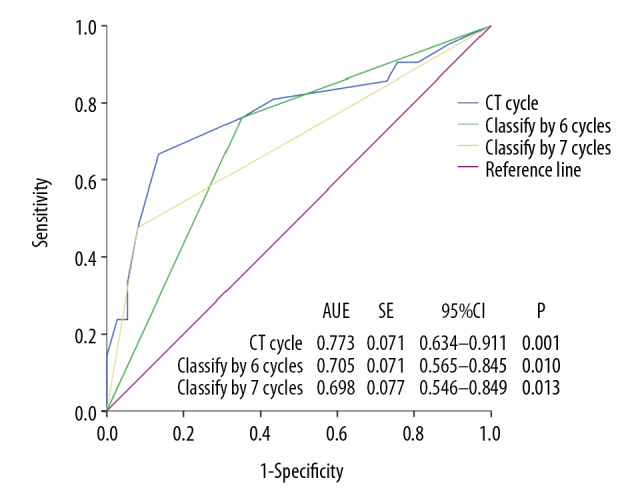
ROC curve for NPC patient survival with bone metastasis based on number of chemotherapy cycles.
Based on the ROC curve analysis results, 6 chemotherapy cycles were set as the optimal cut-off value in this study. Based on the number of chemotherapy cycles, patients were then randomly grouped if they received <6 or ≥6 cycles of chemotherapy. Each group had 29 patients. No statistically significant differences were observed for sex, age, KPS scores, T stage, N stage, pathological type, number of bone metastases, or the combination of nasopharyngeal radiotherapy with chemotherapy cycles (P<0.05) (Tables 2, 3).
Table 2.
Comparison of patient characteristics between the 2 groups.
| Variables | <6-cycle (N=29) | ≥6-cycle (N=29) | P-value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 26 | 26 | |
| Female | 3 | 3 | |
| Age | |||
| >60 | 7 | 3 | 0.164 |
| ≤60 | 22 | 26 | |
| Karnofsky performance status | 0.487 | ||
| >80 | 23 | 25 | |
| ≤80 | 6 | 4 | |
| T stage | 0.089 | ||
| T1–2 | 6 | 12 | |
| T3–4 | 23 | 17 | |
| N stage | 0.487 | ||
| N0–1 | 4 | 6 | |
| N2–3 | 25 | 23 | |
| Histology (WHO) | 1.000 | ||
| Type I/II | 9 | 9 | |
| Type III | 20 | 20 | |
| Number of bone metastatic sites | 1.000 | ||
| >3 | 16 | 16 | |
| ≤3 | 13 | 13 | |
| Nasopharyngeal radiotherapy | 0.063 | ||
| Yes | 13 | 20 | |
| No | 16 | 9 |
Table 3.
Comparison of the different chemotherapy cycles between the 2 groups.
| Chemotherapy | Cycle(N) | <6-cycle N(%) | ≥6-cycle N(%) | P-value |
|---|---|---|---|---|
| Palliative | 0.421 | |||
| TPF | ≤4 | 19 (65.5%) | 16 (55.2%) | |
| >4 | 10 (34.5%) | 13 (44.8%) | ||
| Concurrent | 1.000 | |||
| DDP | 0 | 20 (69.0%) | 21 (72.4%) | |
| 1 | 2 (6.9%) | 2 (6.9%) | ||
| 2 | 3 (10.3%) | 2 (6.9%) | ||
| 3 | 4 (13.8%) | 4 (13.8%) |
TPF – docetaxel, cisplatin and 5-fluorouracil; DDP – cisplatin.
Survival outcomes
The median OS for the whole group was 24 months, and the 1-, 2-, and 3-year OS rates were 78.5%, 49.4%, and 26.8%, respectively. The median OS for the <6-cycle chemotherapy group was 21 months, and the 1-, 2-, and 3-year OS rates were 64.8%, 34.3%, and 17.2%, respectively. The median OS for the ≥6-cycle chemotherapy group was 26 months. The 1-, 2-, and 3-year OS rates were 92.6%, 54.9%, and 30.9%, respectively. The differences between the groups were statistically significant (P<0.005). The results are shown in Table 4, and the survival curves are shown in Figures 2 and 3.
Table 4.
OS rates in the 2 patient groups.
| Group | N | OS(%) | P-value | ||
|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | |||
| <6-cycle | 29 | 64.8% | 34.3% | 17.2% | 0.042 |
| ≥6-cycle | 29 | 92.6% | 54.9% | 30.9% | |
Figure 2.
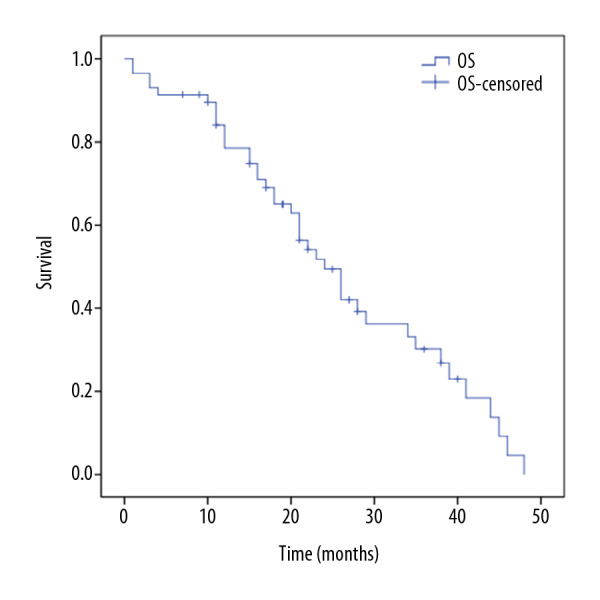
Overall survival for the entire patient cohort.
Figure 3.
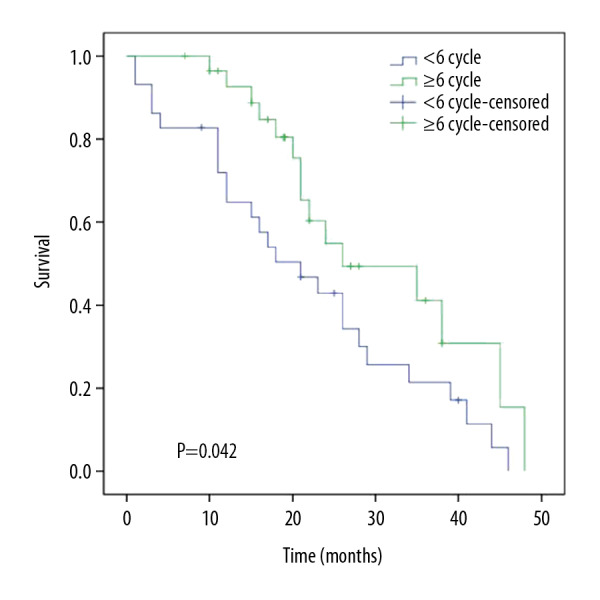
Overall survival for the 2 patient groups.
Univariate analysis
Univariate analysis demonstrated that the number of bone metastatic sites (<3 vs. ≥3) and chemotherapy cycles (<6 vs. ≥6) were able to predict OS. Multivariate analysis demonstrated both to be independent prognostic factors for NPC patients with initial bone-only metastasis. The results of univariate and multivariate analysis are shown in Tables 5 and 6.
Table 5.
Univariate analysis of clinical factors for overall survival.
| Variables | HR | 95% CI for HR | P-value |
|---|---|---|---|
| Sex (Female vs. Male) | 1.681 | 0.582–4.855 | 0.337 |
| Age (>60 vs. ≤60 years) | 1.212 | 0.568–2.590 | 0.619 |
| KPS (≤80 vs. >80 years) | 0.957 | 0.884–1.036 | 0.279 |
| Histology (Type I/II vs. Type III) | 0.953 | 0.492–1.846 | 0.888 |
| T stage (T1–2 vs. T3–4) | 1.112 | 0.782–1.581 | 0.554 |
| N stage (N0–1 vs. N2–3) | 0.777 | 0.521–1.159 | 0.216 |
| Number of bone metastatic sites (<3 vs. ≥3) | 1.434 | 1.024–2.008 | 0.036 |
| Nasopharyngeal radiotherapy (receipt vs. nonreceipt) | 0.522 | 0.273–1.000 | 0.050 |
| Chemotherapy cycles (<6 vs. ≥6) | 0.516 | 0.267–0.996 | 0.049 |
HR – hazard ratio; CI – confidence interval.
Table 6.
Independent prognostic factors from multivariate analysis for overall survival.
| Factors | HR | 95% CI for HR | P-value |
|---|---|---|---|
| Number of bone metastatic sites (<3 vs. ≥3) | 1.513 | (1.076~2.129) | 0.017 |
| Chemotherapy cycles (<6 vs. ≥6) | 0.462 | (0.237–0.899) | 0.023 |
Discussion
This study demonstrated that patients with advanced metastatic NPC with bone metastases have different prognoses based on the number of chemotherapy cycles that were administered. Based on T stage comparisons, patients who received more than 6 cycles of intensive chemotherapy had longer survival times.
Numerous studies have shown that predicting the prognosis of patients with metastatic NPC with bone metastases is very different [10,21,22]. Lan et al. retrospectively analyzed clinical data from 178 patients with newly diagnosed NPC with bone metastases; the 5-year overall survival rate was 25.5%. Shen et al. reviewed and analyzed 312 patients with bone metastases from NPC and showed that the median survival time was 23.4 months, with 1-, 3-, and 5-year overall survival rates of 79.1%, 34.0%, and 22.1%, respectively. At present, there are no standardized treatments for metastatic NPC. With the widespread application of docetaxel in China, more and more clinical trials have confirmed the safety and effectiveness of a 2-drug or 3-drug combination regimen containing docetaxel in metastatic nasopharyngeal carcinoma. Clinical studies began to use TP (Docetaxel+Platinum) or TPF (Docetaxel+Platinum+Fluorouracil) instead of PF as first-line chemotherapy. Chinese scholars and experts have reached a consensus suggesting that a platinum-based 2-drug or 3-drug combination plan is still recommended for newly-diagnosed metastatic nasopharyngeal carcinoma [14]. Based on the 2017 edition of the NCCN guidelines, concurrent chemoradiotherapy or induction chemotherapy with platinum-based chemotherapy is recommended for NPC. Radiotherapy and treatment of the primary neck lesion are recommended [23]. Therefore, based on the consensus of Chinese experts and the latest treatment guidelines, patients in our study who were diagnosed with NPC and initial bone-only metastases received concurrent chemoradiotherapy or induction chemotherapy and treatment of the primary neck lesion, and none of the patients received radiation therapy for bone metastases. Systemic chemotherapy and local radiotherapy are crucial for the initial treatment of metastatic NPC. However, no clear guidelines are available for the appropriate chemotherapy intensity and intervention time of radiotherapy.
Several studies have suggested that adequate systemic chemotherapy should be the first-line treatment strategy for metastatic NPC. Multiple retrospective studies have demonstrated that ≥4 cycles of intensive chemotherapy significantly prolong OS and PFS for patients with metastatic NPC compared to <4 cycles of intensive chemotherapy [24–27]. However, several studies have shown that long-term chemotherapy may reduce patient tolerance and delay local treatment of the primary lesion. Chen et al. [17] performed a retrospective analysis of 406 cases of newly-diagnosed metastatic NPC, showing no significant survival benefit for patients who received >6 chemotherapy cycles versus ≤6 chemotherapy cycles (MST: 27.6 months: 23.2 months, P=0.099). Tian et al. [18] performed a retrospective analysis of patients with NPC oligometastasis and showed that 4 to 5 cycles of chemotherapy were better than >6 cycles or <4 cycles for overall survival benefit (P<0.01). In the present study, the median OS for patients who received <6-cycles of chemotherapy was 21 months, and the 1-, 2-, and 3-year OS rates were 64.8%, 34.3%, and 17.2%, respectively. The median OS for patients who received ≥6-cycle chemotherapy was 26, and the 1-, 2-, and 3-year OS rates were 92.6%, 54.9%, and 30.9%, respectively. Similar to previous studies, Wang et al. [11] and others showed that patients with metastatic NPC treated with ≥6 cycles had 1-, 2-, and 3-year survival rates of 88.9%, 66.7%, and 22.2%, respectively. Our univariate and multivariate analysis suggested that patients who received <6 cycles of chemotherapy had a worse prognosis, and this agrees with previous studies [11,16,28]. Hence, for NPC patients with initial bone metastases, 6 cycles of intensive chemotherapy are recommended and consensus has been reached with local Chinese medical institutions [14].
The number of bone metastases is an independent risk factor for prognosis. A retrospective analysis of 312 NPC patients with bone metastases showed that if the number of bone metastases was >3, then the median OS was only 16.2 months; however, the median OS was 32.4 months when the number of metastatic sites was ≤3. Patient mortality increased by 1.80 times when the number of metastatic sites was >3 (95% CI: 1.29–2.50, P<0.05) [18]. In our study, the median OS for patients with >3 metastatic sites was 21 months, while the median OS for patients with metastatic sites ≤3 was 26 months. Both univariate and multivariate analyses showed that the number of bone metastatic sites was associated with prognosis, consistent with previous studies [21,29–31]. Hence, aggressive treatment is required for patients with multiple bone metastases, and results in significant survival benefits.
Recent studies have shown that histological subtype determines the long-term survival outcomes of patients with NPC. Pan et al. [32] demonstrated that the NKC subtype has the best prognosis, while the KSCC subtype has the worst prognosis. Wu et al. [33] found that within a follow-up period over 5 years, patients with DNKC had poorer NPC-specific survival (NPC-SS) compared to UNKC, and had comparable NPC-SS between the 2 subtypes after more than 5 years of follow-up. Moreover, within the follow-up periods of 1, 2, and 3 years, patients with KSCC experienced poorer NPC-SS compared to UNKC, but there was comparable NPC-SS between KSCC and UNKC patients after more than 3 years of follow-up. However, in our study, multivariate analysis showed that the histology was not an independent prognostic factors for OS, probably because the pathological type in both subgroups of our study was mainly WHO III.
Several recent studies have demonstrated that primary radiotherapy was associated with prognosis. Hu et al. [34] demonstrated that 679 newly-diagnosed metastatic NPC patients who underwent local radiotherapy had a 50% reduction in mortality rates. These findings were similar to the results published by Chen et al. [35]. The 3-year OS for patients who received primary radiotherapy was 51.7%. Univariate and multivariate analysis demonstrated that primary radiotherapy was a prognostic factor associated with survival. Rusthoven et al. [36] analyzed 718 metastatic NPC patients from the NCDB database and found that chemoradiotherapy after systemic chemotherapy could achieve longer survival benefits compared to chemotherapy alone. Numerous studies have demonstrated that the addition of radiotherapy for the treatment of the primary lesion could significantly improve the prognosis and curative effects in some patients [18,26,37–39].
In this study, patients who had their primary tumor treated with radiotherapy had a better median OS compared to patients who did not – 28 versus 17 months (P=0.043). However, Cox multivariate analysis demonstrated no significant correlation with survival. We believe this may be due to the small cohort size in this study.
The results of this study indicated that initial high-intensity chemotherapy treatment of NPC patients with bone metastases could prolong patient survival time. The number of bone metastatic sites and ≥6 cycles of chemotherapy were factors that influenced prognosis, both of which could help guide treatment choice and improve overall survival.
There were several limitations to our study. First, this was a single-center retrospective study with a small patient cohort and a limited follow-up period. Second, all adverse reactions were not recorded due to the limited follow-up period.
Conclusions
In summary, NPC patients with ≤3 bone metastatic sites and who received ≥6 cycles of intensive chemotherapy had better OS and prognosis.
Footnotes
Ethics statement
This retrospective study was approved by the Ethics Committee of the Affiliated Tumor Hospital of Guangxi Medical University.
Conflicts of interest
None.
Source of support: This work was supported by the Guangxi Key R&D Program (GuikeAB18221007)
References
- 1.Xing-Li Y, Yan W, Shao-Bo L, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: Analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18(1):606. doi: 10.1186/s12885-018-4419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S, Pan J, Han L, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110(3):385–89. doi: 10.1016/j.radonc.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Pan J, Wu J, et al. Factors associated with overall survival in 1706 patients with nasopharyngeal carcinoma: Significance of intensive neoadjuvant chemotherapy and radiation break. Radiother Oncol. 2010;96(1):94–99. doi: 10.1016/j.radonc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Peng G, Wang T, Yang K, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–93. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Sun P, Chen Y, Feng X, et al. High-dose static and dynamic intensity-modulated radiotherapy combined with chemotherapy for patients with locally advanced nasopharyngeal carcinoma improves survival and reduces brainstem toxicity. Med Sci Monit. 2018;24:8849–59. doi: 10.12659/MSM.910465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad A, Stefani S. Distant metastases of nasopharyngeal carcinoma: A study of 256 male patients. J Surg Oncol. 2010;33(3):194–97. doi: 10.1002/jso.2930330310. [DOI] [PubMed] [Google Scholar]
- 7.Ai Q, Hu CW, Bhatia KS, et al. Nasopharyngeal carcinoma: Relationship between invasion of the prevertebral space and distant metastases. Eur Arch Otorhinolaryngol. 2018;275(2):497–505. doi: 10.1007/s00405-017-4825-z. [DOI] [PubMed] [Google Scholar]
- 8.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13(7):1007–15. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Ng W, Chan L, et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncology. 2012;48(10):1007–13. doi: 10.1016/j.oraloncology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Lan YH, Tian MY, Bai L, et al. [Post-treatment prognostic score model establishment and stratified therapy for newly diagnosed metastatic nasopharyngeal carcinoma]. China Journal of Radiation Oncology. 2015;24(4):421–26. [in Chinese] [Google Scholar]
- 11.Wang CC, Cao KJ, Li X, et al. [Prognosis analysis of nasopharyngeal carcinoma patients with distant metastasis]. Ai Zheng. 2007;26(2):212–15. [in Chinese] [PubMed] [Google Scholar]
- 12.Han L, Lin SJ, Li YM, et al. [Therapeutic results of 46 patients with initially diagnosed metastatic nasopharyngeal carcinoma]. China Journal of Radiation Oncology. 2009;18(3):170–72. [in Chinese] [Google Scholar]
- 13.Lee V, Kwong D, Leung TW, et al. Palliative systemic therapy for recurrent or metastatic nasopharyngeal carcinoma – How far have we achieved? Crit Rev Oncol Hematol. 2017;114:13–23. doi: 10.1016/j.critrevonc.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Chen XZ, Hu CS, Li JG, et al. [Expert consensus on the treatment of metastasis nasopharyngeal carcinoma]. China Journal of Radiation Oncology. 2018;27(1):23. [in Chinese] [Google Scholar]
- 15.Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: Patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124(1):139–46. doi: 10.1016/j.radonc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Bai Y, Wu M, et al. Combined CT-guided radiofrequency ablation with systemic chemotherapy improves the survival for nasopharyngeal carcinoma with oligometastasis in liver: Propensity score matching analysis. Oncotarget. 2016;8(32):52132–141. doi: 10.18632/oncotarget.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604–13. doi: 10.5732/cjc.013.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian YH, Zou WH, Xiao WW, et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: A subset with better overall survival. Head Neck. 2016;38(8):1152–57. doi: 10.1002/hed.24345. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Leslie HS, Mary KG, Christian W. TNM classification of malignant tumours (UICC International Union Against Cancer) 7th ed. Hoboken: Wiley-Blackwell; 2009. [Google Scholar]
- 21.Shen L, Dong J, Li S, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist. 2015;20(3):291–98. doi: 10.1634/theoncologist.2014-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang XJ, Wang XY, Yang ZN, et al. [Prognostic analysis of 68 patients with initially diagnosed bone-only metastatic nasopharyngeal carcinoma]. China Journal of Radiation Oncology. 2017;26(10):1137–40. [in Chinese] [Google Scholar]
- 23.NCCN. NCCN clinical practice guidelines in oncology: Head and neck cancers [EB/OL] 2017. Feb 06, [2017-06-10]. http://guide.medlive.cn/guideline/preview/1/12707?token=0232f55bfc6a990c30a7370f0bee8026.
- 24.Li W, Bai Y, Wu M, et al. Combined CT-guided radiofrequency ablation with systemic chemotherapy improves the survival for nasopharyngeal carcinoma with oligometastasis in liver: Propensity score matching analysis. Oncotarget. 2017;8(32):52132–41. doi: 10.18632/oncotarget.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun M, Zeng L, Chen BB, et al. [Prognosis and treatment of newly diagnosed nasopharyngeal carcinoma with oligometastases]. China Journal of Radiation Oncology. 2016;25(11):1156. [in Chinese] [Google Scholar]
- 26.Hu SX, He XH, Dong M, et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32(9):224. doi: 10.1007/s12032-015-0663-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Li J, Zou S, et al. [Identify independent prognostic factors and optimize treatment of initial nasopharyngeal carcinoma with distant metastasis]. Journal of Sun Yat-sen University (Medical Sciences) 2014;35(6):880–88. [in Chinese] [Google Scholar]
- 28.Hong RL, Sheen TS, Ko JY, et al. Induction with mitomycin C, doxorubicin, cisplatin and maintenance with weekly 5-fluorouracil, leucovorin for treatment of metastatic nasopharyngeal carcinoma: a phase II study. Br J Cancer. 1999;80(12):1962–67. doi: 10.1038/sj.bjc.6690627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu YF, Ding F, Chen B, et al. [Prognostic impact of degree of bone metastasis in patients with nasopharyngeal carcinoma after radiochemotherapy]. China Journal of Radiation Oncology. 2013;22(4):299–302. [in Chinese] [Google Scholar]
- 30.Aera Y, Chel Hun C, Tae-Hyun K, et al. Bone metastasis in primary endometrial carcinoma: Features, outcomes, and predictors. Int J Gynecol Cancer. 2014;24(1):107–12. doi: 10.1097/IGC.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 31.Lu T, Guo Q, Cui X, et al. Prognostic evaluation of nasopharyngeal carcinoma with bone-only metastasis after therapy. Yonsei Med J. 2016;57(4):840–45. doi: 10.3349/ymj.2016.57.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan XX, Liu YJ, Yang W, et al. Histological subtype remains a prognostic factor for survival in nasopharyngeal carcinoma patients. Laryngoscope. 2020;130(3):E83–88. doi: 10.1002/lary.28099. [DOI] [PubMed] [Google Scholar]
- 33.Wu SG, Lian CL, Wang J, et al. The effect of histological subtypes on survival outcome in nasopharyngeal carcinoma after extensive follow up. Ann Transl Med. 2019;7(23):768. doi: 10.21037/atm.2019.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Kong L, Gao J, et al. Use of radiation therapy in metastatic nasopharyngeal cancer improves survival: A SEER analysis. Sci Rep. 2017;7(1):721. doi: 10.1038/s41598-017-00655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Ye D, Liu KT, et al. [Investigation of prognostic influencing factors and treatment option in treatment-naive patients with metastatic nasopharyngeal carinoma]. Chinese General Practice. 2018;21(14):51–57. [Google Scholar]
- 36.Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: Patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124(1):139–46. doi: 10.1016/j.radonc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for, de novo, metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117–26. doi: 10.1016/j.ejca.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Jiang R, You R, Pei X, et al. Development of a ten-signature classifier using a support vector machine integrated approach to subdivide the M1 stage into M1a and M1b stages of nasopharyngeal carcinoma with synchronous metastases to better predict patients’ survival. Oncotarget. 2016;7(3):3645–57. doi: 10.18632/oncotarget.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Y, Bu J, Cheng J, et al. Selective radiotherapy after distant metastasis of nasopharyngeal carcinoma treated with dose-dense cisplatin plus fluorouracil. Asian Pac J Cancer Prev. 2015;16(14):6011–17. doi: 10.7314/apjcp.2015.16.14.6011. [DOI] [PubMed] [Google Scholar]


