Abstract
Background.
Cognitive impairment associated with lifetime major depressive disorder (MDD) is well-supported by meta-analytic studies, but population-based estimates remain scarce. Previous UK Biobank studies have only shown limited evidence of cognitive differences related to probable MDD. Using updated cognitive and clinical assessments in UK Biobank, this study investigated population-level differences in cognitive functioning associated with lifetime MDD.
Methods.
Associations between lifetime MDD and cognition (performance on six tasks and general cognitive functioning [g-factor]) were investigated in UK Biobank (N-range 7,457–14,836, age 45–81 years, 52% female), adjusting for demographics, education, and lifestyle. Lifetime MDD classifications were based on the Composite International Diagnostic Interview. Within the lifetime MDD group, we additionally investigated relationships between cognition and (a) recurrence, (b) current symptoms, (c) severity of psychosocial impairment (while symptomatic), and (d) concurrent psychotropic medication use.
Results.
Lifetime MDD was robustly associated with a lower g-factor (β = −0.10, P FDR = 4.7 × 10−5), with impairments in attention, processing speed, and executive functioning (β ≥ 0.06). Clinical characteristics revealed differential profiles of cognitive impairment among case individuals; those who reported severe psychosocial impairment and use of psychotropic medication performed worse on cognitive tests. Severe psychosocial impairment and reasoning showed the strongest association (β = −0.18, P FDR = 7.5 × 10−5).
Conclusions.
Findings describe small but robust associations between lifetime MDD and lower cognitive performance within a population-based sample. Overall effects were of modest effect size, suggesting limited clinical relevance. However, deficits within specific cognitive domains were more pronounced in relation to clinical characteristics, particularly severe psychosocial impairment.
Key words: Cognition, depression, psychosocial functioning, medication, UK Biobank
Introduction
Major depressive disorder (MDD) is a highly prevalent condition, affecting around one in five people over their lifetime globally [1–3]. Previous clinical research has shown that individuals with MDD show cognitive deficits, particularly in executive functioning, working memory, attention, and processing speed [4–7], as well as affect-related cognitive biases, including negative information biases for perception, attention, and memory [8,9]. Additionally, there is evidence that residual cognitive deficits are present in remitted cases [4,10–12]. A recent systematic review and meta-analysis that investigated studies including individuals remitted from MDD revealed significant small to moderate deficits in the same domains of cognitive functioning, and showed worse cognitive functioning associated with recurrent episodes [12].
Meta-analyses are invaluable for investigating patterns of results from smaller studies that are heterogenous in nature. One disadvantage, however, is that included studies show differential procedures of participant inclusion and recruitment, of which some will be more focused on certain clinical populations (e.g., allowing bipolarity or including participants after antidepressant treatment). As a result, meta-analytically derived effect sizes may overestimate cognitive impairment related to MDD compared with the general population. Understanding the degree of impairment associated with lifetime MDD (current or past diagnosis) in the general population thus requires further investigation within a sufficiently powered community-based sample. This summarizes the added value of population-based studies which are not biased by specific clinical characteristics and that are sufficiently powered to detect potentially modest effect sizes.
UK Biobank is a large-scale adult population-based study that is well-suited to investigate population-level cognitive differences related to lifetime MDD. Previous population-based studies that investigated baseline assessments of the UK Biobank cohort study indicated modestly decreased visuospatial memory performance in participants with lifetime MDD [13], but showed comparable cognitive performance to controls for other cognitive measures of reasoning, reaction time, and memory [13,14]. Robust evidence from population-based studies is currently limited, so that the degree and patterns of cognitive impairment in community-based individuals with lifetime MDD remain uncertain.
Of note, UK Biobank follow-up assessments were recently extended to include updated cognitive measures with improved reliability [15,16]. These tests also cover a broader range of cognitive domains previously implicated in MDD and show sufficient performance variability within the healthy population. Furthermore, previous mood disorder groupings relied on a combination of self-report and relatively unstructured questionnaire items, whereas the more recent lifetime MDD assessments in UK Biobank were based on a Structured Diagnostic Interview Questionnaire, that is the Composite International Diagnostic Interview (CIDI-SF) [17]. In the current study, we used the opportunity provided by these updated assessments to further investigate patterns of cognition functioning in lifetime MDD within the large population-based UK Biobank sample.
Furthermore, the novelty and clinical relevance of this study was increased by additionally conducting a population-based investigation of clinical characteristics associated with cognitive functioning. The first clinical variable of interest was recurrence of depressive episodes, given the usually highly recurrent nature of MDD and the ongoing discussions with regard to “scar theories” (which propose that disease-related psychological or biological changes may result in a predisposition to future depressive episodes) [18]. Although earlier research has not consistently reported recurrence of MDD being associated with lower cognitive functioning [10,19], recent evidence suggests that cognitive deficits do accumulate with repeated episodes [12]. Furthermore, although no optimal measure of MDD remission was available from UK Biobank assessments, we investigated associations with the putative presence of current depressive symptoms. There is a general consensus that low mood can negatively affect cognitive performance, and more pronounced cognitive impairment for symptomatic individuals is supported by previous meta-analytic studies [6,10,20]. However, another meta-analysis showed similar effect sizes for the current and remitted state [4], and associations with depressive symptoms may be attenuated within the general population [21]. Third, we addressed associations between psychosocial functioning during the depressive episode and subsequent cognitive deficits. Previous research suggests that cognitive deficits contribute to impaired psychosocial functioning [22–24], which impacts on quality of life. Furthermore, cognitive deficits were found to mediate of decreased work performance, contributing to the overall cost attributable to MDD [25,26]. The fourth clinical variable of interest was use of psychotropic medication concurrent with cognitive assessment. Pharmacological treatment is expected to have a complex influence on cognitive functioning, although reliable evidence is limited [27]. Meta-analyses suggest that some antidepressants may improve cognition [28,29], but these results appear to be specific to particular treatments [30–32], while for other treatments, medication side-effects may potentially affect cognitive performance negatively [6,14,33]. Given the variety of psychotropic medications, we did not aim to investigate their differential effects, but rather investigated the overall association between psychotropic medication and cognitive functioning within the general population.
In summary, the aim of the current study was to investigate cognitive functioning in the context of lifetime MDD within the general population, as conducted within the large-scaled population-based UK Biobank sample. Robustness of associations was tested via inclusion of different combinations of demographic, education, and lifestyle (smoking, alcohol consumption, and body mass index [BMI]) [34,35] covariates. We also examined clinical characteristics that are considered relevant to cognitive functioning in the context of lifetime MDD and may therefore influence the association: (a) recurrence of depressive episodes, (b) current MDD symptomatology, (c) severe psychosocial impairment (while symptomatic), and (d) use of psychotropic medication (at time of assessment). These characteristics were hypothesized to distinguish case subgroups with more severe cognitive impairment, or specifically for psychotropic medication, either increased or reduced impairment.
Methods
Participants
Adults (40–69 years) were recruited for participation in UK Biobank (http://www.ukbiobank.ac.uk) between 2006 and 2010 [36]. The present study included participants with data for at least one of the six neuropsychological test scores currently investigated from the third UK Biobank assessment (2014 onwards; N = 28,480). Participants with self-reported neurological conditions were excluded from all analyses. A g-factor, representing general cognitive function, was derived from all remaining participants with complete data for included cognitive test measures (N = 13,589). When assessing individual tasks, complete cognitive data was not required. Participants with specific psychiatric disorders or an unclassified/missing lifetime MDD status were excluded from further analyses (Supplementary Materials 1, Figure S1). Following exclusion criteria, N = 14,877 individuals (n cases = 4,486, M age = 63.5, SDage = 7.4, age range 45–81 years, 52% female) were included across all analyses, with sample sizes ranging from N = 7,457 to N = 14,386 per case–control analysis.
Measures and procedure
At the third assessment, participants visited the assessment center where they provided demographic and health information in response to a series of touchscreen questions and completed computerized cognitive tests on the touchscreen. Medical history and current medication use were assessed during interviews led by health professionals. Furthermore, participants completed a web-based Mental Health Questionnaire (MHQ) at home [37], which informed lifetime MDD classifications. Most participants (all those with complete cognitive data) completed the MHQ between 2.2 years before and 0.4 years after their assessment center visit. UK Biobank received Research Ethics Committee (REC) approval for the center assessments (ref 11/NW/0382), also later amended to cover the MHQ, and all participants provided informed consent before assessment (http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=200).
Classification of lifetime MDD
Case–control classification was based on the short form of the Composite International Diagnostic Interview (CIDI-SF) [17], administered as part of the MHQ [37]. This included two binary screening questions regarding ever having experienced (a) depressive feelings or (b) loss of interest, for a period of two or more weeks. Participants who responded “Yes” to either question then answered a question about the lifetime number of depressive episodes, followed by six binary questions on experience of other DSM-IV MDD symptoms during their worst episode, including: (c) feelings of worthlessness, (d) tiredness, (e) difficulty concentrating, (f) suicidal thoughts, (g) changes in sleeping pattern, and (h) changes in weight. Summed responses to all eight depression symptom questions provided a symptom score (range 0–8). Participants were also asked how often they had experienced depressive feelings/loss of interest during their worst episode, how long these feelings lasted, and whether they interfered with their “roles, life or activities” (psychosocial impairment). Participants were classified as lifetime MDD cases if all of the following applied [37]:
Summed symptom score ≥ 5.
At worst, symptoms experienced “almost every day” or “every day,” and lasted “most of the day” or “all day.”
Symptoms impaired psychosocial functioning “somewhat” or “a lot.”
Clinical characteristics
Within individuals who were classified with lifetime MDD as described above, we further identified four clinical characteristics: (a) recurrent MDD, reflecting more than one depressive episode, (b) putative current MDD symptoms concurrent with the cognitive assessment, derived from responses on the touchscreen questionnaire (Supplementary Materials 2), (c) severe psychosocial impairment (at the time of the depressive episode), indicated by the maximum CIDI-SF symptom score of eight in combination with the maximum score for psychosocial impairment [37]; and (d) concurrent use of psychotropic medication, which was derived from reported use of antidepressant (94.6%), antipsychotic (3.9%), or anxiolytic (1.5%) medication as assessed during the nurse-led interview (Supplementary Materials 3, Figure S2).
Cognitive assessment
In the current study, we considered cognitive tests from the third UK Biobank assessment. We included data from the following cognitive tests:
Digit symbol substitution test (DSST)
Trail making test, alphanumeric trail (TMT-B)
Numeric memory (NM)
Matrix pattern completion (Matrix)
Verbal numeric reasoning (VNR)
Tower rearranging (Tower)
This selection comprises all updated cognitive test data available at time of analysis (DSST, TMT-B, Matrix, and Tower), and additionally included two cognitive tests repeated at this assessment that were also administered at earlier UK Biobank assessments (VNR and NM). Further details on each cognitive test can be found in Table 1. Psychometric properties, advantages, and limitations of the UK Biobank cognitive tests have been discussed in detail elsewhere [16].
Table 1.
Descriptions of cognitive tests
| Test | Description | Cognitive domains | Variable | Range |
|---|---|---|---|---|
| DSST | Presented with a key grid containing digit-symbol pairs, the participant had one minute to indicate which number corresponded to each of the symbols in the task grid. | Processing speed and attention | Total correct score | 0–89 |
| TMT-B | Digits and letters were scattered around the screen in circles. The participant was asked to click on them sequentially, alternating between digits and letters (1-A-2-B-3-C, etc.) | Executive functions (mental flexibility, complex attention) and processing speed | Time to completion (s) reversed in analyses | 20–300 |
| NM | Every trial, a sequence of digits was briefly presented on screen, after which the participant was asked to recollect it from memory. If answered correctly, the sequence length increased with one digit. | Working memory and attention | Maximum digits remembered correctly | 2–12 |
| Matrix | Presented with a series of matrix pattern blocks that each missed one element, the participant was asked to select the element that best completed the pattern from a range of choices. | Reasoning ability (abstract) | Total correct score | 0–15 |
| VNR | The participant had 2 min to complete as many multiple-choice reasoning questions as possible. | Reasoning ability (verbal, numeric) and processing speed | Total correct score | 0–13 |
| Tower | Presented with a series of illustrations displaying three differently colored hoops on three pegs, the participant was asked how many moves it would take to re-arrange the hoops into another specific position. | Executive functions (strategy, planning) and working memory | Total correct score | 0–18 |
Abbreviations: DSST, digit symbol substitution task; Matrix, matrix pattern completion; NM, numeric memory; TMT-B, trail making test B (alphanumeric trail); Tower, tower rearranging; VNR, verbal numeric reasoning.
Education, lifestyle, and parental illness history
Measures of education, alcohol consumption, and smoking were derived from touchscreen questionnaire responses (Supplementary Materials 4). For education, a multiple-choice question allowed participants to report all qualifications. Participants were classified into one of five categories depending on the highest qualification they had attained. Alcohol consumption was measured as alcohol units per week [38]. Past smokers were categorized by pack years [39] quartiles, with participants who had never regularly smoked in a fifth category. BMI (in kg/m2) was derived by the UK Biobank team from physical measurements. Participants also reported for a number of listed illnesses whether their biological mother or father ever experienced the illness. Parental history of severe depression (from either parent) was derived from all available assessment responses.
Statistical analysis
Statistical analyses were performed in R (version 3.2.3). Continuous variables were visually inspected and log-transformed where necessary to more closely approximate normal distribution. We reversed the TMT-B variable before statistical modeling, so that for all tests, higher scores represented better performance. For all outcome variables, negative regression coefficients thus indicated negative associations with cognitive performance. Regression coefficients are standardized throughout. Significance level was determined by a two-tail threshold of α = 0.05.
Derivation of g-factor
We derived a g-factor as single measure of general cognitive functioning [40] by application of Principal Component Analysis (R function “prcomp”) including only complete observations (n = 13,589), extracting scores on the first principal component. This derived g-factor accounted for 45.0% of the test score variance. Component loadings of the model were all in the expected direction (loadings range 0.34–0.47; Supplementary Materials 5, Table S1, and Figure S3).
Statistical modeling
Associations between lifetime MDD and cognitive performance were tested using linear models. Each model included lifetime MDD as the predictor of interest and a cognitive score as the outcome variable. Primary models included age and sex as confounds (Tables 2 and 3). Additional models also included (a) education, (b) lifestyle factors (alcohol, smoking, and BMI), and (c) both education and lifestyle factors (full model) as potential confounds. These results were false discovery rate (FDR) corrected across the 24 test-specific models and separately across the four g-factor models. Additional explorative analyses assessed age by MDD interactions or effects of parental history of severe depression on cognitive functioning.
Table 2.
Demographic variables for participants who completed all cognitive tasks
| Control n = 5,221 | Case n = 2,236 | Effect size | |
|---|---|---|---|
| Age (years), mean (SD) a | 64.7 (7.3) | 62.5 (7.1) | 0.30 A *** |
| Sex female, n (%) b | 2,379 (45.6) | 1,503 (67.2) | 0.20 B *** |
| Education b | n.s. | ||
| Incomplete, n (%) | 263 (5.0) | 87 (3.9) | |
| Compulsory, n (%) | 600 (11.5) | 267 (11.9) | |
| Continued, n (%) | 304 (5.8) | 121 (5.4) | |
| College, n (%) | 1,327 (25.4) | 561 (25.1) | |
| University, n (%) | 2,710 (51.9) | 1,188 (53.1) | |
| Missing data, n (%) | 17 (0.3) | 12 (0.5) | |
| Alcohol units c | 0.007 C *** | ||
| Median (IQR) | 10.7 (15.6) | 9.0 (14.9) | |
| Missing data, n (%) | 317 (6.1) | 162 (7.2) | |
| Smoking | |||
| Lifetime regular smoker, n (%) b | 1,079 (20.7) | 624 (27.9) | 0.08 B *** |
| Pack years, median (IQR) c | 14.8 (17.5) | 16.5 (22.4) | 0.005 C ** |
| Missing data, n (%) | 86 (1.6) | 34 (1.5) | |
| BMI c | 0.006 C *** | ||
| Median (IQR) | 25.5 (5.0) | 26.2 (6.0) | |
| Missing data, n (%) | 204 (4.6) | 112 (5.0) | |
| Patental history severe depression b | 0.14 B *** | ||
| Yes, n (%) | 364 (7.0) | 357 (16.0) | |
| No, n (%) | 4,494 (86.1) | 1,715 (76.7) | |
| Missing data, n (%) | 363 (7.0) | 164 (7.3) |
Abbreviations: BMI, body mass index; IQR, interquartile range; n.s., nonsignificant; SD, standard deviation.
Independent t-test.
Chi-squared test.
Kruskal–Wallis test.
Hedge’s g.
Cramer’s V.
η 2.
p < 0.01.
p < 0.001.
Table 3.
Linear model coefficients for the association between lifetime mood disorder and cognitive performance
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Primary model | (+Education) | (+Lifestyle) | Full model | |
| g-factor | ||||
| β-coefficient | −0.10 *** | −0.10 *** | −0.08 *** | −0.10 *** |
| (95% CI) | (−0.15, −0.05) | (−0.15, −0.06) | (−0.14, −0.03) | (−0.15, −0.05) |
| DSST | ||||
| β-coefficient | −0.13 *** | −0.13 *** | −0.12 *** | −0.13 *** |
| (95% CI) | (−0.18, −0.09) | (−0.18, −0.09) | (−0.17, −0.08) | (−0.18, −0.08) |
| TMT-B | ||||
| β-coefficient | −0.08 *** | −0.08 *** | −0.08 ** | −0.09 *** |
| (95% CI) | (−0.13, −0.04) | (−0.13, −0.04) | (−0.13, −0.03) | (−0.14, −0.04) |
| NM | ||||
| β-coefficient | −0.03 | −0.03 | −0.02 | −0.03 |
| (95% CI) | (−0.08, 0.02) | (−0.08, 0.01) | (−0.07, 0.03) | (−0.08, 0.02) |
| Matrix | ||||
| β-coefficient | −0.03 | −0.03 | 0.00 | −0.01 |
| (95% CI) | (−0.07, 0.02) | (−0.08, 0.02) | (−0.05, 0.06) | (−0.06, 0.04) |
| VNR | ||||
| β-coefficient | −0.06 *** | −0.06 *** | −0.05 * | −0.07 *** |
| (95% CI) | (−0.10, −0.03) | (−0.10, −0.03) | (−0.09, −0.01) | (−0.10, −0.03) |
| Tower | ||||
| β-coefficient | −0.06 * | −0.06 * | −0.05* | −0.06 * |
| (95% CI) | (−0.11, −0.01) | (−0.11, −0.01) | (−0.11, 0.00) | (−0.11, −0.01) |
Bold values represent PFDR < 0.05.
Abbreviations: CI, confidence interval; DSST, digit symbol substitution task; g-factor, derived measure of general cognitive performance; Matrix, matrix pattern completion; NM, numeric memory; TMT-B, trail making test (alphanumeric trail); Tower, tower rearranging; VNR, verbal numeric reasoning.
p < 0.05.
p < 0.01.
p < 0.001.
With the aim of investigating whether cognitive performance related to specific clinical features of depression, we also examined relationships, within the case group only, with recurrent depression, putative current depressive symptoms, impact on psychosocial functioning, and psychotropic medication use. First, we tested a linear model including participants classified with lifetime MDD only (n = 2,179; Figure 2A) that predicted g-factor from all four clinical characteristics, including age and sex as covariates. Given the large degree of overlap between clinical characteristics within our sample, these variables were entered as simultaneous predictors in order to model their unique contributions to cognitive functioning. Second, we further explored whether the four clinical characteristics were associated with differential profiles of cognitive performance. Using the same approach, we tested another six univariate linear models (applying FDR-correction over six tests) that predicted each cognitive test scores from all four clinical characteristics, including age and sex as covariates.
Figure 2.
(A) Venn diagram of all participants classified with lifetime MDD and each of the four the clinical characteristics recurrent depression, putative current symptoms, severe psychosocial impairment, and psychotropic medication. These clinical characteristics were investigated within the group of case participants. (B–E) Cognitive profiles associated with the clinical characteristics (B) recurrent depression, (C) putative current symptoms, (D) severe psychosocial impairment (while symptomatic), and (E) use of psychotropic medication (at time of assessment). Points represent points estimates of the β-coefficient within the case models, whereas lines reflect the 95% confidence interval of the β-coefficient. *p < 0.05, ***p < 0.001. Abbreviations: DSST, digit symbol substitution task; Matrix, matrix pattern completion; NM, numeric memory; TMT-B, trail making test (alphanumeric trail); Tower, tower rearranging; VNR, verbal numeric reasoning.
Results
Sample characteristics
Table 2 summarizes demographic and lifestyle information on participants who completed all cognitive tests. The MDD group included a higher proportion of females (45.6% of controls, 67.2% of cases, χ 2(1) = 293.2, p < 2.2 × 10−16) and individuals with parental history of severe depression (7.0% of controls, 16.0% of cases, χ 2(1) = 146.7, p < 2.2 × 10−16). MDD cases were also younger (M difference = 2.2 years, t (4357.9) = 12.1, p < 2.2 × 10−16), had higher BMI (Mdn difference = 0.7 kg/m2, H (1) = 40.1, p = 2.4 × 10−10), were more often lifetime regular smokers (20.7% of controls, 27.9% of cases, χ 2(1) = 46.0, p = 1.2 × 10−11), and among smokers, they had smoked more cigarettes (Mdn difference = 1.8 pack years, H (1) = 7.8, p = 5.3 × 10−3). Conversely, control group participants reported more alcohol consumption (Mdn difference = 1.7 units/week, H (1) = 46.6, p = 8.7 × 10−12). Education did not significantly differ between groups (χ 2(4) = 5.6, p = 0.23). Case and control sample sizes for individual cognitive tests, as well as test descriptive statistics, can be found in Table S2 (Supplementary Materials 6).
Associations between lifetime MDD and cognitive performance
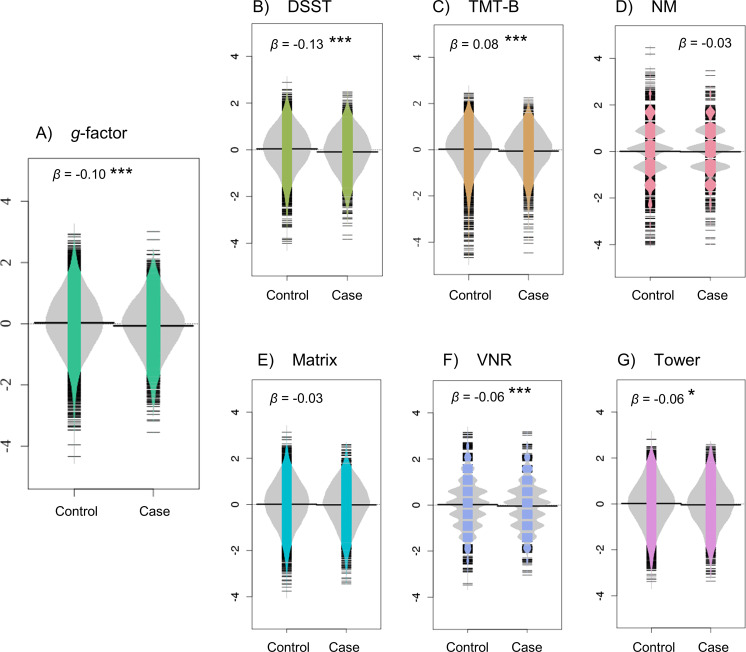
Results indicated significant associations between lifetime MDD and cognitive performance (Table 3). The primary models consistently showed lower cognitive performance for the case group relative to controls with small effect sizes in terms of the g-factor (β = −0.10, P FDR = 4.7 × 10−5) and four of the six cognitive tests (DSST, β = −0.13, P FDR = 1.1 × 10−7; TMT-B, β = −0.08, P FDR = 1.1 × 10−3; VNR, β = −0.06, P FDR = 2.0 × 10−3; Tower, β = −0.06, P FDR = 3.4 × 10−2) (Figure 1). These results were robust over subsequent models that additionally included confounding variables, and all significant results (except for the Tower lifestyle model) survived multiple testing correction. Associations between cognitive performance and covariates are reported in Table S3 (Supplementary Materials 7). Explorative analyses did not suggest any age by MDD interaction effects (Supplementary Materials 8, Table S4) or effects of parental history of severe depression (Supplementary Materials 9, Table S5) on cognitive functioning.
Figure 1.
Visualization of the primary model results for (A) g-factor, (B) DSST, (C) TMT-B, (D) NM, (E) Matrix, (F) VNR, and (G) Tower. Graphs display case-control group differences in cognitive performance after adjustment for confounders (i.e., age and sex were regressed out). Specifically, they show significant but modest associations of lifetime MDD classification with lower general cognitive performance (g-factor), and with lower performance on DSST, TMT-B, VNR, and Tower. *p < 0.05, **p < 0.01, ***p < 0.001. Abbreviations: DSST, digit symbol substitution task; g-factor, derived measure of general cognitive performance; Matrix, matrix pattern completion; NM, numeric memory; TMT-B, trail making test (alphanumeric trail); Tower, tower rearranging; VNR, verbal numeric reasoning.
Associations between clinical characteristics and cognitive performance
For the associations between clinical characteristics and cognitive impairment, as measured by g-factor, we found no effect of recurrent depression (β = 0.02, p = 0.56), and a very modest, nonsignificant point estimate for putative current symptoms at time of assessment (β = −0.07, p = 0.15). Conversely, retrospectively reported severe psychosocial impairment during the depressive episode was significantly associated with lower g-factor (β = −0.14, p = 1.5 × 10−2). Use of psychotropic medication showed a small, nonsignificant point estimate for its association with general cognitive functioning (β = −0.10, p = 0.10).
Further exploration within the lifetime MDD case group revealed differential cognitive profiles related to clinical characteristics (Figure 2B–E and Supplementary Materials 10, Table S6). Severe psychosocial impairment was associated with worse VNR performance (β = −0.18, p = 1.2 × 10−5, P FDR = 7.5 × 10−5). Furthermore, results indicated nominally significant associations with lower TMT-B performance for severe psychosocial impairment (β = −0.11, p = 2.3 × 10−2, P FDR = 0.07) and psychotropic medication (β = −0.13, p = 2.7 × 10−2, P FDR = 0.16).
Discussion
The findings of the current study describe a robust association between lifetime MDD and lower general cognitive performance within a population-based sample. The DSST, TMT-B, VNR, and Tower results further indicate that executive functioning, processing speed, and aspects of reasoning were predominantly affected. These effects were of modest overall effect size (β = −0.10 for general cognitive performance), suggesting that they are of limited clinical relevance, although such differences may have substantial consequences for whole populations. Comparisons within the case group, however, showed that psychosocial functioning (while symptomatic) and use of psychotropic medication (at time of assessment) predicted the lowest measures of cognitive performance. These clinical characteristics also showed differential profiles of cognitive impairment, whereby severe psychosocial impairment was associated with reasoning (VNR) deficits. Severe psychosocial impairment, along with use of psychotropic medication, was also related to moderately lower mental flexibility and processing speed (TMT-B). These results highlight (i) that cognitive functioning is impaired among individuals with current, but also past MDD, and (ii) that deficits within specific cognitive domains may be more pronounced—and therefore of potential clinical relevance—in relation to retrospectively reported psychosocial impairment (during the depressive episode) and concurrent use of psychotropic medication.
Meta-analyses of small case–control studies have previously indicated cognitive deficits associated with current and remitted MDD within the domains of processing speed, executive functioning, memory, and attention [4,12]. This study corroborates such previous findings, but we also note that these population-level effect sizes are smaller than in traditional case–control studies. Of note, psychosocial impairment while symptomatic was also significantly associated with greater cognitive deficits in individuals with lifetime MDD, and we found some evidence for an association between psychotropic medication use and lower processing speed. This implicates that clinical studies that recruit from treatment centers, and thereby include participants from a patient group more likely to experience severe psychosocial impairment and/or use psychotropic medication, may show inflated effect sizes in comparison with the general population because of their sample characteristics.
Psychosocial impairment during the depressive episode was found to be associated with lower cognitive functioning at subsequent assessment. Because other clinical characteristics such as the putative presence of current depressive symptoms were taken into account, this suggests that the impact of a depressive episode on quality of life may not be limited to the symptomatic phase. This result is consistent with previous adult clinical studies [23] and may reflect a subgroup of remitted individuals vulnerable to cognitive impairment, who potentially also experienced greater cognitive deficits during the depressive episode. Previous clinical research also showed that cognitive impairment in remitted MDD was associated with psychosocial dysfunction in multiple domains [24]. Future research will need to address the underlying causality of the relationship between severe psychosocial impairment and impaired cognitive functioning in the context of lifetime MDD. If functional impairment persists specifically in remitted individuals who experience residual cognitive deficits, quality of life could be increased with interventions that target cognitive symptoms [24,41–43].
Findings of the current study did not indicate robust associations of cognitive impairment with recurrent depressive episodes or putative current MDD symptoms, and less robust associations with psychotropic medication. Thus, within the healthy and nonclinical UK Biobank population, these MDD characteristics were less relevant to the association between lifetime MDD and cognitive impairment.
Of note, however, the current investigation was limited by the phenotypes available in UK Biobank. Classification of lifetime MDD was based on the CIDI-SF, which, although based on diagnostic criteria and well validated, is still limited by reliance on retrospective self-report. As a consequence of the interval between cognitive and clinical assessment, no certain measure of remission or continuous measure of depressive symptoms at time of cognitive assessment could be established. Constrained by these limitations, the characteristic of putative current symptoms was locally derived from responses to the touchscreen questionnaire to explore the effect of depressive symptoms, but one should note that this variable was not previously validated. Therefore, it remains unclear whether differences in cognitive functioning within the UK Biobank sample depend on the state of current depression, or rather reflect a trait that also persists after remission. Given the aim of the current study to investigate (residual) cognitive impairment associated with lifetime MDD in the general adult population, the mid-late life age range of UK Biobank participants at the time of the third assessment could also be considered a limitation. However, the cohort has been previously described as a relatively healthy mid-late life cohort [37], from which we excluded individuals with reported neurological or degenerative conditions from the current study.
The current study benefited from the availability of newer, more reliable cognitive assessments in UK Biobank, but as such also imposed the limitation of a cross-sectional design. Although explorative analyses did not suggest any age-interaction effects indicative of accelerated cognitive ageing, nor any parental history effects suggesting cognitive vulnerability, it remains unclear when the MDD-related cognitive differences emerged. Future large-scale longitudinal research will be needed to unravel the relationship between MDD and cognitive impairment over the disease course as well as the lifespan.
In summary, the present findings suggest that lifetime MDD relates to impaired cognitive functioning among adults in their mid-late life, with most prominent deficits in the cognitive domain of processing speed (DSST). Severe psychosocial impairment during the depressive episode was associated with greater overall cognitive impairment, and specifically on tasks of reasoning (VNR) and mental flexibility and processing speed (TMT-B). Furthermore, clinical characteristics showed differential profiles of impairment that were of modest effect. These findings add to meta-analytic evidence by providing accurate population-level estimates, which is an important foundation for future studies addressing cognitive functioning in the context of MDD. Longitudinal trajectories of cognitive performance in lifetime MDD and the differential influences of pharmacological treatments on these trajectories are important targets for further research. The longitudinal association between psychosocial impairment while symptomatic and subsequent cognitive impairment, also when remitted, reflects their likely impact on quality of life and suggests that both cognitive and psychosocial functioning should be key targets in the treatment of MDD.
Acknowledgments.
This research has been conducted using the UK Biobank resource. We would like to thank all of the participants from UK Biobank who have shown their continued commitment, as well as the UK Biobank team for collecting and providing the data.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2020.19.
click here to view supplementary material
The R scripts of our data preparation procedures and statistical analyses are available for download via https://osf.io/f76du/.
Financial Support
This work was supported by Wellcome Trust (grant number 104036/Z/14/Z) and the Medical Research Council (grant numbers MC_PC_17209, MR/M013111/1, MR/R024065/1). The funders had no role in the design or analysis of this study, decision to publish, or preparation of the manuscript. UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has also had funding from the Welsh Government, British Heart Foundation, Cancer Research UK and Diabetes UK.
Conflict of Interest
AMM has received research support from Eli Lilly, Janssen, and The Sackler Trust. AMM has also received speaker fees from Illumina and Janssen.
Data Availability Statement
There are restrictions prohibiting the provision of data in this manuscript. The data were obtained from a third party, UK Biobank, upon application. Interested parties can apply for data from UK Biobank directly, at http://www.ukbiobank.ac.uk. UK Biobank will consider data applications from bona fide researchers for health-related research that is in the public interest. By accessing data from UK Biobank, readers will be obtaining it in the same manner as we did.
References
- 1.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;24:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, Girolamo G, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(90):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;15(2):16065. [DOI] [PubMed] [Google Scholar]
- 4.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44:2029–2040. [DOI] [PubMed] [Google Scholar]
- 5.Wagner S, Müller C, Helmreich I, Huss M, Tadić A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry. 2015;24:5–19. [DOI] [PubMed] [Google Scholar]
- 6.Lee RSC, Hermens DF, Porter MA, Redoblado-hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140:113–124. [DOI] [PubMed] [Google Scholar]
- 7.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18:139–149. [DOI] [PubMed] [Google Scholar]
- 9.Lemoult J, Gotlib IH. Depression: a cognitive perspective. Clin Psychol Rev. 2019;69:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 2011;134:20–31. [DOI] [PubMed] [Google Scholar]
- 11.Bora E, Harrison BJ, Yu M. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43:2017–2026. [DOI] [PubMed] [Google Scholar]
- 12.Semkovska M, Quinlivan L, Grady TO, Johnson R, Collins A, Connor JO, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:851–861. [DOI] [PubMed] [Google Scholar]
- 13.Cullen B, Smith DJ, Deary IJ, Pell JP, Keyes KM, Evans JJ. Understanding cognitive impairment in mood disorders: mediation analyses in the UK Biobank cohort. Br J Psychiatry. 2019;215(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B, Nicholl BI, Mackay DF, Martin D, Ul-haq Z, McIntosh A, et al. Cognitive function and lifetime features of depression and bipolar disorder in a large population sample: cross-sectional study of 143,828 UK Biobank participants. Eur Psychiatry. 2015;30:950–958. [DOI] [PubMed] [Google Scholar]
- 15.Lyall DM, Cullen B, Allerhand M, Smith DJ, Mackay D, Evans J, et al. Cognitive test scores in UK Biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One. 2016;11(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. medRxiv [preprint]. 2019;1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler RC, Andrews G, Mroczek D, Ustün TB, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF). Int J Methods Psychiatr Res. 1998;7(4):171–185. [Google Scholar]
- 18.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Am Psychol Assoc. 2010;24(1):9–34. [DOI] [PubMed] [Google Scholar]
- 20.Ahern E, Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology. 2017;31(1):52–72. [DOI] [PubMed] [Google Scholar]
- 21.Schweizer S, Kievit RA, Emery T, Cam-CAN, Henson RN. Symptoms of depression in a large healthy population cohort are related to subjective memory complaints and memory performance in negative contexts. Psychol Med. 2018;48:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans VC, Iverson GL, Yatham LN, Lam RW. The relationship between neurocognitive and psychosocial functioning in major depressive disorder: a systematic review. J Clin Psychiatry. 2014;75(12):1359–1370. [DOI] [PubMed] [Google Scholar]
- 23.Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157–171. [DOI] [PubMed] [Google Scholar]
- 24.Knight MJ, Air T, Baune BT. The role of cognitive impairment in psychosocial functioning in remitted depression. J Affect Disord. 2018;235:129–134. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates and treatment interventions. Depress Anxiety. 2013;30:515–527. [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RMA, et al. The prevalence and effects of mood disorders on work performance in a nationally representative sample of US workers. Am J Psychiatry. 2006;163(9):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarty T, Lam RW. Cognitive dysfunction in major depressive disorder: assessment, impact and management. Neuropsychology. 2016;31(1):52–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keefe RSE, McClintock SM, Roth RM, Doraiswamy PM, Tiger S, Madhoo M. Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Affect Disord. 2014;75(8):864–876. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre RS, FLorea I, Tonnoir B, Loft H, Lam RW, Cronquist Christensen M. Efficacy of vortioxetine on cognitive functioning in patient with major depressive disorder. J Clin Psychiatry. 2017;78(1):115–121. [DOI] [PubMed] [Google Scholar]
- 31.Baune BT, Brignone M, Larsen KG. A network meta-Analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. 2018;21(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nierenberg AA, Loft H, Olsen CK. Treatment effects on residual cognitive symptoms among partially or fully remitted patients with major depressive disorder: a randomized, double-blinded, exploratory study with vortioxetine. J Affect Disord. 2019;250:35–42. [DOI] [PubMed] [Google Scholar]
- 33.Nagane A, Baba H, Nakano Y, Maeshima H, Hukatsu M, Ozawa K, et al. Comparative study of cognitive impairment between medicated and medication-free patients with remitted major depression: class-specific influence by tricyclic antidepressants and newer antidepressants. Psychiatry Res. 2014;218:101–105. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Back JH, Kim J, Kim S, Na DL, Cheong H-K, et al. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. 2010;22(2):174–187. [DOI] [PubMed] [Google Scholar]
- 35.Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11:651–657. [DOI] [PubMed] [Google Scholar]
- 36.Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: current status and what it means for epidemiology. Heal Policy Technol. 2012;1:123–126. [Google Scholar]
- 37.Davis KAS, JR C, Adams M, Allen N, Breen G, Cullen B, et al. Mental health in UK Biobank revised—development, implementation and results from an online questionnaire completed by 157,366 participants. BJPsych Open. 2019;4(3):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Clarke T-K, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol Psychiatry. 2017;22:1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Artigas MS, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. 2015;3:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson W, Te Nijenhuis J, Bouchard TJ. Still just 1 g: consistent results from five test batteries. Intelligence. 2008;36:81–95. [Google Scholar]
- 41.Kaser M, Zaman R, Sahakian BJ. Cognition as a treatment target in depression. Psychol Med. 2017;47:987–989. [DOI] [PubMed] [Google Scholar]
- 42.Solé B, Jiménez E, Martinez-Aran A, Vieta E. Cognition as a target in major depression: new developments. Eur Neuropsychopharmacol. 2015;25:231–247. [DOI] [PubMed] [Google Scholar]
- 43.Bortolato B, Miskowiak KW, Köhler CA, Maes M, Fernandes BS, Berk M, et al. Cognitive remission: a novel objective for the treatment of major depression? BMC Med. 2016;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2020.19.
click here to view supplementary material
Data Availability Statement
There are restrictions prohibiting the provision of data in this manuscript. The data were obtained from a third party, UK Biobank, upon application. Interested parties can apply for data from UK Biobank directly, at http://www.ukbiobank.ac.uk. UK Biobank will consider data applications from bona fide researchers for health-related research that is in the public interest. By accessing data from UK Biobank, readers will be obtaining it in the same manner as we did.