Abstract
Obesity is the major contributing factor for the increased prevalence of type 2 diabetes (T2D) in recent years. Sustained positive influx of lipids is considered to be a precipitating factor for beta cell dysfunction and serves as a connection between obesity and T2D. Importantly, fatty acids (FA), a key building block of lipids, are a double-edged sword for beta cells. FA acutely increase glucose-stimulated insulin secretion through cell-surface receptor and intracellular pathways. However, chronic exposure to FA, combined with elevated glucose, impair the viability and function of beta cells in vitro and in animal models of obesity (glucolipotoxicity), providing an experimental basis for the propensity of beta cell demise under obesity in humans. To better understand the two-sided relationship between lipids and beta cells, we present a current view of acute and chronic handling of lipids by beta cells and implications for beta cell function and health. We also discuss an emerging role for lipid droplets (LD) in the dynamic regulation of lipid metabolism in beta cells and insulin secretion, along with a potential role for LD under nutritional stress in beta cells, and incorporate recent advancement in the field of lipid droplet biology.
Keywords: FFAR1, lipotoxicity, lipolysis, lipid droplets, PLIN
Introduction
Nutritional overload is the major trigger for the development of type 2 diabetes (T2D).1 Insulin resistance associated with nutritional overload is first compensated by increasing insulin secretion from pancreatic beta cells. However, eventual failure of the compensation results in the development of T2D.1 Importantly, the pathology behind the failure of pancreatic islets in T2D is not merely “insufficient” compensation. The loss of beta cell mass and function in T2D is a progressive change that continues after the initial clinical manifestation of T2D, and is largely responsible for progressive deterioration in glycemic control and increased dependency on insulin therapy in T2D.2,3 As overnutrition precedes the development of islet dysfunction in T2D, excess exposure of beta cells to lipids is an early event during the development of T2D and considered to contribute to the loss of beta cell mass and function (lipotoxicity).4 At the same time, lipids are indispensable for beta cells as an energy source, building blocks for biological membranes, and signaling molecules.5 In addition, fatty acids (FA) have a unique functional role in beta cells, as they acutely augment glucose-stimulated insulin secretion (GSIS).6 Thus, knowledge regarding islet lipid metabolism is critically important to understand the two-sided relationship between beta cells and lipids.
Here, we aim to present a comprehensive and up-to-date review of the regulation of lipid metabolism in beta cells and the response of beta cells to lipid overload with the following focus areas. We discuss recent advances in the understanding of molecular mechanisms by which FA support insulin secretion through receptor- and cell-mediated pathways. Although the concept of lipotoxicity was originally proposed over 20 years ago, a sequence of events that initiates beta cell dysfunction and drives progressive beta cell failure in human T2D remains poorly defined. We also discuss the current understanding of lipotoxicity in beta cells by incorporating recent publications. Finally, the recent advancement in lipidology has revealed that lipid droplets (LD) play a key role in the spatial and temporal regulation of intracellular lipid metabolism; we review recent research that has begun to define the nature and function of LD in pancreatic islets.
How do FA and lipid metabolites support GSIS?
Exogenous FA do not trigger insulin secretion from beta cells in the absence of glucose. However, a certain level of FA is essential for normal GSIS, and the acute rise in FA potently augments GSIS in vivo and in vitro.7,8 While both the chain length and saturation of FA affect the potency of the stimulation, palmitic acids (16:0, PA) and oleic acids (18:1, OA), two FA abundant in the circulation and cells, are both potent stimulators of GSIS.9,10 The augmentation of GSIS by FA is partially mediated by the activation of the cell surface FFA1/GPR40 receptor (FFAR1).6 In addition, intracellular metabolism of FA in beta cells is considered to generate metabolic coupling factors (MCF) that contribute to insulin secretion.6
The regulation of insulin secretion by cell surface fatty acid receptors
Glucose-dependent augmentation of insulin secretion by FFAR1 activation.
FFAR1 is a G protein–coupled receptor highly expressed in beta cells and activated by medium- to long-chain FA.11 The receptor primarily signals by Gαq-mediated activation of phospholipase C (PLC) and increases insulin secretion in the presence of glucose. This glucose dependency makes FFAR1 an attractive therapeutic target for T2D, as the receptor will not trigger insulin secretion at low glucose levels, thereby reducing the risk of hypoglycemia.11 More than 50% of the augmentation of insulin secretion by FA is considered to be mediated by FFAR1, based on genetic and pharmacological suppression of FFAR1 signaling, while the remainder is considered to require intracellular metabolism of FA.12 FFAR1 activation phosphorylates protein kinase D1 (PKD1) through diacylglycerol (DAG) generated by PLC, resulting in F actin remodeling and insulin secretion. FFAR1 activation also increases intracellular calcium ([Ca2+]i) by releasing calcium from the endoplasmic reticulum (ER) through inositol triphosphate (IP3) receptor. However, the overall significance of ER calcium ([Ca2+]ER) release in GSIS under FFAR1 activation remains unclear, as influx of calcium from outside of cells appears to contribute to insulin secretion more than calcium released from the ER.11 Physiological ligands for FFAR1 in vivo have been debated since fatty acid concentration in the circulation is higher when insulin secretion is suppressed during fasting; one proposed mechanism is that chylomicron-derived FA activate FFAR1 postprandially,13 though activation of FFAR1 by 20-hydroxyeicosatetraenoic acid (20-HETE) has emerged as an alternative mechanism for postprandial activation of FFAR1.14
20-HETE is an omega-hydroxyl fatty acid generated by arachidonic acid (AA) that activates FFAR1 more potently than FA abundant in the circulation, such as PA in mouse and human beta cells.14 Although circulatory levels of 20-HETE are too low to stimulate FFAR1 in vivo, glucose has been shown to increase the production of 20-HETE by beta cells. Thus, 20-HETE can activate FFAR1 in beta cells through an autocrine/paracrine loop in a glucose-dependent manner. Interestingly, the production of 20-HETE is reduced in islets from mouse models of T2D and human islets from type 2 diabetic donors, indicating that lower production of 20-HETE may contribute to reduced GSIS in T2D. Identification of 20-HETE as a ligand for FFAR1 provides a mechanism by which FFAR1 is activated without an increase in fatty acid availability.15 Further studies are necessary to determine whether FFAR1 is primarily activated by 20-HETE or by FA in beta cells, since the reduction in GSIS in the absence of added FA has been noted in some,16 but not in others,17,18 studies when FFAR1 signaling is inhibited in mice in vivo and isolated islets in vitro.
Palmitic acid hydroxystearic acid (PAHSA) is a branched PA that is another potential endogenous ligand of FFAR1.18 Found in an adipose tissue and in the circulation, PAHSA has been shown to reduce insulin resistance and adipose inflammation.18 While its contribution to GSIS in vivo requires further study, PAHSA has been shown in vitro to activate FFAR1 directly in human islets and MIN6 cells.18
FFAR1 as a target to improve glucose homeostasis in T2D.
After its discovery in 2003 as a stimulator of insulin secretion,19 strong interest developed to use FFAR1 activation for diabetic therapy. It was first questioned whether chronic activation of FFAR1 by hyperlipidemia in T2D positively or negatively affects beta cell health and function. An early study showed that mice with pancreatic and duodenal homeobox 1 (Pdx1)-cre-mediated deletion of FFAR1 were protected against hyperglycemia, while overexpression of FFAR1 using Pdx1 promoter impaired islet function, indicating that chronic activation of FFAR1 impairs islet function and glucose homeostasis in mice.20 However, later studies did not persistently show the benefit of FFAR1 deficiency under chronic fatty acid loading in mouse models in vivo and in vitro.11 In addition, FFAR1 activators were shown to improve glucose homeostasis and insulin secretion in mouse models of T2D.11
In humans, FFAR1 is not among the T2D risk loci in genome-wide association study (GWAS), and nonsynonymous variants of FFAR1 have not shown correlation with the development of T2D.11 However, the expression level of FFAR1 in human islets is reported to correlate with insulin secretion and T2D status, indicating that a reduction of FFAR1 may play an active role in impaired insulin secretion in human T2D.21,22 Collectively, current evidence suggests that FFAR1 is a legitimate target to improve insulin secretion and glucose homeostasis in T2D. Indeed, the first-generation FFAR1 agonists, such as TAK-875 and MK-8666, showed promise in human clinical trials by improving hyperglycemia. Unfortunately, trials were eventually stopped because of hepatotoxicity due to the lipophilic nature of the agonists rather than a direct consequence of FFAR1 activation by agonists.23 A new generation of agonists with better safety profiles has been actively in development and may soon become a new class of therapeutic agents for T2D.11 Newer ligands that bind a different site within FFAR1 (ago-allosteric ligands) engage Gαs in addition to Gαq, leading to increased incretin secretion from enteroendocrine cells (L and K cells), allowing further enhancement of insulin secretion.15
A proposed role for GPR119 in the regulation of glucose hemostasis.
GPR119 is a Gαs-coupled receptor that was originally proposed to stimulate insulin secretion in response to oleoylethanolamine.24 While activation of GPR119 in isolated islets increases insulin secretion, the deletion of the gene GPR119 from beta cells in mice surprisingly did not reduce insulin secretion or blunt response to GPR119 agonists, indicating that GPR119 in beta cells is dispensable for GPR119-mediated insulin secretion in vivo.25 It was proposed that GPR119 increases GSIS indirectly through incretin release mediated by the GPR119 receptor in enteroendocrine cells.25
Intracellular lipid metabolites as regulators of insulin secretion
Prior to the identification of FFAR1, much research was focused on identifying an intracellular pathway by which FA augment insulin secretion.8,26 Two key concepts that emerged were that intracellular lipid metabolism is tightly intertwined with glucose metabolism and, second, that esterification and lipolysis of neutral lipids (glycerolipids/fatty acid cycling) support GSIS in beta cells. Below, we present the current view of these concepts, incorporating newly available information from studies of both beta cells and lipid biology.
Fatty acid uptake and release by beta cells.
Fatty acid uptake serves as the first step when FA regulate GSIS through intracellular targets. Unlike glucose, which is hydrophilic and needs a transporter for uptake, FA have a low energy barrier to cross the phospholipid (PL) bilayer, enabling their easy passage.27 Although plasma membrane proteins, such as CD36, and fatty acid transport protein (FATP) facilitate crossing of FA through the plasma membrane, FA appear to cross the plasma membrane on the order of milliseconds, even in the absence of these proteins.27,28 CD36/SR-B2 is a class B scavenger receptor that shows high affinity for long-chain FA.29 CD36 is considered to concentrate FA within specific plasma membrane domains and increase fatty acid availability for subsequent membrane translocation. Several studies have shown that CD36 increases fatty acid uptake in beta cells.30,31 Although not studied in beta cells extensively, it is important to note that CD36 modulates fatty acid signaling in addition to facilitating fatty acid transfer across the plasma membrane.32 FATPs are acyl-coenzyme A (CoA) synthases at the plasma membrane that promote movement of FA into aqueous cytosol by increasing water solubility through the addition of a Co-A moiety.27 In general, total uptake of FA by cells is primarily determined by the concentration gradient of FA across the plasma membrane. Thus, the increase in the extracellular concentration of FA and “metabolic trapping” of FA within cells through the formation of acyl-CoA (FATP), fatty acid esterification, and fatty acid oxidation all promote cellular fatty acid uptake.27 It is likely that these multiple factors, in addition to CD36, affect uptake of FA by beta cells. Reflecting ease of passage through the plasma membrane, FA can be released from the plasma membrane and the intracellular pool of beta cells in a glucose concentration–dependent manner.33 While it has been known that efficient GSIS requires exposure of beta cells to a certain concentration of FA,8 a recent study highlighted that FA released from the plasma membrane to the extracellular space play an important role in GSIS.34 Extensive washing of beta cells, or sequestration of secreted FA by fatty acid–free bovine serum albumin (BSA), reduced [Ca2+]i oscillation within seconds and blunted insulin release in MIN6 cells and mouse beta cells.34 FA released from the plasma membrane appear to increase GSIS by activating FFAR1, considering that FFAR1 antagonist caused the reduction of [Ca]i.34
The regulation of lipid metabolism by glucose in beta cells.
The availability of glucose alters intracellular metabolism of FA rapidly and significantly in beta cells.26,35 Two key changes brought by glucose in beta cells include the increase in malonyl Co-A and the formation of glycerol-3-phosphate (glycerol-3-p).35 Although both changes are not unique to beta cells and are seen in other types of cells including myocytes and hepatocytes, these changes are proposed to play a key role in regulating GSIS in beta cells.
Glucose increases malonyl Co-A by activating acetyl-CoA carboxylase (ACC) and increasing acetyl-CoA through citrate production from glucose (Fig. 1). Thus, malonyl Co-A has been regarded as a “fuel-sensor” in a wide range of cells.36,37 In beta cells, a function of malonyl-CoA appears to be integrated into the regulation of GSIS; beta cell–specific knockout of ACC1 impairs insulin secretion both in vivo and in vitro.26,38 It has been proposed that malonyl-CoA impacts GSIS in beta cells by its well-known function as a suppressor of carnitine palmitoyl transferase 1 (CPT1). Metabolic labeling of beta cells has confirmed that fatty acid oxidation is reduced upon glucose loading in beta cells, and blockade of beta-oxidation by etomoxir (CPT1 inhibitor) does not impair GSIS, indicating that beta-oxidation does not provide energy source for GSIS.39,40 In contrast, the promotion of fatty acid oxidation by either overexpression of CPT1 or by expression of a mutant form of CPT1 impaired GSIS in INS1 cells and rat islets.41 The reduction of beta-oxidation at high glucose in beta cells is proposed instead to support GSIS by increasing the intracellular pool of long-chain FA available for esterification and protein modification.35 Malonyl-CoA can be elongated by fatty acid synthase (FAS) to provide an endogenous source of long-chain FA as well. However, there are conflicting data whether the suppression or reduction of FAS impairs GSIS.42,43 Thus, it remains to be determined whether long-chain FA directly derived from malonyl-CoA, or long-chain FA from the circulation, play a significant role in supporting GSIS.
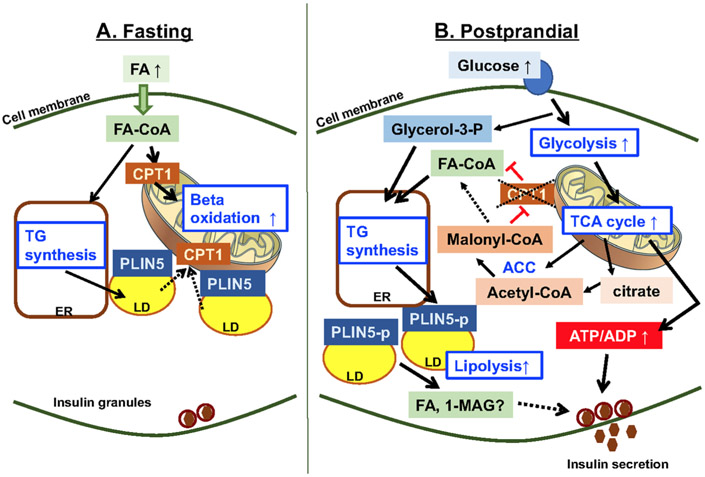
Figure 1.
Cross talk between lipid metabolism and glucose metabolism in beta cells. (A) Fasting increases fatty acids (FA) in the circulation due to lipolysis in adipose tissues. FA are transported into mitochondria by carnitine palmitoyl transferase 1 (CPT1) for beta-oxidation and used as the primary source of energy during fasting in beta cells.5 In addition, a part of FA is esterified to triglycerides (TG) at the endoplasmic reticulum (ER) to form lipid droplets (LD).131 PLIN5 is a lipid droplet protein whose expression is upregulated during fasting in beta cells and considered to coat LD during fasting along with PLIN2.132 Although not shown in beta cells, LD are also known to aid the transfer of FA to mitochondria for beta-oxidation during starvation.124 (B) Glucose plays a central role in triggering insulin secretion by increasing ATP/ADP through upregulation of glycolysis and the TCA cycle.6 In addition, glucose increases citrate and activates acetyl-CoA carboxylase (ACC), resulting in the increase of malonyl-CoA, which in turn suppresses CPT1 and reduces transport of fatty acid coenzyme A (FA-CoA) into mitochondria and beta-oxidation.26 While this pathway is not necessarily unique to beta cells, the resultant increase in long-chain FA is considered to support insulin secretion. Glucose also provides glycerol-3 phosphate (glycerol-3-p) that is used for TG synthesis.35 In addition to TG synthesis, glucose is proposed to increase lipolysis in beta cells.35 Although there may potentially be multiple pathways involved, phosphorylation of PLIN5 is one proposed mechanism that mediates the increase in lipolysis postprandially in beta cells.124 The increase in TG synthesis and lipolysis is proposed to support insulin secretion through GL/FFA cycling. Metabolites generated by lipolysis, such as FA and 1-monoacylglycerol (1-MAG), are proposed to augment insulin secretion.6
The formation of glycerol-3-p from glucose provides a glycerol backbone for esterification of endogenous and exogenous FA to form glycolipids, such as PLs, DAG, and triacylglycerol (TG) (Fig. 2).40,44 Earlier studies demonstrated that synthesis of DAG by esterification of FA increases quickly in response to glucose in beta cells.45,46 Recent metabolic studies provide further support that glycerol-3-p generated from glucose is used for synthesis of glycerolipids in beta cells.33,40,44 While the increase in glycerolipid formation with increased flux of glucose is not a unique feature of beta cells, the formation of glycerolipids from glycerol-3-p is considered to facilitate GSIS through several pathways. Glycerol-3-p generation consumes nicotinamide adenine dinucleotide (NADH) and produces NAD+ that can accelerate glycolysis. The glycerolipids that are generated, such as DAG, are proposed to augment insulin secretion (discussed further below; Fig. 2). At high glucose concentrations, the formation of glycerol-3-p allows removal of excess glucose by incorporation into TG or secretion as glycerol after removal of phosphate from glycerol-3-p.33 Interestingly, a recent study in INS1 cells showed that the activation of FFAR1 also promotes glycerolipid formation in beta cells, indicating that increased GSIS by FFAR1 may not totally be independent from intracellular fatty acid metabolism.40
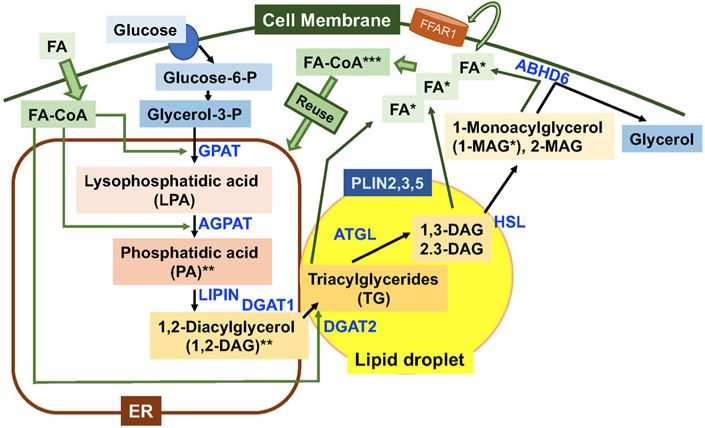
Figure 2.
Triacylglycerol (TG) synthesis and lipolysis in beta cells. Fatty acids (FA) enter cells with or without the aid of facilitators and form FA-CoA.27 Glucose transporter 1 and 2 mediate uptake of glucose into beta cells.6 Glycerol-3-phosphate (glycerol 3-p) generated from glucose undergoes sequential reactions mediated by glycerol-3-phosphate acyltransferase (GPAT) and 1-acylglycerol-3-phosphate-O-acyltransferase (AGPAT) to attach two fatty acid chains to form phosphatidic acids at the endoplasmic reticulum (ER).125 Phosphate of phosphatidic acids is removed by lipin to form 1,2-diacylglycerol (1,2-DAG). The addition of a third fatty acid chain to 1,2-DAG within the bilayer of the ER membrane by diacylglycerol transferase (DGAT) 1 and 2, or at lipid droplets (LD) by DGAT2 forms triacylglycerol (TG).125 TG created within the bilayer of the ER membrane will bud off as LD or will be transferred to LD, as LD often maintain a connection with the (ER).125 Thus, TG primarily resides in LD that are covered by a single phospholipid layer and studded with lipid metabolism enzymes and a perilipin family of lipid droplet proteins. Human beta cells express PLIN2, 3, and 5.131 Adipose triglyceride lipase (ATGL) is a major TG lipase that initiates lipolysis by removing FA from Sn1 or Sn2 position.47 Hormone-sensitive lipase (HSL) has high activity against DAG and preferentially releases FA from Sn3.47 In beta cells, membrane-bound ABHD6 is reported to function as a monoacylglycerol lipase and releases the last FA from monoacylglycerol (MAG).49 FA being released can be secreted from beta cells and activate cell surface fatty acid receptors, such as FFAR1, or are converted to FA-CoA and reused for TG synthesis.6 *, Metabolites that have known targets to increase insulin secretion; **, metabolites that potentially increase insulin secretion but are not confirmed to be directly released from the ER; and ***, metabolites implicated for insulin secretion but that do not have confirmed targets.
Glycerolipid/free fatty acid cycle and insulin secretion.
The glycerolipid/free fatty acid (GL/FFA) cycle consists of esterification of FA to synthesize TG and then lipolysis of TG to release glycerol and FA that can be re-esterified to continue cycling (Fig. 2).6 In beta cells, it has been proposed that de novo glycerolipid synthesis in response to glucose is coupled with lipolysis to form the GL/FFA cycle, and that the GL/FFA cycle plays an active role in promoting GSIS based on evidence extensively discussed in previous reviews.6,35 In brief, three key supporting arguments include (1) rapid formation of glycerolipids in response to glucose (as discussed above), (2) generation of metabolites known to modulate insulin secretion by the cycle, and (3) reduction of GSIS by suppression of lipolysis.
To understand the GL/FFA cycles, it is important to note that the sequential steps of lipolysis is not an exact reversal of TG synthesis in most mammalian cells, both for products and locations of reactions (Fig. 2). Sequential esterification of glycerol-3-p produces lysophosphatidic acids (LPA) and phosphatidic acids primarily at the ER. Phosphatidic acids are then converted to Sn-1,2 DAG by the action of lipin/phosphatidate phosphatase at the ER. Thereafter, diglyceride acyltransferase (DGAT)1 and DGAT2 will convert DAG to TG that will be incorporated into LD. Lipolysis of TG will be initiated at LD by adipose-triglyceride lipase (ATGL), which preferentially removes FA from Sn-1 or 2, producing Sn-1,3 DAG and Sn-2,3 DAG.47 Sn-1,3 DAG and Sn-2,3 DAG are further metabolized to 1-monoacylglycerol (MAG) or 2-MAG by hormone-sensitive lipase (HSL), which preferentially removes FA from Sn-3.47 This reaction is considered to occur at LD in general, but the expression of HSL in insulin granules is reported in beta cells, indicating that MAG may be generated in the vicinity of insulin granules.48 MAG is further hydrolyzed to produce glycerol and FA by lipases including alpha/beta-hydrolase containing domain 6 (ABHD6) that resides at the plasma membrane in beta cells.49
Several metabolites generated by the GL/FFA cycles are implicated in regulating insulin secretion. Phosphatidic acids are reported to augment GSIS by reducing guanosine triphosphatase (GTPase) activity associated with insulin granules.50 Sn-1,2 DAG is another metabolite that has been proposed to increase GSIS by activating protein kinase C (PKC) and PKD.51 Sn-1,2 DAG is also considered to increase exocytosis by activating Munc13-1.52 However, phospholipase D, which is known to increase GSIS,53 can generate phosphatidic acids from plasma membrane PL. Similarly, PLC, the downstream target of both FFAR1 and muscarinic receptor in beta cells, can generate Sn-1,2 DAG from plasma membrane PL. It remains to be determined whether phosphatidic acids and Sn-1,2 DAG generated during TG synthesis at the ER directly contribute to GSIS to the same extent as those generated at the plasma membrane by PLC and phospholipase D. For the lipolysis arm, TG, Sn-1,3 DAG, Sn-2,3 DAG, and 2-MAG do not have proposed targets to regulate insulin secretion. 1-MAG generated by ABHD6 has been reported to promote exocytosis by interacting with Munc 13-1 like Sn-1,2 DAG in mouse and human islets.54 At the same time, the pharmacological inhibition of MAG lipase reduced GSIS in INS1 cells and rat islets despite the increase in MAG.55 Further study is required to determine whether the location and type of enzymes, species of MAG generated, or host species contributes to differences between the two studies. Although FA and its activated form FA acyl-CoA have been proposed to augment insulin secretion, a precise mechanism regarding their contribution to GSIS remains elusive.35 One possible mechanism involves secretion of FA and activation of FFAR1 (Fig. 2).
Another proposed mechanism involves acylation of proteins, a widely seen posttranslational modification.26 A variety of proteins highly relevant to GSIS, such as Cavβ2a calcium channel and synaptosome-associated protein 25 (SNAP25), require palmitoylation for membrane targeting.56,57 While dynamic regulation of protein palmitoylation during GSIS has been suggested, it remains unclear which proteins are palmitoylated in a glucose-dependent manner and aid in insulin secretion.58 The ATP-sensitive potassium channel (KATP channel) is an attractive candidate as it is palmitoylated. However, the activity of the KATP channel increases by palmitoylation.26
Another proposed mechanism by which FA increase GSIS is activation of atypical PKC.59 Although it may not acutely regulate GSIS, the activation of nuclear receptors by FA is a known mechanism by which lipolysis regulates gene expression in cardiomyocytes, liver, and adipose tissues,60,61 and may be relevant in modulating beta cell function in the long term.
Finally, the evidence for the contribution of lipolysis to GSIS benefits from careful review. Classically, linear increase in glycerol secretion in response to glucose has been taken as a key piece of evidence for the upregulation of lipolysis by glucose in beta cells.62 However, a recent study indicated that glycerol-3-p generated from glucose can be converted back to glycerol directly by glycerol-3-p phosphatase in beta cells, which questions the accuracy of glycerol as an index of lipolysis in beta cells.63 When the time course of changes in TG and DAG species in response to glucose was determined by lipidomics, it is implicated that lipolysis of TG produces DAG with 16:0 and 18:0 fatty acid chains in beta cells after glucose exposure.64 Beyond this, the acute reduction of GSIS by pan lipase inhibitor orlistat in rat islets and beta cell lines is the strongest evidence supporting an active role of lipolysis in the acute regulation of GSIS.6,65 As orlistat suppresses multiple neutral lipases, it has yet to be determined which lipase plays a primary role in supporting GSIS in beta cells.66 Beta cell–specific knockout and knockdown of the ATGL gene and mRNA, respectively, in mice and INS1 cells have been associated with TG accumulation and impaired insulin secretion, supporting the notion that ATGL is the major TG lipase in beta cells and that sustained suppression of TG lipolysis negatively affects insulin secretion.67,68 In support of 1-MAG’s role in promoting exocytosis, islets from mouse insulin promoter (MIP)-Cre-mediated ATGL knockout mice contained less 16:0 and 18:0 MAG and showed lower GSIS, which is partly recovered by the presence of 1-MAG.68 Interestingly, this MIP-Cre-mediated ATGL knockout mice showed improved insulin sensitivity along with lower circulatory insulin, indicating interplay between beta cells and whole-body energy homeostasis.68 In a separate model, rat insulin promoter (RIP)-Cre-mediated ATGL knockout mice on a high-fat diet also showed impaired GSIS in isolated islets that is attributed to mitochondrial dysfunction due to reduced activation of peroxisome proliferator-activated receptor (PPAR)δ by FA.67 Glucose homeostasis and insulin secretion from isolated islets were studied in several models of HSL knockout mice. Results were somewhat mixed but showed a certain degree of islet dysfunction in general.62 Thus, chronic reduction of ATGL and HSL in beta cells impairs GSIS through multiple targets depending on nutritional conditions or genetic background. However, it requires a further study with a specific inhibitor to suppress each enzyme to clarify actual contribution of each lipase in supporting GSIS acutely.
Collectively, significant circumstantial evidence exists for the contribution of the GL/FFA cycle to insulin secretion. However, the extent of contribution to insulin secretion, the regulation of the cycle, and molecular targets of the cycle are not firmly established yet.
Lipid signaling that regulates glucagon secretion
Glucagon secreted from alpha cells prevents hypoglycemia through its action primarily on the liver by promoting gluconeogenesis as a hormone. However, glucagon also fine tunes beta cell function in a paracrine manner.69 Newly emerging data have revealed that lipid metabolism and fatty acid signaling in alpha cells regulate alpha cell function.
While contribution of fatty acid oxidation for insulin secretion by beta cells is small, glucagon secretion under low glucose was shown to depend on fatty acid oxidation in both mouse and human islets.70 Fatty acid receptors in alpha cells also have an active role in the regulation of glucagon secretion. FFAR1 in alpha cells is reported to respond to increases in plasma FA during fasting and augment glucagon secretion.15 GPR119 in alpha cells may augment glucagon release under hypoglycemia.71
In summary, both fatty acid receptors and intracellular lipid metabolism are an integral part of the regulation of insulin and glucagon secretion and allow dynamic changes in their secretion in response to availability of glucose and FA.
The regulation of beta cell proliferation by FA
The increase in insulin secretion upon nutritional load is important not only for the maintenance of glucose homeostasis but also for the storage of nutrition for future use. To support the increase demands of insulin secretion for a sustained period, the expansion of beta cell mass becomes critical. While it is well accepted that glucose stimulates beta cell proliferation, there have been conflicting data about whether FA directly increase beta cell proliferation.72,73 There are several reports indicating that cultures of rat islets with FA, and the infusion of FA over 2–4 days in rats, increases beta cell proliferation.72-75 The activation of mammalian target of rapamycin complex 1 (mTORC1) has been implicated in supporting proliferation by FA in one study of rat beta cells.76 However, in another report, fatty acid infusion blocked glucose-induced proliferation of beta cells in mice.77 In human islet in culture, both positive and negative results have been reported, depending on types of FA added.75,76,78 As there are numerous variables, including length of treatment, species and strains of rodents, age, and type of FA, it is currently difficult to reach consensus. Further studies, especially those that focus on humans, will be valuable.
Lipotoxicity and lipid metabolism
Continuous exposure to excess lipids, especially those rich in saturated FA, is proposed to be the major contributing factor for the initiation and the progression of beta cell failure in T2D.79 The negative effect of lipid exposure on beta cells is readily seen in vitro when clonal beta cells and islets are exposed to high concentration of FA, such as PA and OA. Numerous studies collectively have shown that prolonged (over 24–72 h) incubation of beta cells and islets with a high concentration of FA (0.2–0.5 mM) in the presence of glucose causes apoptosis and reduces insulin secretion (glucolipotoxicity) through activation of multiple stress pathways, including ceramide generation, ER stress, oxidative stress, mitochondrial dysfunction, and inflammation.79-81 While evidence exists for each stress pathway’s contribution to lipotoxicity, it remains inconclusive how these relate to the beta cell failure that occurs over decades in humans. Below, we review evidence connecting beta cell dysfunction in humans with the exposure of beta cells to lipids through recent clinical and metabolomic studies, the understanding of lipotoxicity in beta cells, and the gap in knowledge that requires future studies.
Review of clinical studies that connect pancreatic islet exposure to lipids and beta cell failure
As fatty acid uptake does not require transporters, the increase in environmental FA is expected to create positive balance of FA within cells and trigger lipotoxicity in beta cells. Hyperlipidemia is one potential source that exposes beta cells to excess lipids during T2D development in humans.1 Indeed, many human studies, including those with prediabetes, have demonstrated that the concentration of FA in circulation negatively correlates with parameters of beta cell function in both adults and youths from different ethnic populations;82-84 however, it should be noted that absence of a correlation between the two has also been reported.85 Local accumulation of lipids in the pancreas also increases exposure of islets to lipids. Historically, lipotoxicity as a precipitating factor for beta cell failure was first proposed in a rodent model of T2D in which the accumulation of pancreatic fat precedes beta cell dysfunction.86 Advancement of imaging studies such as ultrasound, computer tomography, and magnetic resonance imaging enables measurement of pancreatic fat in humans and has revealed increased pancreatic lipid contents with obesity, metabolic syndrome, and T2D.87,88 Although it is not established whether pancreatic fat accumulation is sufficient for the development of beta cell dysfunction, or if reduction of pancreatic fat reverses beta cell dysfunction in humans,89-91 local fat accumulation is a possible source of lipids that affect beta cell health in humans. Collectively, human clinical studies provide a certain degree of evidence that the increase in circulatory and local lipids triggers beta cell failure in T2D.
Metabolomics implicates incomplete beta-oxidation as a culprit for beta cell failure in T2D
Acylcarnitines are intermediary metabolites of fatty acid oxidation that are increased in the circulation of insulin-resistant subjects and in T2D.92 Long-chain acylcarnitines (>C14), generated in mitochondria from long-chain fatty acid coenzyme A, are proposed to reduce insulin sensitivity in insulin target tissues, especially in skeletal muscle, by affecting ion channels and phosphorylation of the insulin receptor at the plasma membrane.92 A recent study implicated that the accumulation of acylcarnitines due to incomplete beta-oxidation of long-chain FA is a culprit of beta cell failure in T2D.93 Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI–MSI) based analysis of metabolites in islets from diabetic humans and two mouse models of T2D (Tally ho and db/db mice) showed an increase of acylcarnitines, including stearoylcarnitine, palmitoylcarnitine, linoleolcarnitine, and acetylcarnitine in islets.93 Stearoylcarnitine progressively increases as insulin content decreases in islets during the development of diabetes during both prediabetic and diabetic stages of mice.93 Combining metabolomic data and in vitro treatment of MIN6 cells with long-chain acylcarnitines, the study proposed that incomplete utilization of FA in the mitochondria increases acylcarnitines first in mitochondria and then in the cytosol, impairing both oxidative phosphorylation in mitochondria and insulin synthesis in cytosol.93 In a separate study in which mouse islets were incubated with FA and high glucose in vitro, the accumulation of carnitine ester 3-hydroxytetradecenoic acids was noted.94 Interestingly, medium-chain acylcarnitines (C6–C10) that are known to rise prior to long-chain acylcarnitines in mitochondria are increased in the blood of recent-onset T2D and subjects with gestational diabetes mellitus (GDM). Moreover, exposure to medium-chain acylcarnitines at the levels seen in GDM blunted insulin secretion in mice in vivo and in human and mouse islets in vitro.95 Collectively, data suggest that accumulation of acylcarnitines appears to be an early event during the development of beta cell dysfunction in T2D. While acute glucose challenge reduces fatty acid transport into mitochondria by suppressing CPT1 in beta cells (Fig. 1), beta cell dysfunction under chronic lipid exposure appears to be associated with incomplete fatty acid oxidation within mitochondria that likely stems from an influx of FA to mitochondria beyond their capacity for beta-oxidation.
Stress pathways that contribute to beta cell dysfunction under nutritional stress
Multiple stress pathways known to be activated by beta cell exposure to lipids in vitro provide evidence of their activation in human pancreatic islets affected by T2D.79,80,96 Since the activation of one stress pathway can induce other stress pathways, progressive beta cell demise in T2D likely is a result of simultaneous activation of multiple stress pathways. While it is beyond the scope of this review to detail each stress pathway implicated in lipotoxic damage of beta cells, below we briefly describe key information focused on recent research.
Ceramides are biologically active sphingolipids that can provoke ER stress, oxidative stress, and c-Jun N-terminal kinase (JNK) activation, reduce insulin gene expression, and dysregulate intracellular signaling, including protein kinase B (PKB) inhibition and Ras-related C3 botulinum toxin substrate 1 (Rac1) activation.97-99 However, the contribution of ceramide to beta cell demise in human T2D is not firmly established, as its accumulation is not necessarily seen in all models of T2D, especially at early stages.98 The prooxidant nature of FA, combined with low expression of antioxidative defense enzymes, is believed to provoke oxidative stress in beta cells and produce peroxidated lipids and proteins that cause inflammation and damage organelles in beta cells.100,101
Recently, ferroptosis was identified as one form of regulated cell death that depends on iron and reactive oxygen species.102 While there still is not direct demonstration of ferroptosis by lipotoxic stimuli in beta cells, the contribution of iron overload for the development of beta cell failure in human T2D has significant evidence making ferroptosis a potential pathway involved in beta cell demise under overnutrition.103
Insulin resistance associated with overnutrition pressures ER to meet increased demand for insulin production.104 High concentrations of FA have been shown to deplete [Ca2+]ER in beta cells105,106 by reducing expression of pumps that regulate [Ca2+]ER homeostasis, such as sarco/ER Ca2+-ATPase 2B (SERCA2B),107 sorcin,108 and stromal interaction molecule 1 (STIM1).109 As ER maintains higher calcium concentration compared with cytosol to support protein folding and to regulate [Ca2+]i, [Ca2+]ER depletion results in ER stress and dysregulates insulin secretion. Overload of FA, especially that of saturated FA, is reported to alter the composition of membrane PLs and contribute to ER stress, mitochondrial dysfunction, and production of proinflammatory metabolites such as AA.110-112 Interestingly, diet rich in omega-3 polyunsaturated eicosapentaenoic acids (EPA) was shown to change the cell membrane composition of pancreatic islets, reduce AA production, and improve beta cell function in BTBR leptinob/ob mice, indicating a significant contribution of membrane lipids for beta cell failure in diabetes.113 Dilation of the ER and swelling of mitochondria were reported in an electron micrscopic study of human beta cells in T2D.114 In a study of INS1 cells, PA increased dynamin-related protein 1 (DRP1) phosphorylation and led to ER enlargement and mitochondrial fragmentation, providing one potential pathway by which FA alter the ER and mitochondria morphology.115 Human islets from T2D show signs of inflammation.96 PA has been shown to provoke an inflammatory response in beta cells and recruit inflammatory macrophages into islets.96,116,117 Interleukin 1 beta (IL-1β) has been proposed as a key upstream molecule to initiate islet inflammation during the development of T2D.117 However, it should be noted that a recent large human trial of an IL-1β inhibitor, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), did not demonstrate prevention of diabetes over a medium period of 3.7 years despite the reduction in cardiovascular events also considered to have inflammatory basis.118 IL-1β may therefore not be a critical node governing progression of beta cell failure during the development of T2D.
Despite the wealth of information regarding molecular pathways by which lipotoxicity causes beta cell demise, a case like IL-1β illustrates that we still lack critical information regarding the hierarchy of multiple stress pathways or the identity of early stress response that can be targeted to prevent further exacerbation of stress response. Thus, future studies that address sequence of events during the development of beta cell failure are important for identifying effective targets for prevention or reversal of beta cell dysfunction in human T2D. For this goal, it is critical to recognize a limitation in simulating the development of beta cell failure in animal models, especially in mice that show significant difference in islet structure, gene expression, and functions compared with humans.119-121 When responses to high-fat diet are compared between mouse and human islets being transplanted to immunodeficient NOD/scid/gamma (NSG) mice, basal insulin secretion was increased in both mouse and human islets, but GSIS was reduced only in human islets.119 While mouse islets showed increases in beta cell proliferation and unfolded protein response, human islets did not but did develop oxidative stress.119 Interestingly, two histological differences noted between mouse and human islet transplants were amyloid accumulation and the formation of large LD.119 While the lack of amyloid formation in mouse islets is well known, the study implicated a potential significant difference between lipid metabolism and lipid droplet formation under chronic nutritional stress in human beta cells that might contribute to blunting in GSIS seen only in human islets.119 We next discuss the currently available knowledge regarding islet LD.
Islet LD in the regulation of lipid metabolism, insulin secretion, and defense against lipotoxic stress
General knowledge of LD from studies of nonbeta cells
LD are an intracellular organelle consisting of a neutral lipid core of TG and sterol esters (cholesterol ester and retinol ester) surrounded by a PL monolayer.122 The lipid droplet surface is studded by the perilipin (PLIN) family of proteins (PLIN1–5) that play an important role in stabilization of LD, lipolysis, and interaction of LD with other proteins and organelles (Table 1).123 LD provide temporal and spatial regulation of lipid metabolism and have diverse functions beyond lipid metabolism through interaction with a wide array of proteins and intracellular organelles, including the ER, mitochondria, the nucleus, and lysosomes.122 The best characterized function of LD is to regulate mobilization of stored lipids through interaction with lipases and lysosomes (autophagy), as recently reviewed.60,123 LD are coated with Rab proteins and are mobile within cells. High-resolution imaging of mouse embryonic fibroblasts labeled with a fluorescent fatty acid probe revealed that LD serve as a shuttle to transfer FA from lysosomes to the mitochondria.124 Thus, a proposed role of the GL/FFA cycling in supporting GSIS makes LD an organelle highly relevant for regulation of GSIS (Fig. 2).
Table 1.
Perilipin family of proteins
| Name | Tissue expression145 | Characteristics |
|---|---|---|
| PLIN1 | Adipose and steroidogenic tissues | Contains six serine residues that are phosphorylated by PKA.123 Prevents lipolysis at nonstimulated state.123 Interacts with ATGL and HSL in a PKA-dependent manner to increase lipolysis.123 |
| PLIN2 | Ubiquitous Found on the surface of LD |
Considered to serve as a barrier for lipolysis123 No known association with ATGL123 Recognized by HSPA8/Hsc7 for degradation by chaperon-mediated autophagy.123 |
| PLIN3 | Ubiquitous | Can be found as a cytosolic protein and on the surface of LD.145 No known association with ATGL.123 Recognized by HSPA8/Hsc7 for degradation by chaperon-mediated autophagy.123 |
| PLIN4 | High expression in adipocytes. Low expression in the heart and skeletal muscles | No known association with ATGL.123 |
| PLIN5 | High expression in oxidative tissues Increased expression upon fasting in islets131 |
Associates with ATGL and an ATGL coactivator CGI58.110 One molecule of PLIN5 is considered to bind either ATGL or CGI58, not both simultaneously.110 Has one PKA consensus site, and PKA-mediated phosphorylation increases lipolysis.123 Phosphorylation also promotes nuclear translocation and activation of PGC-1alpha.139 Associates with mitochondria.140 |
LD are also a potential target to mitigate lipotoxicity in beta cells as LD have been implicated in cellular processes highly relevant to lipotoxicity. LD generate from the ER, where triglyceride synthesis occurs, and often maintain contact with the ER.125 ER stress from overnutrition and the accumulation of unfolded protein has been noted to increase lipid droplet formation in yeasts and mammalian cells.122 In hepatocytes treated with tunicamycin and cardiomyocytes treated with PA, the promotion of lipid droplet formation mitigates ER stress.122 On the other hand, reduction of lipid droplet formation has been associated with lower ER stress in the liver of mice fed a high-fat diet, indicating that the contribution of LD to stress mitigation depends on the context of stress and cell types.126
An increase in lipid droplet formation is also noted under oxidative stress in cancer cells, hepatocytes, and glial cells.122 Similar to ER stress, both positive and negative effects of lipid droplet formation on oxidative stress have been reported indicating the importance of cell-specific studies.127,128 LD often reside in the vicinity of mitochondria in cells with a high capacity for fatty acid oxidation.124,129 This close association is proposed to facilitate transfer of FA from LD to mitochondria for beta-oxidation124 and/or transfer of energy from the mitochondria to LD for lipid droplet expansion;129 two opposite directions of communication that likely occur depending on cell types and energy status. In addition, it is proposed that LD protect mitochondria by sequestering FA and preventing fatty acid overload of mitochondria.130 Collectively, LD are often incorporated in ER stress, oxidative stress, and mitochondrial dysfunction pathways, but cell type–specific studies are required to determine whether LD play a positive or negative role under a specific type of stress.
A potential role of LD in beta cell function and health
Despite its potential importance, the role of LD in beta cells has been underappreciated because mouse beta cells form very small LD. However, LD of rat and human beta cells are readily visible using bodipy 493/503 neutral lipid dye (Fig. 3).76,131 More importantly, the formation of LD in beta cells is dynamically regulated in mice, rats, and humans.76,131,132 Rat and human islets cultured with FA accumulate LD in beta cells and increase expression of PLIN2 in an mTORC1–dependent manner.76 The expression of PLIN2 and PLIN5 in mouse islets is regulated by nutritional cues such as high-fat feeding and fasting in vivo.131,132 Human islets transplanted into immune-deficient mice accumulate LD in beta cells in response to a high-fat diet.119 Proteomic analysis of LD isolated from INS1 cells confirmed that LD are enriched with proteins that are known to regulate lipid metabolism, including PLIN2, long-chain fatty acid–CoA ligase (ACLS) 3, ACLS4, and ATGL.133 In addition, proteins functionally associated with vesicle formation, mitochondria, and ER have been shown to be present in the proteome of LD from INS1 cells, providing further support that LD are a metabolically active organelle in beta cells.133
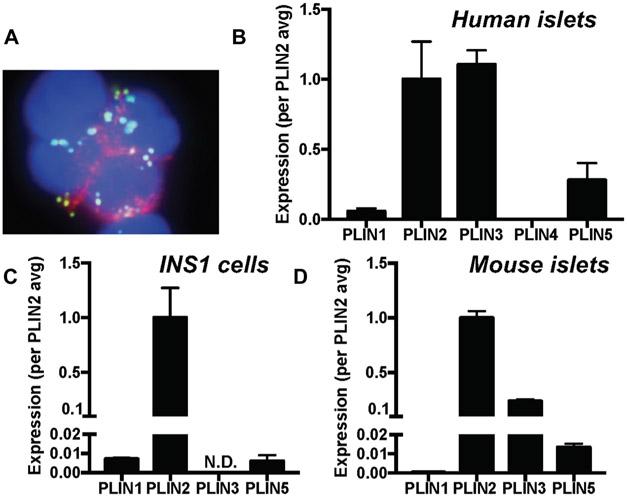
Figure 3.
Lipid droplet formation and perilipin expression in beta cell models. (A) Dispersed human islets were stained for insulin (red), neutral lipid (bodipy 493/405, green), and nuclei (DAPI), and the image was captured using a fluorescent microscope using methods published.131 Expression levels of perilipin (PLIN) 1–5 were compared by qPCR using TaqMan primers using methods published131 in human islets from nondiabetic donors (n=7–14) (B), INS1 cells (n=3–4) (C), and islets from a 4-month-old male ad libitum fed C57BL6NJ mouse (n = 6) (D). Data are mean ± SEM and expressed taking the average expression of PLIN2 as 1. N.D., not detected.
PLIN expression profile in pancreatic islets and beta cells
It is helpful to address the role of LD from the point of PLIN because unique molecular characteristics of each PLIN on the surface of LD play a critical role in determining the function of LD (Table 1).123 As shown in Figure 3, the profile of PLIN expression differs between beta cell lines and pancreatic islets. However, PLIN2 is the most abundantly expressed PLIN in both beta cell lines (MIN6 cells132 and INS1 cells) and pancreatic islets132 (Fig. 3). In human islets, the expression of PLIN3 is as high as that of PLIN2. PLIN3 is expressed at significant level in mouse islets as well, but its expression is not detectable in INS1 cells. PLIN5 is the third most abundant PLIN in both human and mouse islets. While it is unclear how different PLIN expression profiles affect lipid metabolism and lipid droplet formation in beta cell lines and pancreatic islets, recent studies of each PLIN in beta cells and pancreatic islets have begun to provide important clues regarding the role of LD in the regulation of lipid metabolism and beta cell function.
PLIN2, the most abundant PLIN in beta cells
PLIN2 is widely expressed in nonadipocytes and shows a strong correlation with cellular triglyceride pool size partly because TG prevents ubiquitin-mediated degradation of PLIN2.134 Unlike PLIN1 that regulates lipolysis through protein kinase A (PKA)–dependent interaction with HSL, PLIN2 does not have a known association with lipases or a consensus site for phosphorylation by PKA, and it is considered to serve as a passive barrier for lipolysis.123 It has reportedly been demonstrated that overexpression of PLIN2 is sufficient to increase lipid droplet formation, while the downregulation of PLIN2 reduces lipid droplet formation in a wide range of cells, indicating that PLIN2 can actively determine lipid droplet pool size.123 In nonbeta cells, the modulation of PLIN2 expression is reported to affect multiple aspects of cellular lipid handling, including FA uptake, beta-oxidation, and activation of nuclear receptors in the liver, heart, macrophages, and white adipocytes.123,135,136
PLIN2 protein levels closely correlate with cellular triglyceride contents in beta cells as well. PLIN2 protein is increased in beta cell lines, mouse islets, and human islets after fatty acid loading in vitro and in islets from obese mice (high-fat diet, ob/ob mice) and fasted mice.133,137
In MIN6 cells, the reduction of PLIN2 by antisense oligo nucleotide (ASO) significantly reduced TG contents, FA uptake, and FA oxidation indicating that PLIN2 in beta cells regulates lipid metabolism as well.133 While GSIS itself was relatively unaffected, augmentation of GSIS by PA was significantly blunted in MIN6 cells treated by ASO against PLIN2, indicating that PLIN2-coated LD are required for acute augmentation of GSIS by FA in MIN6 cells.133 To test whether PLIN2 protects beta cells from the cytotoxic effect of chronic lipid overload, PLIN2 was downregulated by shRNA in RINm5F and INS1 cells;138 the reduction did not exacerbate or prevent cell death under chronic lipid overload, indicating that PLIN2 and LD formation have little effect on cell viability under lipotoxic stress in these beta cell lines.138 However, the impact of PLIN2 reduction for beta cell function under lipotoxic stress was not studied.138 The role of PLIN2 in beta cells under ER stress was studied using Akita mice that develop marked ER stress due to an insulin 2 gene mutation.137 Whole-body PLIN2 deficiency in Akita mice improved blood glucose levels compared with PLIN2-sufficient Akita mice, indicating that the loss of PLIN2 reduces ER stress in beta cells.137 In the study, the authors propose that the loss of PLIN2 reduces ER stress by upregulating autopahgy.137 Future work will be important to determine whether the loss of PLIN2 positively or negatively affects beta cell function in vivo under other stresses, especially overnutrition, considering that LD are reported to protect or exacerbate stress responses depending on type of stress.122
PLIN5, a potential regulator of lipolysis in beta cells
PLIN5 is proposed to control lipolysis of nonadipocytes in a PKA-dependent manner by interacting with both ATGL and the lipase activator CGI-58 (comparative gene identification-58, aka ABHD5).123 In addition, PLIN5 is proposed to translocate to nuclei upon PKA activation and upregulate genes important for mitochondrial function by activating PPAR-γ-coactivator 1-alpha (PGC1-α) through Sirtuin 1–mediated deacetylation.139 In accordance with a proposed function in facilitating lipolysis and mitochondrial fatty acid utilization, the expression of PLIN5 is high in cells that actively oxidize FA, such as cardiomyocytes, oxidative muscle, and brown adipose tissue.123 PLIN5 has a mitochondrial target sequence that is proposed to facilitate close association of LD with mitochondria.140 The expression of PLIN5 is very low in many cultured cells, including MIN6 and INS1 cells (Fig. 3); PLIN5 expression in islets from fed mice is also relatively low (Fig. 3). However, gene and protein expression of PLIN5 increases drastically during fasting in mice.131 Triglyceride contents are also increased in islets of fasted mice, indicating that circulatory FA released from adipose tissue during fasting accumulates in mouse islets and forms LD enriched with PLIN5.131 Although mRNA levels of PLIN2 do not increase upon fasting in mouse islets, PLIN2 protein levels do increase due to posttranslational stabilization by an increase in TG driven by PLIN5.132 It is known that the rise of circulatory FA is important for subsequent postprandial insulin secretion, since the prevention of a rise in circulatory FA reduces insulin secretion upon refeeding.7,141 Thus, upregulation of PLIN5 during fasting provides a mechanism to trap circulatory FA as TG within islets during fasting, and subsequently mobilizes stored TG upon refeeding when cyclic AMP (cAMP) levels increase (Fig. 1). The postprandial rise of cAMP contrasts beta cells from adipocytes and hepatocytes in which the rise in cAMP and lipolysis occurs during fasting.60 In support of PLIN5’s role in the regulation of lipolysis, overexpression of PLIN5 in MIN6 cells increases lipolysis. Moreover, overexpression of PLIN5 in MIN6 cells and mouse islets augments GSIS in response to FA and cAMP.132 As FFAR1 antagonism markedly reduces augmentation of GSIS by FA after PLIN5 overexpression, FA released by lipolysis appear to be secreted and increases GSIS through the activation of FFAR1.132 Importantly, PLIN5 expression in human islets is higher than that in mouse islets.131 Thus, PLIN5 may impact lipid metabolism and beta cell function significantly in human islets compared with mouse islets.
Other PLIN in beta cells
PLIN1 is selectively expressed in adipocytes and steroidogenic cells and serves as an on–off switch for lipolysis in these cells by preventing lipolysis at the nonstimulated condition and increasing lipolysis in response to PKA activation through recruitment of ATGL and HSL to the surface of LD.123 While there are reports of PLIN1 expression in rat and human beta cells, the expression level of PLIN1 is low compared with PLIN2, 3, and 5 in mouse and human islets, making the assessment of functional contribution of PLIN1 by downregulation in beta cells challenging (Fig. 3).131,138,142
PLIN3 is the second abundant PLIN in mouse and human islets, but it is virtually absent in beta cell lines, including MIN6 and INS1 cells (Fig. 3).131,132 A recent study of PLIN3-deficient mice indicates that PLIN3 prevents lipolysis in adipose tissue, and its deficiency promotes beige adipocyte formation through PPARα activation, presumably by increased generation of FA by lipolysis.61 However, little is currently known about the specific function of PLIN3 in beta cells. Considering the low expression levels of PLIN3 in beta cell lines, an assessment of PLIN3 function in beta cells will require rodent or human islet models. PLIN4 has three times larger molecular weight compared with other PLINs, and the least studied among PLINs in nonbeta cells. Whole-body PLIN4-deficient mice had little metabolic phenotypes except for the reduced fatty accumulation in the heart.143 Recently, PLIN4 was found to inhibit mitophagy in a neuroblastoma cell line.144 There currently is little information regarding the role of PLIN4 in beta cells. Of note, the expression of PLIN4 in beta cells and pancreatic islets is very low (Fig. 3).
Unanswered questions for the role of LD and PLIN in beta cells
With increased awareness of the multifaceted nature of LD, more information is emerging for the role played by LD in lipid metabolism and stress responses in multiple cell sytstems.122,123 However, there are still unanswered questions regarding the function of LD in beta cells. The GL/FFA cycle proposes that a rapid upregulation in lipolysis contributes to GSIS.35 However, further study is required to determine how glucose regulates lipolysis in beta cells. While PLIN5 is a potential mediator by which glucose upregulates lipolysis in beta cells, its expression is very low in beta cell lines, indicating that additional mechanisms need to be considered if a unified mechanism exists to regulate the GL/FFA cycle in beta cell lines and islets. Marked differences in lipid droplet size and expression profiles of PLIN occur in mouse and human beta cells. Thus, appropriate models need to be used to understand the regulation of lipid metabolism by LD and the mitigation of stress responses under chronic nutritional stress in human islets.119 It is known that autophagy contributes to the mobilization of lipids through macroautophagy, chaperon-mediated autophagy, and microautophagy.60 Considering that acute upregulation of lipolysis by glucose is required for the GL/FFA cycle to contribute to GSIS, autophagy may have a limited contribution to the GL/FFA cycle in beta cells. However, cross talk between LD and autophagosomes may have a role in the progression of beta cell dysfunction under nutritional stress, requiring future studies.
Concluding remarks
Significant progress has been made in recent years in our understanding of the regulation of insulin secretion by lipids and the response of beta cells to nutritional overload. Cell surface FFAR1 is likely activated by FA from environment and by endogenous FA and non-FA (20-HETE) secreted by beta cells. Currently, FFAR1 is one of the most promising targets in beta cells for T2D therapy. Recent metabolomic studies have provided strong confirmation that glucose acutely upregulates fatty acid esterification, which feeds into the GL/FFA cycle. However, it requires future studies to determine how lipolysis is regulated in beta cells to generate MCF from the GL/FFA cycle. In vitro models of lipotoxicity and mouse models of obesity have identified that nutritional stress activates stress pathways including oxidative stress, mitochondrial dysfunction, ER stress, and inflammation in beta cells. Further studies are required to determine what key molecular targets are effective to prevent or reverse the progression of beta cell demise in humans under nutritional stress. Of note, recent studies identified incomplete fatty acid oxidation as an early defect in beta cells, providing a promising lead to target early impairment in lipid metabolism in T2D. With a role in temporal and spatial regulation of lipid metabolism, further understanding of the function of LD in pancreatic islets is expected to aid answering some of unanswered questions, especially considering that LD are a prominent organelle in human beta cells.
Supplementary Material
Acknowledgments
This work was financially supported by the National Institutes of Health (R01-DK090490) and the American Diabetes Association to Y.I. (1-17-IBS-132). Authors used human pancreatic islets provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope (2UC4DK098085). Y.I. drafted the review. R.C., S.L., B.P., and J.P. contributed to the production of figures and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Competing interests
The authors declare no competing interests.
References
- 1.Prentki M & Nolan CJ. 2006. Islet beta cell failure in type 2 diabetes. J. Clin. Invest 116: 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halban PA, Polonsky KS, Bowden DW, et al. 2014. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab 99: 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alejandro EU, Gregg B, Blandino-Rosano M, et al. 2015. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol. Aspects Med 42: 19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger RH 2003. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144: 5159–5165. [DOI] [PubMed] [Google Scholar]
- 5.Tamarit-Rodriguez J, Vara E & Tamarit J. 1984. Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose. Biochem. J 221:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prentki M, Matschinsky FM & Madiraju SR. 2013. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 18: 162–185. [DOI] [PubMed] [Google Scholar]
- 7.Stein DT, Esser V, Stevenson BE, et al. 1996. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Invest 97: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, et al. 2006. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55(Suppl. 2): S16–S23. [DOI] [PubMed] [Google Scholar]
- 9.Stein DT, Stevenson BE, Chester MW, et al. 1997. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest 100: 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cen J, Sargsyan E & Bergsten P. 2016. Fatty acids stimulate insulin secretion from human pancreatic islets at fasting glucose concentrations via mitochondria-dependent and -independent mechanisms. Nutr. Metab. (Lond.) 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghislain J & Poitout V. 2017. The role and future of FFA1 as a therapeutic target. Handb. Exp. Pharmacol 236: 159–180. [DOI] [PubMed] [Google Scholar]
- 12.Kebede M, Alquier T, Latour MG, et al. 2008. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57: 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husted AS, Trauelsen M, Rudenko O, et al. 2017. GPCR-mediated signaling of metabolites. Cell Metab. 25: 777–796. [DOI] [PubMed] [Google Scholar]
- 14.Tunaru S, Bonnavion R, Brandenburger I, et al. 201820-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun 9: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trauelsen M, Luckmann M, Frimurer TM, et al. 2018. The HETE is on FFAR1 and pancreatic islet cells. Cell Metab. 27: 273–275. [DOI] [PubMed] [Google Scholar]
- 16.Alquier T, Peyot ML, Latour MG, et al. 2009. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58: 2607–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda-Nagasumi K, Takami-Esaki R, Iwachidow K, et al. 2013. Lack of GPR40/FFAR1 does not induce diabetes even under insulin resistance condition. Diabetes Obes. Metab 15:538–545. [DOI] [PubMed] [Google Scholar]
- 18.Syed I, Lee J, Moraes-Vieira PM, et al. 2018. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 27: 419–427 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y, Kawamata Y, Harada M, et al. 2003. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 20.Steneberg P, Rubins N, Bartoov-Shifman R, et al. 2005. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 1: 245–258. [DOI] [PubMed] [Google Scholar]
- 21.Del Guerra S, Bugliani M, D’Aleo V, et al. 2010. G-protein-coupled receptor 40 (GPR40) expression and its regulation in human pancreatic islets: the role of type 2 diabetes and fatty acids. Nutr. Metab. Cardiovasc. Dis 20: 22–25. [DOI] [PubMed] [Google Scholar]
- 22.Tomita T, Masuzaki H, Iwakura H, et al. 2006. Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion. Diabetologia 49: 962–968. [DOI] [PubMed] [Google Scholar]
- 23.Kaku K, Enya K, Nakaya R, et al. 2016. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: a 52-week open-label phase III study. Diabetes Obes. Metab 18: 925–929. [DOI] [PubMed] [Google Scholar]
- 24.Moran BM, Abdel-Wahab YH, Flatt PR, et al. 2014. Activation of GPR119 by fatty acid agonists augments insulin release from clonal beta-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol. Chem 395: 453–464. [DOI] [PubMed] [Google Scholar]
- 25.Panaro BL, Flock GB, Campbell JE, et al. 2017. Beta-cell inactivation of Gpr119 unmasks incretin dependence of GPR119-mediated glucoregulation. Diabetes 66: 1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaney GC & Corkey BE. 2003. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 46: 1297–1312. [DOI] [PubMed] [Google Scholar]
- 27.Pownall H & Moore K. 2014. Commentary on fatty acid wars: the diffusionists versus the translocatists. Arterioscler. Thromb. Vasc. Biol 34: e8–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton JA & Kamp F. 1999. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 48:2255–2269. [DOI] [PubMed] [Google Scholar]
- 29.Glatz JFC & Luiken J. 2018. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res 59: 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noushmehr H, D’Amico E, Farilla L, et al. 2005. Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 54: 472–481. [DOI] [PubMed] [Google Scholar]
- 31.Khan S & Kowluru A. 2018. CD36 mediates lipid accumulation in pancreatic beta cells under the duress of glucolipotoxic conditions: novel roles of lysine deacetylases. Biochem. Biophys. Res. Commun 495: 2221–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepino MY, Kuda O, Samovski D, et al. 2014. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr 34: 281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugabo Y, Zhao S, Lamontagne J, et al. 2017. Metabolic fate of glucose and candidate signaling and excess-fuel detoxification pathways in pancreatic beta-cells. J. Biol. Chem 292: 7407–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauke S, Keutler K, Phapale P, et al. 2018. Endogenous fatty acids are essential signaling factors of pancreatic beta-cells and insulin secretion. Diabetes 67: 1986–1998. [DOI] [PubMed] [Google Scholar]
- 35.Prentki M & Madiraju SR. 2012. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol. Cell. Endocrinol 353: 88–100. [DOI] [PubMed] [Google Scholar]
- 36.Ruderman NB, Saha AK, Vavvas D, et al. 1999. Malonyl-CoA, fuel sensing, and insulin resistance. Am. J. Physiol 276: E1–E18. [DOI] [PubMed] [Google Scholar]
- 37.Sugden MC, Bulmer K & Holness MJ. 2001. Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem. Soc. Trans 29: 272–278. [DOI] [PubMed] [Google Scholar]
- 38.Cantley J, Davenport A, Vetterli L, et al. 2019. Disruption of beta cell acetyl-CoA carboxylase-1 in mice impairs insulin secretion and beta cell mass. Diabetologia 62: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corkey BE, Deeney JT, Yaney GC, et al. 2000. The role of long-chain fatty acyl-CoA esters in beta-cell signal transduction. J. Nutr 130: 299S–304S. [DOI] [PubMed] [Google Scholar]
- 40.El-Azzouny M, Evans CR, Treutelaar MK, et al. 2014. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J. Biol. Chem 289: 13575–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrero L, Rubi B, Sebastian D, et al. 2005. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the beta-cell impairs glucose-induced insulin secretion. Diabetes 54: 462–471. [DOI] [PubMed] [Google Scholar]
- 42.Joseph JW, Odegaard ML, Ronnebaum SM, et al. 2007. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J. Biol. Chem 282: 31592–31600. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald MJ, Hasan NM, Dobrzyn A, et al. 2013. Knockdown of pyruvate carboxylase or fatty acid synthase lowers numerous lipids and glucose-stimulated insulin release in insulinoma cells. Arch. Biochem. Biophys 532: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenz MA, El Azzouny MA, Kennedy RT, et al. 2013. Metabolome response to glucose in the beta-cell line INS-1 832/13. J. Biol. Chem 288:10923–10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter-Riesch B, Fathi M, Schlegel W, et al. 1988. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J. Clin. Invest 81: 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf BA, Easom RA, Hughes JH, et al. 1989. Secretagogue-induced diacylglycerol accumulation in isolated pancreatic islets. Mass spectrometric characterization of the fatty acyl content indicates multiple mechanisms of generation. Biochemistry 28: 4291–4301. [DOI] [PubMed] [Google Scholar]
- 47.Eichmann TO, Kumari M, Haas JT, et al. 2012. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem 287: 41446–41457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindvall H, Nevsten P, Strom K, et al. 2004. A novel hormone-sensitive lipase isoform expressed in pancreatic beta-cells. J. Biol. Chem 279:3828–3836. [DOI] [PubMed] [Google Scholar]
- 49.Zhao S, Mugabo Y, Iglesias J, et al. 2014. Alpha/beta-hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 19: 993–1007. [DOI] [PubMed] [Google Scholar]
- 50.Kowluru A 2010. Small G proteins in islet beta-cell function. Endocr. Rev 31: 52–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang QJ 2006. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci 27: 317–323. [DOI] [PubMed] [Google Scholar]
- 52.Kang L, He Z, Xu P, et al. 2006. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 3: 463–468. [DOI] [PubMed] [Google Scholar]
- 53.Hughes WE, Elgundi Z, Huang P, et al. 2004. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J. Biol. Chem 279: 27534–27541. [DOI] [PubMed] [Google Scholar]
- 54.Zhao S, Poursharifi P, Mugabo Y, et al. 2015. Alpha/beta-hydrolase domain-6 and saturated long chain monoacylglycerol regulate insulin secretion promoted by both fuel and non-fuel stimuli. Mol. Metab 4: 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamontagne J, Al-Mass A, Nolan CJ, et al. 2017. Identification of the signals for glucose-induced insulin secretion in INS1 (832/13) beta-cells using metformin-induced metabolic deceleration as a model. J. Biol. Chem 292: 19458–19468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazim AS, Storm P, Zhang E, et al. 2017. Palmitoylation of Ca(2+) channel subunit CaVbeta2a induces pancreatic beta-cell toxicity via Ca(2+) overload. Biochem. Biophys. Res. Commun 491: 740–746. [DOI] [PubMed] [Google Scholar]
- 57.Gonelle-Gispert C, Molinete M, Halban PA, et al. 2000. Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells. J. Cell. Sci 113 (Pt 18): 3197–3205. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Ghany M, Sharp GW& Straub SG.2010. Glucose stimulation of protein acylation in the pancreatic beta-cell. Life Sci. 87: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaney GC, Korchak HM & Corkey BE. 2000. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal beta-cells. Endocrinology 141: 1989–1998. [DOI] [PubMed] [Google Scholar]
- 60.Zechner R, Madeo F & Kratky D. 2017. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol 18: 671–684. [DOI] [PubMed] [Google Scholar]
- 61.Lee YK, Sohn JH, Han JS, et al. 2018. Perilipin 3 deficiency stimulates thermogenic beige adipocytes through PPARalpha activation. Diabetes 67: 791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fex M & Mulder H. 2008. Lipases in the pancreatic betacell: implications for insulin secretion. Biochem. Soc. Trans 36: 885–890. [DOI] [PubMed] [Google Scholar]
- 63.Mugabo Y, Zhao S, Seifried A, et al. 2016. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic beta-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 113: E430–E439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson GL, Mellett N, Chu KY, et al. 2016. A comprehensive lipidomic screen of pancreatic beta-cells using mass spectroscopy defines novel features of glucose-stimulated turnover of neutral lipids, sphingolipids and plasmalogens. Mol. Metab 5:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fex M, Haemmerle G, Wierup N, et al. 2009. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia 52: 271–280. [DOI] [PubMed] [Google Scholar]
- 66.Iglesias J, Lamontagne J, Erb H, et al. 2016. Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors. J. Lipid Res 57:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang T, Abbott MJ, Ahmadian M, et al. 2013. Desnutrin/ATGL activates PPARdelta to promote mitochondrial function for insulin secretion in islet beta cells. Cell Metab. 18: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attane C, Peyot ML, Lussier R, et al. 2016. A beta cell ATGL-lipolysis/adipose tissue axis controls energy homeostasis and body weight via insulin secretion in mice. Diabetologia 59: 2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. 2018. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 27: 549–558 e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briant LJB, Dodd MS, Chibalina MV, et al. 2018. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. 23: 3300–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li NX, Brown S, Kowalski T, et al. 2018. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67: 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moulle VS, Ghislain J & Poitout V. 2017. Nutrient regulation of pancreatic beta-cell proliferation. Biochimie 143: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma RB & Alonso LC. 2014. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr. Diab. Rep 14:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milburn JL Jr.,Hirose H, Lee YH, et al. 1995. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J. Biol. Chem 270: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 75.Moulle VS,Vivot K, Tremblay C, et al. 2017. Glucose and fatty acids synergistically and reversibly promote beta cell proliferation in rats. Diabetologia 60: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vernier S, Chiu A, Schober J, et al. 2012. beta-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets 4: 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pascoe J, Hollern D, Stamateris R, et al. 2012. Free fatty acids block glucose-induced beta-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes 61: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maedler K, Oberholzer J, Bucher P, et al. 2003. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52: 726–733. [DOI] [PubMed] [Google Scholar]
- 79.Poitout V & Robertson RP. 2008. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev 29: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bensellam M, Laybutt DR & Jonas JC. 2012. The molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directions. Mol. Cell. Endocrinol 364: 1–27. [DOI] [PubMed] [Google Scholar]
- 81.Doliba NM, Liu Q, Li C, et al. 2017. Inhibition of cholinergic potentiation of insulin secretion from pancreatic islets by chronic elevation of glucose and fatty acids: protection by casein kinase 2 inhibitor. Mol. Metab 6: 1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller MR, Pereira RI, Langefeld CD, et al. 2012. Levels of free fatty acids (FFA) are associated with insulin resistance but do not explain the relationship between adiposity and insulin resistance in Hispanic Americans: the IRAS Family Study. J. Clin. Endocrinol. Metab 97: 3285–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morita S, Shimajiri Y, Sakagashira S, et al. 2012. Effect of exposure to non-esterified fatty acid on progressive deterioration of insulin secretion in patients with type 2 diabetes: a long-term follow-up study. Diabet Med. 29:980–985. [DOI] [PubMed] [Google Scholar]
- 84.Johnston LW, Harris SB, Retnakaran R, et al. 2018. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia 61: 821–830. [DOI] [PubMed] [Google Scholar]
- 85.Rebelos E, Seghieri M, Natali A, et al. 2015. Influence of endogenous NEFA on beta cell function in humans. Diabetologia 58: 2344–2351. [DOI] [PubMed] [Google Scholar]
- 86.Lee Y, Hirose H, Ohneda M, et al. 1994. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catanzaro R, Cuffari B, Italia A, et al. 2016. Exploring the metabolic syndrome: nonalcoholic fatty pancreas disease. World J. Gastroenterol 22: 7660–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia TS, Rech TH & Leitao CB. 2017. Pancreatic size and fat content in diabetes: a systematic review and meta-analysis of imaging studies. PLoS One 12: e0180911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Staaf J, Labmayr V, Paulmichl K, et al. 2017. Pancreatic fat is associated with metabolic syndrome and visceral fat but not beta-cell function or body mass index in pediatric obesity. Pancreas 46: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tushuizen ME, Bunck MC, Pouwels PJ, et al. 2007. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 30: 2916–2921. [DOI] [PubMed] [Google Scholar]
- 91.Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, et al. 2018. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab. 28: 547–556 e543. [DOI] [PubMed] [Google Scholar]
- 92.McCoin CS, Knotts TA & Adams SH. 2015. Acylcarnitines–old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol 11: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aichler M, Borgmann D, Krumsiek J, et al. 2017. N-acyl taurines and acylcarnitines cause an imbalance in insulin synthesis and secretion provoking beta cell dysfunction in type 2 diabetes. Cell Metab. 25: 1334–1347 e1334. [DOI] [PubMed] [Google Scholar]
- 94.Doliba NM, Liu Q, Li C, et al. 2015. Accumulation of 3-hydroxytetradecenoic acid: cause or corollary of glucolipotoxic impairment of pancreatic beta-cell bioenergetics? Mol. Metab 4: 926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batchuluun B, Al Rijjal D, Prentice KJ, et al. 2018. Elevated medium-chain acylcarnitines are associated with gestational diabetes mellitus and early progression to type 2 diabetes and induce pancreatic beta-cell dysfunction. Diabetes 67: 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imai Y, Dobrian AD, Morris MA, et al. 2013. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol. Metab 24: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kowluru A & Kowluru RA. 2018. RACking up ceramide-induced islet beta-cell dysfunction. Biochem. Pharmacol 154: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boslem E, Meikle PJ & Biden TJ. 2012. Roles of ceramide and sphingolipids in pancreatic beta-cell function and dysfunction. Islets 4: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veret J, Bellini L, Giussani P, et al. 2014. Roles of sphingolipid metabolism in pancreatic beta cell dysfunction induced by lipotoxicity. J. Clin. Med 3: 646–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lenzen S 2017. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic beta-cells. Biochim. Biophys. Acta. Gen. Subj 1861: 1929–1942. [DOI] [PubMed] [Google Scholar]
- 101.Sasson S 2017. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free Radic. Biol. Med 111: 102–109. [DOI] [PubMed] [Google Scholar]
- 102.Latunde-Dada GO 2017. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta. Gen. Subj 1861:1893–1900. [DOI] [PubMed] [Google Scholar]
- 103.Backe MB, Moen IW, Ellervik C, et al. 2016. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu. Rev. Nutr 36: 241–273. [DOI] [PubMed] [Google Scholar]
- 104.Biden TJ, Boslem E, Chu KY, et al. 2014. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab 25: 389–398. [DOI] [PubMed] [Google Scholar]
- 105.Gwiazda KS, Yang TL, Lin Y, et al. 2009. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am. J. Physiol. Endocrinol. Metab 296: E690–E701. [DOI] [PubMed] [Google Scholar]
- 106.Zarain-Herzberg A, Garcia-Rivas G & Estrada-Aviles R. 2014. Regulation of SERCA pumps expression in diabetes. Cell Calcium 56: 302–310. [DOI] [PubMed] [Google Scholar]
- 107.Tong X, Kono T, Anderson-Baucum EK, et al. 2016. SERCA2 deficiency impairs pancreatic beta-cell function in response to diet-induced obesity. Diabetes 65: 3039–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marmugi A, Parnis J, Chen X, et al. 2016. Sorcin links pancreatic beta-cell lipotoxicity to ER Ca2+ stores. Diabetes 65: 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kono T, Tong X, Taleb S, et al. 2018. Impaired store-operated calcium entry and STIM1 loss lead to reduced insulin secretion and increased endoplasmic reticulum stress in the diabetic beta cell. Diabetes 67: 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Granneman JG, Moore HP, Mottillo EP, et al. 2009. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem 284: 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boslem E, Weir JM, Macintosh G, et al. 2013. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic beta-cells. J. Biol. Chem 288:26569–26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brookheart RT, Michel CI & Schaffer JE. 2009. As a matter of fat. Cell Metab. 10: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neuman JC, Schaid MD, Brill AL, et al. 2017. Enriching islet phospholipids with eicosapentaenoic acid reduces prostaglandin E2 signaling and enhances diabetic beta-cell function. Diabetes 66: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masini M, Martino L, Marselli L, et al. 2017. Ultrastructural alterations of pancreatic beta cells in human diabetes mellitus. Diabetes Metab. Res. Rev 33 10.1002/dmrr.2894. [DOI] [PubMed] [Google Scholar]
- 115.Wikstrom JD, Israeli T, Bachar-Wikstrom E, et al. 2013. AMPK regulates ER morphology and function in stressed pancreatic beta-cells via phosphorylation of DRP1. Mol. Endocrinol 27:1706–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. 2012. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 15:518–533. [DOI] [PubMed] [Google Scholar]
- 117.Donath MY, Dalmas E, Sauter NS, et al. 2013. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 17: 860–872. [DOI] [PubMed] [Google Scholar]
- 118.Everett BM, Donath MY, Pradhan AD, et al. 2018. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol 71: 2392–2401. [DOI] [PubMed] [Google Scholar]
- 119.Dai C, Kayton NS,Shostak A, et al. 2016. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest 126: 1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Skelin Klemen M, Dolensek J, Slak Rupnik M, et al. 2017. The triggering pathway to insulin secretion: Functional similarities and differences between the human and the mouse beta cells and their translational relevance. Islets 9: 109–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arrojo e Drigo R, Ali Y, Diez J, et al. 2015. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia 58: 2218–2228. [DOI] [PubMed] [Google Scholar]
- 122.Welte MA & Gould AP. 2017. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids 1862: 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sztalryd C & Brasaemle DL. 2017. The perilipin family of lipid droplet proteins: gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids 1862: 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rambold AS, Cohen S & Lippincott-Schwartz J. 2015. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32: 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pol A, Gross SP & Parton RG. 2014. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J. Cell Biol 204: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Najt CP, Senthivinayagam S, Aljazi MB, et al. 2016. Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol 310: G726–G738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bailey AP, Koster G, Guillermier C, et al. 2015. Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell 163: 340–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu L, Zhang K, Sandoval H, et al. 2015. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benador IY,Veliova M, Mahdaviani K, et al. 2018. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27: 869–885 e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen TB, Louie SM, Daniele JR, et al. 2017. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell. 42: 9–21 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trevino MB, Machida Y, Hallinger DR, et al. 2015. Perilipin 5 regulates islet lipid metabolism and insulin secretion in a cAMP-dependent manner: implication of its role in the postprandial insulin secretion. Diabetes 64: 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faleck DM, Ali K, Roat R, et al. 2010. Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am. J. Physiol. Endocrinol. Metab 299: E249–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larsson S, Resjo S, Gomez MF, et al. 2012. Characterization of the lipid droplet proteome of a clonal insulin-producing beta-cell line (INS-1 832/13). J. Proteome Res 11: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 134.Masuda Y, Itabe H, Odaki M, et al. 2006. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J. Lipid Res 47: 87–98. [DOI] [PubMed] [Google Scholar]
- 135.Ueno M, Suzuki J, Hirose M, et al. 2017. Cardiac overexpression of perilipin 2 induces dynamic steatosis: prevention by hormone-sensitive lipase. Am. J. Physiol. Endocrinol. Metab 313: E699–E709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Libby AE, Bales ES, Monks J, et al. 2018. Perilipin-2 deletion promotes carbohydrate-mediated browning of white adipose tissue at ambient temperature. J. Lipid Res 59: 1482–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen E, Tsai TH, Li L, et al. 2017. PLIN2 is a key regulator of the unfolded protein response and endoplasmic reticulum stress resolution in pancreatic beta cells. Sci. Rep 7: 40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Plotz T, Hartmann M, Lenzen S, et al. 2016. The role of lipid droplet formation in the protection of unsaturated fatty acids against palmitic acid induced lipotoxicity to rat insulin-producing cells. Nutr. Metab. (Lond.) 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gallardo-Montejano VI, Saxena G, Kusminski CM, et al. 2016. Nuclear perilipin 5 integrates lipid droplet lipolysis with PGC-1alpha/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat. Commun 7:12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang H, Sreenevasan U, Hu H, et al. 2011. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res 52: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dobbins RL, Chester MW, Daniels MB, et al. 1998. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 47: 1613–1618. [DOI] [PubMed] [Google Scholar]
- 142.Borg J, Klint C, Wierup N, et al. 2009. Perilipin is present in islets of Langerhans and protects against lipotoxicity when overexpressed in the beta-cell line INS-1. Endocrinology 150:3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen W, Chang B, Wu X, et al. 2013. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab 304: E770–E779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han X, Zhu J, Zhang X, et al. 2018. Plin4-dependent lipid droplets hamper neuronal mitophagy in the MPTP/p-induced mouse model of Parkinson’s Disease. Front. Neurosci 12: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Imai Y, Trevino MB & Ahima RS. 2015. Lipid droplet proteins and hepatic lipid metabolism In Hepatic Lipogenesis and Regulation of Metabolism. Ntambi J, Ed.: 165–188. Switzerland: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.